Abstract
In order to achieve the comprehensive quality control of Chinese herbal medicine (CHM), the conventional practice of selecting a single marker for testing has been gradually replaced by the determination of multiple active components based on the characteristics of the synergistic interaction of CHM and applicability of sophisticated analytical techniques. However, with a huge number of CHM in the market and more complex preparations, the limited availability of various standard substances for quantitative analysis has been a major bottleneck in realizing the goal. To overcome these uprising problems, quantitative analysis of multi-components by single marker (QAMS) was proposed and accepted as a new method to reflect the internal quality of CHM. In this review, the current knowledge about QAMS is systematically summarized, including the general content of QAMS, current status, and general procedure. Additionally, speculation is proposed about the future applications of QAMS approaches in the modernization and standardization of CHM.
Keywords: Chinese herbal medicine, quantitative analysis of multi-components by single marker, relative correction factor
1. Introduction
Nowadays, Chinese herbal medicine (CHM) has been attracting attention increasingly due to its long-standing clinical application and reliable therapeutic efficacy.1, 2 Despite the great advances in modern pharmaceutical research, CHM continues to play an important role in the prevention and treatment of various diseases.3 Therefore, the quality control of CHM is highly significant because it directly decides the curative effect of CHM.4 However, the internal quality control of CHM is more difficult than that of western drugs considering the myriad of chemicals consisted in CHM and the interactions of multiple phytochemicals contributing to the curative effect.5, 6 Therefore, the internal quality control of CHM remains as the research focus and is difficult in CHM study. Thus, reliable methods are required to assay the amounts of active or toxic compounds in CHM to ensure the safety and internal quality control of CHM.7
Currently, there are two main methods for the quantitative determination of components in CHM used for quality control. One method involves the use of an active component or a marker compound as the internal standard substance for internal quality control. Nevertheless, it is far from sufficient to assess the other effective components in CHM.8, 9 The other method involves the use of multiple reference substances for quality assessment. However, high-purity internal standard substances or reference substances of herbal medicines are expensive and insufficient, especially when the component is in a low content and difficult to be purified from CHM.10, 11 Furthermore, some components of CHM become unstable when purified from a complicated matrix.12 Considering these reasons, the scarcity and high cost of the reference substances have been the two major bottlenecks in the implementation of multi-components in routine quality control. Therefore, it is urgently necessary to develop a convenient and low-cost approach for the internal quality control of CHM.
In order to tackle this urgency, a rational method for quality control of CHM called quantitative analysis of multi-components by single marker (QAMS) was first systematically proposed by Wang et al13 in 2006. It only determines one of the components which are inexpensive and easy to get to realize simultaneous determination of multiple components, according to the internal relationship between the efficacy of CHM and proportion of the other effective ingredients.14 This method is also called simultaneous determination of multiple components or single standard to determine multi-components. QAMS has been gradually recognized and approved by researchers since proposed. For instance, in the United States Pharmacopoeia (the 37 version), QAMS was applied in establishing the quality standards of part natural medicines, such as red clover, Echinacea angustifolia, Ginkgo, Hypericum perforatum L., and milk thistle.15 The European Pharmacopoeia Standards also adopted QAMS to build the quality control specification of herbal medicine, such as ginkgo leaf and garlic powder.16 In 2010, this method was adopted by the Chinese Pharmacopoeia.17 Thus, QAMS has been widely accepted and applied in the quality control of herbal medicine.
QAMS is considered as a potential method to reflect the internal quality of CHM. Being widely used in the researches of CHM, it is indispensable in the process of modernization and internationalization of CHM.18 Therefore, in this study, the general content of QAMS, current status, and future applications have been systematically elaborated to aid researchers in mastering this method, encourage further research on CHM, and provide ideas and references for the internal quality control of CHM.
2. Rationale and general content of QAMS studies
2.1. Rationale for QAMS
According to the principle that within a concentration range, the absorption of an analyte (chemical component) is linearly proportional to sample concentration (or mass) and their relations could be described with the equation: W = f.A (where W is the sample concentration, A is the response peak area of the analyte, and f is the correction factor).13 The value of f is a constant related with the detected substance and the sensitivity of the detector. Supposed several components coexisting in the CHM sample, each chemical component could be described in the equation (1).19
| (1) |
where Ai is the peak area of component under test and Wi is the concentration of the component under test.
To obtain the relative correction factor (RCF), supposing selected “k” as the internal reference substance, “i” as one of the other investigated components, RCF between components “k” and “i” is established through equation (2).
| (2) |
With the results of fki, the quantification of other investigated substances in the CHM sample could be carried out according to the following equation (3).9, 20
| (3) |
Therefore, the quantitative analyses of other components can be performed by detecting the content of internal reference substance and then calculating the contents of multi-components by RCFs.
2.2. General content of QAMS studies
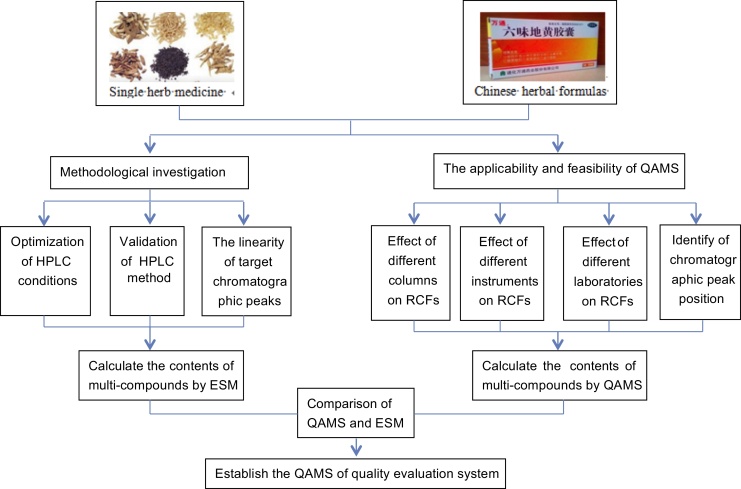
In the external standard method (ESM), the content of all the reference standards corresponding to analytes needs to be determined. In comparison, in the QAMS method, only the content of internal standard substance needs to be determined. Therefore, QAMS can not only greatly reduce the detection time and the cost of the experiment but also improve the practicability of the method and control the quality of herbal or botanical products more effectively and comprehensively. With further elaborate deepening research on QAMS, a prototype had been formed, and the process of QAMS study on CHM is divided into three sections.9, 13, 20, 21, 22, 23
The first section is high-performance liquid chromatography (HPLC) method optimization and validation. To optimize the HPLC method, it is critical to select a suitable mobile phase, elution mode, and detection wavelength. Besides, according to the established HPLC method, method precision, repeatability, stability, and recovery methodology were carried out to investigate the reliability and repeatability of the method. Additionally, standard linear calibrations curve with the r2, linear range, limit of detection, and limit of quantification were determined for each investigated compound in order to calculate the content of each investigated compound by ESM.
