Abstract
Background
Advances in molecular genetic technologies have improved our understanding of genetic causes of rare neurological disorders with features of myoclonus.
Case Report
A family with two affected siblings, presenting with multifocal polymyoclonus and neurodevelopmental delay, was recruited for whole-exome sequencing following unyielding diagnostic neurometabolic investigations. Compound heterozygous mutations in TBC1D24, a gene previously associated with various epilepsy phenotypes and hearing loss, were identified in both siblings. The mutations included a missense change c.457G>A (p.Glu157Lys), and a novel frameshift mutation c.545del (p.Thr182Serfs*6).
Discussion
We propose that TBC1D24-related diseases should be in the differential diagnosis for children with polymyoclonus.
Keywords: TBC1D24, myoclonus
Introduction
Myoclonus is defined as a sudden, brief (less than 100 ms), shock-like muscle contraction involving agonist and antagonist muscles, leading to a sudden jerky movement.1 The lifetime prevalence of myoclonus is estimated to be 8.6 cases per 100,000 population.2 Physiological myoclonus, in the form of hiccoughs or hypnic jerks, is easily recognized. Pathological myoclonus has a wide range of etiologies including acquired/hypoxic neurological injury, as well as neoplastic, infectious, post-infectious, metabolic, and genetic causes. There are a number of ways in which myoclonus is classified, including by anatomical/physiological origin (cortical, subcortical, spinal, peripheral), or by etiology. In the approach to pathological myoclonus, it is useful to consider the distribution of myoclonus, age of onset, insidious/acute onset, triggering/alleviating factors and associated signs or symptoms (Supplementary Table 1).
Recently, advances in molecular genetic technologies have enabled accelerated gene discovery, adding to our understanding of rare neurological disorders, including myoclonus (Supplementary Table 1).
We describe two siblings with infantile-onset multifocal polymyoclonus at rest who were found on whole-exome sequencing to have mutations in TBC1D24, a gene previously associated with various epilepsy phenotypes (including familial infantile myoclonic epilepsy, migrating partial seizures of infancy),3–13 hearing impairment,14–17 and DOORS (deafness, onychodystrophy, osteodystrophy, mental retardation, and seizures) syndrome.18 Prolonged electroencephalograms (EEGs) revealed no epileptiform features; furthermore, multiple EEGs captured episodes of polymyoclonus and demonstrated no EEG correlation.
To our knowledge, this is the first report of TBC1D24 mutations in a phenotype with prolonged, multifocal polymyoclonus as the main presenting feature with no discernible features of epilepsy.
Case report
A family with two affected children was ascertained and recruited for molecular genetic analysis. The participating family gave written informed consent and the study was performed in accordance with the Declaration of Helsinki. Genomic DNA from the affected individuals, and both parents was extracted from peripheral lymphocytes by standard techniques.
Whole-exome sequencing was carried out in both affected individuals using Illumina’s TruSeq Exome Enrichment kit (Illumina, Inc San Diego, California, USA), according to manufacturer’s recommendations. Sequencing was performed on Illumina HiSeq2000 using 100-bp paired-end reads. Data were analyzed following Genome Analysis Toolkit’s (GATK) Best Practices (PMIDs, 20644199, 21478889, 25431634). To confirm the identified TBC1D24 variants direct Sanger sequencing was performed. The appropriate exon amplified by polymerase chain reaction (PCR) (primer sequences and PCR conditions on request) was directly sequenced by the Big Dye Terminator Cycle Sequencing System (Applied Biosystems Inc.) on an ABI PRISM 3730 DNA Analyzer (Applied Biosystems Inc.) and analyzed using Chromas (http://www.technelysium.com.au/chromas.html).
We present two siblings born to non-consanguineous parents of Polish descent. There is no significant family history.
