Abstract
Purpose
In this review we will discuss recent developments in optogenetics and their potential applications in ophthalmology to restore vision in retinal degenerative diseases.
Recent findings
In recent years, we have seen major advances in the field of optogenetics providing us with novel opsins for potential applications in the retina. Microbial opsins with improved light-sensitivity and red shifted action spectra allow for optogenetic stimulation at light levels well below the safety threshold in the human eye. In parallel, remarkable success in the development of highly efficient viral vectors for ocular gene therapy led to new strategies of using these novel optogenetic tools for vision restoration.
Summary
These recent findings show that novel optogenetic tools and viral vectors for ocular gene delivery are now available providing many opportunities to develop potential optogenetic strategies for vision restoration.
Introduction
Optogenetics is a technique to control or to monitor neural activity with light? which is achieved by the genetic introduction of light-sensitive proteins [1], [2]. Optogenetic activators like channelrhodopsin (ChR), halorhodopsin, and archaerhodopsin (Arch) are used to control neurons, whereas monitoring of neuronal activity can be performed with genetically encoded sensors for ions (e.g. calcium) or membrane voltage. The effector in this system is light that has the advantage to operate with high spatial and temporal resolution at multiple wavelengths and locations [3]. One of the first steps in the development of the optogenetic technology was the discovery in 1971 by Oesterhelt and Stoeckenius [4] that bacteriorhodopsin, a rhodopsin-like protein from the purple membrane of Halobacterium halobium that pumps protons under illumination. This was followed by identification of other members of the opsin family - halorhodopsin in 1984 by Sugiyama and Mukohata [5] and channelrhodopsin in 2002 by Nagel et al. [6]. Some other early approaches were developed and applied by the groups of Boris Zemelman and Gero Miesenböck at the Sloan-Kettering Cancer Center in New York City [7], and Dirk Trauner, Richard Kramer and Ehud Isacoff at the University of California, Berkeley [8], among other teams. A major breakthrough was the discovery that upon introduction of a microbial opsin gene without any other components, neurons became responsive to light [9]. First introduced in neuroscience, today, optogenetics represents a groundbreaking technology that enables optical modulation of selected cells within variety of complex tissues via introduction of natural or engineered proteins containing a photoreceptive domain coupled to biological function. Even though the classic optogenetic proteins have a number of weak points, including expression in mammalian cells and low light-sensitivity, significant diversification of the optogenetic toolbox has been achieved in the past several years, ensuring improved protein properties needed for optogenetic vision restoration.
Optogenetic tools
“Opsins” represent a major optogenetic tool. They are a family of retinal-binding, seven-transmembrane, light-sensitive proteins encoded by opsin genes. Opsins function as light-responsive ion pumps or sensory receptors and can be found ubiquitously in all organisms, including eukaryotes and bacteria (reviewed in [10]). Opsin genes are divided into two distinct families: microbial opsins (type I; found in prokaryotes, algae and fungi; more typically encode proteins that utilize retinal in the all-trans configuration) and animal opsins [type II; present only in higher eukaryotes and responsible mainly for vision; encode G protein–coupled receptors (GPCRs) and, in the dark, bind retinal in the 11-cis configuration].
“Microbial opsins” capture light energy and use it to either actively pump ions across cell membrane or to open channels allowing a passive flow of ions across cell membrane. Introduced into non-light-sensitive cells, microbial opsins enable rapid optical control of specific cellular processes. Microbial opsins offer high speed neural activation and silencing, without requiring the use of chemicals in the mammalian brain. The most commonly used microbial opsins in optogenetics include ChRs and light-driven pumps, such as halorhodopsin and Archs. ChRs are blue light-activated nonspecific cation channels from green algae. The first described ChR, channelrhodopsin-1 (ChR1), was identified in Chlamydomonas reinhardtii [6]. A second ChR - ChR2 - was later characterized from the same organism [11]. Both ChRs exhibit fast kinetics and allow selective depolarization of genetically targeted cells upon illumination [11]. Halorhodopsins are light-driven inward chloride pumps from archaeal species. When expressed in the targeted cell and illuminated with yellow light, they pump chloride ions from the extracellular medium into the cell and mediate hyperpolarization with consecutive silencing of the target cell. First to be used in neurons was halorhodopsin from the archaea Natronomonas pharaonis (NpHR) (reviewed in [12]). ChR2 and NpHR respond optimally to 470 nm and 580 nm light, respectively [11], [13]. Based on their different activation maxima, they can be co-expressed in the same cell to activate or silence the cell activity in an independent manner, with millisecond precision and rapid reversibility [13], [14]. Archs, for example Arch-3, are light-driven outward proton pumps from Halorubrum sodomense. When expressed in neurons and illuminated with yellow or green light, Arch pumps positive charge out of the cells, hyperpolarizing them. A molecule with increased light-sensitivity – ArchT - has been recently introduced [15] and used for silencing of large brain regions. Excitation maxima for Arch and ArchT are at approximately 566 nm [16], [15].
