Abstract
Background
Noninvasive and easy-to-use surface electromyography (EMG) is frequently utilized for the diagnosis of temporomandibular disorders (TMDs). However, few EMG parameters that consider TMDs in addition to the cranio-cervical-mandibular system have been regarded as important in traditional Korean medicine.
Methods
This clinical trial will be conducted as an assessor-blinded cross-sectional study. The participants will be classified based on the Diagnostic Criteria for TMDs Symptom Questionnaire (DC/TMD SQ) and 30 TMD patients and 30 healthy controls will be enrolled. The primary outcome will be the percentage overlapping coefficient (POC; %) in the masseter and sternocleidomastoid muscles between the patient group and healthy control group in clenching and cervical side flexion. The secondary outcomes include the score from temporomandibular joint-related questionnaires, the difference in the absolute values of EMG for the healthy group and TMD group before/after wearing intraoral appliances, and the change in the location of the temporomandibular joint as determined by X-ray imaging and 3D face photography.
Discussion
This study will provide information about the objective diagnostic method for TMD using surface EMG and will verify the effectiveness of surface EMG in diagnosing TMD. Furthermore, the method or device for diagnosis TMD will improve the expansion of treatment area to TMD by accumulating evidence for the efficacy of TKM treatment.
Keywords: diagnosis, electromyography, study protocol, temporomandibular disorder, traditional Korean medicine
1. Introduction
Temporomandibular disorders (TMDs) include a cluster of symptoms related to a number of disease entities, including pain in the masticatory muscles and temporomandibular joint (TMJ), headache, disturbances in jaw movements, and sounds in the joints while opening and closing the mouth.1, 2, 3. In particular, TMDs are caused by the impaired function of the neuromuscular system are accompanied by diverse symptoms, including depression, sleep disorders, and fatigue. Moreover, these disorders often become chronic, thereby prolonging patient pain.4, 5
In general, the anatomic structure of TMDs is diagnosed by X-ray imaging, computed tomography, and magnetic resonance imaging. However, these examinations are expensive, frequently not readily available and have limitations in recording clinical symptoms. Thus, the International Research Diagnostic Criteria (RDC) for TMDs Consortium utilized clinical symptoms to record and diagnose TMDs.1, 6 In addition, various studies have reported that mandibular kinesiography, electromyography (EMG), and sonography hold potential as diagnostic techniques for TMDs.7, 8, 9, 10, 11
Among these diagnostic techniques, EMG senses and records the electrical potential created when muscular cells are activated by neural or electrical stimulation, and the results provide information on muscular contraction, muscular tone and, muscle fatigue.10, 12 In particular, it is easy to adjust the attachment position for EMG electrodes, and this approach provides time-series data with high temporal resolution.8 Moreover, surface-type EMG, unlike needle-type EMG, is noninvasive and can analyze the total synergistic activity of a muscular movement unit as a whole withoutany pain.13 Thus, surface-type EMG has been widely used in the diagnosis of repetitive strain injuries,14 work-related musculoskeletal disorders,15 myofascial pain syndrome,16 chronic fatigue,17 fibromyalgia,15 and rehabilitation training.18
In traditional Korean medicine (TKM), doctors of Korean medicine (DKM) regard the effect of imbalance of the TMJ on the cranio-cervical-mandibular system as important and thus consider the balance of the TMJ and the location of the mandible as critical factors for treating not only TMD but also a wide range of diseases of the cranial nerve and musculoskeletal system.19, 20 However, few methods used to diagnose TMDs consider the relationship among cranio-cervical-mandibular systems, which can be easily utilized by DKM.
Thus, we aimed to design a protocol to develop EMG indicators that also considered the cranio-cervical-mandibular system, in order to objectively and scientifically diagnose TMD using noninvasive and easy-to-use surface EMG measurement equipment. We will further confirm the effectiveness of this approach by analyzing the change in EMG indicators after the application of the TMJ balance appliance (TBA).
2. Methods
2.1. Study design
This clinical study will be conducted as an assessor-blinded cross-sectional study. Individuals who agree to participate in the study and provide written informed consent will be eligible for inclusion.