The older sibling, A1, was diagnosed with cardiac arrhythmia antenatally, and was born via elective caesarean section at term. At the age of 5 weeks, his mother noted he had intermittent mouth-twitching movements while breastfeeding. This progressed to paroxysmal myoclonic twitching and jerking movements involving varying muscle groups, including those of his eyelids, face, lips, abdomen, and limbs. Myoclonic episodes ranged from short, spontaneously resolving tolerated events with small amplitude twitches to prolonged distressing events with larger amplitude jerks (Video 1A,B). He had preserved awareness with most episodes, but some prolonged episodes resulted in autonomic disturbance such as pallor, sweating, and reduced responsiveness. In casualty, prolonged episodes of myoclonus were often treated as status epilepticus. Benzodiazepines and rectal paraldehyde were reported to terminate some events. Although his symptoms were initially paroxysmal, by the age of 2 years he had almost constant myoclonus involving varying muscle groups. These symptoms were exacerbated by fatigue and abolished by sleep.
Video 1. (A) Patient A1 at various ages. The video demonstrates orolingual and facial myoclonus.
(B) Patient A1 at various ages. The video demonstrates limb myoclonus, abdominal myoclonus and widespread polymyoclonus. Towards the end of the video distal choreoathetoid movements are also present in the upper limbs.
(C) Patient A2 at approximately 3 months of age. The video demonstrates myoclonus in the right arm and orolingual myoclonus.
At the age of 10 months, A1 received a pacemaker for third-degree atrioventricular block. His development was delayed. He started walking unsteadily at 3 years and said his first word at 3 years of age. At 16 months of age, he was diagnosed with extreme hypermetropia. An early hearing screen was normal, but at the age of 5 years, he developed profound sensorineural hearing loss. On examination, he had dysplastic ears, downslanting palpebral fissures, silvery pigmentation to his hair, and a full philtrum. His weight was on the 25th centile and his head circumference was between the 9th and 25th centile. He had bilateral rotatory nystagmus. He had truncal hypotonia but increased peripheral tone, with a dynamic component, in all four limbs. Deep tendon reflexes were brisk in his lower limbs, with flexor plantar responses bilaterally. Asynchronous migratory myoclonus involving various muscle groups was easily observed throughout the examination.
Patient A2 had an unremarkable perinatal history. She started having myoclonic twitching and jerking in her limbs at the age of 6 weeks. Like her brother, this progressed to paroxysmal episodes of myoclonus, most often involving the abdomen or face. As she grew older, her myoclonus also became more widespread and constant.
A2 did not have cardiac symptoms. There have been no concerns regarding her vision or hearing. Currently, at the age of 3 years, she has poor balance and coordination in addition to speech delay. She does not have abnormal eye movements. She has normal tone and deep tendon reflexes.
Extensive neurometabolic investigations performed on blood, cerebrospinal fluid (CSF) and urine for both siblings revealed nothing of note apart from a mildly reduced CSF glucose on one occasion for A1 (1.9 mmol/l, plasma glucose 4.4 mmol/l) and two occasions for A2 (1.7 mmol/l, plasma 4.5 mmol/l; and 1.9 mmol/l, plasma 4.7 mmol/l). For both individuals, these normalized on repeat testing.
A1 had a normal male karyotype and normal microarray results. Testing for mutations in candidate genes for GLUT1 deficiency syndrome and mitochondrial disorders, including SLC2A1, POLG, and DGOUK, yielded negative results.
Repeated EEGs including prolonged EEGs encompassing sleep for both siblings showed a mild excess of slow activity, but no evidence of epileptiform activity. Polygraphic electromyogram (EMG) recordings of right and left deltoids, biceps, forearm, and quadriceps muscles demonstrated myoclonias of approximately 80–110 ms duration in all the EMG channels without any evidence of spread from one to another. A few myoclonias were more hypersynchronous, approximately 40–60 ms in duration (Supplementary Figure 2). Back-averaging did not reveal any preceding EEG change in either child. Nerve conduction studies and somatosensory evoked potential tests were normal.
