Abstract
Background
The aim of this study was to evaluate the hypoglycemic, hypolipidemic, and anti-inflammatory potentials of ethanolic extract of leaves of Amaranthus paniculatus linn. (EEAP) on alloxan-induced diabetic rats scientifically. Hyperglycemia induces the generation of free radicals which can affect antioxidant defenses, thus leading to the disruption of beta cellular functions, oxidative damage to membranes, leading to the release of C-reactive protein and altered lipid metabolism.
Methods
Diabetes was induced by intraperitoneal injection of ice-cold aqueous alloxan monohydrate at the dose of 150 mg/kg body weight.
Results
After a daily single oral administration of the EEAP for 28 days starting from the study protocol, the blood glucose, serum glutamic pyruvic transaminase (SGPT), serum glutamic oxaloacetic transaminase (SGOT), total cholesterol (TC), triglyceride (TG), and C-reactive protein (CRP) levels were assessed. The results obtained from the study administration of daily dose of EEAP significantly reduced the blood glucose, SGPT, SGOT, TC, TG, and CRP in a dose-dependent manner. The results obtained were comparable to those of glibenclamide. The serum levels of TC, TG, and CRP were significantly altered in the diabetic control group, but it was significantly decreased in the extract-treated group and standard glibenclamide-treated group, except at a dose of 100 mg/kg where there was no significant effect on the TG level. The finding obtained suggests that EEAP acts through molecular level, modifying the altered pathways in diabetes and associated complications.
Conclusion
The results obtained suggest that EEAP possesses a potential for the management of diabetes and associated complications in experimentally-induced diabetic rats.
Keywords: antidiabetic, C-reactive protein, hypolipidemic, total cholesterol, triglycerides
1. Introduction
A number of plant species worldwide are known to have hypoglycemic, hypolipidemic, or both activities. Despite the presence of known antidiabetic medicines in the pharmaceutical market, screening for new antidiabetic sources from natural plants is still attractive because they contain substances that have an alternative and safe effect on diabetes mellitus.1 The herb Amaranthus paniculatus Linn. belongs to the family Amaranthaceae; in Ayurveda, it is called as Rajgira. It is a small tree that attains a height of 10 feet. Branches are small, thick, and thorny.2 In Ayurveda, the various parts of A. paniculatus Linn., such as fruits, seeds, roots, and leaves, are used for various indications like indigestion, joint pains, body pain, inflammation, appetizers, liver-related problems, worm infestation, and heart-related problems.3 Recently, very few pharmacological screenings were performed in vitro such as in the treatment of Alzheimer's disease and antioxidant property.4 Estrogenic effect of petroleum ether extract of seeds of A. paniculatus Linn. is observed in albino rats.5 Till date, no in vivo scientific study has been performed regarding Amaranthus paniculatus Linn. to evaluate antidiabetic and hypolipidemic activity. The objective of the present investigation is to explore the antidiabetic, hypolipidemic, and free radical scavenging potentials of A. paniculatus Linn.
2. Methods
2.1. Plant materials
The leaves of A. paniculatus were collected from Osmanabad district of Maharashtra, herbarium authenticated at Botanical Survey of India, Pune, and head of department of Manjara Charitable Ayurvedic Hospital, Latur, where voucher specimen is deposited (MCT/AUR/2015-017). The leaves of A. paniculatus were shed dried and powdered for extraction. The air dried powder was extracted using Soxhlet extraction apparatus. Fifty grams of powder was extracted with 450 mL of ethanol for 48 hours. The extract obtained was evaporated under a vacuum pump evaporator. The yield of crude extract of A. paniculatus leaves was found to be 11%. The extract was preserved in the refrigerator at 10 °C during the experimental procedure. The ethanolic extract of A. paniculatus (EEAP) was prepared in 5% of gum acacia solution for oral administration
2.2. Chemical reagents and drugs
All the reagents used in this study were of analytical grade, and the diagnostic kits used for the estimation of serum glutamic pyruvic transaminase (SGPT), serum glutamic oxaloacetic transaminase (SGOT), lipid profile, and C-reactive protein (CRP) were obtained from Span Diagnostic, India (Span diagnostic India, Sigma-Aldrich, India and Wockhardt Pharmaceutical, Aurangabad). Alloxan was purchased from Sigma-Aldrich, India and standard glibenclamide was supplied by Wockhardt Pharmaceutical, Aurangabad as gratis.
