Abstract
Objective
The aims of this study were to investigate whether fertilization could induce the resumption of meiosis in mouse oocytes arrested at metaphase I (MI) after in vitro maturation (IVM), and to investigate the effect of Ca2+ chelator treatment at the time of fertilization on the transition from MI to metaphase II (MII).
Methods
MII-stage and arrested MI-stage mouse oocytes after IVM were fertilized, and then embryonic development was monitored. Blastocysts from each group were transferred into 2.5 days post-coitum pseudo-pregnant ICR mice. MI oocytes after IVM were treated with a Ca2+ chelator to investigate the effect of Ca2+ oscillations on their maturation.
Results
As insemination time increased, the number of oocytes in the MI group that reached the MII stage also increased. The blastocyst rates and total cell numbers in the MII group were significantly higher than in the MI group. No pregnancy occurred in the MI group, but 10 pregnancies were achieved (10 of 12) in the MII group. The proportion of MI oocytes that matured to MII oocytes after fertilization was significantly higher in the non-treated group than in the Ca2+ chelator-treated group.
Conclusion
The findings that a higher proportion of MI-arrested oocytes progressed to MII after fertilization and that the MI-to-MII transition was blocked by Ca2+ chelator treatments before fertilization indicate that the maturation of MI oocytes to MII oocytes is associated with intracellular Ca2+ oscillations driven by fertilization.
Keywords: Arrested metaphase I oocyte, Calcium oscillations, Fertilization, In vitro oocyte maturation
Introduction
In vitro maturation (IVM) of human oocytes is a feasible alternative to controlled ovarian hyperstimulation cycles, but the pregnancy rates of IVM cycles are still low because of the low number of mature oocytes [1]. In addition, 15% to 20% of the oocytes collected in controlled ovarian hyperstimulation cycles are immature, and some of these oocytes are arrested at the germinal vesicle (GV) and metaphase I (MI) stages, even after prolonged culture [2]. In the normal IVM and in vitro fertilization cycles, oocytes are exposed to spermatozoa after reaching metaphase II (MII), and immature oocytes (GV and MI) are discarded. The potential of using the immature oocytes in these cycles is important because doing so would allow more embryos to be obtained in each cycle, which may lead to an increased number of embryos being available for transfer or cryopreservation and, therefore, to an increased probability of achieving pregnancy.
Oocyte maturation is divided into nuclear maturation and cytoplasmic modification [3]. Nuclear maturation starts with GV breakdown (GVBD), and progresses to MI and then to MII. Cytoplasmic maturation is a more complex process, and includes the accumulation of essential maternal factors, changes in the expression profile of cell cycle control proteins, modification of plasma membrane permeability, and differentiation of the Ca2+ signaling machinery [3,4]. In most mammals, oocytes are arrested in MII after nuclear and cytoplasmic maturation and are then exposed to spermatozoa. However, it has been reported that the maturation of immature oocytes is enhanced by co-incubation with spermatozoa, even before fertilization [5]. Niwa et al. [6] found that the maturation of bovine oocytes was not required for sperm penetration and Chian et al. [7] reported that bovine immature oocytes could promote oocyte maturation as a result of sperm penetration. Moreover, Van Blerkom et al. [8] reported that human oocytes fertilized at the GV stage resumed meiosis until the pronucleus (PN) stage and appeared to develop to the 2-cell stage. These reports suggest that sperm can penetrate immature oocytes, and then assist in the completion of the final step of maturation. However, no study has confirmed whether the final maturation of arrested oocytes at the time of insemination occurs spontaneously without sperm penetration or is initiated by sperm penetration into the cytoplasm. Moreover, Ca2+ oscillations derived by spermatozoa may influence the maturation process in which MI arrest is overcome. Therefore, this study was designed to examine whether MI oocytes were completely arrested after IVM, whether sperm insemination was capable of completing meiotic maturation in MI-arrested oocytes, and the developmental potential of oocytes fertilized at the MII or MI stage after IVM.
Methods
1. Retrieval of immature oocytes and IVM
All animal procedures were performed in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The animal studies were also approved by the Animal Research Ethics Committee of the Maria Research Center. The B6D2-F1 female mice were given an intraperitoneal treatment of 10 IU of pregnant mare serum gonadotropin (Intervet, Boxmeer, the Netherlands) and were euthanized by cervical dislocation 48 hours later. Their ovaries were excised and immediately placed in pre-warmed MRC#D01 medium (Maria Medical Foundation, Seoul, Korea) buffered with 3-(N-Morpholino) propanesulfonic acid (MOPS) [9]. Cumulus-enclosed oocytes at the GV stage were collected by puncturing the large antral follicles and then cultured for 18 hours in maturation medium in an incubator with 6% CO2, 5% O2, and 89% N2 at 37℃. The medium used for IVM was MRC#D46 (Maria Medical Foundation) with 20% serum substitute supplement (Irvine Scientific, Santa Ana, CA, USA), supplemented with 10 ng/mL of human recombinant epidermal growth factor (Invitrogen, Carlsbad, CA, USA), 0.7 IU/mL of recombinant follicle stimulating hormone (Fostimon; Institut Biochimique SA, Lugano, Switzerland), and 0.1 IU/mL of recombinant luteinizing hormone (Luveris; Merck Serono, Darmstadt, Germany).