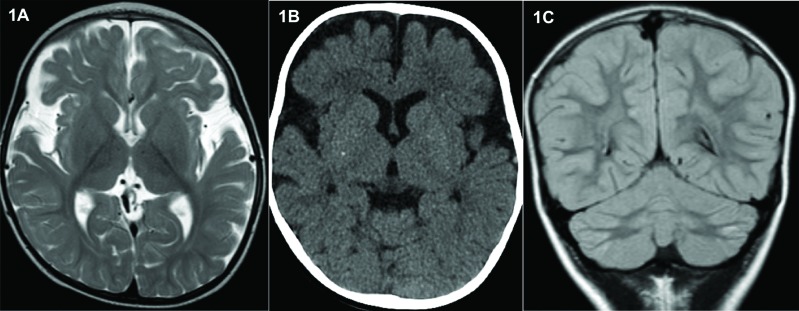
A1 had serial cranial magnetic resonance imaging (MRI) scans from the age of 2 months to 19 months. These demonstrated hypoplasia of the frontal and temporal lobes. The deep grey matter appeared normal with no evidence of cortical dysplasia (Figure 1A). A computed tomography (CT) head scan showed tiny non-specific foci of calcification in the basal ganglia (Figure 1B).
Figure 1. Magnetic Resonance Imaging Features in Sibship with TBC1D24 Mutations. (A) MRI brain scan (Axial T2-weighted) of A1 age 19 months showing underdevelopment of the frontal and temporal lobes. (B) CT head scan of A1 20 months showing small non-specific foci of calcification within the basal ganglia. (C) MRI head scan (Coronal T2-weighted) of A2 at 3 years 10 months showing symmetrical signal abnormalities and atrophy of the lateral aspects of the cerebellar hemispheres.
Cranial MRI and MR spectroscopy performed at 6 weeks of age for A2 were normal. However, a repeat MRI scan at 3 years 10 months showed atrophy of the lateral aspects of the cerebellar hemispheres and symmetrical signaling abnormalities (Figure 1C).
A1 received trials of treatment with an extensive range of medication including anti-epileptics (valproate, carbamazepine, nitrazepam, levetiracetam, clobazam), metabolic supplements (coenzyme Q10, biotin, riboflavin, thiamine), levodopa, and piracetam. None of these achieved sustained benefit, although levetiracetam and clobazam each controlled myoclonus briefly. Immunotherapy had no significant effect.
Levetiracetam exacerbated A2’s symptoms and pyridoxine was ineffective. She did not tolerate the ketogenic diet. Bromocriptine resulted in dyskinetic movements, which terminated with discontinuation of the medication. Carbamazepine afforded her some symptomatic relief.
For both siblings as symptoms were abolished by sleep, the best therapeutic strategy seemed to be chloral hydrate used, as required, to induce sleep during periods of distress.
Variants identified through whole-exome sequencing were filtered by the following criteria: 1) very low frequency in control populations (exclusion of variants with a minor allele frequency > 0.1% in the established databases and 2,000 in-house analyzed exomes); 2) presentation of homozygous or compound heterozygous changes considering an autosomal recessive inheritance pattern; and 3) prediction of putative pathogenicity based on mutation type or in silico prediction of effects on protein function and/or structure. Using these criteria, two heterozygous variants confirmed by Sanger sequencing were identified in TBC1D24 (Supplementary Figure 1): 1) a previously reported missense change c.457G>A (p.Glu153Lys; rs376712059)6,13,15 and 2) a previously unreported frameshift mutation, c.545del (p.Thr182Serfs*6) predicted to cause nonsense-mediated RNA decay or result in a truncated protein. Both mutations showed appropriate familial segregation.
Other possible disease-causing mutations identified in the two subjects that passed selection criteria were excluded (Supplementary Table 2). Mutations found in two genes (NOTCH4 and PRR21) could not be verified as it was not possible to design primers for confirmatory Sanger sequencing and segregation studies because of highly repetitive sequences. We note that whole-exome sequencing false-positive rates for indels can be as high as nearly 50%.19 Considering their phenotype, TBC1D24 was thus deemed to be the most likely candidate gene accounting for our patients’ symptoms.
Discussion
Myoclonus is rare and confirmation of a definitive diagnosis can often prove challenging. A definitive genetic diagnosis facilitates pre-pregnancy counseling and ends an often long diagnostic odyssey. Identification of causative genes can also contribute to the understanding of disease mechanisms and may facilitate development of novel targeted therapies. However, obtaining a genetic diagnosis is often complicated by genetic heterogeneity and phenotypic pleiotropy. Our report further illustrates how molecular genetic advances can facilitate gene discovery and clinical diagnosis.