The second section is the ruggedness and robustness of QAMS. The ruggedness and robustness test was designed to determine all the potential and changeable factors on RCF, which mainly rely on the consistency of the environmental and operational parametric variables, such as different columns, HPLC instruments, environment conditions, flow rate, and column temperature. To ensure the validity of this newly developed method, validation was assessed by calculating the relative standard deviations (RSD; generally, when RSD < 5%, the results showed that the errors had a small influence on RCF). Furthermore, the position of investigated compounds—for better authentication as well as convenience to quantitative determination of CHM the next time, the relative retention time of the other investigated compounds with internal reference standard by different columns, instruments need to be investigated.
The final section is to evaluate and verify the QAMS feasibility for the determination of multi-compounds in CHM. First, calculate the content of internal reference standard and then acquire the other content of reference standards according to their RCFs. Second, calculate the content of all reference standards by ESM. It is indicated that the development of QAMS is successful when RSD of the results in triplicate using the QAMS and ESM are less than 5%. Finally, establish the QAMS quality evaluation system, which can accurately calculate the content of effective components and reflect the internal quality of CHM. The general content of QAMS studies can be summarized as shown in Fig. 1.
Fig. 1.
General content of QAMS studies.
ESM, external standard method; HPLC, high-performance liquid chromatography; QAMS, quantitative analysis of multi-components by single marker; RCF, relative correlation factor.
3. QAMS research progress on CHM
There is a rising trend in the amount of published research work related to QAMS studies on CHM in recent years. Studies on QAMS started to appear around 2006, and the research achievements in this area have been increasing since the last 10 years. Beyond the Chinese core journals, articles pertaining to research on CHM have been published in Science Citation Index-cited journals as well, such as in Analytical and Bioanalytical Chemistry, Food Chemistry and Journal of Chromatography A.60, 73, 74 Moreover, the 2010 edition of Chinese Pharmacopoeia has included the QAMS method for determining the content of berberine, epiberberine, coptisine, palmatine, and jatrorrhizine in Rhizoma coptidis.75
Summarized from the above published papers, the research objects of QAMS mainly include single-herb medicine, Chinese herbal prescription, and Chinese medicine preparations, among which single herb accounts for the vast majority (Table 1). The main cause for this situation is that the compounds in the prescriptions or preparations of Chinese medicine are so complicated that it is not easy to determine enough markers for quality control and evaluation. Thus, the application of QAMS method in the analysis of Chinese herbal prescription or Chinese medicine preparations needs to be further improved and optimized to ensure the safety and efficacy of CHM in clinical use.
Table 1.
Study on the quantitative analysis of multi-components by single marker for Chinese herbal medicine.
| Names | Composition determination | Internal content | Relative correction Factor | References |
|---|---|---|---|---|
| Coptidis Rhizoma | Berberine hydrochloride (1); jatrorrhizine hydrochloride (2); columbamine hydrochloride (3); epiberberine hydrochloride (4); coptisine hydrochloride (5); palmatine hydrochloride (6) | Berberine hydrochloride | f1/2 = 1.131; f1/3 = 0.999; f1/4 = 1.011; f1/5 = 1.076; f1/6 = 1.025 | 24 |
| Epimedii Herba | Icariin (1); epimendin A (2); epimendin B (3); epimendin C (4) | Icariin | f1/2 = 1.352; f1/3 = 1.384; f1/4 = 1.340 | 25 |
| Zhilining tablets | Dehydroandrographolide (1); andrographolide (2); costunolide (3); dehydrocostuslactone (4) | Dehydroandrographolide | f1/2 = 0.872; f1/3 = 1.424; f1/4 = 1.337 | 26 |
| Danshen mixture | Sodium danshensu (1); protocatechuic aldehyde (2); caffeic acid (3); rosemary acid (4); Dan phenolic acid B (5) | Sodium danshensu | f2/1 = 7.292; f3/1 = 4.265; f4/1 = 3.519; f5/1 = 2.749 | 27 |
| Yinqiao Jiedu serial preparations | Chlorogenic acid (1); neochlorogenic Acid (2); 4-caffeoylquinic acid (3); caffeic acid (4); isochlorogenic acid B (5); isochlorogenic acid A (6); isochlorogenic acid C (7) | Chlorogenic acid | f2/1 = 1.088; f3/1 = 1.109; f4/1 = 0.592; f5/1 = 0.940; f6/1 = 0.871; f7/1 = 0.799 | 28 |
| Rhei Radix et Rhizome | Rhein (1); gallic acid (2); catechin (3); emodin-8-O-glucoside (4); aloe-emodin (5); emodiu (6); chryopbauol (7); physcion (8) | Rhein | f1/2 = 3.75; f1/3 = 3.08; f1/4 = 0.89; f1/5 = 0.75; f1/6 = 1.79; f1/7 = 1.57; f1/8 = 0.42 | 29 |
| Alismatis Rhizoma | Alisol B 23-acetate (1); alisol A (2);alisol A 24-acetate (3); alisol B (4) | Alisol B 23-acetate | f1/2 = 0.9874; f1/3 = 1.097; f1/4 = 0.9777 | 30 |
| Aristolochia contorta Bge | Aristolochic acid A (1); aristolochic acid B (2); aristolochic acid C (3); aristolochic acid D (4) | Aristolochic acid A | f2/1 = 0.8389; f3/1 = 0.7822; f4/1 = 0.7782 | 31 |
