Abstract
Background
To determine the phytochemical constituents, antioxidant, and anticancer activities of Leea indica leaf extracts on DU-145 and PC-3 human prostate cancer cell lines.
Methods
Leaf sample was subjected to Soxhlet extraction method with increasing polarity of solvents, namely, chloroform, ethyl acetate, methanol, ethanol, and aqueous. Phytochemical screening was done using different biochemical tests. Quantitative analysis for phenol was determined by Folin–Ciocalteu reagent method. The antioxidant activity was tested using 2,2-diphenyl-1-picrylhydrazyl, ferric ion reducing power assay, and phosphomolybdenum assay. In vitro anticancer activity on DU-145 and PC-3 human prostate cancer cell lines was evaluated by (3-(4, 5-dimethyl thiazole-2yl)-2, 5-diphenyl tetrazolium bromide) MTT assay.
Results
Phytochemical screening confirmed the presence of phyto-constituents like alkaloids, flavonoids, glycosides, phenols, lignins, saponins, sterols, tannins, anthraquinone, and reducing sugar. Methanol and ethanol extracts exhibited higher phenolic content as compare to aqueous extract. Antioxidant capacities were shown highest in methanol and ethanol extracts based on the test performed. The methanol and ethanol leaf extracts were found to be selectively cytotoxic in vitro to (DU-145 and PC-3) prostate cancer cell lines with IC50 values 529.44 ± 42.07 μg/mL and 677.11 ± 37.01 μg/mL for DU-145 and 547.55 ± 33.52 μg/mL and 631.99 ± 50.24 μg/mL for PC-3 respectively, while it had no cytotoxic effect on normal mice embryo fibroblast cells.
Conclusion
The results indicate that Leea indica was a promising antioxidant and anticancer agent for DU-145 and PC-3 human prostate cancer cell lines. However, further studies are needed to conclude its therapeutic use.
Keywords: anticancer, antioxidant, Leea indica, phytochemical, prostate cancer
1. Introduction
Prostate cancer is the most common forms of malignancy in men, particularly in developed countries where the majority of cases are diagnosed in men aged above 50 years.1 Present treatments options available for prostate cancer includes surgical treatment, hormone therapy, radiation therapy, and chemotherapy; these treatments have shown improvement in patients but generally ends up with adverse and toxic side effects.2 There has also been a wide use of dietary supplements such as vitamin E, selenium, soy products, lycopene, and green tea catechin in the treatment of prostate cancer.3, 4, 5 Evidence from the World Health Organization, states that about 65% of the population across the globe prefer to use traditional and herbal medicines to treat disease.6 The use of complementary alternative medicines has dramatically increased in India along with USA, in the last 2 decades.7 Approximately 60% of anticancer agents are derived from medicinal plants and other natural resources; however, there are still a number of plants that have an anticancer potential but they have not yet been fully investigated.8 Thus, the alternate solution for the harmful effects of synthetic drugs is the use of complementary alternative medicines as very few studies have been reported on the use of herbal medicine in treatment of prostate cancer.9
Leea indica (Burm.f.) Merr (Leeaceae), is a large evergreen shrub native to tropical India, Bangladesh, China, Bhutan, and Malaysia.10 The leaves are claimed to have medicinal values such as anticancer, antidiabetic, antidiarrheal, antidysenteric, and antispasmodic based on folktale practices.11 However, this plant has not been studied for anticancer activity on prostate cancer and there are very few available studies on antioxidant properties of this plant. Thus, we have made an attempt to use this herbal plant to check the efficacy against prostate cancer cell lines. The present study was aimed to evaluate preliminary phytochemical, in vitro antioxidant properties, and in vitro anticancer potential activities against DU-145 and PC-3 human prostate cancer cell lines.
2. Methods
2.1. Collection and authentication of plant part
L. indica plant was collected from Dandeli in Western Ghats of North Karnataka region in April 2015. The plant was identified and authenticated by Dr K. Kotresha, Department of Botany, Karnataka Science College, Dharwad, Karnataka. A voucher specimen was deposited in the Department of Botany, Karnataka Science College, Dharwad, Karnataka. Fresh plant material was washed with water, air dried, and then blended to a fine powder. The powder was stored in airtight containers at 4 °C for further use.
2.2. Preparation of plant materials
About 100-g dried leaves were crudely powdered and subjected to extraction by a Soxhlet extractor. The extraction was done with different solvents in their increasing order of polarity such as chloroform, ethyl acetate, methanol, ethanol, and aqueous. Each time fresh plant material was taken and later extracted with other solvents. All the extracts were concentrated by a rotary vacuum evaporator and the left-over solvent was evaporated to dryness using a water bath.