TBC1D24 (OMIM 613577) encodes Tre2/Bub2/Cdc16 (TBC) 1 domain family member 24, a member of a family of Rab-specific GTPase-activating proteins.12 These have a role in coordinating Rab proteins and other GTPases for transport of intracellular vesicles. TBC1D24 interacts with ADP ribosylation factor 6 (ARF6), a GTPase with an essential role in membrane trafficking.5,20 The Rab GTPases control neuronal cell morphology and migration. In addition, TBC1D24 has a TBC lysine motif catalytic (TLDc) domain, thought to have a role in oxidative stress resistance.18,21 Loss of TBC1D24 function may result in abnormal vesicle trafficking, abnormal neuronal migration/maturation, and neurodegeneration.12,18,21
Mutations in TBC1D24 have been described in an array of disorders summarized in Table 1. Overall, among disorders associated with TBC1D24 mutations, recurring phenotypes include seizures, myoclonus, neurodevelopmental impairment, sensorineural hearing impairment, visual impairment, and cerebral and/or cerebellar atrophy on brain imaging. Movement disorders including dystonia, choreoathetosis, and dyskinesia have also been reported.12,13 Our sibship shares a number of clinical features with previously reported TBC1D24 cases. These include myoclonus, cerebellar atrophy, and neurodevelopmental impairment. A1 also had visual impairment and developed sensorineural hearing impairment.
Table 1. Phenotypes Associated with TBC1D24 Mutations.
| Clinical Phenotype | Familial Infantile Myoclonic Epilepsy (OMIM 605021) | Focal Epilepsy with Cerebrocerebellar Malformation | MMPSI (OMIM 615338) | Progressive Myoclonic Epilepsy with Dystonia (OMIM 615338) | EOEE and Hearing Loss |
|---|---|---|---|---|---|
| References | Zara et al.3, de Falco et al.4, Falace et al.5, Poulat et al.6 | Corbett et al.7, Afawi et al.8 | Milh et al.9 | Duru et al.10, Guven and Tolun11 | Stražišar et al.12 |
| Reported mutations/genotype | c.439G>C (p.Asp147His) c.1526C>T (p.Ala509Val) c.457G>A (p.Glu157Lys) |
c.751C>T (p.Phe251Leu) | c.468C>A (p.Cys156*) c.686T>A (p.Phe229Ser) |
c.969_970delGT (p.Ser324Thrfs*3) |
c.32A>G (p.Asp11Gly) c.1008delT (p.His336GInfs*12) |
| Inheritance | AR | AR | AR | AR | AR |
| Clinical features | Seizures Normal psychomotor development and neurological examination to moderate intellectual and psychomotor impairment Bulbous nose and flat nasal root | Seizures Myoclonus Moderate intellectual disability Ataxic with cerebellar signs Dystonia Dysarthria |
Seizures Psychomotor regression Axial hypotonia Loss of visual contact |
Seizures Post-ictal hemiparesis Dystonic episodes Myoclonus with startle responses to auditory and tactile stimuli Axial hypotonia Pyramidal signs Severe neurodevelopmental impairment Vulnerability to infection Bilateral optic atrophy, macular degeneration and visual impairment in one individual |
Seizures Profound sensorineural deafness Myoclonic jerks Acquired microcephaly Dyskinetic movements Axial hypotonia Poor visual contact |
| Types of seizures | GTC Myoclonic: trigger sensitive | Focal seizures with auras Tonic–clonic Myoclonic |
Focal prolonged migrating clonic seizures | Focal/unilateral Clonic seizures Myoclonic Tonic |
Clonic seizures Tonic seizures |
| EEG/EMG findings | Preserved background. 1 individual with slow background activity in occipital region. Interictal multiple diffuse spikes and slow waves. Ictal EEG with low amplitude spikes at vertex Jerk-locked back averaging confirmed cortical myoclonus | Slow background rhythms. No epileptiform discharges. Ictal EEG not available |