2. Maturity assessment and in vitro fertilization
After IVM, adherent cumulus cells were removed from oocytes mechanically by gentle micropipetting using a fine-drawn glass pipette. The in vitro matured oocytes were divided into two groups according to their maturity: oocytes that displayed a distinct first polar body (PB) were classified as the MII group, while those without a PB and a visible GV nucleus were classified as the MI-arrested group. All oocytes from the MII group and some from the MI group were inseminated using sperm collected from the cauda epididymis of B6D2-F1 males in MRC#D01 medium. The remaining MI oocytes were cultured for a further 4 hours to investigate the spontaneous maturation rate in insemination medium. Throughout the fertilization period, the maturity of MI oocytes was re-evaluated by monitoring the appearance of the first PB every hour for up to 4 hours. After insemination, oocytes were washed and placed in insemination medium. The fertilization rate was assessed by the presence of the 2-cell stage at 20 hours following insemination. The cleaved embryos were cultured sequentially in cleavage and blastocyst medium (MRC#D13 and 46, Maria Medical Foundation) for 96 hours.
3. Embryo transfer
CD-1 females were used as embryo recipients. Females were mated with vasectomized males to induce pseudo-pregnancy, and successful mating was confirmed by a copulation plug check on the following morning. The day on which a vaginal plug was found was defined as day 1 of pregnancy. Pseudo-pregnant mice at 2.5 days postcoitum were randomly assigned to the MII and MI groups. Prior to embryo transfer (ET), recipients were anesthetized by being placed in a chamber filled with 2% to 3% isoflurane for 2 to 3 minutes (Hana Pharm, Seoul, Korea) in oxygen. ET using a non-surgical embryo transfer device (NSET) (ParaTechs Co., Lexington, KY, USA) was carried out according to the manufacturers' instructions. Briefly, small and large specula were placed sequentially into the vagina to open and expose the cervix. The NSET device tip, loaded with eight blastocysts, was inserted through the cervix and the blastocysts were transferred into the uterus. The recipients were allowed to deliver at term to evaluate the pregnancy and live pup birth rates.
4. Differential staining
Differential staining of the inner cell mass and trophectoderm cells was carried out as described previously [10]. Briefly, zona-intact or partially hatching blastocysts were incubated in 1 mL of MOPS-buffered mouse tubal fluid (MTF) medium with 1% Triton X-100 and 100 µg/mL of propidium iodide (Sigma, St. Louis, MO, USA) for up to 10 seconds. Then, the blastocysts were immediately transferred to 1 mL of fixative solution (100% ethanol with 25 µg/mL bisbenzimide [Hoechst 33258, Sigma]) and stored at 4℃ overnight. The blastocysts were then mounted onto a glass microscope slide in a drop of glycerol, gently flattened with a coverslip, and visualized for cell counting. Cell counts were determined from digital photographs of images obtained using an inverted microscope (Nikon, Otawara, Japan) with an ultraviolet lamp.
5. Ca2+ chelator treatment
Arrested MI oocytes after IVM were treated with 1 or 10 µg of a Ca2+ chelator (1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetra-acetic acid acetoxymethyl ester [BAPTA-AM; Molecular Probes, Eugene, OR, USA]) to investigate the effects of Ca2+ oscillations on the MI-to-MII transition. The oocytes were incubated in Ca2+-free MTF medium containing 5 mg/mL of human serum albumin and BAPTA-AM for 30 minutes in the presence of Pluronic F-127 (Molecular Probes) to aid in loading. Control oocytes were incubated without BAPTA-AM in medium containing an equivalent volume of dimethyl sulfoxide and Pluronic F-127. After BAPTA-AM treatment, those oocytes were inseminated as described above and then their maturity was evaluated by monitoring the appearance of the first PB.
6. Statistical analyses
Statistical analyses were conducted using the t-test to compare the fertilization rate, embryo development, total cell number, and pregnancy rate of the two groups. The effect of Ca2+ chelator treatment was evaluated by analysis of variance. Data were analyzed using SPSS ver. 17.0. (SPSS Inc., Chicago, IL, USA), and p-values <0.05 were considered to indicate statistical significance.