2.3. Phytochemical analysis
The crude powder of L. indica was extracted using water and different organic solvents to ensure obtaining polar and nonpolar constituents. Qualitatively tested for different phytochemical constituents namely alkaloids, flavonoids, glycosides, phenols, lignins, saponins, sterols, tannins, anthraquinones, and reducing sugar by following the standard procedure of Deepti et al.12
2.4. Estimation of total phenolic content
The total phenolic content of L. indica was determined using the Folin–Ciocalteu reagent method of Wolfe et al,13 with slight modification. A volume of 200 μL of extract is mixed with an equal volume of Folin–Ciocalteu reagent and incubated for 10 minutes. Then, 1.25 mL of aqueous sodium carbonate is added and the reaction mixture is incubated for 90 minutes at 37 °C after addition of 1-mL distilled water. The absorbance of the blue color was read at 760 nm spectrophotometrically using distilled water as a blank. Gallic acid is used as standard and the total phenolic content was expressed as mg/g gallic acid equivalent (GAE).
2.5. Determination of antioxidant activity by using in vitro methods
2.5.1. Ferric ion reducing antioxidant power assay
Ferric reducing/antioxidant power (FRAP) assay was used to measure the total antioxidant power of the extracts. Antioxidant activity assays were performed by the method described by Benzie and Strain14 with a slight modification. Methanol, ethanol, and aqueous extract of L. indica in different concentrations ranging from 100 μL to 500 μL were mixed with 2.5 mL of 0.2 mM phosphate buffer (pH 7.4) and 2.5 mL of potassium ferricyanide [1% weight/volume (W/V)]. The resulting mixture is incubated at 50 °C for 20 minutes, followed by the addition of 2.5 mL of trichloroacetic acid (10% W/V) and centrifuged at 3000 rpm for 10 minutes. Then, 2.5 mL of distilled water was added and later 0.5 mL of ferrous chloride (0.1% W/V). Finally, the absorbance was measured at 700 nm. Ascorbic acid was used as positive reference standard.
2.5.2. Phosphomolybdenum assay
The total antioxidant activity was estimated by phosphomolybdenum (PM) assay using the standard procedure of Prieto et al.15 Methanol, ethanol, and aqueous extract of L. indica in different concentration ranging from 100 μL to 500 μL were added to each test tube individually containing 3 mL of distilled water and 1 mL of molybdate reagent solution. These tubes were kept incubated at 95 °C for 90 minutes. After incubation, they are kept at room temperature for 20–30 minutes and the absorbance was measured at 695 nm. Ascorbic acid was used as the reference standard.
2.5.3. 2, 2-Diphenyl-1-picrylhydrazyl radical scavenging ability assay
Free radical scavenging effect of L. indica leaf extract was determined using the stable scavenger 2, 2-diphenyl-1-picrylhydrazyl (DPPH) with slight modifications of the method described by Brand-Williams et al.16 Briefly, the concentrations (100–500 μL) of extracts were prepared in ethanol. DPPH solution (0.004%) was prepared in ethanol and 1 ml of this solution was mixed with the same volume of methanol, ethanol, and aqueous leaf extracts and standard ascorbic acid solution separately. The mixture was incubated for 30 minutes in the dark at room temperature and the absorbance was measured at 517 nm. The degree of DPPH purple decolorization to DPPH yellow indicated the scavenging efficiency of the extract. Lower absorbance of the reaction mixture indicated higher free radical-scavenging activity.
The scavenging activity against DPPH was calculated using the equation:
where Ac is the absorbance of the control reaction (1 ml of ethanol with 1 ml of DPPH solution), and At is the absorbance of the test sample. The results were analyzed in triplicate. The IC50 value is the concentration of sample required to inhibit 50% of the DPPH free radical.
2.6. Culturing of cell lines
The cell lines DU-145, PC-3, and MEF-L929 were procured from the National Centre for Cell Science, Pune, India. The cells were subcultured in Dulbecco modified eagle medium supplemented with 10% fetal bovine serum, 1% penicillin–streptomycin, 1% nonessential amino acids in tissue culture flasks, and incubated in a CO2 incubator in a 5% CO2 and 95% humidity atmosphere. After trypsinization, the cell count was done and the cell viability was tested by trypan blue using a hemocytometer. A known number of cells (2 × 103 cell/well in 100 μl of medium) were seeded into 96-well plates respectively for carrying out a MTT assay.
2.7. Treatment groups
DU-145, PC-3, and MEF-L929 cell lines were treated with L. indica leaf methanol, ethanol, and aqueous extract (5 mg/mL). Desired concentrations of test compounds were prepared in di-methyl sulfoxide prior to the experiment. The reactant mixtures were diluted with media and cells were treated with different concentration ranges of the extract (3.125–200 μg/mL) and incubated for 72 hours, respectively, which was the optimal treatment time of the extracts in each of the cell lines. The effect induced was also compared with the standard drugs used, namely, paclitaxel for moderately (DU-145) and highly metastatic (PC-3) prostate cancer cell lines. The following treatment groups are set up of the study. Negative control: cells alone. Positive control: cells + paclitaxel. Test groups: cells + methanol extract; cells + ethanol extract; and cells + aqueous extract. The same treatment group was followed for mice embryo fibroblast (MEF-L929) normal cell lines.