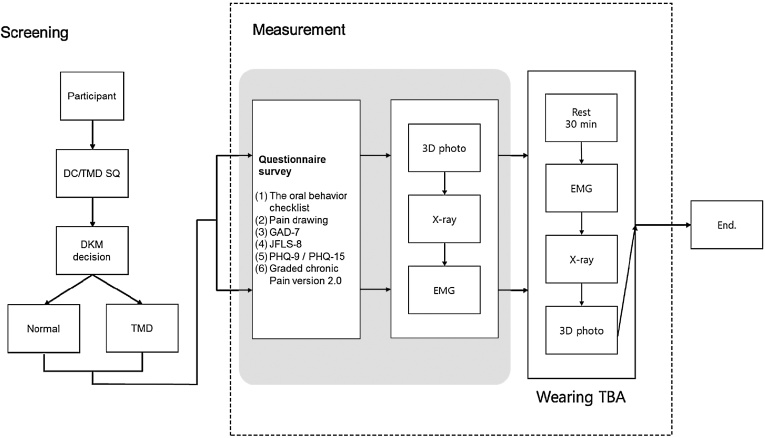
Participants will be classified into the normal and TMD groups based on the diagnostic criteria for TMDs symptom questionnaire (DC/TMD SQ, available at http://www.rdc-tmdinternational.org/TMDAssessmentDiagnosis/DCTMD.aspx), as recommended by the International RDC Consortium, and the final diagnosis of TMD or imbalance of TMJ will be confirmed by DKM. Next, all of the participants will be investigated for TMJ-related functional, behavioral, and psychological factors. Facial asymmetry, the TMJ position, and differences in muscle power between the left and right masseter and sternocleidomastoid muscles during clenching and cervical side flexion, repectively, will be measured using 3D photography, X-ray imaging, and surface EMG, respectively. In addition, patients will wear the TBA between the upper and lower teeth to secure the TMJ free space and then will be retested using 3D photography, X-ray imaging, and surface EMG. The flow chart of this study is summarized in Fig. 1.
Fig. 1.
Flow chart of study.
DC/TMD SQ, diagnostic criteria for temporomandibular disorders symptom questionnaire; DKM, doctors of Korean medicine; EMG, electromyography; GAD, Generalized Anxiety Disorder; JFLS, Jaw Functional Limitation Scale; PHQ, Patient Health Survey; TBA, temporomandibular joint balance appliance; TMD, temporomandibular disorders.
Subjects will be gathered at the Woosuk University Korean Medicine Hospital in Jeonju, South Korea, in accordance with the Declaration of Helsinki and Guidelines for Good Clinical Practice. This protocol has been registered with the Clinical Research Information Service, Republic of Korea, which is a registry in the World Health Organization Registry Network.
2.2. Types of participants
2.2.1. Inclusion criteria for all participants
-
1.
Males and females aged 19–59 years.
-
2.
Individuals who agree to participate in the study and sign the written consent after receiving a clear explanation of the objectives and characteristics of the clinical study.
2.2.2. Exclusion criteria for all participants
-
1.
Individuals with neurological disorders.
-
2.
Individuals with involuntary muscle movements.
-
3.
Individuals with skin disease or trauma in the area to be measured.
-
4.
Individuals with a history of wearing an intraoral device.
-
5.
Individuals who cannot use a TBA.
-
6.
Individuals who have intraoral inflammation diseases and dental diseases and/or wear intraoral equipment such as dentures and implants.
-
7.
Pregnant women.
-
8.
Individuals who are unable to fill out the form related to the study.
-
9.
Other persons who are judged inappropriate for the study by the investigators.
2.3. Sample size
This study aims to verify a TMD-specific EMG indicator, i.e., the percentage overlapping coefficient (POC), between the left and right masseter muscle and sternocleidomastoid muscle during maximum voluntary clenching and cervical side flexion. However, there are no previous data available to undertake a formal power calculation to determine the required sample size. Thus, we designed the current study as a pilot to provide the initial data needed to perform the power calculation required for a large-scale, randomized controlled trial. The sample size of 30 individuals in each group was chosen as the minimum sample size for statistical significance for univariate analysis.
2.4. Assessor blinding
The designated assessor will screen participants and divide them into the patient group or healthy control group. Screening results will not be disclosed to the participants and other investigators until the end of the study. Only the assessor will acknowledge whether a specific individual is included this study and to which group he/she belongs.