“Animal opsins” such as rhodopsin and melanopsin belong to the large family of naturally occurring light-driven GPCRs and they are important tools for optogenetic applications (reviewed in [2], [12]). In contrast to microbial opsins, the light sensitivity of animal opsins is much higher, because the light-signal is amplified by G-protein coupled signalling cascades. Previous studies have shown that vertebrate rhodopsins [17], [18] or vertebrate cone opsins [19] can be used as optogenetic tools to control neuronal excitability at low light levels.
Optogenetics for vision restoration
The expression of light-sensitive microbial opsins is a promising approach to restore vision in retinal degenerative diseases without the need for invasive surgery. Optogenetic tools can be genetically expressed in various sub-populations of retinal neurons using viral vectors. The key idea of optogenetics is to convert light-insensitive retinal neurons into ‘artificial photoreceptors’.
Cell type specific targeting of microbial opsins
Different optogenetic strategies can be used, depending on the anatomical and functional state of the retina. If cones are still alive but they lack outer segments, they can be targeted with optogenetic inhibitors, such as halorhodopsin. The rational for this therapeutic strategy relies on the observation that in some cases, the retina retains cone cell bodies with shortened or absent outer segments. These cone photoreceptors survive, but they lose their sensitivity to light [20], [21], [22]. Alternatively, it is possible to bypass the photoreceptors and to target ON and OFF bipolar cells (with activators and inhibitors, respectively). Finally, when bipolar cells degenerate, ‘artificial photoreceptors’ can be made from ganglion cells (reviewed in [23]***).
Bi et al. showed first that light sensitivity can be restored through expression of ChR2 in retinal ganglion cells after complete photoreceptor degeneration [24], which was followed by other studies [25], [26], [27], [28]. However, the non-selective expression of optogenetic tools in retinal ganglion cells does not allow for image processing mediated by retinal interneurons.
Cell type specific targeting of microbial opsins to retinal bipolar cells, that utilize neural circuits upstream of ganglion cells, can elicit ganglion cell responses that are closer to natural activity patterns. In a pioneering study, Lagali et al. [29] used electroporation to express ChR2 under the control of the ON bipolar cell promoter in ON bipolar cells of the blind mice (rd1). ChR2 mediated activation ON bipolar cells induced spiking responses in retinal ganglion cells and resulted in recovery of visually evoked potentials in the cortex and visually guided behaviours. However, electroporation had to be replaced by viral gene delivery that is suitable for future clinical applications. A major drawback was that natural occurring adeno-associated viryses (AAVs) could not transduce effectively the inner nuclear layer of the retina. Therefore, AAV technology had to be developed in order to provide stronger gene expression in retinal bipolar cells. First, it was shown that a tyrosine capsid-mutated serotype - AAV8-Y733F - could provide some gene expression to bipolar cells via sub-retinal administration in the rd1 mouse model [30]. More recently, AAV-based vectors with better retinal diffusion properties have been developed by using a “directed evolution” approach [31]*. Two studies in 2014 showed that engineered AAVs can efficiently deliver depolarizing ChR2 variants to ON bipolar cell population [32]*, [33]*. Importantly, ChR targeted to ON bipolar cells with AAVs restored both ON and OFF component of the visual responses, which is based on retinal processing in inner plexiform layer (illustrated in Fig. 1). The restoration of the ON/OFF pathway was observed in the retina and in the visual cortex. Furthermore, light-induced locomotory behaviour was restored in these treated blind mice [33]*.
Figure 1.
(A) Fundus image of a rd1 mouse retina expressing ChR2(H134R)-GFP under the control of a ON-bipolar specific promotor. (B) Live two-photon images of ON-bipolar cells types. Examples of different ON-bipolar cell types expressing ChR2(H134R)-GFP fusion: (left) ON cone bipolar cell type 7, (middle) ON cone bipolar cell type 9, and (right) rod bipolar cell. (C) Wiring diagram illustrates the classical rod-cone bipolar cell pathway mediated by AII amacrine cells: rod bipolar cells release glutamate onto AII amacrine cells upon depolarization. AII amacrine cells form electrical synapses with axon terminals of ON cone bipolar cells and glycine-ergic (sign-inverting) synapses with those of OFF cone bipolar cells. These cone bipolar cells form glutamate-ergic synapses with ganglion cells. This synaptic circuitry is the basis for channelrhodopsin expressing ON-cone/rod-bipolar cells (both shown in green) triggering spiking of ON ganglion cells at light increments and in OFF ganglion cells at light decrements. (Reproduced with permission from Mace et al., Mol Ther. 2015 Jan;23(1):7–16. [33]*).