2.8. MTT cell viability assays
After 72 hours, the media of treated cells (100 μL), were removed and the cell culture were incubated with 50 μL of MTT at 37 °C for 4 hours. After incubation, the formazan produced was then solubilized by the addition of 100 μL di-methyl sulfoxide. The suspension was placed on a microvibrator for 5 minutes and then the absorbance was recorded at 540 nm by an enzyme-linked immunosorbent assay reader and the results were analyzed in triplicate and the percentage was calculated.17
2.9. Statistical analysis
The results were expressed as mean ± standard deviation. Descriptive statistics was used to analyze the mean, standard deviation, variation, and level of statistical significance between groups. When p < 0.05 and p < 0.01, it was considered statistically significant for analysis of percent inhibition of cell growth.
3. Results
3.1. Total yield of crude extract
The total yield of crude extracts from L. indica leaves by using the solvents, namely, chloroform, ethyl acetate, methanol, ethanol, and aqueous were 12.8 g, 12.48 g, 17.12 g, 16.52 g, and 18.64 g (weight/weight), respectively, with reference to the air-dried plant material.
3.2. Phytochemical analysis
Preliminary screening of L. indica extracts (chloroform, ethyl acetate, methanol, ethanol, and aqueous) showed the presence of diversity of phytochemical constituents. The presence of alkaloids was detected in methanol and aqueous extract, flavonoids were present in methanol, ethanol, and aqueous extracts, the presence of glycosides was shown in all five solvents, phenols were detected in methanol, ethanol, and aqueous extracts, lignins were present in chloroform and ethyl acetate extract, saponins were present in methanol and aqueous extracts, sterols in methanol and aqueous, tannins in ethyl acetate, methanol, ethanol, and aqueous, anthraquinone in ethanol and aqueous, phlobatannins and reducing sugar in methanol, ethanol, and aqueous extracts, and volatile oil was absent in all five solvents (Table 1).
Table 1.
Preliminary phytochemical screening of Leea indica leaves extracts.
| Constituent | Test | Chloroform | Ethyl acetate | Methanol | Ethanol | Aqueous |
|---|---|---|---|---|---|---|
| Alkaloids | Iodine | – | – | – | – | – |
| Wagner’s | – | – | + | – | ++ | |
| Dragendroff’s | – | – | + | – | ++ | |
| Flavonoids | Pew’s | – | – | + | + | + |
| Shinoda | – | – | ++ | + | ++ | |
| NaOH | – | – | – | + | – | |
| Glycosides | Keller-Killani | + | ++ | ++ | ++ | + |
| Glycosides | – | + | – | – | – | |
| Conc. H2SO4 | – | ++ | ++ | ++ | ++ | |
| Molisch | ++ | ++ | ++ | ++ | ++ | |
| Phenols | Ellagic acid | – | – | ++ | ++ | ++ |
| Phenol | – | – | ++ | ++ | ++ | |
| Lignins | Labat | + | ++ | – | – | – |
| Saponins | Foam test | – | – | – | + | + |
| Sterols | Salkowski’s | + | – | + | ++ | – |
| Tannins | Gelatine | – | – | ++ | + | ++ |
| Lead acetate | – | + | ++ | ++ | ++ | |
| Anthraquinone | Bomtrager’s test | – | – | – | ++ | + |
| Phlobatannins | – | – | + | + | ++ | |
| Reducing sugar | – | + | ++ | ++ | ++ | |
| Volatile oil | – | – | – | – | – |
–, absent; +, moderately present; ++, high presence.
3.3. Total phenol content
In the present study, total phenolic content of different extracts of leaves of L. indica was determined by the Folin–Ciocalteu reagent method and expressed as GAE/g of plant extracts. Methanol extract exhibited the maximum amount of phenolic content among the extracts, i.e., (65.20 ± 0.15) mg/g GAE followed by (60.97 ± 0.23) mg/g GAE in ethanol extract, (53.04 ± 0.15) mg/g GAE in aqueous extract, and (84.59± 0.52) mg/g GAE in standard gallic acid. All the calculations were made using the r2 values from the graphs.
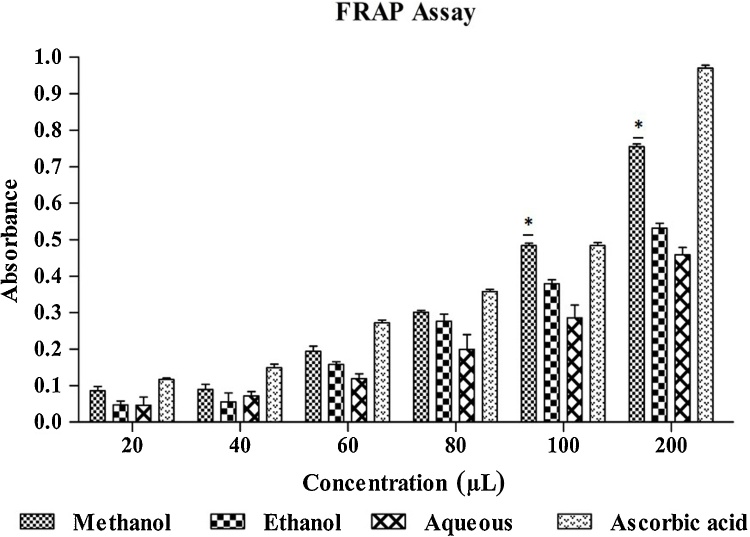
3.4. FRAP assay
In the present study, the presence of antioxidants in the sample would result in the reduction of ferri cyanide Fe3+ to ferro cyanide Fe2+ by donating an electron. Methanol, ethanol, and aqueous extracts were subjected to FRAP assay along with standard ascorbic acid. In the results obtained, methanol extract showed higher activity than aqueous followed by ethanol extract which was comparable to standard ascorbic acid (Fig. 1).