| Magnoliae Flos extract | Magnolin (1); pinoresinol dimethylether (2); 1irioresinol B dimethylethe (3);epi-magnoli A (4) | Magnolin | f1/2 = 0.9968; f1/3 = 0.9735; f1/4 = 1.0068 | 32 |
| Schisandra chinensis | Rchizandrol A (1); schizandrol B (2); schisantherin A (3);deoxyschizandrin (4); schizandrin B (5); schizandrin C (6) | Rchizandrol A | f1/2 = 0.8175; f1/3 = 0.8233; f1/4 = 0.9758; f1/5 = 0.9652; f1/6 = 0.6732 | 33 |
| Yao-Bi-Tong capsules | Ginseno-side Rg1(1); notoginsenoside R1(2); ginsenoside Re (3); ginsenoside Rb1(4); ginsenoside Rd (5) | Ginseno-side Rg1 | f1/2 = 0.999; f1/3 = 1.218; f1/4 = 0.990; f1/5 = 1.094 | 34 |
| Phellodendri Cortex | Berberine (1); magnolia alkali (2); cortex phellodendri (3); alkali (4); Martin (5) | Berberine | f1/2 = 0.999; f1/3 = 1.218; f1/4 = 0.990; f1/5 = 1.094 | 35 |
| Mentha haplocalyx Briq. | Hesperidin (1); diosmin (2); didymin (3); buddleoside (4) | Hesperidin | f2/1 = 2.358; f3/1 = 1.560; f4/1 = 1.571 | 23 |
| Liuwei Dihuang preparations | Paeonol (1);morroniside (2); loganin (3); paeoniflorin (4) | Paeonol | f1/2 = 1.174; f1/3 = 1.186; f1/4 = 0.814 | 36 |
| Abelmoschus manihot | Hyperoide (1); rutin (2); isoquercetin (3); hibifolin (4); myricetin (5); quercetin −3'-O-glucoside (6); quercetin (7) | hyperoide | f2/1 = 0.828; f3/1 = 1.312; f4/1 = 0.494; f5/1 = 0.989; f6/1 = 1.608; f7/1 = 1.516 | 37 |
| Dabaidu capsules | Phosphoric acid (1); rhein (2); chrysophanol (3); physcion (4) | Phosphoric acid | f1/2 = 1.18; f1/3 = 1.40; f1/4 = 1.05 | 38 |
| Honeysuckle preparations | Chlorogenic acid (1); neochlorogenic acid (2); cryptochlorogenic acid(3) | Chlorogenic acid | f1/2 = 1.055; f1/3 = 1.031 | 39 |
| Eriobotryae Folium | Ursolic acid (1);euscaphic acid (2); tormentic acid (3); maslinic acid (4); corosolic acid (5); oleanolic acid (6) | Ursolic acid | f2/1 = 1.050; f3/1 = 1.061; f4/1 = 0.969; f5/1 = 0.980; f6/1 = 0.991 | 40 |
| Gycyrrhizae Radix et Rhizoma | Glycyrrhizic acid (1); liquiritin (2); liquiritiyenin (3); isoliquiritiyenin (4); glycyrrhetinic acid (5); licochalcone A (6) | Glycyrrhizic acid | f2/1 = 2.1853; f3/1 = 5.2407; f4/1 = 9.0947; f5/1 = 4.5847; f6/1 = 0.3266 | 41 |
| Celery seed extract | 3-n-butylphthalide (1); sedenenolide (2); sedanolide (3) | 3-n-butylphthalide | f1/2 = 0.226; f1/3 = 0.702 | 42 |
| Sophora flavescens | Oxymatrine (1); sophocarpine (2); Matrine (3); oxysophocarpine (4); sophoridine (5) | Oxymatrine | f1/2 = 1.653; f1/3 = 1.057; f1/4 = 1.083; f1/5 = 1.0067 | 43 |
| Sanhuang tablets | Emodin (1); rhein (2); chrysophanol (3); physcion (4) | Emodin | f2/1 = 1.13; f3/1 = 1.46; f4/1 = 1.01 | 44 |
| Yinhuang tablets | Chlorogenic acid (1);3-O-caffeoylquinic acids (2); 4-O-caffeoylquinic acids (3); 4,5-O-caffeoylquinic acids (4); 3,5-O-caffeoylquinic acids (5); 3,4-O-caffeoylquinic acids (6) | Chlorogenic acid | f2/1 = 1.011; f3/1 = 1.023; f4/1 = 1.054; f5/1 = 1.1190; f6/1 = 1.223 | 45 |
| Angelica dahurica var. formosana | Imperatorin (1); isoimperatorin (2); oxyimperatorin (3) | Imperatorin | f1/2 = 0.7254; f1/3 = 0.6839 | 46 |
| Zingiberis Rhizoma | 6-gingerol (1); 6-shogaol (2); 10-gingerol (3); 8-gingerol (4) | 6-gingerol | f1/2 = 0.711; f1/3 = 1.286; f1/4 = 1.299 | 47 |
| Suxiao Jiuxin pills | D-borneol (1); isoborneol (2); L-borneol (3) | D-borneol | f1/2 = 0.9651; f1/3 = 1.0148 | 48 |
| Yinhuang preparations | Baicalin (1); wogonoside (2); baicalein (3); wogonin (4) | Baicalin | f2/1 = 1.20; f3/1 = 1.62; f4/1 = 1.68 | 49 |
| Schisandra chinensis | Schisandrin (1); schisantherin A (2); deoxyschizandrin (3); γ-schizandrin (4) | Schisandrin | f1/2 = 0.706; f1/3 = 1.086; f1/4 = 0.876 | 50 |
| Akebiae Fructus | Saponins PD (1); saponin X (2); saponin B (3); saponin A (4) | Saponins PD | f1/2 = 1.180; f1/3 = 1.045; f1/4 = 0.893 | 51 |
| Paeoniae Radix Alba | Paeoniflorin (1);albiflorin (2) | Paeoniflorin | f2/1 = 0.940 | 52 |
| Phellodendri Amurensis Cortex | Berberine (1); palmatine (2); Jatrorrhizine (3) | Berberine | f1/2 = 1.2068; f1/3 = 1.0028 | 53 |
| Fraxini cortex | Aesculin (1); asculetin (2); fraxin (3); fraxetin (4) | Aesculin | f2/1 = 1.74; f3/1 = 0.80; f4/1 = 1.40 | 54 |
| Paeonia veitchii | Paeoniflorin (1); albiflorin (2); benzoylpaeoniflorin (3); gallic acid (4); methyl-gallate (5); pentagalloylglucose (6) | Paeoniflorin | f1/2 = 0.330; f1/3 = 0.357; f1/4 = 1.248; f1/5 = 0.445; f1/5 = 0.820 | 55 |
| Dangzuo | Piperine (1);gallic acid (2); hydroxysafflor yellow A (3); cinnamic aldehyde (4) | Piperine | f1/2 = 1.3686; f1/3 = 0.2620; f1/4 = 3.1333 | 56 |
| Salvia miltiorrhiza | Sodium danshensu (1); protocatechuic aldehyde (2); rosmarinic acid (3); lithospermicacid (4); salvianolic acid B (5) | Sodium danshensu | f2/1 = 7.559; f3/1 = 2.901; f4/1 = 1.825; f5/1 = 1.746 | 57 |
| Danshen | Tanshinone IIA (1); tanshinone I (2); cryptotanshinone (3) | Tanshinone IIA | f1/2 = 1.32; f1/3 = 1.18 | 58 |
| Houttuynia cordata | Chlorogenic acid (1); new chlorogcnic acid (2); cryptochlorogenic acid (3); rutin (4); hyperin (5); isoquercitrin (6); quercitrin (7) | Chlorogenic acid | f1/2 = 1.004; f1/3 = 0.995; f1/4 = 0.599; f1/5 = 0.856; f1/6 = 0.859; f1/7 = 0.62 | 59 |