Focal migrating EEG discharges during seizures Interictal EEG: disorganized |
Slow background in EEG Multifocal or bilateral generalized multiple spikes and spike waves in EEG associated with myoclonias. |
Generalized spike-wave discharges with frontocentral predominance during seizures No clear EEG correlate for myoclonic jerks |
| Imaging findings | Normal 1 individual with nodular periventricular heterotopia 1 individual had MRI abnormalities in lentiform nuclei, ventricular dilatation and white matter changes post-cardiac arrest. An earlier MRI was normal | Selective atrophy and signal abnormality in cerebellum Cerebral cortical thickening most marked in cingulate regions and occipital poles |
Global cerebral atrophy sparing the posterior fossa | Thin corpus callosum Delayed myelination Diffuse cerebral atrophy (asymmetrical for one patient) Cerebellar atrophy |
Prominent frontotemporal atrophy |
| Clinical Phenotype | Spectrum of Epilepsy Phenotypes Including DOORS Syndrome | DOORS Syndrome (OMIM 220500) | Non-syndromic Deafness (DFNB86) (OMIM 614617) | Non-syndromic Hearing Loss (DFNA65) (OMIM 616044) | Migrating Paroxysmal Myoclonus and Cerebellar Signs |
|---|---|---|---|---|---|
| References | Balastrini et al.13 | Campeau et al.18 | Rehman et al.14, Bakhchane et al.15 | Azaiez et al.16, Zhang et al.17 | Doummar et al.22 |
| Reported mutations/genotype | c.32A>G (p.Asp11Gly) c.58C>T (p.Gln20*) c.115G>C (p.Ala39Pro) c.118C>T (p.Arg40Cys) c.119G>T (p.Arg40Leu) c.277C>T (p.Pro93Ser) c.313T>C (p.Cys105Arg) c.328G>A (p.Gly110Ser) c.439G>C (p.Asp147His) c.457G>A (p.Glu153Lys) c.468C>A (p.Cys156*) c.533C>G (p.Ser178Trp) c.619C>T (p.Gln207*) c.679C>T (p.Arg227Trp) c.680G>A (p.Arg227Gln) c.686T>C (p.Phe229Ser) c.724C>T (p.Arg242Cys) c.731C>T (p.Ala244Val) c.751T>C (p.Phe251Leu) c.809G>A (p.Arg270His) c.845C>G (p.Pro282Arg) c.919A>G (p.Asn307Asp) c.957G>C (p.Lys319Asn) c.969_970delGT(p.Ser324Thrfs*3) c.999G>T (p.Leu333Phe) c.1008delT (p.His336Glnfs*12) c.1126G>C (p.Gly376Arg) c.1384del (p.Glu462Serfs*61) c.1460dup (p.His487Glnfs*71) c.1079G>T (p.Arg360Leu) c.1499C>T (p.Ala500Val) c.1544C>T (p.Ala515Val) c.1661_1667del (p.Gln554Leufs*12) |
c.724C>T (p.Arg242Cys) c.118C>T (p.Arg40Cys) c.119G>T (p.Arg40Leu) c.1008delT (p.His336GInfs*12) c.1206+5G>A (Splice site) c.58C>G (p.Gln20Glu) c.328G>A (p.Gly110Ser) c.999G>T (p.Leu333Phe) |
c.208G>T (p.Asp70Tyr) c.878G>C (p.Arg293Pro) c.641G>A (p.Arg214His) c.1316insG (p.Val439Valfs*32) c.457G>A (p.Glu153Lys) c.798G>T (p.Lys266Asn) |
c.533C>T (p.Ser178Leu) | c.809G>A (p.Arg270His) |
| Inheritance | AR | AR | AR | AD | AR |
| Clinical features | Seizures In some Axial hypotonia Acquired microcephaly Poor visual contact, cortical blindness, bilateral optic atrophy, macular degeneration Sensorineural deafness Dysmorphia including bulbous nose with flat nasal root, thin or prominent philtrum, synophrys, up or down slanting palpebral fissures Acral abnormalities: hypoplastic terminal phalanges, brachydactyly Skeletal abnormalities tibial torsion, scoliosis, etc. Movement disorders: dystonic episodes, tremor, dyskinesia Ataxia Feeding difficulties Heart defects Autism spectrum disorder Psychosis Hyperactivity Peripheral neuropathy Renal anomalies |
Seizures Sensorineural deafness Small or absent nails Hypoplastic terminal phalanges 2-Oxoglutaric aciduria Neurodevelopmental Impairment Bulbous nose with flat nasal root In some individuals: Microcephaly in one-third Occasional craniosynostosis Autistic spectrum disorder Eyes: colobomas, visual impairment Heart defects (ASD/VSD), double outlet right ventricle) Kidneys, adrenal glands, and genitalia malformations |