2.3. Phytochemical screening
The preliminary phytochemical study was performed to detect the various chemical constituents, such as bioflavonoids, glycosoids, and other various phytochemical constituents in the extract.6, 7
2.4. Acute toxicity and dose fixation study
The acute toxicity study was performed as per the Organization for Economic Co-operation and Development (OECD) 423 guidelines. The lethal dose of A. paniculatus was set as 3000 mg/kg and 2000 mg/kg. The Wistar rats (Wochardt research centre Aurangabad) (n = 6) were given two different doses (3000 mg/kg and 2000 mg/kg). The different dose of ethanolic extract of A. paniculatus was given orally daily for 7 days, and the animals were observed for any physical signs of toxicity, such as writhing, gasping, palpitation, decreased respiratory rate, and mortality.
2.5. Animals
Healthy male and female rats weighing 250 g and 300 g were procured from Wockhardt Research Centre, Aurangabad (Maharashtra, India) and maintained in standard animal cages at a temperature of 25 ± 1 °C and relative humidity of 50–60% with 12-hour light and dark cycle. The animal experiments were conducted as per the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), India. The experimental protocol was approved by the Institutional Animal Ethics Committee (CPCSEA/IAEC/YBCOP/2014-15/07) (Wochardt research centre Aurangabad). During the animal experiments, animal were fed with standard pellet diet and free water ad libitum.
2.6. Induction of diabetes and experimental design
Diabetes was induced by intraperitoneal injection of ice-cold aqueous alloxan monohydrate at a dose of 150 mg/kg body weight in animals fasted overnight.8, 9 After 48 hours, blood samples were collected from retro-orbital puncture and the blood sugar level was determined by glucometer ((AccuChek, Johnson and Johnson, India). The animals showing blood sugar level more than 200 mg/dL were considered as hyperglycemic and selected for the studies. The animals are divided into five groups containing six animals in each group (n = 6) as follows: Group 1 – Normal control; Group 2 – Diabetic-untreated control; Group 3 – Diabetic rats treated with 100-mg/kg body weight of EEAP; Group 4 – Diabetic rats treated with 150-mg/kg body weight of EEAP; and Group 5 – Diabetic rats treated with 10-mg/kg body weight of EEAP. Glibenclamide was used as the reference standard during the studies.
After 72 hours of induction of diabetes, the plant extract suspended in 5% of gum acacia solution was administered for 28 days using an oral feeding needle. Blood samples were collected on 7th day, 14th day, 21st day, and 28th day from starting of study for the measurement of blood glucose via the tail vein. Blood sugar levels were measured by glucometer. After 28 days, blood sample was collected through the tail vein and analyzed for total cholesterol (TC), total triglycerides (TG), CRP, SGPT, and SGOT.
2.7. Biochemical analysis
SGPT and SGOT were determined by Reitman and Frankel colorimetric method. TC was estimated using the modified Triender method. The serum (0.01 mL) was added to 1 mL of standard reagent and incubated at 37 °C for 5 minutes; the absorbance was measured at 500 nm. The content was expressed as mg/dL. TG was determined using the glyceryl phosphate oxidase method. To 0.01 mL of the serum, 1 mL of standard reagent was added and incubated at 37 °C for 5 minutes; the absorbance was measured at 500 nm.10, 11, 12 The content was expressed as mg/dL. CRP was measured by the colorimetric principle.13
2.8. Statistical analysis
Statistical analyses of all the results were performed by one-way analysis of variance, followed by Dunnet's multiple comparison tests using the GraphPad InStat V software. Results were expressed as mean ± standard error of mean. Statistical significance level was set at p < 0.01.
3. Results
3.1. Phytochemical analysis
The preliminary phytochemical study revealed that EEAP shows the presence of glycosides, bioflavonoids, amino acids, and fibers.
3.2. Acute toxicity study
The acute toxicity study was performed as per OECD 423 guidelines. Both the doses of EEAP were found safe and nontoxic because rats did not show any signs of writhing, gasping, palpitation, decreased respiratory rate, or mortality.