| Green tea extracts | Epigallocatechin gallate (1); gallic acid (2); gallocatechin (3); epigallocatechin (4); caffeine (5); catechin (6); epicatechin (7); gallocatechin gallate (8); epicatechingallate (9); catechin gallate (10) | Epigallocatechin gallate | f1/2 = 0.54; f1/3 = 6.79; f1/4 = 7.37; f1/5 = 0.55; f1/6 = 1.81; f1/7 = 1.87; f1/8 = 0.88; f1/9 = 0.69; f1/10 = 0.69 | 60 |
| Psoralea corylifolia L | Neobavaisoflavone (1); isobavachin (2); bavachin (3); bavachinin (4); isobavachalcone(5);corylifol A(6); 4/-O-methylbavachalcone (7) | Neobavaisoflavone | f2/1 = 0.683; f3/1 = 2.92; f4/1 = 0.833; f5/1 = 1.98; f6/1 = 0.763; f7/1 = 1.43 | 61 |
| Qingkailing injection | Gly (1); Asp (2); Glu (3); Ser (4); Arg (5); Ala (6); Pro (7); Tyr (8); Val (9); Ile (10); Leu (11); Phe (12); Orn (13); Lys (14) | Gly | f1/2 = 0.546; f1/3 = 0.499; f1/4 = 0.688; f1/5 = 0.448; f1/6 = 0.892; f1/7 = 0.735; f1/8 = 0.470; f1/9 = 0.669; f1/10 = 0.668; f1/11 = 0.636; f1/12 = 0.519; f1/13 = 0.927; f1/14 = 0.986 | 62 |
| Compound danshen preparations | Danshensu (1);Protocatechuic acid (2); Protocatechuicaldehyde (3); Caffeic acid (4); Rosmarinic acid (5); Lithospermic acid (6); Notoginsenoside R1 (7); Salvianolic acid B (8) | Danshensu | f1/2 = 7.237; f1/3 = 7.119; f1/4 = 6.272; f1/5 = 3.272; f1/6 = 1.917; f1/7 = 0.980; f1/8 = 1.694 | 63 |
| Chrysanthemum Indicum Flower | Linarin (1); chlorogenic acid (2); Luteolin-7-O-β-D-glucoside (3); 3,4-di-O-caffeoylquinic acid (4); 3,5-di-O-caffeoylquinic acid (5); 3,4-di-O-caffeoylquinic acid (6) | Linarin | f1/2 = 0.71; f1/3 = 1.01; f1/4 = 0.66; f1/5 = 0.61; f1/6 = 0.60 | 64 |
| Houttuynia cordata | Chlorogcnic acid (1); neochlorogenic acid (2); cryptochlorogenic acid (3); rutin (4); hyperin (5); isoquercitrin (6); quercitrin (7) | Chlorogcnic acid | f1/2 = 1.004; f1/3 = 0.995; f1/4 = 0.599; f1/5 = 0.856; f1/6 = 0.859; f1/7 = 0.862 | 65 |
| Ilex Pubescens | Ilexgenin A (1); ilexoside O (2); ilexsaponin B3 (3); ilexsaponin B2 (4); ilexsaponin B1 (5); ilexsaponin A1 (6) | Ilexgenin A | f2/1 = 0.336; f3/1 = 0.318; f4/1 = 0.455; f5/1 = 0.574; f6/1 = 0.509 | 66 |
| Angelicae Sinensis Radix | Ferulic acid (1); senkyunolide A (2); Z-ligustilide (3) | Ferulic acid | f2/1 = 1.103; f3/1 = 1.612 | 67 |
| Dysosma versipellis | Podophyllotoxin (1); 4′-demethylepipodophyllotoxin (2); 4′-demethyl-4-deoxypodo phyllotoxin (3); 4-deoxypodophyllotoxin (4) | Podophyllotoxin | f1/2 = 1.0300; f1/3 = 0.6574; f1/4 = 0.9 | 68 |
|
Radix Aconiti Lateralis |
Aconitine (1); benzoylmesaconine (2); benzoylhypaconine (3); hypaconitine (4); mesaconitine (5) | Aconitine | f2/1 = 1.015; f3/1 = 1.039; f4/1 = 1.074; f5/1 = 0.803 | 69 |
| Hawthorn leaves | Vitexin-4-O-glucoside (1); Chlorogenic acid (2); vitexin-2-O-rhamnoside (3); vitexin (4); rutin (5); hyperoside (6) | Vitexin-4-O-glucoside | f1/2 = 1.119; f1/3 = 1.009; f1/4 = 0.706; f1/5 = 1.063; f1/6 = 0.830 | 70 |
| P. cuspidatum | Emodin (1); resveratrol (2); emodin-8-O-β-D-glucopyranoside (3); poly-datin (4) | Emodin (1); resveratrol (2) | f1/3 = 1.119; f2/4 = 1.78 | 71 |
4. Important factors to be considered in the establishment of QAMS
QAMS can overcome the insufficiency of reference substance and save the high test cost, showing prominent advantages and good application prospect, which makes it possible to be applied in the quality control and evaluation model with multi-components. However, many problems still need to be further studied and discussed in the process of QAMS, such as the selection of internal standard substance, the influence factors of RCF, the positioning of investigated components, and the choice of QAMS rationality evaluation method.58 The following sections will discuss those important factors that aim to provide the reference for researchers when operating QAMS.
4.1. Selection of internal standard substance
According to the rules of QAMS, it was essential to select a suitable internal standard substance. Generally, the selected internal standard substance should not only have definite therapeutic effect and stable chemical property but should also be easily available and inexpensive. In addition, it needs to have an appropriate content in CHM.19 For instance, Li et al76 selected tanshinone IIA as the internal standard substance to build the QAMS of Salvia miltiorrhiza because it is not only one of the main effective components of Salvia miltiorrhiza but also a high-content ingredient and easy to get.76 The choice of the internal standard substance would be regarded as deviated from the QAMS thought when the internal standard substance cannot embody the above fundamental principles. Hence, choosing an appropriate internal standard substance is very important for the establishment of the QAMS method.
4.2. Factors influencing RCF
In the quality evaluation of QAMS, the key is to establish the RCF between internal standard substance and other effective ingredients. The main factors that influence RCF include different laboratories, chromatographic instrument systems, packing, and the models of chromatographic column.77, 78 In the process of establishing QAMS, all of the above factors can influence the value of RCF. Therefore, it is necessary to thoroughly investigate the factors influencing RCF because a good reproducibility of RCF is the key to the precision of analysis results.