Fig. 1.
Ferric reducing/antioxidant power assay for extracts of Leea indica.
Data is presented as mean standard error of the mean (n = 3). Statistical significance was assessed using one-way as compared with standard.
*p < 0.05.
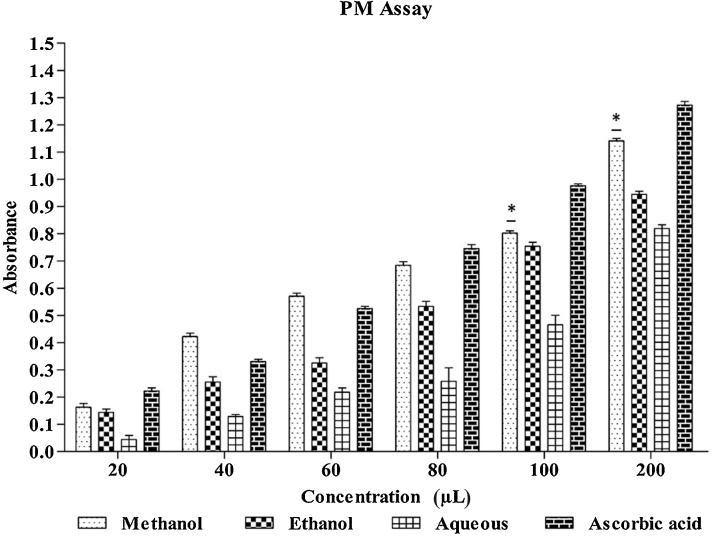
3.5. PM assay
In the present study, methanol, ethanol, and aqueous extracts were subjected to PM assay along with standard ascorbic acid. Methanol extract showed the highest activity among ethanol and aqueous extract and standard ascorbic acid (Fig. 2).
Fig. 2.
Phosphomolybdenum assay for extracts of Leea indica.
Data is presented as mean standard error of the mean (n = 3). Statistical significance was assessed using one-way as compared to standard group.
*p < 0.05.
3.6. DPPH assay
In the present study, the different concentrations of methanol, ethanol, and aqueous extract of leaves of L. indica were subjected to DPPH free radical scavenging assay. The antioxidant capacity of the extract was compared with ascorbic acid as the standard antioxidant. Methanol extract exhibited higher antioxidant activity than the ethanol and aqueous extract (Table 2).
Table 2.
Determination of percentage inhibition of 2, 2-diphenyl-1-picrylhydrazyl radical scavenging activity of Leea indica (%).
| Concentration (mg) | Percentage of inhibition |
|||
|---|---|---|---|---|
| Methanol | Ethanol | Aqueous | Standard: ascorbic acid | |
| 100 | 57.11 ± 0.43 | 43.87 ± 0.16 | 33.76 ± 0.14 | 62.45 ± 0.17 |
| 200 | 64.15 ± 0.49 | 45.05 ± 0.21 | 44.06 ± 0.18 | 66.96 ± 0.25 |
| 300 | 73.24 ± 0.29 | 67.75 ± 0.29 | 62.05 ± 0.32 | 75.03 ± 0.19 |
| 400 | 78.16 ± 0.15 | 70.84 ± 0.12 | 73.69 ± 0.11 | 82.15 ± 0.14 |
| 500 | 82.86 ± 0.25 | 80.01 ± 0.33 | 77.40 ± 0.12 | 90.78 ± 0.12 |
The values presented are mean ± standard deviation, n = 3. Results were analyzed using descriptive statistics.