3.3. Effect on blood sugar level
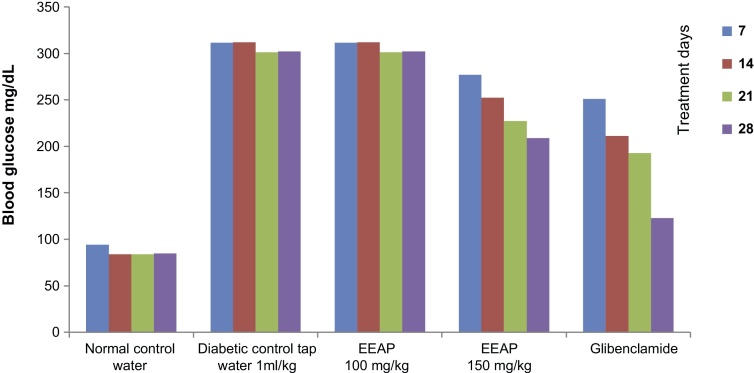
The effects of different dosages of EEAP on blood glucose levels in diabetic rats are illustrated in Table 1 (Fig. 1). The EEAP leaves showed hypoglycemic activity in diabetic rats in a dose-dependent manner. The group treated with ethanolic extract dose at 150 mg/kg showed significant hypoglycemic activity on 7th day, 14th day, 21st day, and 28th day compared with the diabetic control group. While the group received ethanolic extract dose at 100 mg/kg also showed the hypoglycemic activity but not comparable to the group treated with 150 mg/kg of ethanolic extract. The standard group received glibenclamide dose at 10 mg/kg which significantly reduced the blood glucose level during the study period.
Table 1.
Effect of ethanolic extract of Amaranthus Paniculatus on blood glucose level of alloxan-induced diabetic rats
| Groups | Dose/treatment | Effect on blood glucose level (d) |
|||
|---|---|---|---|---|---|
| 7 | 14 | 21 | 28 | ||
| Normal control | Normal saline (1 mL/kg) | 94.16 ± 0.98* | 84 ± 2.0* | 84 ± 2.45* | 84.83 ± 0.7* |
| Diabetic control | Tap water (1 mL/kg) | 311.6 ± 0.84 | 312.1 ± 2.11 | 301 ± 1.2 | 302.16 ± 2.4 |
| Ethanolic extract | 100 mg/kg | 292.8 ± 0.40* | 278.1 ± 1.45* | 251 ± 1.69* | 222.7 ± 3.1* |
| Ethanolic extract | 150 mg/kg | 277 ± 2.8* | 252.16 ± 4.3* | 227.16 ± 1.4* | 209.8 ± 1.7* |
| Glibenclamide | 10 mg/kg | 251 ± 1.83* | 211 ± 3.9* | 192.83 ± 1.014* | 123.5 ± 0.71* |
Values are expressed as mean ± SEM; n = 6.
p < 0.01 significant vs. diabetic control.
Fig. 1.
Effect of ethanolic extract of Amaranthus paniculatus on blood glucose level of alloxan-induced diabetic rats.
3.4. Effect on SGOT and SGPT levels
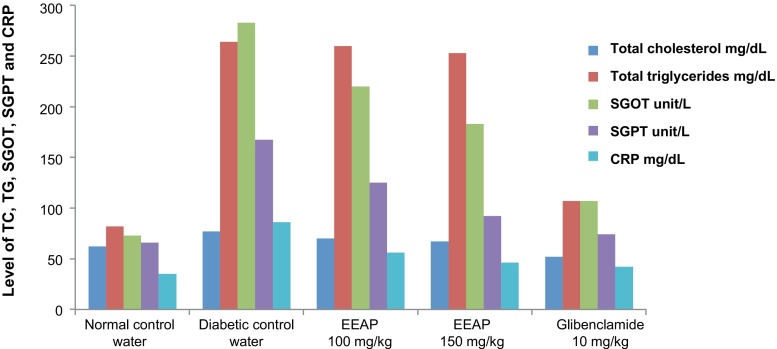
The SGOT and SGPT activities are known to be cytosolic marker enzymes reflecting hepatocellular necrosis as they are released into the blood after cell membrane damage.14 Therefore, in the present study, both the enzyme activities were used as indicators of hepatic damage. SGOT and SGPT levels were determined at the end of the experimental study. Table 2 and (Fig. 2) shows the activities of SGOT and SGPT in experimental rats. When compared with normal rats, diabetic rats showed more activities of serum SGOT and SGPT than the extract-treated groups and normal group. There was a significant difference between two administration doses of extract, each of which showed different effects on the SGOT and SGPT levels. The EEAP at dose of 150 mg/kg effectively reduced the serum SGOT and SGPT level more significantly than the diabetic control group.