4.2.1. Influence of different laboratories on the RCF
Even in the same laboratory and test condition, there are still small differences in the values of RCF when re-tested on account of different liquid laboratories and operating personnel. Consequently, it is required to establish an accurate methodology to investigate whether the error introduced by the above factors is within permission. Generally, a methodology is allowed to be used in different laboratories when the RSD < 5%, which means different laboratories have little influence on the RCF.79 Wu et al80 have established the internal quality of Zhong-Jie-Feng injection by QAMS method and verified a good reproducibility of the RCF in different laboratories.
4.2.2. Influence of different HPLC instruments and different detectors on RCF
As one of the most important influencing factors, a suitable chromatography instrument system is essential to the RCF value. Because different HPLC instruments and different detectors affect the calculation of peak area, which are mainly related with the slit of detector, bandwidth value, slope sensitivity, peak width, and ways of integration, all of these factors could affect the value of RCF. At present, Waters, Agilent, and Shimadzu are the most commonly used analytical instruments. Duan et al80 adopted three kinds of instruments (Waters, DIONEX, and Shimadzu) to investigate the influence on RCF in the process of Dangyao by QAMS, demonstrating that different instruments have less influence on the RCF. Moreover, in the research process of QAMS, it is also crucial to choose the most suitable one from all the commonly used detectors, which mainly include ultraviolet-visible detector, photodiode array detector, fluorescence detector, evaporative light-scattering detector, and differential refractive index detector.82
In general, in the process of establishing QAMS, it is better to choose the same type of detector so as to ensure a consistent responsivity to various compounds. Furthermore, ultraviolet-visible detector is often used in HPLC; however, compounds with different structures have different optimal absorption wavelength. Therefore, it must be combined with the structure of the components which were under test and appropriate detection wavelength must be selected to decrease the measurement error.83 To establish a method for the quality control of Shenxiong Yangxin Granule by QAMS, the best absorption wavelengths of puerarin, ferulic acid, hesperidin, salvianolic acid B, ammonium glycyrrhizinate, and schisandrin are 250 nm, 321 nm, 283 nm, 286 nm, 237 nm, and 250 nm, respectively. Zhang et al84 selected 250 nm as the maximum absorption wavelength to ensure a better peak absorption.
4.2.3. Influence of different columns on RCF
The choice of chromatographic column is another important factor influencing the RCF value. Because different HPLC columns differ in the granularity of stationary phase, surface area, end capping, inner diameter, and length, the results of the tailing factor or symmetry factor, resolution, and theoretical plate number could affect the value of RCF.85 Stationary phase is one of the gist to choose column because different stationary phases have different absorption capacity. For instance, particle-based C18 column is commonly used for QAMS, particularly reversed chromatographic column, such as Agilent ZORBAX SB-C18, Eclipse XOB-C18, because their packing, packing size, and carbon loads are different. The above factors can cause the adsorption capacity of compounds to differ, which will further affect the RCF value. Hence, the influence of different chromatographic columns on RCF must be investigated.
Apart from the three factors referred above, there are still other influencing factors, such as the distribution proportion of the mobile phase, flow rate, and column temperature, all of which can lead to the deviation of RCF.31 These deviations can be effectively controlled by improving the chromatographic system and optimizing the chromatography conditions, which lay a solid foundation for the reproducibility of RCF.
4.3. Positioning of the investigated components
In the application of established QAMS method, it is difficult to accurately and directly identify the position of other components under test since only the internal standard substance is used as the reference substance. Therefore, method to locate other components under the test by only using the internal standard substance is the key problem in QAMS. According to the reported literature, there are two main positioning methods of the peaks, the retention time ratio and the relative retention time ratio between the effective components under test and the internal standard substance.86
4.4. The choice of QAMS rationality evaluation method
In order to further investigate the accuracy and feasibility of QAMS, it is necessary to choose a reasonable evaluation method to verify the rationality. ESM, as one of the most commonly used evaluation method, has been applied in many researches.87 When the QAMS calculated value has no significant difference with ESM, it suggests that the establishment of RCF has a good reproducibility and credibility. Hence, we can achieve the multi-constituent determination by QAMS, especially when the reference substances are in short supply. In addition, there are also other methods, such as internal standard method and linear regression method.88
5. Advantages and limitations of QAMS
After 10 years of development, the advantages and limitations of QAMS have been gradually revealed. The following sections will discuss the advantages and disadvantages of QAMS to promote further enrichment of the method, eventually making it more systematic and perfect.
5.1. Advantages of QAMS
5.1.1. Reflecting the multi-component and multi-target characteristics of CHM
Currently, there is a common practice among natural products’ analysis to select multiple components as markers for quality control because the diversity of CHM makes it impossible to only use one single reference substance to accurately and fully reflect the internal quality of CHM. However, the multi-component quality evaluation model requires plenty of reference substances. The application of ESM and internal standard method would be limited when the reference substance is insufficient or unstable, especially in multi-component quantitative and quality control. By using the internal function relationship and proportion relationship between other effective components and internal reference substance, QAMS makes it possible to realize synchronous measurement of multiple components by only determining one component. It represents a comprehensive qualitative approach for the quality evaluation of CHM and embodies the multi-target characteristics of CHM as well.28
5.1.2. Economy, saving, and environmental protection
After establishing QAMS method, the content of multiple components can be determined through RCF and used for the quality control of CHM. It means that with the help of RCF, the content of multiple components can be accurately calculated even if the reference substance is unavailable or unstable, which can largely improve the work efficiency and reduce the test cost. Besides, QAMS method consumes less organic solvents and reduces the pollution to the environment.89 For example, to perform quantitative analyses for determining the content of epigallocatechin gallate, gallic acid, gallocatechin, epigallocatechin, caffeine, catechin, epicatechin, gallocatechin gallate, epicatechingallate, and catechin gallate in green tea, we only have to detect the content of a single epigallocatechin gallate and subsequently calculate the contents of other components with RCF, thereby reducing the amount of solvent consumption and operating costs.60
5.1.3. Making up the fuzziness of fingerprints
Compared with conventional analytical approaches, chromatographic fingerprints can give an overall view of all components in CHM and demonstrate both the “sameness” and “differences” among various samples clearly.6 However, one of the drawbacks is that it can only show results of similarity calculated based on the relative value using preselected marker compound as a reference standard, and minor differences between similar chromatograms may not be distinguished. On the basis of fingerprints, the QAMS method lays more emphasis on the content of main ingredients or effective components, makes further quantitative analysis of fingerprints data, and defines the content of specific active ingredients. Compared with fingerprints data, QAMS provides a more advanced instrument for quality control on CHM.90
5.2. Limitations of QAMS
5.2.1. Limitation of component under test and detection wavelength
Because ultraviolet-visible detector is the most commonly used detector in QAMS, the components under test should be similar in structure to ensure that the ultraviolet absorption for all the components are in the maximum wavelength. There are two solutions when there is a difference between internal reference component and other components under test in ultraviolet absorption spectrum. One is to choose the shared absorption wavelength region of all components, and the other is to adopt variable wavelength detection in the process of gradient elution according to the corresponding maximum absorption wavelength of the components under test.91 For example, Bai et al78 studied the guizhi decoction by QAMS using 230 nm to detect paeoniflorin, 250 nm to detect glycyrrhizic acid, and 280 nm to detect liquorice glycosides, cinnamic acid, and cinnamic aldehyde.