Results
1. Effects of sperm insemination on meiotic maturation and embryo development
Schultz et al. [11] reported that the fertilization of GV oocytes was frequently associated with polyspermy. Therefore, a preliminary study was conducted to compare the proportion of abnormal fertilization between MI-arrested oocytes and MII oocytes. No significant difference in the rate of normal fertilization was found between the two groups (Table 1). To obtain MII and MI-arrested oocytes, 1,145 GV oocytes were cultured in maturation medium for 18 hours. The proportions of MII, MI, and GV oocytes were 42.4%, 56.7%, and 0.9%, respectively (Table 2). Additionally, the formation rate of 2PN embryos was similar to the 2-cell incidence rate presented in Table 3. Therefore, the formation rate of 2-cell embryos was regarded as the fertilization rate, and a separate investigation of 2PN formation was not performed in this experiment.
Table 1. Comparison of the proportions of abnormal fertilization between MI-arrested oocytes and MII oocytes.
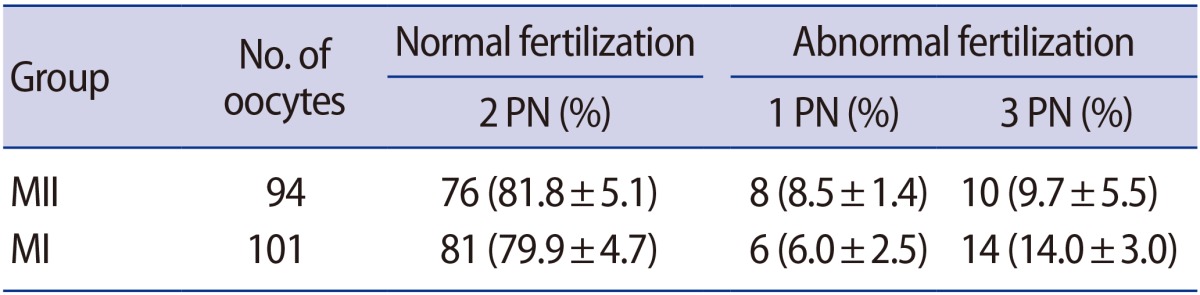
Values are presented as number (mean±standard deviation).
MI, metaphase I; MII, metaphase II; PN, pronucleus.
Table 2. Maturation rates of GV oocytes after 18 hours of culture.
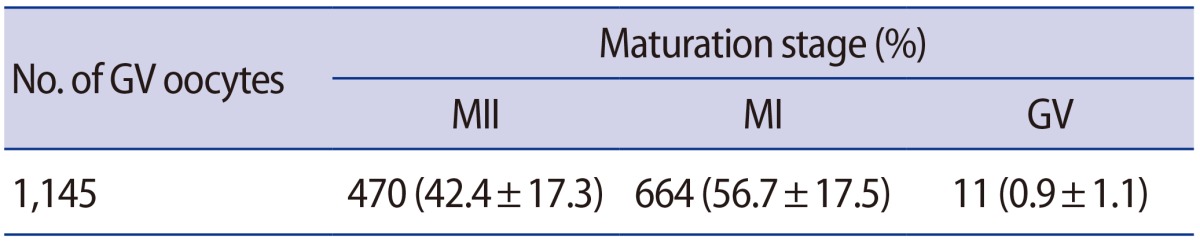
Values are presented as number (mean±standard deviation).
GV, germinal vesicle; MII, metaphase II; MI, metaphase I.
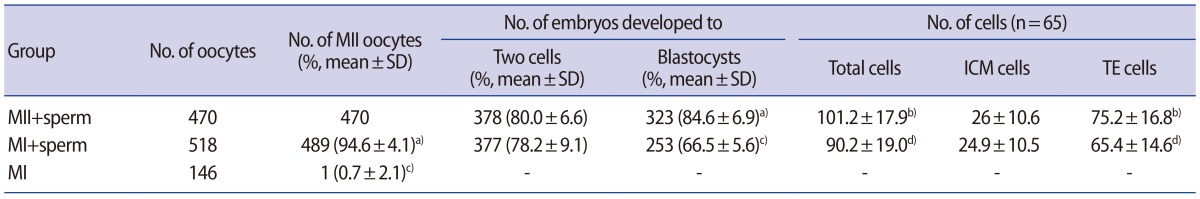
Table 3. Effects of sperm insemination of MI-arrested oocytes on embryonic development and cell numbers.
Values are presented as mean±standard error of the mean unless otherwise indicated.