Another strategy is to express halorhodopsin in non-functional but surviving “dormant” cone photoreceptors. The feasibility of this approach was shown by Botond Roska’s group at the Friedrich Miescher Institute for Biomedical Research, Basel, Switzerland [34]. Halorhodopsin was able to substitute for the impaired phototransduction cascade in treated blind mice (Fig. 2). These reactivated cones could drive sophisticated retinal circuit functions, including lateral inhibition and directional selectivity, and they mediated cortical processing as well as visually guided behaviour. In collaboration with Serge Picaud’s team at the Institut de la Vision, Paris, France, human retinal explants were used to reactivate light-insensitive photoreceptors with halorhodopsin, demonstrating the functionality of a microbial opsin in the human retina (Fig. 3) [34]. High-resolution imaging techniques, such as optical coherence tomography (OCT) and adaptive optics, allow direct non-invasive examination of the retinal architecture and offer possibility to observe cone photoreceptor mosaic, to detect the earliest occurrence of retinal dystrophic events and document the progression of the retinal degenerative disease [35]. With these techniques it became possible to identify surviving retinal cells and determine patients’ suitability for optogenetics treatment [23]**, [34]. Recently developed in-vitro post-mortem human retina preparation allowed the investigation of gene expression from viral vectors [36]. A vector with known human compatibility was able not only to express NpHR in cones of human retinal explants with no intrinsic, rod- or cone-mediated, photosensitivity, but NpHR targeted to human photoreceptors restored light responses in photoreceptor cells [34]. These results clearly demonstrated that reactivation of the surviving retinal structures and phototransduction cascade required for vision is possible. Patients with preserved layer of cone bodies despite significant retinal degeneration (visual acuity below light perception and no visual field) thus could be eligible for optogenetic functional restoration of cone photoreceptors [23]**.
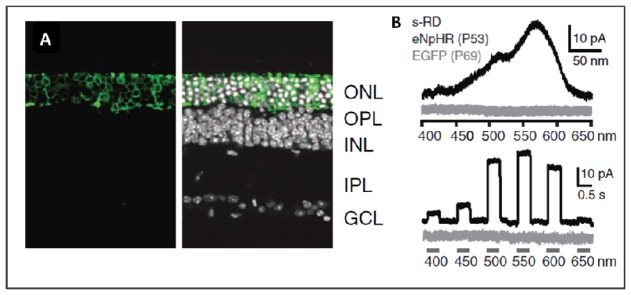
Figure 2.
(A) AAV mediated expression of a light-sensitive chloride pump halorhodopsin in persisting photoreceptors of a blind mouse (Cnga3−/ −; Rho−/ −) (B) Halorhodopsin induced light responses in photoreceptors of the blind mouse (Cnga3−/ −; Rho−/ −). (from Busskamp et al., Science. 2010 Jul 23;329(5990):413–7.).
Figure 3.
Translational aspects of halorhodopsin mediated reactivation of photoreceptors. (A) Retinal slice from a human retinal explant (24 hours postmortem). Scale bar, 30 μm. (B) Fluorescent live image of a lentivirus-transfected area from a human retina after 7 days in culture and 2 days after lentiviral administration. Scale bar, 20 μm. (C) Action spectrum of a halorhodopsin expressing human photoreceptor stimulated with full-field light flashes ranging from 450 to 650 nm (top, current response; bottom, voltage response). Gray bars indicate the timing of the stimulus. (D) Representative OCT scan covering the foveal region of a healthy individual. (E) Magnified image. IS, inner segments; RPE, retinal pigment epithelium. Scale bar, 200 μm. (F) OCT from the left eye of a 40-year-old male patient with sporadic retinitis pigmentosa (loss of vision since the age of 15). Outer segments are undetectable. (Reproduced with permission from Busskamp et al., Science. 2010 Jul 23;329(5990):413–7. [34]).
Gene delivery of optogenetic tools
One of the major advantages of applying optogenetics to the retina is the availability of a number of efficient viral vectors for targeted gene delivery to the eye’s retina. Vectors derived from AAVs are currently the most promising vehicles for gene delivery to the neural retina [37]. Importantly, subretinal administration of AAVs has been demonstrated to be safe and effective in patients in a number of clinical trials supporting clinical relevance of this vector [38], [39], [40], [41]. The safety aspects of AAV as a vector are further strengthened by the great versatility of AAV as a vector platform providing many opportunities to engineer this virus to suit particular applications [42]. As there are a large number of naturally occurring AAVs [43] with unique transduction characteristics useful for targeting different cell types, the applications of AAVs grew rapidly in the retina. Sub-retinally delivered naturally occurring AAV serotypes are able to deliver genes to diverse cell types including glia, epithelium and many types of neurons [44]. To fill remaining gaps in gene delivery to the neural retina, the diverse library of naturally occurring AAVs have been further diversified by rationally designed [45] or laboratory evolved AAV vectors [31]*, [46], [47]. This continuous and creative development of AAV vectors provided opportunities to overcome existing challenges in retinal gene therapy such as targeting of specific cells within the retina by the virtue of the AAV capsid [46] or transduction of photoreceptors following surgically simple intravitreal injections [31]*.