Non-syndromic sensorineural deafness Of 15 affected individuals assessed for epilepsy in Rehman et al.16, 1 individual had a history of seizures: attributed to coincidence Family history of epilepsy |
Non-syndromic hearing loss with onset in the third decade | Paroxysmal migrating myoclonus with preserved awareness Ataxia Progressive cognitive decline |
| Clinical Phenotype | Spectrum of Epilepsy Phenotypes Including DOORS Syndrome | DOORS Syndrome (OMIM 220500) | Non-syndromic Deafness (DFNB86) (OMIM 614617) | Non-syndromic Hearing Loss (DFNA65) (OMIM 616044) | Migrating Paroxysmal Myoclonus and Cerebellar Signs |
|---|---|---|---|---|---|
| Types of seizures | Infantile spasms Febrile seizures Myoclonic Tonic seizures Clonic seizures Tonic–clonic with/without focal onset Focal seizures |
GTC Myoclonic Infantile spasms Absence seizures Focal seizures |
Not mentioned | NA | NIL |
| EEG/EMG findings | Focal epileptiform discharges: frontocentral, temporal, occipital Multifocal discharges Migrating focal discharges Generalized spike-waves |
Not mentioned | Normal | Not done | Interictal EEG: slow waves in occipital region |
| Imaging findings | Cerebellar atrophy Global cerebral atrophy Cerebral atrophy sparing the posterior fossa Hippocampal atrophy Basal ganglia atrophy Hippocampal sclerosis Delayed myelination Thin corpus callosum Hyperintensity of basal ganglia Hyperintensity in cerebellar cortex and white matter |
Thin corpus callosum Corpus callosum agenesis Dandy walker malformation Cerebellar atrophy Hyperintense T2 in cerebellar hemispheres Cortical atrophy Delayed myeliniation Increased T2 signal in frontal region Increased flair in occipital horn |
Normal | Not mentioned | Progressive hemispheric cerebellar atrophy with hypersignal of the cerebellar cortex and white matter on T2 and fluid-attenuated inversion recovery sequences |
Abbreviations: AD, Autosomal Dominant; AR, Autosomal Recessive; ASD, Atrial Septal Defect; EEG, Electroencephalogram; EOEE, Early-onset Epileptic Encephalopathy; GTC, Generalized Tonic Clonic; MMPSI, Malignant Migrating Partial Seizures of Infancy; NA, Not Available; VSD, Ventricular Septal Defect.
To our knowledge, TBC1D24 mutations have not been reported in a phenotype involving prolonged, almost continuous multifocal myoclonus as the main presenting feature with no discernible epileptiform features on repeated interictal and ictal EEG recordings. Doummar et al.22 reported a case with similar multifocal myoclonus and homozygous c.809G>A (p.Arg270His) TBC1D24 mutations. However, unlike our sibship, interictal EEGs of this case presented paroxysmal epileptiform abnormalities and there was evidence suggesting his myoclonus had cortical origin. The myoclonia our sibship present with are more variable, asynchronous, and isolated, with a multifocal segmental pattern, than Doummar’s case, who had synchronous, generalized bursts of myoclonia. Interestingly, both siblings also had frequent abdominal myoclonus, which is rare and associated with myoclonus of spinal origin.
In conclusion, our report expands the TBC1D24 mutation spectrum by describing a novel mutation and extends the gene’s phenotypic spectrum. It suggests TBC1D24 should be considered amongst candidate genes in children with myoclonus and neurodevelopmental impairment even in the absence of clear epileptiform features.