MI, metaphase I; MII, metaphase II; SD, standard deviation; ICM, inner cell mass; TE, trophectoderm.
a,c)Significant differences within each column (p<0.01); b,d)Significant differences within each column (p<0.05).
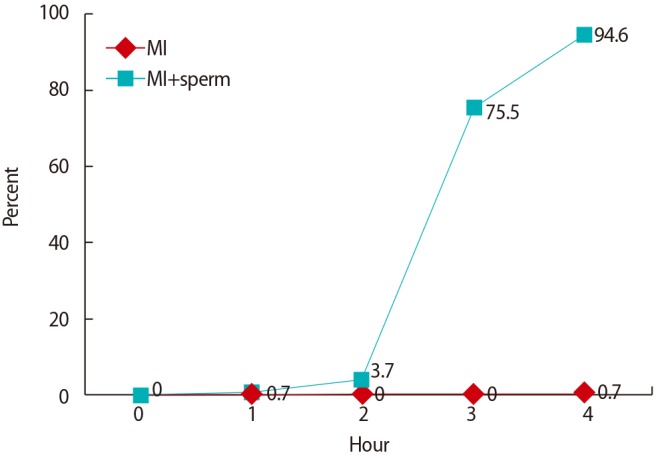
To evaluate the effect of sperm addition on oocyte maturation, MI oocytes in the presence or absence of sperm were monitored every hour for 4 hours during fertilization. Over time, the proportions of oocytes reaching the MII stage among the MI oocytes with spermatozoa were 3.7% at 2 hours, 75.5% at 3 hours, and 94.6% at 4 hours (Figure 1). However, the MI oocytes cultured in fertilization medium without spermatozoa did not proceed to the MII stage, but remained arrested in MI (1 of 146, 0.7%) (Table 3, Figure 1). Moreover, the spontaneous maturation rate (2.1%) of the MI oocytes further cultured for up to 20 hours was not significantly different from that of MI oocytes cultured for 4 hours. These results suggest that sperm induces the resumption of meiosis or the MI-to-MII transition in MI-arrested oocytes. The data comprise the pooled results of nine independent experiments.
Figure 1. Maturation rates of MI oocytes after sperm addition.
There were no significant differences in the fertilization rate of oocytes between the MII (378 of 470, 80.0%±6.6%) and MI (377 of 489, 78.2%±9.1%) groups. However, the rate of blastocyst formation was significantly higher in the MII group (323 of 378, 84.6%±6.9%) than in the MI group (253 of 377, 66.5%±5.6%) (p<0.01). Similarly, the numbers of total cells and trophectoderm cells in the MII group (101.2±17.9 and 75.2±16.8, respectively) were significantly higher than those in the MI group (90.2±19.0 vs. 65.4±14.6, respectively) (p<0.05). However, the numbers of inner cell mass cells did not significantly differ between the MII and MI groups (26.0±10.6 vs. 24.9±10.5, respectively).
Ninety-six blastocysts of each group were transferred to 12 pseudopregnant recipients. The rate of live pups in the MII group was significantly higher than in the MI group (p<0.01) (Table 4). No pregnancy was obtained from the MI group. In contrast, 10 pregnancies were achieved (10 of 12, 83.3%) in the MII group, and the rate of live pups per transferred embryo was 32.3% (31/96). All live pups were morphologically normal.
Table 4. Live pups produced following the transfer of blastocysts derived from MII or MI oocytes.
MII, metaphase II; MI, metaphase I.
a,b)Significant differences within each column (p<0.01).
2. Effects of Ca2+ oscillations on overcoming MI arrest
MI oocytes after IVM were treated with 1 or 10 µg of BAPTA-AM to investigate the role of Ca2+ derived from sperm during the MI-to-MII transition. Following incubation with or without BAPTA-AM, the oocytes were fertilized with sperm. The vast majority of oocytes in the BAPTA-AM-treated groups remained at MI without formation of the first PB (Table 5). The proportion of MI oocytes that matured to MII oocytes after fertilization was significantly higher in the non-treated group (165 of 177, 93.2%±5.7%) than in the 1 or 10 µg BAPTA-AM-treated group (5 of 166, 3.3%±3.2%; and 2 of 166, 1.3%±1.8%, respectively) (p<0.01). Moreover, those oocytes were unable to form 2 cells (Table 5). This result indicates that induction with BAPTA-AM was sufficient to block the intracellular Ca2+ oscillations, and that the maturation of MI oocytes to MII after fertilization was related to the intracellular Ca2+ oscillations driven by fertilization. The data comprise the pooled results of five independent experiments.
Table 5. Effects of BAPTA-AM treatment on the MI-to-MII transition.