Challenges and new strategies
Recent studies have demonstrated that insertion of microbial opsins into retinal neurons is a feasible optogenetic approach to restore vision. However, the potential immune response to microbial opsins in human retina is an important issue. Therefore, tolerability of microbial opsins has to be evaluated carefully in in vivo in the non-human primate retina. These experiments are an essential prerequisite for future clinical trials. Concerning viral delivery, challenges remain in restricting expression to specific sub-types of neurons as cell-type specific promoters are still lacking for the large number of different neuronal subtypes in the retina. Natural AAVs are suitable for targeting retinal ganglion cells with a pan-retinal expression pattern in mice but they are less efficient in primates. New developments are needed to make AAV as efficient as it is in the mouse retina in larger animals such as dogs and primates [31]*.
An important aspect concerns the fact that the light intensity for optogenetic stimulation is very high. In order to stimulate ChR2, a prosthetic device would be necessary that projects an intensified image on the retina. Under normal light conditions ChR2 will not respond without the intensifier glasses. Thus, if the regained visual sensation may cause side effects after long light exposure, optogenetic stimulation could be turned off by removing the stimulation goggles. In such a stimulator goggle an adaptive light sensor and a light-emitting diode array facing the eye would be necessary to amplify low light intensities. However, to avoid the risk of potential photochemical damage, the light intensity should not be too high. Therefore, there has been great interest in the generation of more light-sensitive ChR variants. A multitude of new mutants with improved light sensitivity and expression level compared with the native channelrhodopsin have been engineered (reviewed in [12], [10], [48]). These variants are maximally activated by blue and green light and they include for example the variant CatCh (L132C) with improved light sensitivity [49] and other ChR2 mutations at L132 [50]. Very recently, conversion of channelrhodopsin into a light-gated chloride channel was reported [51]*, [52]*. These directly light-gated anion channels could be used as an alternative to Arch or halorhodopsin in those retinal cell types where light-induced hyperpolarization would be necessary, such as photoreceptors or OFF bipolar cells. Step-function opsins (SFOs) [53] were generated to induce prolonged depolarization making them suitable to produce long-term behavioural effects or influence animal development [54]; but because of their long open-state lifetimes up to several minutes, SFOs are not applicable for restoring vision. In general, increased light-sensitivity correlates with a decrease in temporal kinetics; i.e. there is consistently a trade-off between light-sensitivity and temporal resolution that can be achieved with these mutants.
Finally, the wavelength that is need for optogenetic stimulation is an important parameter concerning safety issues. In the case of halorhodopsin, which is optimally stimulated with orange light at 580 nm, the required light intensity is well below the safety threshold in the human eye [34]. In contrast, ChR needs stimulation with blue light, which has a much higher potential of inducing photochemical damage in the eye. Recent efforts led to the generation of novel depolarizing and hyperpolarizing optogenetic tools with red-shifted action spectra. The first ChR variant (VChR1) with significant red spectral shift was isolated from the alga Volvox carteri (maximal activation at 540 nm) [55]. Membrane-trafficking enhancement was achieved with the variant C1V1 [56]. In the more recent red-shifted variant ReaChR, the sensitivity and response kinetics have been significantly improved [57]*. ReaChR is optimally excited with orange to red light (590–630 nm). Compared to the previously described red-shifted variants ChRs (VChR1, MChR1, and C1V1 [58], [59]), ReaChR has improved membrane trafficking and expression level in mammalian cells, more robust spectral response above 600 nm, and faster kinetics compared with existing red-shifted channelrhodopsins [57]*. In 2014, Chrimson - a red light activated ChR - has been discovered through sequencing of multiple species of alga. This variant can be still activated at far red shifted wavelengths (>650 nm) [60]*. In the same year, a new inhibitory opsin Jaws-cruxhalorhodopsin - derived from Halobacterium salinarum - has been engineered to result in red light induced (630 nm) photocurrents allowing for optogenetic inhibition deeper layers of neural tissue [61]. Jaws enabled transcranial inhibition of brain neurons of mice in response to red light and induced robust inhibition of sensory-evoked neural activity in the cortex. It also suppressed visually evoked neural activity in mice and restored photosensory responses in retinas of retinitis pigmentosa model mice [61]. These novel red-shifted opsins have strong therapeutic potential for vision restoration.
Conclusions
In less than ten years since the first experiments using light to control genetically defined cell populations, optogenetics has been used in many fields of neuroscience generating remarkable progress in understanding fundamental principles of neuronal circuitry in health and disease. In vision research, optogenetics made it possible to confer light-sensitivity to distinct retinal cell-types, thus offering new perspectives for vision restoration in a wide range of inherited retinal degenerative diseases (e.g. retinitis pigmentosa), as well as age-related eye diseases (e.g. age-related macular degeneration). Although the therapeutic potential of optogenetics remains to be determined, the first steps towards its clinical application have already been undertaken. Ongoing development of opsins with increased photosensitivity, vectors and promoters able to target specific retinal cells and suitable light-delivery devices will very likely result in future translation of the optogenetic therapeutic approach to the clinic.
Key points.
Optogenetics enables optical modulation of selected cells within variety of complex tissues, via introduction of proteins containing a light-sensitive domain coupled to biological function.