5.2.2. Limitation of linear scope
Generally speaking, a number of effective compounds are used to assess the quality of CHM. However, different harvesting time, origins, climate, etc, all have impact on the content of effective components in CHM. The established QAMS cannot maintain accuracy if the content of one component is not in the range of linear scope, which has posed a challenge for the establishment of the CHM quality control standards and the standardization of QAMS.19
6. QAMS corresponding chromatographic techniques
Suitable for the analysis of most CHM, HPLC has been the most commonly used technique in the establishment of QAMS and been greatly improved in several fields over these years. For further convenience, HPLC can be coupled with different detectors, such as ultraviolet detector, diode array detector, and evaporative light-scattering detector, which can support the determination of different chemical structures and expand its application in the QAMS to a larger extent.92 Gas chromatography, a technique mainly used for the analysis of volatile compounds, is commonly used in the quality control of the CHM containing volatile oil combined with QAMS method.93 Although there are no reports of adopting gas chromatography in QAMS till date, this method will be used for quality control of the CHM containing volatile oil in the future.
Beyond that, there are some other chromatographic techniques recommended to apply in QAMS, such as high-performance capillary electrophoresis, ultra-high performance liquid chromatography, hydrophilic interaction chromatography, and liquid chromatography tandem mass spectrometry. Although there are only a few reports on the application of these chromatographic techniques in QAMS, it is believed that they have a potential and wide application prospect in the QAMS evaluation of CHM.94, 95 Compared with the conventional HPLC method, ultra-high performance liquid chromatography offers the possibility of significantly improving efficiency of chromatographic separation via columns packed with small particles (less than 2 μm), which can withstand high back pressure, enhance chromatographic resolution, improve sensitivity, and shorten operation time notably.96 Furthermore, shorter columns can shorten analysis time and consume less solvent, making less negative impact on the environment.
7. Suggestions for future application of QAMS research on CHM
As a feasible approach to evaluate the effectiveness and internal quality of CHM, QAMS has been considered as a potential method to detect effective constituents in complex mixtures. Although significant research achievement has been obtained in the last 10 years, the QAMS method is still in its preliminary stage, not very mature and stable. Therefore, further work is needed to enrich the QAMS method, and suggestions are as follows.
First, focus on the impact factors of RCF. As a new constant introduced into the quality evaluation approach for CHM, RCF is undoubtedly the core content of constructing QAMS and is bound to be a key error factor of QAMS. All the factors can lead to the change of the detector response values and are likely to have an influence on RCF, especially the chromatographic column, analytical instruments, and the selection of the wavelength.97 However, if RSD is within an acceptable range, generally 5%, it can be accepted as a simple and practical method for the multi-component quality control of CHM, with the advantages of reducing the detection cost and overcoming the difficulty of lacking reference substances.
Second, combine the spectrum-effect relationship with QAMS for overall quality control of CHM.98 Advanced on the basis of fingerprints research, the spectrum-effect relationship can illustrate the relationship between fingerprint and pharmacodynamics effect of CHM through multiple statistical analysis methods and help to screen active characteristic peaks. The chemical structures can be further determined by liquid chromatography tandem mass spectrometry, which can truly provide “markers” for the internal quality of CHM.99 More importantly, when applied in holistic research of CHM, the spectrum-effect relationship is more beneficial to embody the integral view of traditional Chinese medicine theory. The quality of medicinal materials can be more accurately appraised through QAMS by the effective components screened from the spectrum-effect relationship.
Furthermore, combine computer-aided drug design (CADD) with QAMS to better reflect the characteristics of CHM. Due to the complexity of the chemical composition of CHM, there is a trend of applying CADD method in exploiting multi-target drugs, including virtual screening and pharmacophore design.100 The virtual screening is one of the most effective approaches to determine multi-target drugs. It is based on the small molecule database to carry out effective compounds screening, mainly including molecular docking, pharmacophore screening, and fragment search method. The application of CADD method on multi-target natural products has significant advantages compared with conventional methods, such as time and cost efficient, illustrating the multi-targets characteristics of CHM, and revealing corresponding efficacy components. Therefore, the combined application of CADD with QAMS can provide a more appropriate method for the quality control of CHM.
QAMS study is used to put forward new ideas and methods for the quality control of multiple components. It is actually a multidisciplinary and cutting-edge science research because the quality control of CHM needs the crossover of chemistry, pharmacology, molecular biology of the gene, and even statistics to provide a platform. Therefore, further improvement of QAMS study will not only help to guarantee the curative effect and safety of CHM but also accelerate its internationalization.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgments
The authors are grateful to the support of National Science Foundation (81403152), Ministry of Education Research Fund for the Doctoral Program (20130013120001, 20120013130002); Beijing University of Chinese Medicine young teachers of special autonomy issue (2013-QNJSZX008).
Contributor Information
Xiaoping Li, Email: lixiaoping6@yeah.net.
Bing Zhang, Email: zhangbing6@263.net.