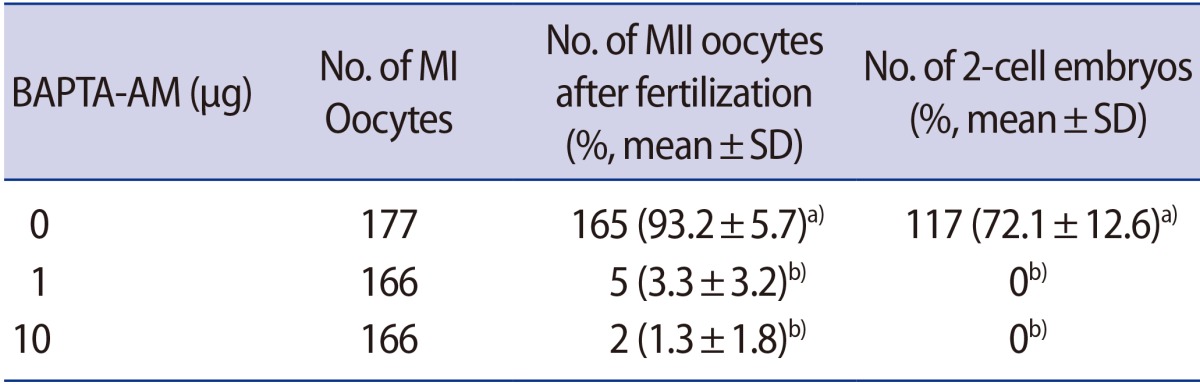
BAPTA-AM, 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetra-acetic acid acetoxymethyl ester; MI, metaphase I; MII, metaphase II; SD, standard deviation.
a,b)Significant differences within each column (p<0.01).
Discussion
MI oocytes that were cultured for a further 4 hours after IVM in fertilization medium without spermatozoa did not complete maturation, but when cultured with spermatozoa, a significant proportion completed maturation to MII (Table 3, Figure 1). The spontaneous maturation rate (2.1%) of the MI oocytes further cultured for up to 20 hours was not significantly different from that of MI oocytes cultured for 4 hours (0.7%). In addition, most MI oocytes incubated for a further 20 hours showed a tendency to degenerate when sperm was introduced (data not shown). Additionally, the oocytes arrested at the GV stage remained arrested after sperm insemination (data not shown). Chian et al. [7] reported similar results for bovine oocytes; only oocytes that reached MI or beyond were stimulated by sperm penetration to complete meiotic maturation, whereas oocytes arrested at the stages before MI remained arrested after sperm penetration. Overall, these results indicated that MI oocytes after IVM were completely arrested at the MI stage, and that sperm could induce the resumption of meiosis or the MI-to-MII transition in MI-arrested oocytes.
Strassburger et al. [12] reported a higher fertilization rate in human IVM MII oocytes after intracytoplasmic sperm injection than in arrested IVM MI oocytes (44% vs. 33%). However, in the present study, the fertilization rate of oocytes arrested in MI was not significantly different from that of those in MII (80.0% vs. 78.2%). Likewise, although the data are not presented here, the fertilization rate in each group showed a tendency similar to the maturation rate from MI to MII. If the fertilization rate was low, the rate of maturation from MI to MII likewise tended to be low. This suggests that sperm penetration affected the progression of oocyte maturation from MI to MII. Despite similar fertilization rates, the rate of blastocyst formation and the number of total cells were significantly higher in the MII group than in the MI group (Table 3). Moreover, no pregnancy was obtained from the MI group (Table 4). This may have been because of their lower developmental competence than the MII group. The reduced developmental competence of MI-arrested oocytes can be further explained by their nuclear and cytoplasmic immaturity. Liu et al. [4] reported that cytoplasmic immaturity decreased the blastocyst rates and cell number by retarding cell proliferation, which may influence post-implantation development and the low efficiency of animal production after ET. Emery et al. [13] suggested that incomplete nuclear maturation and cytoplasmic maturation were associated with poor development and increased embryonic aneuploidy. Their results indicated that nuclear and cytoplasmic immaturities played a role in low developmental competence. Nevertheless, Strassburger et al. [12] demonstrated that human oocytes arrested in MI were fertilized and developed to a healthy neonate with a normal karyotype, although no pregnancy was obtained from MI-arrested oocytes in this study. Further studies are needed to fully understand the physiological and cellular mechanisms involved in the nuclear and cytoplasmic maturation of mammalian oocytes activated by spermatozoa. Additionally, the reason for the failure to achieve pregnancy should be determined. The causes may include problems with cytoplasmic immaturity, oocyte aneuploidy, or differences between humans and mice.