Table 2.
Effect of ethanolic extract of Amaranthus Paniculatus on SGOT, SGPT, and CRP in alloxan-induced diabetic rats
| Groups | Dose/treatment | Level of SGOT (unit/L) | Level of SGPT (unit/L) | Level of CRP (mg/dL) |
|---|---|---|---|---|
| Normal control | Normal saline (1 mL/kg) | 73 ± 0.81* | 65.7 ± 0.84* | 35 ± 1.2* |
| Diabetic control | Tap water (1 mL/kg) | 283 ± 1.2 | 167.5 ± 0.76 | 86 ± 0.56 |
| Ethanolic extract | 100 mg/kg | 220 ± 0.83* | 125 ± 0.97* | 56 ± 0.86* |
| Ethanolic extract | 150 mg/kg | 183 ± 1.33* | 92 ± 0.97* | 46 ± 1* |
| Glibenclamide | 10 mg/kg | 107 ± 1.30* | 74 ± 0.63* | 42 ± 2.2* |
Values expressed as mean ± SEM; n = 6.
p < 0.01 significant vs. diabetic control.
CRP, C-reactive protein; SGOT, serum glutamic oxaloacetic transaminase; SGPT, serum glutamic pyruvic transaminase.
Fig. 2.
Effect of EEAP on total cholesterol, triglyceride, SGOT, SGPT, and CRP in alloxan-induced diabetic rats.
3.5. Effect on TC and TG levels
Hypercholesterolemia and hypertriglyceridemia have been induced in streptozotocin- and alloxan-induced diabetic rats.15 TC and TG levels were determined at the end of the experimental study. In the alloxan-induced diabetic rats, the increase in blood glucose level is accompanied by an increase in serum TC and TG levels.16 In the present study, significant changes were observed in serum TC and TG level between the normal group and diabetic control group as shown in Table 3. The effects of the extract on TG and TC levels were different. There was a significant difference in TC and TG levels between the diabetic control group and diabetic groups treated with EEAP at both doses 100 mg/kg and 150 mg/kg. There was no significant difference observed in the TG level at 100 mg/kg dose.
Table 3.
Effect of ethanolic extract of Amaranthus Paniculatus on serum lipid content in alloxan-induced diabetic rats
| Groups | Dose/treatment | Total cholesterol (mg/dL) | Triglycerides (mg/dL) |
|---|---|---|---|
| Normal control | Normal saline (1 mL/kg) | 62 ± 0.57* | 82.66 ± 0.6* |
| Diabetic control | Tap water (1 mL/kg) | 77 ± 2.1 | 264 ± 2.1 |
| Ethanolic extract | 100 mg/kg | 70 ± 0.6* | 260 ± 2.1 |
| Ethanolic extract | 150 mg/kg | 67 ± 0.54* | 253 ± 0.2* |
| Glibenclamide | 10 mg/kg | 62 ± 0.6* | 107 ± 2.1* |
Values are expressed as mean ± SEM; n = 6.
p < 0.01 significant vs. diabetic control.
3.6. Effect on CRP
CRP is a protein found in the blood. It is produced in the liver, and its level rises in response to inflammation. In the present study, significant changes were observed in CRP as shown in Table 2. When animals received EEAP at both the doses, there was a significant decrease in the CRP level at both the doses.
4. Discussion
Diabetes mellitus causes various complications, such as metabolic, microvascular, macrovascular oxidative stress, and inflammation. Therefore, it is logical to identify the medicinal herbs having antidiabetic with additional antioxidant and anti-inflammatory potentials for the management of diabetes and associated complications. If such medicinal herbs are used adjuvantly with allopathic medicine, the complications may be modulated. Therefore, the present study was undertaken to evaluate the hypoglycemic and hypolipidemic status of EEAP on alloxan-induced diabetic rats. To the best of our knowledge, this is the first report of in vivo antidiabetic and hypolipidemic activities of this plant. Our result obtained in two different doses of EEAP (100 mg/kg and 150 mg/kg) showed significant antidiabetic and hypolipidemic effects. The hypoglycemic effect of EEAP was comparable to that of glibenclamide, which may suggest that EEAP acts by potentiating the insulin effect of plasma by increasing either the pancreatic secretion of insulin from the remnant beta cells of the islets of Langerhans or its responsiveness.