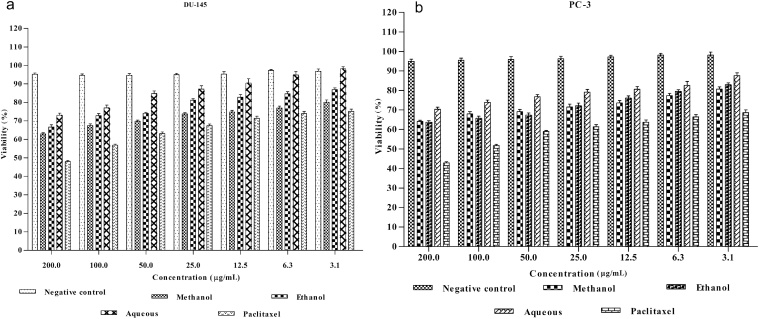
3.7. Effect of L. indica leaf methanol, ethanol, and aqueous extracts on DU-145 and PC-3 prostate cancer cells lines
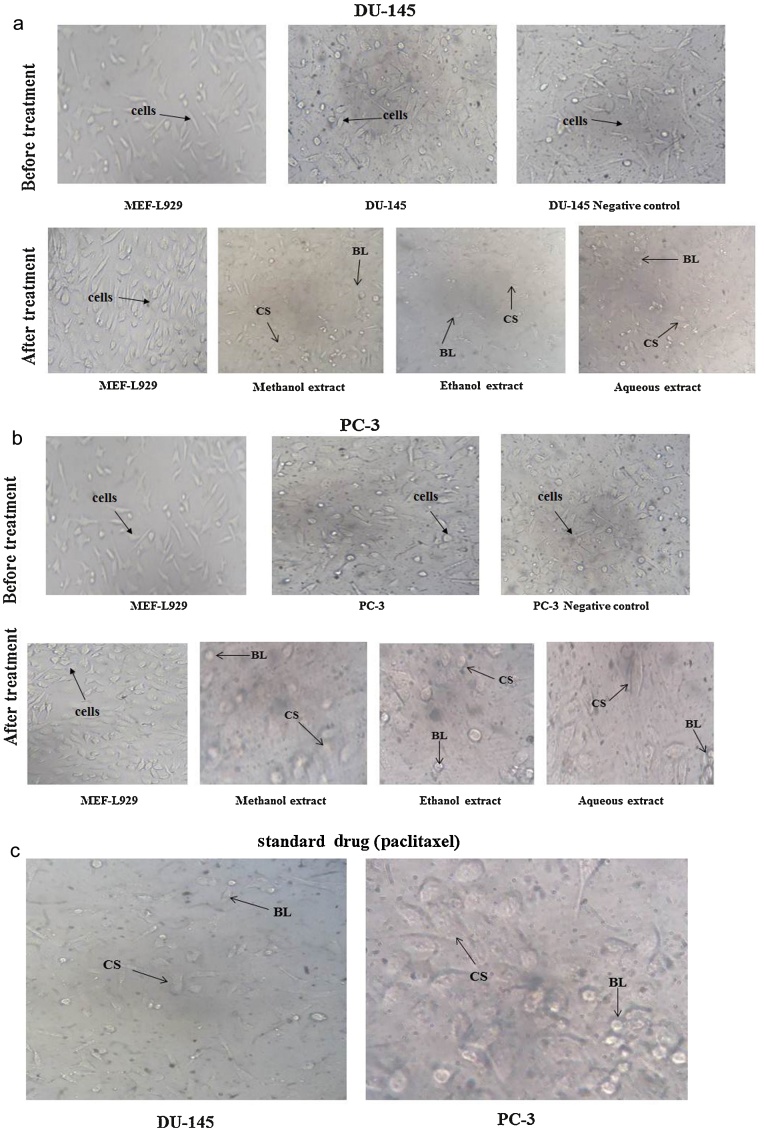
The result of MTT assays revealed that the methanol and ethanol extract of L. indica leaves decreased the percent viability of all the cells but to different extent. Methanol and ethanol extract was found to induce more cytotoxicity towards cancer cell lines DU-145 and PC-3 (Fig. 3A and 3B). All the three extracts showed no cytotoxic effect towards noncancerous normal mice embryo fibroblast (MEF-L929) cell line. The effect induced by the extracts is also similar to that of the standard chemotherapeutic drugs such as paclitaxel, which is commonly used in the treatment of prostate cancer. The combination of the extracts along with the standard drugs also induced more cytotoxicity in cancerous cells. These results revealed morphological changes and shrinkage of cells leading to cell death induced by the extracts in the prostate cancer cell lines (Fig. 4A–C). The IC50 values of methanol, ethanol, and aqueous extracts of L. indica against (DU-145 and PC-3) prostate cancer cell lines are represented in (Table 3).
Fig. 3.
(A) Cytotoxicity of Leea indica methanol, ethanol, aqueous leaf extracts, and standard drug on DU-145 prostate cancer cell lines. (B) Cytotoxicity of Leea indica methanol, ethanol, aqueous leaf extracts, and standard drug on PC-3 prostate cancer cell lines.
Data is presented as mean standard error of the mean (n = 3). Statistical significance was assessed using one-way as compared to the control group and standard.
*p < 0.05.
** p < 0.01.
Fig. 4.
(A) Morphological changes showing inhibition of DU-145 prostate cancer cell lines, whereas no inhibition was observed on MEF-L929 normal cell line for 72 hours. (B) Morphological changes showing inhibition of PC-3 prostate cancer cell lines, whereas no inhibition was observed on MEF-L929 normal cell line for 72 hours. (C) Morphological changes of standard drug paclitaxel on DU-145 and PC-3 prostate cancer cell lines.
BL, membrane blebbing (magnification for DU-145 was 20 × and PC-3 was 40 × ); CS Cellular shrinkage.
Table 3.