To generate the Ca2+ oscillations, a fertilizing spermatozoon introduces phospholipase C-zeta, a sperm-specific isoform of phospholipase C, which activates oocytes [14]. Diffusion of phospholipase C-zeta into the oocytes triggers oscillations of the cytoplasmic free Ca2+ concentration in the oocytes. Kline and Kline [15] reported that incubation of oocytes in >1.0 µg of BAPTA-AM prior to in vitro fertilization blocked the Ca2+ oscillations transiently, so that the oocytes remained at the MII stage and second PB formation did not occur. In the present study, to evaluate the role of Ca2+ oscillations derived from sperm during the MI-to-MII transition, fertilization profiles were analyzed after treating MI-arrested oocytes with 1 µg and 10 µg of a Ca2+ chelator. The vast majority of oocytes in the BAPTA-AM-treated groups remained at MI and did not show formation of the first PB. These findings indicate that treatment with BAPTA-AM was sufficient to block the intracellular Ca2+ oscillations. Moreover, Ca2+ oscillations derived from sperm play an important role in overcoming oocyte arrest after IVM.
Ca2+ plays important roles in oocyte physiology, from oogenesis to maturation and fertilization [3,16]. Murnane and DeFelice [17] reported that plasma membrane Ca2+ currents selectively increased in growing oocytes and that intracellular or plasma membrane Ca2+ currents may mediate the onset of murine oocyte maturation. Lee et al. [18] also showed that GV and GVBD-arrested oocytes had some defects in Ca2+ channel expression or translation. These results suggest that oocytes develop the ability to generate sperm-induced Ca2+ oscillations during meiotic maturation. This requires several cytoplasmic changes, such as reorganization of the endoplasmic reticulum (ER), an increase in the quantity of Ca2+ ions stored in the ER, and the redistribution of ER Ca2+-binding proteins [14].
The ER is the major Ca2+ storage organelle in oocytes. In GV oocytes, the ER forms a fine patch-like network within the cytoplasm [19,20]. At the time of transition from MI to MII, the patch-like ER structure disappears and a distinctive ER cluster forms in the cortical region of the oocyte. FitzHarris et al. [20] reported that ER clusters in the oocyte cortex formed independently of meiotic progression to MII, based on their finding that oocytes spontaneously arrested in MI displayed cortical ER clusters similar to those present in MII eggs. In addition, several ER proteins, such as calreticulin and calnexin, function as Ca2+-binding molecules and as molecular chaperones. The distribution of these proteins changes during the maturation of mammalian oocytes. Balakier et al. [21] reported that calreticulin in human MI oocytes forms patch-like accumulations in the cortex, similar to those observed in MII oocytes. Additionally, calnexin has been found to form three distinct cortical zones in GV oocytes, whereas the three zones of cortical calnexin were redistributed into a single layer in MI/MII oocytes. Similarly, Eppig et al. [22] reported that MI-arrested oocytes exhibited a protein synthesis pattern similar to that of MII-arrested oocytes. Therefore, they concluded that some critical aspects of cytoplasmic maturation can occur in oocytes whose nuclear maturation is arrested at MI. Furthermore, Mehlmann and Kline [19] reported that, similar to mature oocytes, immature mouse oocytes were capable of producing repetitive Ca2+ transient oscillations when fertilized. Moreover, Jones et al. [23] found that oocytes spontaneously arrested in MI that were unable to achieve MII even after prolonged culture produced continuous Ca2+ oscillations similar to those generated in fertilized MII oocytes. These results suggest that the development of the ability of oocytes to generate long-lasing Ca2+ oscillations is more dependent on cytoplasmic changes than on the progression of meiosis.
These previous reports led us to hypothesize that some critical aspects of cytoplasmic maturation occur in oocytes whose nuclear maturation is arrested at MI. To confirm this, GV-arrested oocytes and MI-arrested oocytes after IVM were fertilized, and then their maturation rates were compared (data not shown). The GV-arrested oocytes did not proceed to the MII stage, and most remained arrested at GV, whereas higher proportions of the MI-arrested oocytes progressed to the MII stage. These findings suggest that some cytoplasmic changes, such as ER reorganization and the redistribution of ER Ca2+-binding proteins, are completed in MI-arrested oocytes, similarly to MII oocytes, and can generate sperm-induced Ca2+ oscillations.