Separate assessors involved in the photography, radiography, and EMG will be established.
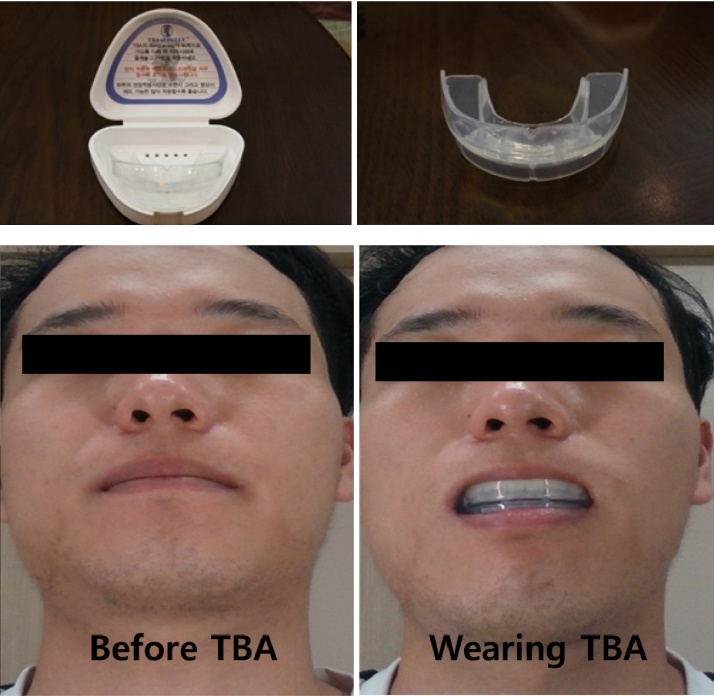
2.5. TMJ balancing appliance
The TBA is an intraoral appliance composed of non-toxic medical silicon that is designed to facilitate medical examination in perioral structures, including the TMJ, and to secure the free space of the TMJ. The TBA will be purchased from a medical device company (JinBiotech Co., Ltd., Cheonan, Korea) where their products are certified by Korea Food and Drug Administration. The size of TBA is distinguished from No. 1 (width 55 mm, length 43 mm) to No. 10 (width 75 mm, length 51 mm) and will be tailored to the area of each participant’s oral cavity.
The TBA is usually placed between the upper and lower teeth by matching the upper and lower sides. After the baseline measurement of face-line, TMJ space, and EMG, we will analyze the change in these three parameters after TBA application (Fig. 2).
Fig. 2.
Temporomandibular joint balancing appliance.
2.6. Data collection
Outcome measurements will be checked by an independent assessor for each patient. These data will be written on the case report form by a certificated clinical research coordinator.
2.7. Measurement
2.7.1. Primary outcome measurement
2.7.1.1. Surface EMG measurement
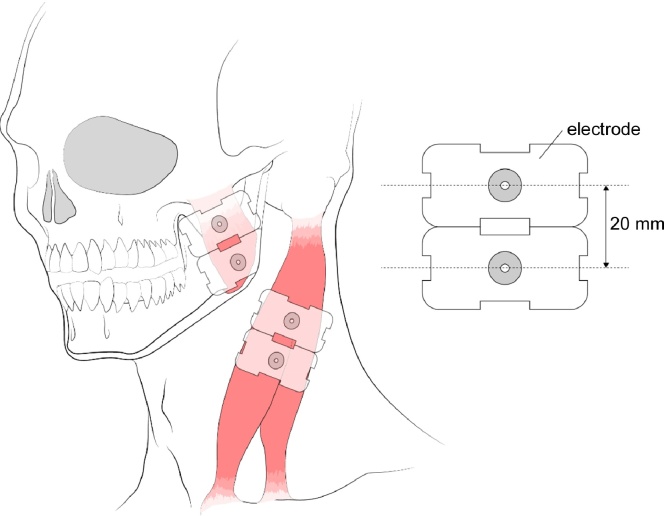
The healthy control and TMD patients shall take a break for 30 minutes before the EMG measurement. EMG will be performed using an eight-channel wireless surface EMG system (LXM5308; LAXTHA, Daejeon, South Korea) in a room with constant humidity and temperature. Four pairs of wet electrodes (T246H; Neuro Medi, Wonju, Kangwondo, South Korea) will be placed with an interelectrode distance of 2 cm on the masseter (3 cm and 5 cm above the mandibular angle) and sternocleidomastoid muscles (4 cm and 6 cm below the mastoid process) of both sides in parallel with the direction of the muscle fibers after cleaning the skin with ethanol swabs (Fig. 3). A plate ground electrode will be positioned in the occipital region. Each electrode will be fixed with hypoallergenic surgical tapes (3 M Micropore Surgical Tape Tan 1533-1; 3 M, St. Paul, MN, USA).