IC50 values of cell proliferation inhibition of Leea indica extracts (μg/mL).
| Cells | Methanol | Ethanol | Aqueous |
|---|---|---|---|
| DU-145 | 529.44 ± 42.07 | 677.11 ± 37.01 | 875.22 ± 38.53 |
| PC-3 | 547.55 ± 33.52 | 631.99 ± 50.24 | 987.88 ± 50.06 |
| Standard: paclitaxel | 0.3 μM/mL | ||
The values presented are mean ± standard deviation, n = 3. Results were analyzed using descriptive statistics.
4. Discussion
In recent years, the use of herbal medicines in cancer treatment has received increasing attention due to their varied phyto-metabolic contents with multiple biological activities.18 The plant collected from Western Ghats was identified according to their taxonomical characters as L. indica and analyzed for the presence of phytochemicals with five solvent extracts. Preliminary phytochemical analysis revealed the presences of secondary metabolites in the selected extracts of the plant. These secondary metabolites are reported to have many biological and therapeutic properties.19 Phyto-constituents were quantified by different biochemical tests. Phenols and flavonoids were detected in methanol, ethanol, and aqueous extract only, whereas alkaloid was present in methanol and aqueous extracts. The presence of glycosides was shown in all five solvents, and tannins in ethyl acetate, methanol, ethanol, and aqueous (Table 1). The studied phytochemicals in leaves of L. indica extracts are pharmaceutically important. Among the different phytochemicals, phenolic compounds have gained attention of different areas of applications such as pharmaceutical, health, food, and cosmetic industries. These compounds are widespread in the plant kingdom as part of our daily diet and are attractive as natural antioxidants.20, 21 In this study, the phenolic content was studied in L. indica where in the methanol extract exhibited the highest total phenolic content (65.20 ± 0.15 mg/g GAE) followed by the ethanol extract (60.97 ± 0.23) mg/g GAE.
Reactive oxygen species are thought to play an important role in many human diseases. Radical scavenging activities are very essential due to the toxic role of free radicals in biological systems. Many secondary metabolites like phenols, polyphenols, and flavonoids serve as sources of antioxidants and perform scavenging activity.22 Reactive oxygen species readily combine and oxidize biomolecules such as carbohydrates, proteins, and lipids and thus making them indolent with subsequent damage to cells, tissues, and organs leading to cancer progression.23, 24 In the present work, three methods were used to evaluate the total antioxidant capacity of methanol, ethanol, and aqueous extracts (FRAP, PM, and DPPH). The ferric ion-reducing antioxidant power assay of the extract may serve as a significant indicator of its potential antioxidant activity. The presence of antioxidants, which have been shown to be an impart antioxidant action by breaking the free radical chain by donating a hydrogen molecule.25 The presence of antioxidants in the extract would result in the reduction of ferri cyanide Fe3+ to ferro cyanide Fe2+ by donating an electron which was measured spectrophotometrically at 700 nm. In this assay, the yellow color of the test solution changes to various shades of green and blue, depending on the reducing power of plant extract. The reducing power increased with the increase in the extract concentrations. This may be served as significant indicator of its potential antioxidant activity. Hence, this study presumed that the methanol extract of L. indica may have a high amount of antioxidant properties which was comparable to that of the synthetic antioxidant standard used (Fig. 1).
The total antioxidant activity of the sample was analyzed by the PM method. It is calorimetric quantitative method which measures the reduction of Phosphate-Mo (VI) to Phosphate-Mo (V) by the sample and subsequent formation of a bluish green colored Phosphate-Mo (V) complex.15 It helps to examine the reduction rate among the antioxidant and molybdenum ligand. In the present study methanol extract exhibited higher absorbance than ethanol and aqueous extract (0.818 ± 0.001; Fig. 2).
DPPH is stable nitrogen centered free radical which is conventionally used to determined free radical scavenging activities of antioxidants present in plant extract or synthetic compound.26, 27 The reduction capability of DPPH radical is determined by the decrease in absorbance at 517 nm induced by the antioxidant. In the present study, the antioxidant activity of L. indica was evaluated using methanol, ethanol, and aqueous extract of the plant and was compared with standard ascorbic acid. The experimental data revealed that the extracts are likely to have the properties of scavenging free radicals with methanol extract showing higher antioxidant capacity (Table 2).