In the retina, optogenetics makes it possible to convert different retinal cells-types into “artificial photoreceptors”. To achieve this, optogenetic tools can be genetically expressed in various sub-populations of retinal cells using viral vectors.
Optogenetic-mediated restoration of visual functions and visually guided behaviors was demonstrated in animal models of retinal degenerative diseases.
Acknowledgments
The authors thank Deniz Dalkara for providing helpful suggestions on viral gene delivery in optogenetics.
Financial support and sponsorship
J.D., K.M., J.A.S.: LABEX LIFESENSES [ANR-10-LABX-65]
J.D.: ERC Starting Grant 309776 (Microbial opsins for mammalian vision: Optogenetics in the retina)
Footnotes
Conflicts of interest
J.A.S. is founder and consultant for Pixium Vision and GenSight Biologics, and consultant for Sanofi-fovea, Genesignal and Vision Medicines. J.D., K.M. declare no conflict of interest.
References
- 1.Deisseroth K, Feng G, Majewska AK, Miesenbock G, Ting A, Schnitzer MJ. Next-generation optical technologies for illuminating genetically targeted brain circuits. J Neurosci. 2006;26:10380–6. doi: 10.1523/JNEUROSCI.3863-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herlitze S, Landmesser LT. New optical tools for controlling neuronal activity. Curr Opin Neurobiol. 2007;17:87–94. doi: 10.1016/j.conb.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 3.Hausser M. Optogenetics: the age of light. Nat Methods. 2014;11:1012–4. doi: 10.1038/nmeth.3111. [DOI] [PubMed] [Google Scholar]
- 4.Oesterhelt D, Stoeckenius W. Rhodopsin-like protein from the purple membrane of Halobacterium halobium. Nat New Biol. 1971;233:149–52. doi: 10.1038/newbio233149a0. [DOI] [PubMed] [Google Scholar]
- 5.Sugiyama Y, Mukohata Y. Isolation and characterization of halorhodopsin from Halobacterium halobium. J Biochem. 1984;96:413–20. doi: 10.1093/oxfordjournals.jbchem.a134852. [DOI] [PubMed] [Google Scholar]
- 6.Nagel G, Ollig D, Fuhrmann M, Kateriya S, Musti AM, Bamberg E, Hegemann P. Channelrhodopsin-1: a light-gated proton channel in green algae. Science. 2002;296:2395–8. doi: 10.1126/science.1072068. [DOI] [PubMed] [Google Scholar]
- 7.Zemelman BV, Lee GA, Ng M, Miesenbock G. Selective photostimulation of genetically chARGed neurons. Neuron. 2002;33:15–22. doi: 10.1016/s0896-6273(01)00574-8. [DOI] [PubMed] [Google Scholar]
- 8.Banghart M, Borges K, Isacoff E, Trauner D, Kramer RH. Light-activated ion channels for remote control of neuronal firing. Nat Neurosci. 2004;7:1381–6. doi: 10.1038/nn1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8:1263–8. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- 10.Zhang F, Vierock J, Yizhar O, Fenno LE, Tsunoda S, Kianianmomeni A, Prigge M, Berndt A, Cushman J, Polle J, Magnuson J, Hegemann P, Deisseroth K. The microbial opsin family of optogenetic tools. Cell. 2011;147:1446–57. doi: 10.1016/j.cell.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagel G, Szellas T, Huhn W, Kateriya S, Adeishvili N, Berthold P, Ollig D, Hegemann P, Bamberg E. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc Natl Acad Sci U S A. 2003;100:13940–5. doi: 10.1073/pnas.1936192100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bernstein JG, Boyden ES. Optogenetic tools for analyzing the neural circuits of behavior. Trends Cogn Sci. 2011;15:592–600. doi: 10.1016/j.tics.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang F, Aravanis AM, Adamantidis A, de Lecea L, Deisseroth K. Circuit-breakers: optical technologies for probing neural signals and systems. Nat Rev Neurosci. 2007;8:577–81. doi: 10.1038/nrn2192. [DOI] [PubMed] [Google Scholar]
- 14.Arenkiel BR, Peca J, Davison IG, Feliciano C, Deisseroth K, Augustine GJ, Ehlers MD, Feng G. In vivo light-induced activation of neural circuitry in transgenic mice expressing channelrhodopsin-2. Neuron. 2007;54:205–18. doi: 10.1016/j.neuron.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han X, Chow BY, Zhou H, Klapoetke NC, Chuong A, Rajimehr R, Yang A, Baratta MV, Winkle J, Desimone R, Boyden ES. A high-light sensitivity optical neural silencer: development and application to optogenetic control of non-human primate cortex. Front Syst Neurosci. 2011;5:18. doi: 10.3389/fnsys.2011.