Fig. 3.
Attached position of electromyography electrodes.
EMG activity will be recorded while each participant maintains a sitting position with his/her eyes open, looking straight ahead, and a head position that allows the Frankfurt plane along the ground. EMG recordings of the masseter and sternocleidomastoid muscles will be executed while the participants perform clenching and cervical side flexion posture tasks as follows.
Clenching and cervical side flexion:
Task 1: the participants relax and close their mouths without clenching their teeth.
Task 2: the participants clench their teeth as much as possible for 5 seconds after taking a 30-second break. This procedure is repeated six times at intervals of 10 seconds (first clenching).
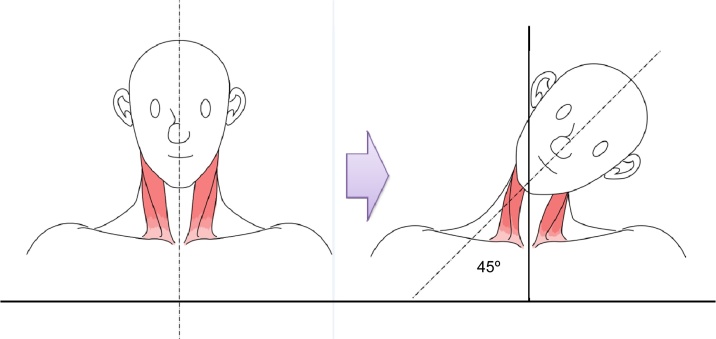
Task 3: the participants bend their heads laterally at an angle of 45 degrees for 5 seconds after taking a 10-second break. This procedure is repeated six times at intervals of 10 seconds (Fig. 4; first side flexion).
Fig. 4.
Cervical side flexion.
Task 4: to induce structural change in the perioral structure, the participants wear the TBA softly without biting it with their teeth and break for 10 minutes.
Task 5: the participants bite the TBA as much as possible for 5 seconds after taking a 30-second break. This procedure is repeated six times at intervals of 10 seconds (second clenching).
Task 6: the participants wear the TBA without biting it for 30 seconds after the third task. Thereafter, they bend their heads laterally at an angle of 45 degrees for 5 seconds. This procedure is repeated six times at intervals of 10 seconds (second side flexion).
2.7.2. Other measurement items
2.7.2.1. Patient’s consent, screening number assignment, and demographic survey
The study participants will be recruited through open recruitment promoted by posters and online advertisements. Participants who are recruited will be given detailed information related to the study (i.e., the purpose and content of the study and the risks and benefits of participating in the clinical trial). Written consent will be obtained from all participants. A screening number will then be allocated to each individual, and a demographic survey of each participant will be conducted and documented. The documentation requirements are as follows: (1) whether the participants provide written informed consent and the date of that consent; (2) the participant’s initials; (3) the date of birth on the participant’s identification card; (4) vital signs (body temperature, blood pressure/pulse rate); (5) demographic and sociological information (gender, age, height, weight, occupation); (6) smoking status; and (7) drinking status.
2.7.2.2. Investigation of the patient’s medical history including medications
At the screening visit, an investigator will examine and record the participant’s medical information [i.e., history of diseases (within the past 3 years), surgeries (within the past 5 years), drugs (within the past 1 month), and other treatments] in detail by taking a history and reviewing medical records. If any medications are taken or treatments are performed without the investigator’s clinical judgement that may influence the results of this study, it may be necessary to remove the participant from the study.
2.7.2.3. Photographing each partcipant’s face
To evaluate the facial asymmetry of the TMD patients, the participants will undergo 3D face photography (RealSense SR300; Intel, Santa Clara, CA, USA).