The ability of oocytes to undergo the exocytosis of cortical granules in response to Ca2+ occurs between MI and MII [24], and is critical for the establishment of the zona block to polyspermy [25]. The fertilization of GV oocytes is frequently associated with polyspermy, possibly due to their inability to undergo cortical granules exocytosis and to mount a zona block [11]. Therefore, a preliminary study was conducted to compare the proportions of abnormal fertilization between MI-arrested oocytes and MII oocytes. No significant difference in the rate of normal fertilization was found between the two groups (Table 1). Additionally, the rates of 1PN and 3PN formation were not significantly different from each other. These data are consistent with those obtained by Strassburger et al. [12] and Shu et al. [26], demonstrating that arrested human MI oocytes exhibited abnormal fertilization patterns similar to those of MII oocytes. It is suggested that the increase in Ca2+ after fertilization in MI-arrested oocytes is sufficient for cortical granule exocytosis, which is responsible for the alteration of the zona pellucida that establishes the zona block to polyspermy.
In conclusion, it was found in this study that oocytes arrested in MI after IVM were activated following fertilization and developed to blastocysts, but their developmental competence was lower than that of MII oocytes. Additionally, the maturation of MI to MII oocytes is associated with the intracellular Ca2+ oscillation driven by fertilization. Further studies are needed to fully understand the mechanisms of overcoming MI arrest by Ca2+. Such studies will be helpful for overcoming oocyte arrest and thus for future IVM practice.
Footnotes
Conflict of interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Goud PT, Goud AP, Qian C, Laverge H, Van der Elst J, De Sutter P, et al. In-vitro maturation of human germinal vesicle stage oocytes: role of cumulus cells and epidermal growth factor in the culture medium. Hum Reprod. 1998;13:1638–1644. doi: 10.1093/humrep/13.6.1638. [DOI] [PubMed] [Google Scholar]
- 2.Heindryckx B, Lierman S, Combelles CM, Cuvelier CA, Gerris J, De Sutter P. Aberrant spindle structures responsible for recurrent human metaphase I oocyte arrest with attempts to induce meiosis artificially. Hum Reprod. 2011;26:791–800. doi: 10.1093/humrep/deq400. [DOI] [PubMed] [Google Scholar]
- 3.Tosti E. Calcium ion currents mediating oocyte maturation events. Reprod Biol Endocrinol. 2006;4:26. doi: 10.1186/1477-7827-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu J, Rybouchkin A, Van der Elst J, Dhont M. Fertilization of mouse oocytes from in vitro-matured preantral follicles using classical in vitro fertilization or intracytoplasmic sperm injection. Biol Reprod. 2002;67:575–579. doi: 10.1095/biolreprod67.2.575. [DOI] [PubMed] [Google Scholar]
- 5.Kim BK, Jabed MA, Kang SR, Kim DE, Han CH, Huh MK, et al. Effects of spermatozoa during in vitro meiosis progression in the porcine germinal vesicle oocytes. Anim Reprod Sci. 2008;104:83–92. doi: 10.1016/j.anireprosci.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 6.Niwa K, Park CK, Okuda K. Penetration in vitro of bovine oocytes during maturation by frozen-thawed spermatozoa. J Reprod Fertil. 1991;91:329–336. doi: 10.1530/jrf.0.0910329. [DOI] [PubMed] [Google Scholar]
- 7.Chian RC, Niwa K, Nakahara H. Effect of sperm penetration in vitro on completion of first meiosis by bovine oocytes arrested at various stages in culture. J Reprod Fertil. 1992;96:73–78. doi: 10.1530/jrf.0.0960073. [DOI] [PubMed] [Google Scholar]
- 8.Van Blerkom J, Davis PW, Merriam J. The developmental ability of human oocytes penetrated at the germinal vesicle stage after insemination in vitro. Hum Reprod. 1994;9:697–708. doi: 10.1093/oxfordjournals.humrep.a138574. [DOI] [PubMed] [Google Scholar]
- 9.Yoon J, Yoon HJ, Juhn KM, Ko JK, Yoon SH, Ko Y, et al. Application of two different synthetic sequential media for the human IVF-ET program: a prospective, randomized, and comparative study. Clin Exp Reprod Med. 2011;38:186–192. doi: 10.5653/cerm.2011.38.4.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thouas GA, Korfiatis NA, French AJ, Jones GM, Trounson AO. Simplified technique for differential staining of inner cell mass and trophectoderm cells of mouse and bovine blastocysts. Reprod Biomed Online. 2001;3:25–29. doi: 10.1016/s1472-6483(10)61960-8. [DOI] [PubMed] [Google Scholar]