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chow BY, Han X, Dobry AS, Qian X, Chuong AS, Li M, Henninger MA, Belfort GM, Lin Y, Monahan PE, Boyden ES. High-performance genetically targetable optical neural silencing by light-driven proton pumps. Nature. 2010;463:98–102. doi: 10.1038/nature08652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li X, Gutierrez DV, Hanson MG, Han J, Mark MD, Chiel H, Hegemann P, Landmesser LT, Herlitze S. Fast noninvasive activation and inhibition of neural and network activity by vertebrate rhodopsin and green algae channelrhodopsin. Proc Natl Acad Sci U S A. 2005;102:17816–21. doi: 10.1073/pnas.0509030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oh E, Maejima T, Liu C, Deneris E, Herlitze S. Substitution of 5-HT1A receptor signaling by a light-activated G protein-coupled receptor. J Biol Chem. 2010;285:30825–36. doi: 10.1074/jbc.M110.147298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Masseck OA, Spoida K, Dalkara D, Maejima T, Rubelowski JM, Wallhorn L, Deneris ES, Herlitze S. Vertebrate cone opsins enable sustained and highly sensitive rapid control of Gi/o signaling in anxiety circuitry. Neuron. 2014;81:1263–73. doi: 10.1016/j.neuron.2014.01.041. [DOI] [PubMed] [Google Scholar]
- 20.Milam AH, Li ZY, Fariss RN. Histopathology of the human retina in retinitis pigmentosa. Prog Retin Eye Res. 1998;17:175–205. doi: 10.1016/s1350-9462(97)00012-8. [DOI] [PubMed] [Google Scholar]
- 21.Li ZY, Kljavin IJ, Milam AH. Rod photoreceptor neurite sprouting in retinitis pigmentosa. J Neurosci. 1995;15:5429–38. doi: 10.1523/JNEUROSCI.15-08-05429.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin B, Masland RH, Strettoi E. Remodeling of cone photoreceptor cells after rod degeneration in rd mice. Exp Eye Res. 2009;88:589–99. doi: 10.1016/j.exer.2008.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sahel JA, Roska B. Gene therapy for blindness. Annu Rev Neurosci. 2013;36:467–88. doi: 10.1146/annurev-neuro-062012-170304. [DOI] [PubMed] [Google Scholar]
- 24.Bi A, Cui J, Ma YP, Olshevskaya E, Pu M, Dizhoor AM, Pan ZH. Ectopic expression of a microbial-type rhodopsin restores visual responses in mice with photoreceptor degeneration. Neuron. 2006;50:23–33. doi: 10.1016/j.neuron.2006.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Y, Ivanova E, Bi A, Pan ZH. Ectopic expression of multiple microbial rhodopsins restores ON and OFF light responses in retinas with photoreceptor degeneration. J Neurosci. 2009;29:9186–96. doi: 10.1523/JNEUROSCI.0184-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tomita H, Sugano E, Yawo H, Ishizuka T, Isago H, Narikawa S, Kugler S, Tamai M. Restoration of visual response in aged dystrophic RCS rats using AAV-mediated channelopsin-2 gene transfer. Invest Ophthalmol Vis Sci. 2007;48:3821–6. doi: 10.1167/iovs.06-1501. [DOI] [PubMed] [Google Scholar]
- 27.Tomita H, Sugano E, Isago H, Hiroi T, Wang Z, Ohta E, Tamai M. Channelrhodopsin-2 gene transduced into retinal ganglion cells restores functional vision in genetically blind rats. Exp Eye Res. 2010;90:429–36. doi: 10.1016/j.exer.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 28.Greenberg KP, Pham A, Werblin FS. Differential targeting of optical neuromodulators to ganglion cell soma and dendrites allows dynamic control of center-surround antagonism. Neuron. 2011;69:713–20. doi: 10.1016/j.neuron.2011.01.024. [DOI] [PubMed] [Google Scholar]
- 29.Lagali PS, Balya D, Awatramani GB, Munch TA, Kim DS, Busskamp V, Cepko CL, Roska B. Light-activated channels targeted to ON bipolar cells restore visual function in retinal degeneration. Nat Neurosci. 2008;11:667–75. doi: 10.1038/nn.2117. [DOI] [PubMed] [Google Scholar]
- 30.Doroudchi MM, Greenberg KP, Liu J, Silka KA, Boyden ES, Lockridge JA, Arman AC, Janani R, Boye SE, Boye SL, Gordon GM, Matteo BC, Sampath AP, Hauswirth WW, Horsager A. Virally delivered channelrhodopsin-2 safely and effectively restores visual function in multiple mouse models of blindness. Mol Ther. 2011;19:1220–9. doi: 10.1038/mt.2011.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dalkara D, Byrne LC, Klimczak RR, Visel M, Yin L, Merigan WH, Flannery JG, Schaffer DV. In vivo-directed evolution of a new adeno-associated virus for therapeutic outer retinal gene delivery from the vitreous. Sci Transl Med. 2013;5:189ra76. doi: 10.1126/scitranslmed.3005708. [DOI] [PubMed] [Google Scholar]