2.7.2.4. Radiography (X-ray)
The participants will undergo X-ray scanning of the left and right TMJ before and after wearing the TBA to confirm the TMD anatomical structure and anatomical status of the TMJ. X-rays of this joint will be carried out in the axiolateral oblique views (Left and Right) .
2.7.2.5. Questionnaire survey
We will survey the participants using AXIS-II instruments developed by the International RDC-TMD Consortium to investigate TMJ-related functional, behavioral, and psychological factors. The included questionnaires in the AXIS-II instruments are the Oral Behavior Checklist, pain drawing, Generalized Anxiety Disorder (GAD-7), Jaw Functional Limitation Scale (JFLS-8), Patient Health Survey (PHQ-9 and PHQ-15), and Graded Chronic Pain version 2.0 examinations. All of the questionnaire surveys are available at http://www.rdc-tmdinternational.org/TMDAssessmentDiagnosis/DCTMD.aspx
2.8. Data monitoring
In order to ensure the accuracy of data, investigators will receive thorough training in questionnaires learning, EMG measurements, and 3D face photography acquisition before starting the trial. Also, an official data monitoring committee will be formed by Korea Institute of Oriental Medicine. Regular monitoring will be confirmed by the committee according to the standing operating procedure. Case report form, informed consent forms, study files, compliance with treatments, serious adverse events, and data records will all be examined.
2.9. Outcome
2.9.1. Primary outcome
The primary outcome will be the POC (%) in the masseter and sternocleidomastoid muscles between the patient group and healthy control group.21 The POC calculation is as follows.
| POC = [1 − ∑(R muscle RMS − L muscle RMS)/∑(R muscle RMS + L muscle RMS)] × 100, |
Where R and L are right and left, respetively. RMS is the root mean square of the EMG signal.
2.9.2. Secondary outcomes
-
1.
Difference in the POC before and after wearing the TBA between the patient group and healthy control group.
-
2.
Difference in the scores of the GAD-7, JFLS-8, PHQ-9, and PHQ-15 and the Graded Chronic Pain version 2.0 examinations between the patient group and healthy control group.
-
3.
Location change in the TMJ measurement by X-ray imaging and 3D face photography.
-
4.
Correlation between the POC and scores of each questionnaire.
-
5.
Correlation between the POC and face asymmetry results from 3D photography.
-
6.
Correlation between the change in the TMJ free space and POC value.
2.10. Adverse events
Any expected or unexpected adverse events will be reported by the participants and practitioners. From the start of participation in the clinical study, investigators will ask the participants about adverse events. In the event of the presence or occurrence of adverse events, the following information will be recorded in detail: (1) temporary pain in the TMJ; (2) temporary pain in the teeth and gingiva; (3) intraoral hypersensitivity reaction (inflammation, allergy); (4) nausea/vomiting; and (5) skin hypersensitivity reaction caused by the EMG electrode attachment (irritant contact dermatitis, skin inflammation).
2.11. Statistical analysis
All of the analyses will be executed using PASW, version 18.0 for Windows (SPSS Inc. Chicago, IL, USA). Statistical analysis will be undertaken as the intent-to-treat and will include all participants except cases with serious protocol violations.
2.11.1. Method of analysis of the primary outcome
-
(1)
The POC of the masseter muscle during clenching between the patient group and healthy control group will be subjected to an independent sample t test.
-
(2)
The POC of the sternocleidomastoid muscles during cervical flexion between the patient group and healthy control group will be subjected to an independent sample t test.
-
(3)
The specificity and sensitivity will also be analyzed to determine the cut-off level of the POC for diagnosing TMD.
2.11.2. Method of analysis of the secondary outcome
-
(1)
To compare each group before and after wearing the TBA, paired t test or repeated measures analysis of variance will be conducted.
-
(2)
Comparison of the scores of the questionnaires (GAD-7, JFLS-8, PHQ-9, and PHQ-15 and Graded Chronic Pain version 2.0 examinations) between the groups will be analyzed by an independent sample t test.
-
(3)
Correlation analysis will be conducted to verify the relationship between the POC, the scores of each questionnaire, and the face asymmetry results from 3D photography, as well as the relationship between the change in the TMJ free space and POC value.