Supplementary Material
All supplementary data referenced in this article is available here: http://dx.doi.org/10.7916/D8QN6CZG.
Footnotes
Funding: A.N. is funded by a Guarantors of Brain Entry Fellowship and an Action Medical Research Training Fellowship. J.B. and R.G.’s work is supported by Fellowships from the Alzheimer’s Society. A.M. and J.N. are funded by Medical Research Council Clinical Research Training Fellowships. A.M. also receives funding from Medical Research Foundation, Child Brain Research and Young Epilepsy; and J.N. is also supported by Great Ormond Street Hospital Children’s Charities. M.A.K. is funded by a Wellcome Trust Intermediate Clinical Fellowship and receives funding from the Rosetrees Trust, Gracious Heart Charity Foundation Great Ormond Street Hospital Children’s Charities (GOSHCC) Child Brain Research and Rachel Marie Trafford Trust.
Financial Disclosures: None.
Conflict of Interest: The authors report no conflict of interest.
Ethics Statement: All patients that appear on video have provided written informed consent; authorization for the videotaping and for publication of the videotape was provided.
References
- 1.Lozsadi D. Myoclonus: a pragmatic approach. Pract Neurol. 2012;12:215–224. doi: 10.1136/practneurol-2011-000107. doi: 10.1136/practneurol-2011-000107. [DOI] [PubMed] [Google Scholar]
- 2.Caviness JN, Alving LI, Maraganore DM, Black RA, McDonnell SK, Rocca WA. The incidence and prevalence of myoclonus in Olmsted County, Minnesota. Mayo Clin Proc. 1999;74:565–569. doi: 10.4065/74.6.565. doi: 10.4065/74.6.565. [DOI] [PubMed] [Google Scholar]
- 3.Zara F, Gennearo E, Stabile M, et al. Mapping of a locus for a familial autosomal recessive idiopathic recessive myoclonic epilepsy of infancy to chromosome 16p13. Am J Hum Genet. 2000;66:1552–1557. doi: 10.1086/302876. doi: 10.1086/302876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Falco FA, Majello L, Santangelo R, Stabile M, Bricarelli FD, Zara F. Familial infantile myoclonic epilepsy: clinical features in a large kindred with autosomal recessive inheritance. Epilepsia. 2001;42:1541–1548. doi: 10.1046/j.1528-1157.2001.26701.x. doi: 10.1046/j.1528-1157.2001.26701.x. [DOI] [PubMed] [Google Scholar]
- 5.Falace A, Filipello F, La Padula V, et al. TBC1D24, an ARF6-interacting protein, is mutated in familial infantile myoclonic epilepsy. Am J Hum Genet. 2010;87:365–370. doi: 10.1016/j.ajhg.2010.07.020. doi: 10.1016/j.ajhg.2010.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poulat AL, Ville D, de Bellescize J, et al. Homozygous TBC1D24 mutation in two siblings with familial infantile myoclonic epilepsy (FIME) and moderate intellectual disability. Epilepsy Res. 2015;111:72–77. doi: 10.1016/j.eplepsyres.2015.01.008. doi: 10.1016/j.eplepsyres.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 7.Corbett MA, Bahlo M, Jolly L, et al. A focal epilepsy and intellectual disability syndrome is due to a mutation in TBC1D24. Am J Hum Genet. 2010;87:371–375. doi: 10.1016/j.ajhg.2010.08.001. doi: 10.1016/j.ajhg.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Afawi Z, Mandelstam S, Korczyn AD, et al. TBC1D24 mutation associated with focal epilepsy, cognitive impairment and a distinctive cerebro-cerebellar malformation. Epilepsy Res. 2013;105:240–244. doi: 10.1016/j.eplepsyres.2013.02.005. doi: 10.1016/j.eplepsyres.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 9.Milh M, Falace A, Villeneuve N, et al. Novel compound heterozygous mutations in TBC1D24 cause familial malignant migrating partial seizures of infancy. Hum Mutat. 2013;34:869–872. doi: 10.1002/humu.22318. doi: 10.1002/humu.22318. [DOI] [PubMed] [Google Scholar]