The evaluation of the anticancer activity of plant extracts is essential for safe treatment. It enables identification of the intrinsic toxicity of the plant and the effects of acute overdose.28, 29 The MTT assay is used in screening the crude extracts as well as in the isolated compounds to assess the toxicity. It could also provide an indication of possible cytotoxic properties of the tested plant extracts. MTT assay is based on the reduction of MTT by mitochondrial dehydrogenase by purple formazan product. It is frequently used as an in vitro model system to measure cytotoxic effects of variety of toxic substances and plant extracts against cancer cell lines.30 In vitro cytotoxicity test using DU-145 and PC-3 prostate cancer cell lines was performed to screen potentially toxic compounds that affect basic cellular functions and morphology. The three extracts (methanol, ethanol, and aqueous) of L. indica showed in vitro growth inhibition effects on the two cancer cell lines (DU-145 and PC-3), while there was no effect on the growth of normal cells (MEF-L929). Such selective effects were concentration as well as, incubation time period dependent. With respect to concentration (3.125 μg/mL, 6.25 μg/mL, 12.5 μg/mL, 25 μg/mL, 50 μg/mL, 100 μg/mL, 200 μg/mL) of each extract were evaluated in triplicates by serial dilution. Among these seven concentrations, 200 μg/mL of methanol and ethanol extract was the most effective in producing percentage growth inhibition. The aqueous extract showed less effect throughout the range of tested concentrations in DU-145 and PC-3 prostate cancer cell lines for a single time point of 72 hours. However, the standard paclitaxel drug showing significant inhibition on the cancer cell lines (Fig. 3A and 3B). The results showed that methanol extract significantly inhibited the (DU-145 and PC-3) cell lines and was the most potent extract with IC50 value 529.44 ± 42.07 μg/mL for DU-145 and 547.55 ± 33.52 μg/mL for PC-3 followed by ethanol (Table 3). The results also confirmed the differential effect induced by the extracts and standard drug in cancerous and normal cells (Fig. 4A–C). Therefore, the inhibition of cell growth by L. indica extracts might be due to the power of the solvent in surpassing effect of several bioactive constituents, the presences of phenolic compounds like gallic acid and other antioxidant agents that are present in L. indica.31, 32
In conclusion, it was observed that the plant L. indica contains a wide variety of secondary metabolites that hold strong antioxidant capacity based on the experiments performed which add scientific evidence to conduct further studies, investigate the lead compounds present in the plant, evaluate its anticancer potential on in vivo animal models and put forward an attempt to carry out trails on human beings.
Conflicts of interest
The authors declare that they have no competing interests.
Acknowledgments
The authors are grateful to Dr Kotresha K, Department of Botany, Karnataka Science College, Dharwad, Karnataka, for identifying the plant. The authors express their sincere gratitude to the authorities of Dr Prabhakar Kore Basic Science Research Center, KLE University, Belagavi, Karnataka, India, for providing the laboratory facilities to execute the Soxhlet extraction and cell culture experiments. The authors also wish to thank the authorities of Department of Biotechnology, K.L.E’s R. L. Science Institute, (Autonomous), Belagavi, Karnataka, India, for providing infrastructure to execute phytochemical analysis and antioxidant assays.
References
- 1.Hariharan K., Padmanabha V. Demography and disease characteristics of prostate cancer in India. Indian J Urol. 2016;32:103–108. doi: 10.4103/0970-1591.174774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harun-ur-Fiashid M., Gafur M., Sadik M.G., Rahman A. Biological activities of a new acrylamide derivative from Ipomoea turpethum. Pakistan J Biol Sci. 2002;5:968–969. [Google Scholar]
- 3.Algotar A.M., Stratton M.S., Ahmann F.R., Ranger-Moore J., Nagle R.B., Thompson P.A., et al. Phase 3 clinical trial investigating the effect of selenium supplementation in men at high-risk for prostate cancer. Prostate. 2013;73:328–335. doi: 10.1002/pros.22573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kristal A.R., Till C., Platz E.A., Song X., King I.B., Neuhouser M.L., et al. Serum lycopene concentration and prostate cancer risk: results from the Prostate Cancer Prevention Trial. Cancer Epidemiol Biomarkers Prev. 2011;20:638–646. doi: 10.1158/1055-9965.EPI-10-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brausi M., Federica R., Bettuzzi S. Chemoprevention of human prostate cancer by green tea catechins: two years later. A follow-up update. Eur Urol. 2008;54:472–473. doi: 10.1016/j.eururo.2008.03.100. [DOI] [PubMed] [Google Scholar]
- 6.Gupta A.K., Tandon N. vol. 2. Indian Council of Medical Research; New Delhi, India: 2004. (Reviews on Indian medicinal plants). [Google Scholar]
- 7.Pandey G., Madhuri S. Medicinal plants: better remedy for neoplasm. Indian Drugs. 2006;43:869–874. [Google Scholar]