For the continuous variables, independent sample t tests will be conducted to assess the average change in the item, and nonparametric analysis will be applied if parametric analysis is unsuitable. If the variables need to be controlled, an analysis of covariance will be performed.
3. Discussion
This cross-sectional study protocol aims to develop an easy and objective diagnostic tool for TMDs and the imbalance of TMJ using EMG. Here, we assumed that TMDs cause the asymmetry of muscular strength between the right and left sides of the jaw.
In particular, the masseter muscle, one of the masticatory muscles, is directly related to jaw motion, and previous studies have reported that TMDs cause asymmetry of muscle strength during the clenching motion.22, 23, 24 In addition, the sternocleidomastoid muscle is associated with the head–neck position and cranio-cervical-mandibular dysfunction.23, 24. Thus, we will evaluate the POC, representing the difference in muscular strength between the left and right sides of the masseter and sternocleidomastoid muscles during clenching and cervical side flexion to diagnose the degree of TMD.
Several previous studies have attempted to diagnose TMDs or confirm their treatment through surface EMG because this method provides objective and quick results compared to questionnaire-based surveys or radiological diagnosis.25, 26, 27, 28 However, most previous studies focused on the masseter and temporalis muscles during jaw motion,27 and few studies were designed to consider the effect of imbalance of the TMJ on the cranio-cervical-mandibular system.23, 29 Therefore, we will apply surface EMG to the sternocleidomastoid muscles during cervical side flexion and confirm the diagnostic effectiveness of surface EMG by utilizing the TBA to secure the free space of the TMJ in TKM.20
We expect that a difference between the POC of the right and left sides of the masseter muscle and sternocleidomastoid muscle will be observed. These differences are expected to indicate the imbalance of TMJ and will be a parameter for diagnosing TMD objectively.
Despite the efficacy of TKM in treatment of various diseases, including TMD,19, 20, 30 many TKM doctors meet a limitation in diagnosis or communication with patients because of the restricted use of medical devices in Korea. The results, expected to be collected from the clinical trials following this protocol, would establish an EMG parameter which may applied to the medical device for TMD diagnosis.
In conclusion, this protocol will provide information about the objective diagnostic method for TMD using surface EMG and will verify the effectiveness of surface EMG in diagnosing TMD. Also, the method or device used for the diagnosis of TMD will improve the treatment for TMD by accumulating evidence for the efficacy of TKM treatment.
3.1. Ethics and dissemination
This study will be conducted after approval is granted by the institutional review board (IRB) of Woosuk University Jeonju Oriental Medicine Hospital (WSOH IRB 1607-04). The study has been registered with the Clinical Research Information Service (CRIS) of the National Research Institute of Health (KCT0002003). At the time of manuscript submission, the study had been in the stage of participant enrollment. The results will be published in a peer-reviewed journal and will be disseminated electronically and in print.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Acknowledgments
This research was funded by the Korea Institute of Oriental Medicine (C16100 and C16090). Funding had no influence on this study.