The mechanism of EEAP is probably similar to that of glibenclamide, which has an insulin-independent mechanism. The plant extract might be acting by decreasing the hepatic glucose production, inhibiting intestinal glucose absorption or correcting insulin resistance; however, possibilities of other mechanisms to exert hypoglycemic effect cannot be ruled out.17, 18
In alloxan-induced diabetic rats, altered lipid metabolism was observed indicated by increases in TC and TG levels. The hypertriglyceridemia observed in alloxan-induced diabetic rats may be due to increased absorption and formation of TG and decreased TG uptake in peripheral tissues. Hypercholesterolemia may be attributed to increased altered enzymatic pathways for the metabolism of cholesterol or due to increased dietary cholesterol absorption. Our study results show that there was a decrease in TG as well as TC levels. It has been also proposed that EEAP acts like glibenclamide by increasing insulin production from the remnant beta cells or by activating response of plasma insulin in alloxan-induced diabetic rats, lowering the TG and TC levels by increasing the activity of enzyme lipoprotein lipase and peripheral tissue utilization of cholesterol.
A previous study showed that insulin activates lipoprotein lipase and hydrolyzes normal conditions.19 Under insulin deficiency, lipoprotein lipase hydrolyzes and fails to perform normal physiological functions, leading to hypercholesterolemia. A similar mechanism of action of EEAP might not be acceptable to demonstrate to TG and TC decreasing activity of EEAP. Henceforth, it might be explained by lowering cholesterol biosynthesis, specifically decreasing the 3-hydroxy-3-methyl-glutaryl coenzyme A reductase activity by reducing the NADPH required for fatty acid and cholesterol synthesis or by increasing glucose utilization by peripheral tissues.20 SGPT and SGOT are the intracellular enzymes released into the blood stream, and they serve as a marker of tissue injury, chiefly hepatocyte as well as renal injury. In addition, increased level of liver function enzymes SGPT and SGOT in serum are not only used for the identification of liver damage but also for the metabolic syndrome diabetes mellitus. Our result obtained suggests that oral administration of EEAP for 28 days in our study showed a significant decrease in the activity of these enzymes, suggesting that EEAP has a hepatoproctive effect and ameliorating alteration in lipid metabolism. Alloxan produces reactive oxygen species which are limited to the pancreatic islets. As a result of the higher concentration of free radicals in plasma, oxidative stress increases which is of particular importance in the pathogenesis of various diseases, including cardiovascular disease and diabetes.21 In our study, it has been observed that following a 28-day administration of EEAP in diabetic rats, EEAP reduces the level of CRP production probably by reducing the level of plasma concentration of interleukin-6 and free radical scavenging activities, minimizing the inflammatory consequences in alloxan-induced diabetic rats.
In conclusion, the present study provides evidences of EEAP as a potential medicinal herb containing certain phytopharmaceuticals, which can be adjuvantly used with modern medicine for integrative management of diabetes and associated complications.
Conflicts of interest
We declare that we have no conflicts of interest.
Acknowledgments
The authors are grateful to Y.B. Chavan College of Pharmacy, Aurangabad, for providing the necessary facilities for conducting the research work. The authors are also thankful to the Botanical Survey of India for the authentication of plant material.