- 10.Duru N, Iseri SA, Selçuk N, Tolun A. Early-onset progressive myoclonic epilepsy with dystonia mapping to 16pter-p13.3. J Neurogenet. 2010;24:207–215. doi: 10.3109/01677063.2010.514368. doi: 10.3109/01677063.2010.514368. [DOI] [PubMed] [Google Scholar]
- 11.Guven A, Tolun A. TBC1D24 truncating mutation resulting in severe neurodegeneration. J Med Genet. 2013;50:199–202. doi: 10.1136/jmedgenet-2012-101313. doi: 10.1136/jmedgenet-2012-101313. [DOI] [PubMed] [Google Scholar]
- 12.Stražišar BG, Neubauer D, Paro Panjan D, Writzl K. Early-onset epileptic encephalopathy with hearing loss in two siblings with TBC1D24 recessive mutations. Eur J Paediatr Neurol. 2015;19:251–256. doi: 10.1016/j.ejpn.2014.12.011. doi: 10.1016/j.ejpn.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 13.Balestrini S, Milh M, Castiglioni C, et al. TBC1D24 genotype-phenotype correlation: Epilepsies and other neurologic features. Neurology. 2016;87:77–85. doi: 10.1212/WNL.0000000000002807. doi: 10.1212/WNL.0000000000002807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rehman AU, Santos-Cortez RL, Morell RJ, et al. Mutations in TBC1D24, a gene associated with epilepsy, also cause nonsyndromic deafness DFNB86. Am J Hum Genet. 2014;94:144–152. doi: 10.1016/j.ajhg.2013.12.004. doi: 10.1016/j.ajhg.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bakhchane A, Charif M, Salime S, et al. Recessive TBC1D24 mutations are frequent in Moroccan non-syndromic hearing loss pedigrees. PLoS ONE. 2015;10:e0138072. doi: 10.1371/journal.pone.0138072. doi: 10.1371/journal.pone.0138072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Azaiez H, Booth KT, Bu F, et al. TBC1D24 mutation causes autosomal-dominant nonsyndromic hearing loss. Hum Mutat. 2014;35:819–823. doi: 10.1002/humu.22557. doi: 10.1002/humu.22557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang L, Hu L, Chai Y, Pang X, Yang T, Wu H. A dominant mutation in the stereocilia-expressing gene TBC1D24 is a probable cause for nonsyndromic hearing impairment. Hum Mutat. 2014;35:814–818. doi: 10.1002/humu.22558. doi: 10.1002/humu.22558. [DOI] [PubMed] [Google Scholar]
- 18.Campeau PM, Kasperaviciute D, Lu JT, et al. The genetic basis of DOORS syndrome: an exome-sequencing study. Lancet Neurol. 2014;13:44–58. doi: 10.1016/S1474-4422(13)70265-5. doi: 10.1016/S1474-4422(13)70265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Belkadi A, Bolze A, Itan Y, et al. Whole-genome sequencing is more powerful than whole-exome sequencing for detecting exome variants. Proc Natl Acad Sci USA. 2015;112:5473–5478. doi: 10.1073/pnas.1418631112. doi: 10.1073/pnas.1418631112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Falace A, Buhler E, Fadda M, et al. TBC1D24 regulates neuronal migration and maturation through modulation of the ARF-6 dependent pathway. Proc Natl Acad Sci USA. 2014;111:2337–2342. doi: 10.1073/pnas.1316294111. doi: 10.1073/pnas.1316294111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Finelli MJ, Sanchez-Pulido L, Liu KX, et al. The evolutionarily conserved Tre2/Bub2/Cdc16 (TBC), lysin motif (LysM), domain catalytic (TLDc) domain is neuroprotective against oxidative stress. J Biol Chem. 2016;291:2751–2763. doi: 10.1074/jbc.M115.685222. doi: 10.1074/jbc.M115.685222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doummar D, Mignot C, Apartis E, et al. A novel homozygous TBC1D24 mutation causing multifocal myoclonus with cerebellar involvement. Mov Disord. 2015;30:1431–1432. doi: 10.1002/mds.26303. doi: 10.1002/mds.26303. [DOI] [PubMed] [Google Scholar]