References
- 1.Schiffman E., Ohrbach R., Truelove E., Look J., Anderson G., Goulet J.P. Diagnostic criteria for temporomandibular disorders (DC/TMD) for clinical and research applications: recommendations of the International RDC/TMD Consortium Network and Orofacial Pain Special Interest Group. J Oral Facial Pain Headache. 2014;28(1):6–27. doi: 10.11607/jop.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wieckiewicz M., Boening K., Wiland P., Shiau Y.Y., Paradowska-Stolarz A. Reported concepts for the treatment modalities and pain management of temporomandibular disorders. J Headache Pain. 2015;16:106–117. doi: 10.1186/s10194-015-0586-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Check J.H. Dextroamphetamine sulfate treatment eradicates long-term chronic severe headaches from temporomandibular joint syndrome–a case that emphasizes the role of the gynecologist in treating headaches in women. Clin Exp Obstet Gynecol. 2016;43(1):119–122. [PubMed] [Google Scholar]
- 4.Yap A.U., Chua E.K., Dworkin S.F., Tan H.H., Tan K.B. Multiple pains and psychosocial functioning/psychologic distress in TMD patients. Int J Prosthodont. 2002;15(5):461–466. [PubMed] [Google Scholar]
- 5.Fuentes Fernandez R., Carter P., Munoz S., Silva H., Oporto Venegas G.H. Evaluation of validity and reliability of a methodology for measuring human postural attitude and its relation to temporomandibular joint disorders. Singapore Med J. 2016;57(4):204–208. doi: 10.11622/smedj.2015159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.John M.T., Dworkin S.F., Mancl L.A. Reliability of clinical ternporomandibular disorder diagnoses. Pain. 2005;118(1-2):61–69. doi: 10.1016/j.pain.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 7.Sato S., Nasu F., Motegi K. Analysis of kinesiograph recordings and masticatory efficiency after treatment of non-reducing disk displacement of the temporomandibular joint. J Oral Rehabil. 2003;30(7):708–713. doi: 10.1046/j.1365-2842.2003.01122.x. [DOI] [PubMed] [Google Scholar]
- 8.Santana-Mora U., Cudeiro J., Mora-Bermudez M.J., Rilo-Pousa B., Ferreira-Pinho J.C., Otero-Cepeda J.L. Changes in EMG activity during clenching in chronic pain patients with unilateral temporomandibular disorders. J Electromyogr Kinesiol. 2009;19(6):e543–9. doi: 10.1016/j.jelekin.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Manfredini D., Favero L., Michieli M., Salmaso L., Cocilovo F., Guarda-Nardini L. An assessment of the usefulness of jaw kinesiography in monitoring temporomandibular disorders: correlation of treatment-related kinesiographic and pain changes in patients receiving temporomandibular joint injections. J Am Dent Assoc. 2013;144(4):397–405. doi: 10.14219/jada.archive.2013.0133. [DOI] [PubMed] [Google Scholar]
- 10.Li B.Y., Zhou L.J., Guo S.X., Zhang Y., Lu L., Wang M.Q. An investigation on the simultaneously recorded occlusion contact and surface electromyographic activity for patients with unilateral temporomandibular disorders pain. J Electromyogr Kinesiol. 2016;28:199–207. doi: 10.1016/j.jelekin.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Ho K.Y., Ho S., Colletti P.M. Use of ultrasonography for assessing treatment efficacy in a case with ankylosis of the temporomandibular joint. J Orthop Sports Phys Ther. 2016;46(3) doi: 10.2519/jospt.2016.0404. 225–5. [DOI] [PubMed] [Google Scholar]
- 12.Baba K., Ai M., Mizutani H., Enosawa S. Influence of experimental occlusal discrepancy on masticatory muscle activity during clenching. J Oral Rehabil. 1996;23(1):55–60. doi: 10.1111/j.1365-2842.1996.tb00812.x. [DOI] [PubMed] [Google Scholar]
- 13.Bonfiglioli R., Botter A., Draicchio F., Calabrese M., Mussoni P., Curti S. Usefulness of surface electromyography of hand muscles in the assessment of myoelectric parameters changes due to repetitive manual tasks. G Ital Med Lav Ergon. 2007;29(Suppl 3):575–578. [PubMed] [Google Scholar]
- 14.Rainoldi A., Gazzoni M., Casale R., Surface E.M.G. signal alterations in Carpal Tunnel syndrome: a pilot study. Eur J Appl Physiol. 2008;103(2):233–242. doi: 10.1007/s00421-008-0694-x. [DOI] [PubMed] [Google Scholar]