- 11.Schultz RM, Montgomery RR, Belanoff JR. Regulation of mouse oocyte meiotic maturation: implication of a decrease in oocyte cAMP and protein dephosphorylation in commitment to resume meiosis. Dev Biol. 1983;97:264–273. doi: 10.1016/0012-1606(83)90085-4. [DOI] [PubMed] [Google Scholar]
- 12.Strassburger D, Friedler S, Raziel A, Kasterstein E, Schachter M, Ron-El R. The outcome of ICSI of immature MI oocytes and rescued in vitro matured MII oocytes. Hum Reprod. 2004;19:1587–1590. doi: 10.1093/humrep/deh236. [DOI] [PubMed] [Google Scholar]
- 13.Emery BR, Wilcox AL, Aoki VW, Peterson CM, Carrell DT. In vitro oocyte maturation and subsequent delayed fertilization is associated with increased embryo aneuploidy. Fertil Steril. 2005;84:1027–1029. doi: 10.1016/j.fertnstert.2005.04.036. [DOI] [PubMed] [Google Scholar]
- 14.Ajduk A, Małagocki A, Maleszewski M. Cytoplasmic maturation of mammalian oocytes: development of a mechanism responsible for sperm-induced Ca2+ oscillations. Reprod Biol. 2008;8:3–22. doi: 10.1016/s1642-431x(12)60001-1. [DOI] [PubMed] [Google Scholar]
- 15.Kline D, Kline JT. Repetitive calcium transients and the role of calcium in exocytosis and cell cycle activation in the mouse egg. Dev Biol. 1992;149:80–89. doi: 10.1016/0012-1606(92)90265-i. [DOI] [PubMed] [Google Scholar]
- 16.Liang SL, Zhao QJ, Li XC, Jin YP, Wang YP, Su XH, et al. Dynamic analysis of Ca2+ level during bovine oocytes maturation and early embryonic development. J Vet Sci. 2011;12:133–142. doi: 10.4142/jvs.2011.12.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murnane JM, DeFelice LJ. Electrical maturation of the murine oocyte: an increase in calcium current coincides with acquisition of meiotic competence. Zygote. 1993;1:49–60. doi: 10.1017/s0967199400001295. [DOI] [PubMed] [Google Scholar]
- 18.Lee JH, Yoon SY, Bae IH. Studies on Ca2+-channel distribution in maturation arrested mouse oocyte. Mol Reprod Dev. 2004;69:174–185. doi: 10.1002/mrd.20162. [DOI] [PubMed] [Google Scholar]
- 19.Mehlmann LM, Kline D. Regulation of intracellular calcium in the mouse egg: calcium release in response to sperm or inositol trisphosphate is enhanced after meiotic maturation. Biol Reprod. 1994;51:1088–1098. doi: 10.1095/biolreprod51.6.1088. [DOI] [PubMed] [Google Scholar]
- 20.FitzHarris G, Marangos P, Carroll J. Changes in endoplasmic reticulum structure during mouse oocyte maturation are controlled by the cytoskeleton and cytoplasmic dynein. Dev Biol. 2007;305:133–144. doi: 10.1016/j.ydbio.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 21.Balakier H, Dziak E, Sojecki A, Librach C, Michalak M, Opas M. Calcium-binding proteins and calcium-release channels in human maturing oocytes, pronuclear zygotes and early preimplantation embryos. Hum Reprod. 2002;17:2938–2947. doi: 10.1093/humrep/17.11.2938. [DOI] [PubMed] [Google Scholar]
- 22.Eppig JJ, Schultz RM, O'Brien M, Chesnel F. Relationship between the developmental programs controlling nuclear and cytoplasmic maturation of mouse oocytes. Dev Biol. 1994;164:1–9. doi: 10.1006/dbio.1994.1175. [DOI] [PubMed] [Google Scholar]
- 23.Jones KT, Carroll J, Merriman JA, Whittingham DG, Kono T. Repetitive sperm-induced Ca2+ transients in mouse oocytes are cell cycle dependent. Development. 1995;121:3259–3266. doi: 10.1242/dev.121.10.3259. [DOI] [PubMed] [Google Scholar]
- 24.Ducibella T, Buetow J. Competence to undergo normal, fertilization-induced cortical activation develops after metaphase I of meiosis in mouse oocytes. Dev Biol. 1994;165:95–104. doi: 10.1006/dbio.1994.1237. [DOI] [PubMed] [Google Scholar]
- 25.Carroll J, Jones KT, Whittingham DG. Ca2+ release and the development of Ca2+ release mechanisms during oocyte maturation: a prelude to fertilization. Rev Reprod. 1996;1:137–143. doi: 10.1530/ror.0.0010137. [DOI] [PubMed] [Google Scholar]
- 26.Shu Y, Gebhardt J, Watt J, Lyon J, Dasig D, Behr B. Fertilization, embryo development, and clinical outcome of immature oocytes from stimulated intracytoplasmic sperm injection cycles. Fertil Steril. 2007;87:1022–1027. doi: 10.1016/j.fertnstert.2006.08.110. [DOI] [PubMed] [Google Scholar]