References
- 1.Kong W.J., Wang J.B., Zang Q.C., Xing X.Y., Zhao Y.L., Liu W. Fingerprint–efficacy study of artificial Calculus bovis in quality control of Chinese materia medica. Food Chem. 2011;127:1342–1347. doi: 10.1016/j.foodchem.2011.01.095. [DOI] [PubMed] [Google Scholar]
- 2.Jin R., Lin Q., Zhou J., Sun B.Y., Zhang B. Construction and application of the multidimensional table for knowledge discovery in ancient Chinese books on materia medica. Engineering. 2013;5:1–6. [Google Scholar]
- 3.World Health Organization; Geneva, Switzerland: 2002. Traditional Medicine Strategy 2002–2005.http://whqlibdoc.who.int/hq/2002/who edm trm 2002 [Google Scholar]
- 4.Gan F., Ye R.Y. New approach on similarity analysis of chromatographic fingerprint of herbal medicine. J Chromatogr A. 2006;1104:100–105. doi: 10.1016/j.chroma.2005.11.099. [DOI] [PubMed] [Google Scholar]
- 5.Liang Y.Z., Xie P.S., Chan K.L. Quality control of herbal medicines. J Chromatogr B. 2004;812:53–70. doi: 10.1016/j.jchromb.2004.08.041. [DOI] [PubMed] [Google Scholar]
- 6.Gong F., Liang Y.Z., Xie P.S., Chau F.T. Information theory applied to chromatographic fingerprint of herbal medicine for quality control. J Chromatogr A. 2003;1002:25–40. doi: 10.1016/s0021-9673(03)00648-4. [DOI] [PubMed] [Google Scholar]
- 7.Xiao P.G., Liu C.X. A re-understanding on the safety matters of Chinese herbal medicines. Chin J Integr Med. 2004;10:242–245. [Google Scholar]
- 8.Alaerts G., Matthijs N., Smeyers-Verbeke J., Vander Heyden Y. Chromatographic fingerprint development for herbal extracts: A screening and optimization methodology on monolithic columns. J Chromatogr A. 2007;1172:1–8. doi: 10.1016/j.chroma.2007.07.080. [DOI] [PubMed] [Google Scholar]
- 9.Fan C.J. Application situation of multi-components quantitation by one marker new method for quality evaluation and control of Chinese herbal medicine. Pharm Clin Chin Mater Med. 2013;4:18–20. [Google Scholar]
- 10.Gao H.M., Wang Z.M., Li Y.J., Qian Z.Z. Overview of the quality standard research of traditional Chinese medicine. Front Med. 2011;5:195–202. doi: 10.1007/s11684-011-0134-x. [DOI] [PubMed] [Google Scholar]
- 11.Yadav A.K., Tiwari N., Srivastava P., Singh S.C., Shanker K., Verma R.K. Iridoid glycoside-based quantitative chromatographic fingerprint analysis: A rational approach for quality assessment of Indian medicinal plant Gambhari (Gmelina arborea) J Pharm Biomed Anal. 2008;47:841–846. doi: 10.1016/j.jpba.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 12.Li S.P., Zhao J., Yang B. Strategies for quality control of Chinese medicine. J Pharm Anal. 2011;55:802–809. doi: 10.1016/j.jpba.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 13.Wang Z.M., Gao H.M., Fu X.T., Wang W.H. Multi-components quantitation by one marker new method for quality evaluation of Chinese herbal medicine. Chin J Chin Mater Med. 2006;31:1925–1928. [PubMed] [Google Scholar]
- 14.Zou G.X., You X.M., Zhang Y., Wang G.H., Jian G.H. New method of multi-components quantitation by one marker new method for quality evaluation of Guanmaikang capsule. Chin J Chin Mater Med. 2008;33:1828–1831. [PubMed] [Google Scholar]
- 15.Wu W.Y., Guo D.A. Strategies for elaboration of comprehensive quality standard system on traditional Chinese medicine. Chin J Chin Mater Med. 2014;39:351–356. [PubMed] [Google Scholar]
- 16.Zhao Y.Y., Guo H.Z., Chen Y.G., Fu X.T., Li Z., Tian L. A comparative study and suggestion on relative correction factor produced by different methods in quantitative analysis of multi-components. Drug Stand Chin. 2014;15:245–251. [Google Scholar]
- 17.National Commission of Chinese Pharmacopoeia . vol. I. China Medical Science Press; Beijing: 2010. p. 285. (Pharmacopoeia of People’s Republic of China). [Google Scholar]
- 18.Tang A.F., Li S., Wang Y.J., Xu Y.Y., Tang H., Wang S.D. Progress and application of quantitative analysis of multi-components by single marker in pharmaceutical chemistry. Cent South Pharm. 2014;12:145–147. [Google Scholar]
- 19.Wen Q.Y., Long W., Yang H., Li P. Advance in multi-components quantitation by one marker in the quality control of Chinese herbal medicine. Chin Pharm. 2014;25:2185–2188. [Google Scholar]
- 20.Zhu J.J., Wang Z.M., Ma X.Y., Feng W.H., Zhang Q.W. A quantitative method for simultaneous determination of four anthraquinones with one marker in Rhei Radix et Rhizoma. Chin Herb Med. 2012;4:157–163. [Google Scholar]
- 21.Shen X.Z., Liu Y., Yang Y.J., Yang F. Research advances in quantitative analysis of multi-components by single-maker. Chin Pharm. 2013;22:1–5. [Google Scholar]
- 22.Wang L.N., Liu H.Y., Zhang J., LI J., Zhang Y.Q. Simultaneous determination of eight bioactive components in Lonicerae japonicae Flos by quantitative analysis of multi-components by single marker. Chin J Exp Tradit Med Formu. 2014;20:57–61. [Google Scholar]
- 23.Xu J.J., Xu C., Liu B. Simultaneous determination of four flavonoid glycosides in Mentha haplocalyx briq. by a quantitative analysis of multi-components by single marker. Chin Pharm J. 2014;49:234–239. [Google Scholar]
- 28.Wang J.J., Zhang L., Guo Q., Kou J.P., Yu B.Y., Gu D.H. Quantitative analysis of seven phenolic acids in eight Yinqiao Jiedu serial preparations by quantitative analysis of multi-components with single-marker. Acta Pharm Sin. 2015;50:480–485. [PubMed] [Google Scholar]
- 31.Huang Q., Xu G., Yang G.H., Yuan J.B., Yang W.L., Chen H.F. Simultaneous quantitative analysis of four aristolochic acids component in Aristolochia contorta bge. J Chin Med Mater. 2014;37:1616–1619. [Google Scholar]
- 58.Hou J.J., Wu W.Y., Da J., Yao S., Long H.L., Yang Z. Ruggedness and robustness of conversion factors in method of simultaneous determination of multi-components with single reference standard. J Chromatogr A. 2011;1218:5618–5627. doi: 10.1016/j.chroma.2011.06.058. [DOI] [PubMed] [Google Scholar]
- 60.Li D.W., Zhu M., Shao Y.D., Shen Z., Weng C.C., Yan W.D. Determination and quality evaluation of green tea extracts through qualitative and quantitative analysis of multi-components by single marker (QAMS) Food Chem. 2016;197:1112–1120. doi: 10.1016/j.foodchem.2015.11.101. [DOI] [PubMed] [Google Scholar]