- 32.Cronin T, Vandenberghe LH, Hantz P, Juttner J, Reimann A, Kacso AE, Huckfeldt RM, Busskamp V, Kohler H, Lagali PS, Roska B, Bennett J. Efficient transduction and optogenetic stimulation of retinal bipolar cells by a synthetic adeno-associated virus capsid and promoter. EMBO Mol Med. 2014;6:1175–90. doi: 10.15252/emmm.201404077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mace E, Caplette R, Marre O, Sengupta A, Chaffiol A, Barbe P, Desrosiers M, Bamberg E, Sahel JA, Picaud S, Duebel J, Dalkara D. Targeting Channelrhodopsin-2 to ON-bipolar Cells With Vitreally Administered AAV Restores ON and OFF Visual Responses in Blind Mice. Mol Ther. 2015;23:7–16. doi: 10.1038/mt.2014.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Busskamp V, Duebel J, Balya D, Fradot M, Viney TJ, Siegert S, Groner AC, Cabuy E, Forster V, Seeliger M, Biel M, Humphries P, Paques M, Mohand-Said S, Trono D, Deisseroth K, Sahel JA, Picaud S, Roska B. Genetic reactivation of cone photoreceptors restores visual responses in retinitis pigmentosa. Science. 2010;329:413–7. doi: 10.1126/science.1190897. [DOI] [PubMed] [Google Scholar]
- 35.Gocho K, Sarda V, Falah S, Sahel JA, Sennlaub F, Benchaboune M, Ullern M, Paques M. Adaptive optics imaging of geographic atrophy. Invest Ophthalmol Vis Sci. 2013;54:3673–80. doi: 10.1167/iovs.12-10672. [DOI] [PubMed] [Google Scholar]
- 36.Fradot M, Busskamp V, Forster V, Cronin T, Leveillard T, Bennett J, Sahel JA, Roska B, Picaud S. Gene therapy in ophthalmology: validation on cultured retinal cells and explants from postmortem human eyes. Hum Gene Ther. 2011;22:587–93. doi: 10.1089/hum.2010.157. [DOI] [PubMed] [Google Scholar]
- 37.Vandenberghe LH, Auricchio A. Novel adeno-associated viral vectors for retinal gene therapy. Gene Ther. 2012;19:162–8. doi: 10.1038/gt.2011.151. [DOI] [PubMed] [Google Scholar]
- 38.Maguire AM, Simonelli F, Pierce EA, Pugh EN, Jr, Mingozzi F, Bennicelli J, Banfi S, Marshall KA, Testa F, Surace EM, Rossi S, Lyubarsky A, Arruda VR, Konkle B, Stone E, Sun J, Jacobs J, Dell’Osso L, Hertle R, Ma JX, Redmond TM, Zhu X, Hauck B, Zelenaia O, Shindler KS, Maguire MG, Wright JF, Volpe NJ, McDonnell JW, Auricchio A, High KA, Bennett J. Safety and efficacy of gene transfer for Leber’s congenital amaurosis. N Engl J Med. 2008;358:2240–8. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bainbridge JW, Smith AJ, Barker SS, Robbie S, Henderson R, Balaggan K, Viswanathan A, Holder GE, Stockman A, Tyler N, Petersen-Jones S, Bhattacharya SS, Thrasher AJ, Fitzke FW, Carter BJ, Rubin GS, Moore AT, Ali RR. Effect of gene therapy on visual function in Leber’s congenital amaurosis. N Engl J Med. 2008;358:2231–9. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- 40.Cideciyan AV, Hauswirth WW, Aleman TS, Kaushal S, Schwartz SB, Boye SL, Windsor EA, Conlon TJ, Sumaroka A, Pang JJ, Roman AJ, Byrne BJ, Jacobson SG. Human RPE65 gene therapy for Leber congenital amaurosis: persistence of early visual improvements and safety at 1 year. Hum Gene Ther. 2009;20:999–1004. doi: 10.1089/hum.2009.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.MacLaren RE, Groppe M, Barnard AR, Cottriall CL, Tolmachova T, Seymour L, Clark KR, During MJ, Cremers FP, Black GC, Lotery AJ, Downes SM, Webster AR, Seabra MC. Retinal gene therapy in patients with choroideremia: initial findings from a phase 1/2 clinical trial. Lancet. 2014;383:1129–37. doi: 10.1016/S0140-6736(13)62117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kwon I, Schaffer DV. Designer gene delivery vectors: molecular engineering and evolution of adeno-associated viral vectors for enhanced gene transfer. Pharm Res. 2008;25:489–99. doi: 10.1007/s11095-007-9431-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu Z, Asokan A, Samulski RJ. Adeno-associated virus serotypes: vector toolkit for human gene therapy. Mol Ther. 2006;14:316–27. doi: 10.1016/j.ymthe.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 44.Allocca M, Mussolino C, Garcia-Hoyos M, Sanges D, Iodice C, Petrillo M, Vandenberghe LH, Wilson JM, Marigo V, Surace EM, Auricchio A. Novel adeno-associated virus serotypes efficiently transduce murine photoreceptors. J Virol. 2007;81:11372–80. doi: 10.1128/JVI.01327-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Srivastava A. Adeno-associated virus-mediated gene transfer. J Cell Biochem. 