- 8.Cragg G.M., Newman D.J. Plants as a source of anti-cancer agents. J Ethnopharmacol. 2005;100:72–79. doi: 10.1016/j.jep.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 9.Rao K.V.K., Schwartz S.A., Nair H.K., Aalinkeel R., Mahajan S., Chawda R., et al. Plant derived products as a source of cellular growth inhibitory phytochemicals on PC-3M, DU-145 and LNCaP prostate cancer cell lines. Curr Sci. 2004;87:1585–1588. [Google Scholar]
- 10.Bais S. A phytopharmacological review on an important medicinal plant: Leea indica. Cancer. 2013;15:16. [Google Scholar]
- 11.Wong Y.H., Habsah A.K., Ling S.K. Bioassay-guided isolation of cytotoxic cycloartane triterpenoid glycosides from the traditionally used medicinal plant Leea indica. Evid Based Complement Altern Med. 2012;5:164689. doi: 10.1155/2012/164689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deepti K.P.U., Vijayalakshmi G. Antimicrobial activity and phytochemical analysis of Morinda tinctoria Roxb. leaf extracts. Asian Pac J Trop Biomed. 2012;2:S1440–S1442. [Google Scholar]
- 13.Wolfe K., Wu X., Liu R.H. Antioxidant activity of apple peels. J Agric Food Chemi. 2003;51:609–614. doi: 10.1021/jf020782a. [DOI] [PubMed] [Google Scholar]
- 14.Benzie I.F.F., Strain J.J. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 15.Prieto P., Pineda M., Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem. 1999;269:337–341. doi: 10.1006/abio.1999.4019. [DOI] [PubMed] [Google Scholar]
- 16.Brand-Williams W., Cuvelier M.-E., Berset C.L.W.T. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol. 1995;28:25–30. [Google Scholar]
- 17.Igarashi M., Miyazawa T. The growth inhibitory effect of conjugated linoleic acid on a human hepatoma cell line, HepG2, is induced by a change in fatty acid metabolism, but not the facilitation of lipid peroxidation in the cells. Biochim Biophys Acta. 2001;1530:162–171. doi: 10.1016/s1388-1981(00)00180-3. [DOI] [PubMed] [Google Scholar]
- 18.Mann J. Natural products in cancer chemotherapy: past, present and future. Nat Rev Cancer. 2001;2:143–148. doi: 10.1038/nrc723. [DOI] [PubMed] [Google Scholar]
- 19.Sumathy R., Sankaranarayanan S., Bama P., Ramachandran J., Vijayalakshmi M., Deecaraman M. Antioxidant and antihemolytic activity of flavanoid extract from fruit peel of Punica granatum. Asian J Pharm Clin Res. 2013;6:211–214. [Google Scholar]
- 20.Sreeramulu D., Raghunath M. Antioxidant activity and phenolic content of roots, tubers and vegetables commonly consumed in India. Food Res Int. 2010;43:1017–1020. [Google Scholar]
- 21.Jaradat N.A., Shawahna R., Hussein F., Al-Lahham S. Analysis of the antioxidant potential in aerial parts of Trigonella arabica and Trigonella berythea grown widely in Palestine: a comparative study. Eur J Integr Med. 2016;8:623–630. [Google Scholar]
- 22.Diplock A.T. Will the good fairies please prove to us that vitamin E lessens human degenerative disease? Free Rad Res. 1997;27:511–532. doi: 10.3109/10715769709065791. [DOI] [PubMed] [Google Scholar]
- 23.Siddique N.A., Mujeeb M., Najmi A.K., Akram M. Evaluation of antioxidant activity, quantitative estimation of phenols and flavonoids in different parts of Aegle marmelos. African J Plant Sci. 2010;4:1–5. [Google Scholar]
- 24.Koksal E., Bursal E., Dikici E., Tozoglu F., Gülçin I. Antioxidant activity of Melissa officinalis leaves. J Med Plant Res. 2011;5:217–222. [Google Scholar]
- 25.Oliveira I., Sousa A., Ferreira I.C., Bento A., Estevinho L., Pereira J.A. Total phenols, antioxidant potential and antimicrobial activity of walnut (Juglans regia L.) green husks. Food Chem Toxicol. 2008;46:2326–2331. doi: 10.1016/j.fct.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 26.Kalaivani T., Mathew L. Free radical scavenging activity from leaves of Acacia nilotica (L.) Wild. ex Delile, an Indian medicinal tree. Food Chem Toxicol. 2010;48:298–305. doi: 10.1016/j.fct.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 27.El-Maati M.F.A., Mahgoub S.A., Labib S.M., Al-Gaby A.M.A., Ramadan M.F. Phenolic extracts of clove (Syzygium aromaticum) with novel antioxidant and antibacterial activities. Eur J Integr Med. 2016;8:494–504. [Google Scholar]
- 28.Padmaja R., Arun P.C., Prashanth D., Deepak M., Amit A., Anjana M. Brine shrimp lethality bioassay of selected Indian medicinal plants. Fitoterapia. 2002;73:508–510. doi: 10.1016/s0367-326x(02)00182-x. [DOI] [PubMed] [Google Scholar]
- 29.Rahman M.A., Akhtar J., Siddiqui S., Arshad M. Evaluation of cytotoxic potential and apoptotic effect of a methanolic extract of Bauhinia racemosa Lam against a human cancer cell line, HeLa. Eur J Integr Med. 2016;8:513–518. [Google Scholar]
- 30.Morshed M.A., Uddin A., Rahman A., Hasan T., Roy S., Amin A.A., et al. In vitro antimicrobial and cytotoxicity screening of Terminalia arjuna ethanol extract. Int J Biosci. 2011;1:31–38. [Google Scholar]
- 31.Rahman M.A., Imran T.B., Islam S. Antioxidative, antimicrobial and cytotoxic effects of the phenolics of Leea indica leaf extract. Saudi J Biol Sci. 2013;20:213–225. doi: 10.1016/j.sjbs.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dalu D., Duggirala S., Akarapu S. Anti-hyperglycemic and hypolipidemic activity of Leea indica. Int J Bioassay. 2014;3:3155–3159. [Google Scholar]