- 73.Gao X.Y., Jiang Y., Lu J.Q., Tu P.F. One single standard substance for the determination of multiple anthraquinone derivatives in rhubarb using high-performance liquid chromatography-diode array detection. J Chromatogr A. 2009;1216:2118–2123. doi: 10.1016/j.chroma.2008.11.104. [DOI] [PubMed] [Google Scholar]
- 74.National Commission of Chinese Pharmacopoeia . vol. I. China Medical Science Press; Beijing: 2010. p. 36. (Pharmacopoeia of People’s Republic of China). [Google Scholar]
- 75.Li Q., Liu W., Luo Z.L., Yang M.H. Simultaneous determination of four tanshinones in Salvia miltiorrhiza by QAMS. Chin J Chin Mater Med. 2012;37:824–828. [PubMed] [Google Scholar]
- 76.Luo Z.L., Qiu F., Wei R.W., Qin Y., Zhang K., Qin J.P. Application of relative correction factor in multi-index determination of Chinese materia medica. Chin Tradit Herb Drug. 2012;43:1448–1452. [Google Scholar]
- 77.Gao H.M., Song Z.H., Wang Z.M., Qian Z.Z., Zhang Q.W. Overview on quantitative analysis of multi-components by single-marker. Chin J Chin Mater Med. 2012;37:405–416. [PubMed] [Google Scholar]
- 78.Bai D., Fan B., Niu X.H., Song J.N. Determination of paeoniflorin, liquiritin, cinnamic acid, cinnamic aldehyde, and glycyrrhizic acid in Guizhi Decoct ion by HPLC method with sample' s internal standard. Chin Traditi Herb Drug. 2014;43:387–390. [Google Scholar]
- 79.Wu T.R., Lu Y.H., Xu Y. Quantitative analysis of six components in Zhong-Jie-Feng injection by QAMS method. Chin J Pharm Anal. 2013;33:1893–1898. [Google Scholar]
- 80.Duan J.P., Feng L., Liu Y.L., Niu X.L. Quantitative Analysis of swertiamarin, sweroside and gentiopicrin in swertia pseudochinensis by QAMS. J Chin Med Mater. 2014;37:624–626. [Google Scholar]
- 82.Liu Y., Xiong W., Tian J., Li C.H., Yang S.Y., Zeng G.G. Development of a novel method combining multi-wavelength HPLC fingerprint and quantitative analysis of multi-component by single marker for quality control of Houttuynia cordata. J Chem Pharm Res. 2014;6:347–356. [Google Scholar]
- 83.Zhang T., Zheng D., Wang W.T., Tao Z.W. Application of fingerprint combined with QAMS in quality evaluation of Shenxiong Yangxin Granule. Chin Traditi Herb Drug. 2015;46:1920–1925. [Google Scholar]
- 84.Zhang M., Chai Y., Ren A.N., Yang Y.L., Zhao Y. Simultaneous determination of ten constituents in Qingqing Granule by quantitative analysis of multi-components by single-marker. Chin J Mod Appl Pharm. 2015;32:318–323. [Google Scholar]
- 85.He B., Yang S.Y., Zhang Y. A new method of calibration and positioning in quantitative analysis of multicomponents by single marker. Acta Pharm Sin. 2012;42:1653–1659. [PubMed] [Google Scholar]
- 86.Ma H.L., Guo Q.M., Zhou F.Q. The QAMS research progress in the quality control of traditional Chinese medicine (TCM) application. J SHANDONG UNIV TCM. 2014;38:282–284. [Google Scholar]
- 87.He B., Liu Y., Yang S.Y., Zhang Y. Simultaneous determination of 10 constituents in Shuangqing Yanhou Tablets by HPLC-QAMS. Chin Tradit Herb Drug. 2013;44:974–981. [Google Scholar]
- 88.Song L.P. Simultaneous determination of 4 phenolic acids in capillary Wormwood herb by a quantitative analysis of multi-components by single marker. J Chin Med Mater. 2015;38:774–776. [Google Scholar]
- 89.Lu T.L., Shi S.M., Cai B.C., Liu H.Z., Chi Y.M., Zhou Y. Advances in studies on multi-component determination of Chinese material medica by QAMS. Chin Tradit Herb Drug. 2012;43:2525–2529. [Google Scholar]
- 90.Lin S.X., Zhang Q.H., Xi P.Y., Lin C.H., Xiao Z.F. Determination of six ingredients in Gardenia Jaminodies fruits with quantitative analysis of multi-components by single marker. J Chin Med Mater. 2015;38:531–535. [PubMed] [Google Scholar]
- 91.Xie W.J., Zhang Y.P., Xu J., Xu W.F. The progress of quantitative analysis of multi-components by single marker in the field of Chinese materia medica. JGCTCM. 2014;36:135–139. [Google Scholar]
- 92.Koek M.M., Jellema R.H., van der Greef J., Tas A.C., Hankemeier T. Quantitative metabolomics based on gas chromatography mass spectrometry: status and perspectives. Metabolomics. 2011;7:307–328. doi: 10.1007/s11306-010-0254-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Huang S.J., Yang Q.W., Shi Y.H., Wang R., Wang Z.T. Simultaneous assay of paeoniflorin and albiflorin in Paeoniae Radix Alba by QAMS. Chin J Chin Mater Med. 2011;36:780–783. [PubMed] [Google Scholar]
- 94.Lu L.L., Qian D.W., Guo J.M., Yan H., Xu B.Y., Sha M. A quantitative method using one marker for simultaneous assay of seven flavonoids in the flowers of Abelmoschus manihot. Chin J Pharm Anal. 2013;33:2028–2087. [Google Scholar]
- 95.Novakova L., Matysova L., Solich P. Advantages of application of UPLC in pharmaceutical analysis. Talanta. 2006;68:908–918. doi: 10.1016/j.talanta.2005.06.035. [DOI] [PubMed] [Google Scholar]
- 96.Hou J.J., Wu W.Y., Da J., Yao S., Long H.L., Yang Z. Ruggedness and robustness of conversion factors in method of simultaneous determination of multi-components with single reference standard. J Chromatogr A. 2011;1218:5618–5627. doi: 10.1016/j.chroma.2011.06.058. [DOI] [PubMed] [Google Scholar]
- 97.Li T.T., Wang X.F., Ma L., Shu Z.H., Fu X.Y. Improved overall quality control standards of Chinese herbs medicine through combining the spectrum effect relationships with the multi-components quantities by one marker. Chin J Exp Tradit Med Formu. 2014;20:225–228. [Google Scholar]
- 98.Zhu C.S., Zhang B., Lin Z.J., Wang X.J., Zhou Y., Sun X.X. Relationship between high-performance liquid chromatography fingerprints and uric acid-lowering activities of Cichorium intybus L. Molecules. 2015;20:9455–9467. doi: 10.3390/molecules20059455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu X., Zhu F., Ma X.H., Shi Z., Yang S.Y., Wei Y.Q. Predicting targeted polypharmacology for drug repositioning and multi-target drug discovery. Curr Med Chem. 2013;20:1646–1661. doi: 10.2174/0929867311320130005. [DOI] [PubMed] [Google Scholar]
- 100.Pevzner Y., Frugier E., Schalk V., Caflisch A., Woodcock H.L. Fragment-based docking: development of the CHARMMing Web User interface as a platform for computer-aided drug design. J Chem Inf Model. 2014;54:2612–2620. doi: 10.1021/ci500322k. [DOI] [PMC free article] [PubMed] [Google Scholar]