2008;105:17–24. doi: 10.1002/jcb.21819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klimczak RR, Koerber JT, Dalkara D, Flannery JG, Schaffer DV. A novel adeno-associated viral variant for efficient and selective intravitreal transduction of rat Muller cells. PLoS One. 2009;4:e7467. doi: 10.1371/journal.pone.0007467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koerber JT, Klimczak R, Jang JH, Dalkara D, Flannery JG, Schaffer DV. Molecular evolution of adeno-associated virus for enhanced glial gene delivery. Mol Ther. 2009;17:2088–95. doi: 10.1038/mt.2009.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fenno L, Yizhar O, Deisseroth K. The development and application of optogenetics. Annu Rev Neurosci. 2011;34:389–412. doi: 10.1146/annurev-neuro-061010-113817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kleinlogel S, Feldbauer K, Dempski RE, Fotis H, Wood PG, Bamann C, Bamberg E. Ultra light-sensitive and fast neuronal activation with the Ca(2)+-permeable channelrhodopsin CatCh. Nat Neurosci. 2011;14:513–8. doi: 10.1038/nn.2776. [DOI] [PubMed] [Google Scholar]
- 50.Pan ZH, Ganjawala TH, Lu Q, Ivanova E, Zhang Z. ChR2 mutants at L132 and T159 with improved operational light sensitivity for vision restoration. PLoS One. 2014;9:e98924. doi: 10.1371/journal.pone.0098924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wietek J, Wiegert JS, Adeishvili N, Schneider F, Watanabe H, Tsunoda SP, Vogt A, Elstner M, Oertner TG, Hegemann P. Conversion of channelrhodopsin into a light-gated chloride channel. Science. 2014;344:409–12. doi: 10.1126/science.1249375. [DOI] [PubMed] [Google Scholar]
- 52.Berndt A, Lee SY, Ramakrishnan C, Deisseroth K. Structure-guided transformation of channelrhodopsin into a light-activated chloride channel. Science. 2014;344:420–4. doi: 10.1126/science.1252367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Berndt A, Yizhar O, Gunaydin LA, Hegemann P, Deisseroth K. Bi-stable neural state switches. Nat Neurosci. 2009;12:229–34. doi: 10.1038/nn.2247. [DOI] [PubMed] [Google Scholar]
- 54.Schultheis C, Liewald JF, Bamberg E, Nagel G, Gottschalk A. Optogenetic long-term manipulation of behavior and animal development. PLoS One. 2011;6:e18766. doi: 10.1371/journal.pone.0018766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang F, Prigge M, Beyriere F, Tsunoda SP, Mattis J, Yizhar O, Hegemann P, Deisseroth K. Red-shifted optogenetic excitation: a tool for fast neural control derived from Volvox carteri. Nat Neurosci. 2008;11:631–3. doi: 10.1038/nn.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O’Shea DJ, Sohal VS, Goshen I, Finkelstein J, Paz JT, Stehfest K, Fudim R, Ramakrishnan C, Huguenard JR, Hegemann P, Deisseroth K. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature. 2011;477:171–8. doi: 10.1038/nature10360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lin JY, Knutsen PM, Muller A, Kleinfeld D, Tsien RY. ReaChR: a red-shifted variant of channelrhodopsin enables deep transcranial optogenetic excitation. Nat Neurosci. 2013;16:1499–508. [Google Scholar]
- 58.Prakash R, Yizhar O, Grewe B, Ramakrishnan C, Wang N, Goshen I, Packer AM, Peterka DS, Yuste R, Schnitzer MJ, Deisseroth K. Two-photon optogenetic toolbox for fast inhibition, excitation and bistable modulation. Nat Methods. 2012;9:1171–9. doi: 10.1038/nmeth.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Packer AM, Peterka DS, Hirtz JJ, Prakash R, Deisseroth K, Yuste R. Two-photon optogenetics of dendritic spines and neural circuits. Nat Methods. 2012;9:1202–5. doi: 10.1038/nmeth.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Klapoetke NC, Murata Y, Kim SS, Pulver SR, Birdsey-Benson A, Cho YK, Morimoto TK, Chuong AS, Carpenter EJ, Tian Z, Wang J, Xie Y, Yan Z, Zhang Y, Chow BY, Surek B, Melkonian M, Jayaraman V, Constantine-Paton M, Wong GK, Boyden ES. Independent optical excitation of distinct neural populations. Nat Methods. 2014;11:338–46. doi: 10.1038/nmeth.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chuong AS, Miri ML, Busskamp V, Matthews GA, Acker LC, Sorensen AT, Young A, Klapoetke NC, Henninger MA, Kodandaramaiah SB, Ogawa M, Ramanlal SB, Bandler RC, Allen BD, Forest CR, Chow BY, Han X, Lin Y, Tye KM, Roska B, Cardin JA, Boyden ES. Noninvasive optical inhibition with a red-shifted microbial rhodopsin. Nat Neurosci. 2014;17:1123–9. doi: 10.1038/nn.3752. [DOI] [PMC free article] [PubMed] [Google Scholar]