References
- 1.Ju J.B., Kim J.S., Choi C.W., Lee H.K., Oh T.K., Kim S.C. Comparison between ethanolic and aqueous extracts from Chinese juniper berries for hypoglycemic and hypolipidemic effects in alloxan-induced diabetic rats. J Ethnopharmacol. 2008;115:110–115. doi: 10.1016/j.jep.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 2.Chandha S.L. Natural source of antioxidants and their adequacy in diet to prevent atheroscelerosis. Mediquest. 1997;14:337–351. [Google Scholar]
- 3.Singh M.P., Panda H., editors. Daya Publishing House; New Delhi: 2005. Medicinal herbs with their formulations. [Google Scholar]
- 4.Conforti F., Giancarlo A.S., Rosa T., Monica R., Francesco M. In vitro activities of Amaranthus paniculatus L. Diamante (Diamante citron) relevant to treatment of diabetes and Alzheimer's disease. Phytother Res. 2007;21:427–433. doi: 10.1002/ptr.2077. [DOI] [PubMed] [Google Scholar]
- 5.Sharangouda S., Patil B. Estrogenic activity of petroleum ether extract of seeds of Amaranthus paniculatus on immature albino rats. IJJP. 2008;2:91–94. [Google Scholar]
- 6.Kokate C.K., Purohit A.P., Gokhale S.B. 26thed. Nirali Publishers; Pune: 2004. Textbook of Pharmacognosy; pp. 311–420. [Google Scholar]
- 7.Evans W.C. 15thed. Elsevier science; New York: 2004. Trease and Evans Pharmacognosy; pp. 253–288. [Google Scholar]
- 8.Rao K.B., Kesavulu M.M., Giri R., Apparao C. Antidiabetic and hypolipidemic effects of Momordica cymbalaria Hook fruit powder in alloxan diabetic rats. J Ethnopharmacol. 1999;67:103–109. doi: 10.1016/s0378-8741(99)00004-5. [DOI] [PubMed] [Google Scholar]
- 9.Rao B.K., Rao C.A. Hypoglycemic and antihyperglycemic activity of Syzygium alternifolium (Wt.) Walp. Seed extracts in normal and diabetic rats. Phytomedicine. 2001;8:88–93. doi: 10.1078/0944-7113-00015. [DOI] [PubMed] [Google Scholar]
- 10.Reitman S., Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. American J Clin Path. 1957;28:56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 11.Triender P. Determination of glucose using glucose oxidase with an alternative oxygen acceptor. Ann Clin Biochem. 1969;6:24–27. [Google Scholar]
- 12.Chan D.C., Watts G.F., Barrett P.H., Beilin L.J., Redgrave T.G., Mori T.A. Regulatory effects of HMG CoA reductase inhibitor and fish oils on apolipoprotein B-100 kinetics in insulin-resistant obese male subjects with dyslipidemia. Diabetes. 2002;51:2377–2386. doi: 10.2337/diabetes.51.8.2377. [DOI] [PubMed] [Google Scholar]
- 13.Rifai N., Bachorik P.S., Albers J.J. Lipids, lipoproteins and apolipoproteins. In: Burtis C.A., Ashwood E.R., editors. Tietz Textbook of Clinical Chemistry. 3rd ed. W.B. Saunders Company; Philadelphia: 1999. pp. 809–861. [Google Scholar]
- 14.Bhatia A.L., Jain M. Amaranthus paniculatus (Linn.) improves learning after radiation stress. J Ethnopharmacol. 2003;85:73–79. doi: 10.1016/s0378-8741(02)00337-9. [DOI] [PubMed] [Google Scholar]
- 15.Ghosh S., Suryawanshi S.A. Effect of Vinca rosea extracts in treatment of alloxan rats. Indian J Exp Biol. 2001;39:748–759. [PubMed] [Google Scholar]
- 16.Sharma S.R., Dwivedi S.K., Swarup D. Hypoglycaemic, antihyperglycaemic and hypolipidemic activities of Caesalpinia bonducella seeds in rats. J Ethnopharmacol. 1997;58:39–44. doi: 10.1016/s0378-8741(97)00079-2. [DOI] [PubMed] [Google Scholar]
- 17.Eddouks M., Jouad H., Maghrani M., Lemhadri A., Burcelin R. Inhibition of endogenous glucose production accounts for hypoglycemic effect of Spergularia purpurea in streptozotocin mice. Phytomedicine. 2003;10:594–599. doi: 10.1078/094471103322331890. [DOI] [PubMed] [Google Scholar]
- 18.Hu X., Sato J., Oshida Y., Xu M., Bajotto G., Sato Y. Effect of Gosha-Jinki-Gan on insulin resistance in streptozotocin-induced diabetic rats. Diabetes Res Clin Pract. 2003;59:103–111. doi: 10.1016/s0168-8227(02)00203-6. [DOI] [PubMed] [Google Scholar]
- 19.Taskinen M.R. Lipoprotein lipase in diabetes. Diabetes/Metabolism Reviews. 1987;3:551–570. doi: 10.1002/dmr.5610030208. [DOI] [PubMed] [Google Scholar]
- 20.Chi M.S. Effects of garlic products on lipids metabolism in cholesterol fed rats. Proceeding of Society of Experimental Biology and Medicine, 1711. 1982:74–178. doi: 10.3181/00379727-171-41494. [DOI] [PubMed] [Google Scholar]
- 21.Pepys M.B., Hirchfield G.M. C-reactive protein: a critical update. J Clin Invest. 2003;12:1805–1812. doi: 10.1172/JCI18921. [DOI] [PMC free article] [PubMed] [Google Scholar]