Abstract
Background
Aromatherapy is used in clinical settings for patients suffering from several chronic and critical diseases such as cancer. Ethyl acetate (EA) is a colorless liquid with a characteristic fruity smell and is naturally present in fruits and wines.
Methods
In the present study, the effect of the aroma of EA was evaluated on human breast cancer cell line MDA-MB-231 and normal cell line, Vero. Cell line viability and mechanism of EA cytotoxicity were determined by Trypan blue dye exclusion assay and phase contrast microscopy.
Results
It was found that EA at a concentration of 0.026 M was effective in causing considerable cytotoxicity in breast cancer cells (without even coming in contact with the culture medium and cells), while showing no effect on normal cells. Mechanism of action of EA on cancer and Vero cells was investigated by DNA fragmentation and dye binding assays using agarose gel electrophoresis (AGE) and fluorescence microscopy/cytometry, respectively. It was found that EA aroma induced predominantly necrosis in the cancer cells exposed to it.
Conclusion
A study such as this has not been attempted before and results need further investigation before EA aroma can be used as a complementary therapy.
Keywords: aromatherapy, breast cancer, cytotoxicity, ethyl acetate, MDA-MB-231 cells
1. Introduction
In aromatherapy, essential oils (also known as volatile oils) are extracted from various aromatic plants for use in palliative care of patients suffering from serious illness in order to improve quality of life.1, 2 The main mode of action of essential oils is through alteration of physiological processes. The volatile constituents of the essential oils are taken up by the body through topical, oral, vaginal, rectal, or olfactory routes.3 Olfactory receptors in the nose respond to the volatile chemical constituents present in the aroma of these oils by sending chemical messages to the limbic system of the brain via neurons.3 This causes a change in mood and emotions by creating a sedative effect for reduction of stress and anxiety, which leads to reduced pain perception.2
Aromatherapy has been demonstrated to be effective in alleviating and relieving the symptoms of stress, anxiety, depression, and nausea in patients undergoing radiotherapy and chemotherapy for cancer.4 Some cancer patients ultimately experience a better quality of life when the pain is controlled through aromatherapy. The National Cancer Institute has also reported the benefits of inhalation of vapors of peppermint, ginger, and cardamom oil on alleviation of the harmful side effects of radiation and chemotherapy.4 Recently, the ability of essential oils to kill cancer cells has been demonstrated with no harm to healthy cells.5
The sense of smell is indispensable to life.6 Our sense of smell usually serves as a warning regarding the safety of food.6 Pleasant aromas also make food more palatable to eat. Esters are a family of organic compounds responsible for many of the pleasant odors of fruits. Esters are formed from carboxylic acids by replacing the acidic hydrogen by an alkyl or aryl group.6 The most common ester, ethyl acetate (EA) occurs as a colorless volatile liquid at room temperature with a pleasant fruity smell, having a boiling point of 77 °C (www.chm.bris.ac.uk). EA is used for flavoring confectionery, ice creams, and cakes, as well as in artificial fruit essences and aroma enhancers and perfume manufacturing industries.6 This colorless liquid has a characteristic sweet smell, similar to pear drops. EA is present in confectionery and perfumes, as well as fruits such as apples. Because of its light and fruity odor, EA is used in perfumes since it confers a fruity smell (as do many esters) and is highly volatile, producing a cooling sensation and leaving the scent of the perfume on the skin.7
EA is inhaled via the nasal route into the body where it is broken down to ethyl alcohol and acetic acid, which are metabolized further before being excreted (www. onlinelibrary.wiley.com). The median lethal dose (LD50) for rats is 5620 mg/kg, which is indicative of low toxicity (www.hazard.com). Since the ester naturally occurs in many organisms, there is little possibility of toxicity. Exposure to concentrations > 1500 mL/m3 causes irritation of the upper respiratory tract and eyes. EA behaves as a narcotic at high concentrations and causes lung, kidney, and liver damage.8 In animal experiments, after repeated exposure to EA at subnarcotic concentrations, the organs most likely to be affected are the lungs, liver, kidneys, and spleen. If applied repeatedly and occlusively to the skin, EA, in its undiluted form causes irritation. It is questionable whether EA causes sensitization.
At high concentrations and under certain culture conditions, EA induces aneuploidy in Saccharomyces cerevisiae.8 Chromosomal aberrations have been reported in the fibroblasts of the Chinese hamsters on EA exposure.8 Salmonella mutagenicity tests and two in vivo micronucleus tests have yielded negative results.8
There are no scientific reports available on the mechanism of action of EA. However, the rapid metabolism of EA to ethanol and acetic acid deserves attention for toxicological evaluation. It has been found that topical application of acetic acid (parent compound) on mucosal or serosal surface causes necrosis of tumor in mouse model of gastric cancer.9 Substituted derivatives of acetic acid like flavone acetic acid (LM975) and flavone acetic acid ester (LM985) are known to exhibit anticancer activities.10, 11 In the present study, the effect of aroma of EA on the viability and survival of breast cancer cells has been studied in vitro. Promising results have indicated that the cytotoxic effect of EA aroma should be studied further with respect to other cancer cell lines and the mechanism of action of EA-induced cytotoxicity in vitro should be investigated in detail.
2. Methods
2.1. Reagents
Trypan blue (0.4%), phosphate-buffered saline (PBS; pH 7.2, 1×), 0.25% trypsin–EDTA (1×), Dulbecco's Modified Eagle's Medium (DMEM/F-12) (1×) and antibiotic/antimycotic solution (100×) were obtained from Gibco, Life Technologies; EA was from Rankem, and fetal bovine serum (FBS) was from Himedia. Agarose, 2,7-dichlorofluorescein diacetate (DCFHDA), acridine orange, and ethidium bromide were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Tali Apoptosis AnnexinV/PI Staining Kit was from Molecular Probes™, Thermo Fisher. All other chemicals used in the study were of analytical grade.