- 15.Elert J.E., Rantapaa-Dahlqvist S.B., Henriksson-Larsen K., Lorentzon R., Gerdle B.U. Muscle performance, electromyography and fibre type composition in fibromyalgia and work-related myalgia. Scand J Rheumatol. 1992;21(1):28–34. doi: 10.3109/03009749209095059. [DOI] [PubMed] [Google Scholar]
- 16.Casale R., Sarzi-Puttini P., Atzeni F., Gazzoni M., Buskila D., Rainoldi A. Central motor control failure in fibromyalgia: a surface electromyography study. BMC Musculoskelet Disord. 2009;10:78–86. doi: 10.1186/1471-2474-10-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chisari C., Bertolucci F., Dalise S., Rossi B. Chronic muscle stimulation improves muscle function and reverts the abnormal surface EMG pattern in myotonic dystrophy: a pilot study. J Neuroeng Rehabil. 2013;10:94–100. doi: 10.1186/1743-0003-10-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu L., Chen X., Lu Z., Cao S., Wu Z.X. Development of an EMG-ACC-based upper limb rehabilitation training system. IEEE Trans Neural Syst Rehabil Eng. 2016 doi: 10.1109/TNSRE.2016.2560906. [DOI] [PubMed] [Google Scholar]
- 19.Yin C.S.K.H., Lee Y.J., Chun S.I., Y.J. Lee Functional Cerebrospinal Therapy (FCST), a new physiologic therapeutics developed as meridian yin-yang balance approach. Korean J. Acupunct. 2005;22:169–1674. [Google Scholar]
- 20.Lee Y.J., Lee J.K., Jung S.C., Lee H.W., Yin C.S., Lee Y.J. Case series of an intraoral balancing appliance therapy on subjective symptom severity and cervical spine alignment. Evid Based Complement Alternat Med. 2013;2013:181769. doi: 10.1155/2013/181769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferrario V.F., Sforza C., Colombo A., Ciusa V. An electromyographic investigation of masticatory muscles symmetry in normo-occlusion subjects. J Oral Rehabil. 2000;27(1):33–40. doi: 10.1046/j.1365-2842.2000.00490.x. [DOI] [PubMed] [Google Scholar]
- 22.Gay T., Piecuch J.F. An electromyographic analysis of jaw movements in man. Electromyogr Clin Neurophysiol. 1986;26(5-6):365–384. [PubMed] [Google Scholar]
- 23.Ries L.G., Alves M.C., Berzin F. Asymmetric activation of temporalis, masseter, and sternocleidomastoid muscles in temporomandibular disorder patients. Cranio. 2008;26(1):59–64. doi: 10.1179/crn.2008.008. [DOI] [PubMed] [Google Scholar]
- 24.Santander H., Miralles R., Perez J., Valenzuela S., Ravera M.J., Ormeno G. Effects of head and neck inclination on bilateral sternocleidomastoid EMG activity in healthy subjects and in patients with myogenic cranio-cervical-mandibular dysfunction. Cranio. 2000;18(3):181–191. doi: 10.1080/08869634.2000.11746131. [DOI] [PubMed] [Google Scholar]
- 25.Ferrario V.F., Sforza C., Tartaglia G.M., Dellavia C. Immediate effect of a stabilization splint on masticatory muscle activity in temporomandibular disorder patients. J Oral Rehabil. 2002;29(9):810–815. doi: 10.1046/j.1365-2842.2002.00927.x. [DOI] [PubMed] [Google Scholar]
- 26.Klasser G.D., Okeson J.P. The clinical usefulness of surface electromyography in the diagnosis and treatment of temporomandibular disorders. J Am Dent Assoc. 2006;137(6):763–771. doi: 10.14219/jada.archive.2006.0288. [DOI] [PubMed] [Google Scholar]
- 27.Suvinen T.I., Kemppainen P. Review of clinical EMG studies related to muscle and occlusal factors in healthy and TMD subjects. J Oral Rehabil. 2007;34(9):631–644. doi: 10.1111/j.1365-2842.2007.01769.x. [DOI] [PubMed] [Google Scholar]
- 28.Tartaglia G.M., Moreira Rodrigues da Silva M.A., Bottini S., Sforza C., Ferrario V.F. Masticatory muscle activity during maximum voluntary clench in different research diagnostic criteria for temporomandibular disorders (RDC/TMD) groups. Man Ther. 2008;13(5):434–440. doi: 10.1016/j.math.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 29.Ferrario V.F., Sforza C., Dellavia C., Tartaglia G.M. Evidence of an influence of asymmetrical occlusal interferences on the activity of the sternocleidomastoid muscle. J Oral Rehabil. 2003;30(1):34–40. doi: 10.1046/j.1365-2842.2003.00986.x. [DOI] [PubMed] [Google Scholar]
- 30.Eom T.M., Lee Y.J., Oh J.M., Park H.J., Park J.S., Yoo H.R. Effect of Intraoral Balancing Appliance Therapy on Tic disorder Non-Randomized, Observational Clinical Study. Integr Med Res. 2015;4(1):98. [Google Scholar]