2.2. Cell lines
MDA-MB-231 (human breast carcinoma, estrogen receptor negative, tumorigenic and invasive cell line) and Vero (ATCC-CCL-81 normal kidney epithelial cell line) cells were obtained from the National Centre for Cell Science (Pune, India), and were maintained by subculturing and passaging as monolayers in 25- and 75-cm2 cell culture flasks (Nest; Tarsons) at 37 °C in the Tissue and Cell Culture Laboratory, Era's Medical College, Lucknow, India, in a 5% CO2 incubator at 95% humidity for producing HCO3 buffering capacity as reported previously.12 The cells were maintained at pH 7.4 in DMEM containing phenol red as a pH indicator and supplemented with 5% FBS.12 The medium, prior to being used in cell culture experiments was vacuum filtered using a Corning filtration system (Corning®, Sigma-Aldrich).
2.3. Experimental setup
Experiments were carried out in 25-cm2 cell culture flasks. Square pieces of sterilized cotton enclosed in cotton mesh were cut and affixed to the inside of the upper boundary of the culture flask by means of a transparent cello tape as shown in Fig. 1. EA in pre-calculated concentrations (0.02 M, 0.026 M, 0.03 M, 0.034 M, 0.04 M, 0.05 M, 0.1 M, 0.2 M, and 0.5 M) was added on to the cotton swabs inside each flask by means of a micropipette. The flasks were immediately closed so as to allow vapors of EA to saturate the flask.
Fig. 1.
Experimental setup.
MDA cells were trypsinized and added to each flask at a density of 105 cells/mL and were allowed to attach. The flasks were placed in the incubator for the next 48 hours. After 24 hours and 48 hours, the cells in the flasks were observed under phase contrast microscopy and photographed (Nikon Eclipse Ti, Japan). After 48 hours, the cells in each flask were trypsinized, centrifuged, and resuspended in PBS, and the viable cells were counted using trypan blue dye exclusion assay.
Each dose of EA was tested in at least three replicate flasks. Results were interpreted as cell viability versus time period graphs. The African green monkey normal kidney epithelial cell line Vero served as a positive control in the study. Briefly, 105 cells/mL were exposed to varying concentrations of EA (0.02–0.5 M) for 48 hours, as described above. Suitable untreated negative controls (only culture medium and MDA/Vero cells, respectively) were also concomitantly used. At the end of the treatment, cells were trypsinized and subjected to trypan blue dye exclusion assay.
2.4. Trypan blue dye exclusion assay
The assay was carried out as reported previously.13
2.5. Acridine orange–ethidium bromide assay
Confirmation of the mechanism of cytotoxicity caused by EA on cancer and normal cells was done as reported previously.14 In separate experiments, MDA and Vero cells were seeded in 25-cm2 cell culture flasks at 105 cells/mL in presence of 0.02 M, 0.026 M, 0.03 M, 0.034 M, 0.04 M, 0.05 M, 0.1 M, 0.2 M, and 0.5 M EA and cultured for 48 hours. Control flasks contained 105 MDA/Vero cells/mL, without exposure to EA. At the end of the treatment, trypsin was added for detachment of the adherent cells, which were pooled with suspended dead cells. Subsequently, cells were centrifuged at 2000 rpm (425 × g) for 5 minutes at room temperature and washed twice with PBS. The cells were subjected to staining using a mixture of acridine orange and ethidium bromide (2 μg/mL) for 10 minutes at 37 °C in a CO2 incubator as reported previously.14 Following this, cells were washed twice with ice-cold PBS and the pellet was resuspended in 100 μL PBS. Thereafter, a drop of cell suspension was placed on a glass slide, with a coverslip and observed under a Leica DM2500 fluorescence microscope (Wetzlar, Germany) equipped with 450–490 nm excitation and 520/570 nm emission filters.14 Morphological characteristics of necrosis and apoptosis were observed at both 10× and 40× magnifications and cells were typed as follows: (1) even nucleated green-colored cells indicated live cells; (2) green-colored cells with condensed or fragmented nuclei indicated early apoptosis; (3) orange–red-colored cells with condensed or fragmented nuclei indicated late apoptosis; and (4) orange–red-colored nucleated cells were indicated as undergoing necrosis.
2.6. Annexin V/propidium iodide staining
Further confirmation of EA-induced cytotoxicity was done using the Tali Apoptosis AnnexinV/PI staining kit. In separate experiments, MDA and Vero cells were seeded in the presence of varying concentrations of EA, as described above, and cultured for 48 hours. The cells were thereafter trypsinized, centrifuged, washed, and pooled with suspended dead cells. The cells were resuspended in Annexin binding buffer (IX) and stained with AnnexinV/PI in the dark at room temperature for 20 minutes. The samples were loaded into Tali cellular analysis slides and read in a Tali image-based cytometer (Invitrogen, Life Technologies). Cell samples were divided into three populations based on the dye labels as apoptotic (green fluorescence), dead (red or yellow fluorescence resulting from a combination of red and green fluorescence), and live (little to no fluorescence).
2.7. Determination of reactive oxygen species
Intracellular reactive oxygen species (ROS) generation was done as reported previously15 using the dye DCFHDA. Nonfluorescent DCFHDA is cell permeable and is converted to fluorescent product dichlorofluorescein (DCF) within a cell subjected to oxidative stress. The fluorescence can be detected by fluorescence microscopy and flow cytometry. In separate experiments, MDA and Vero cells were seeded in cell culture flasks in presence of varying EA concentration, as described above, for 1 hour and 24 hours. At the end of treatment, cells were stained with 10 μM DCFHDA in the dark for 30 minutes at room temperature followed by washing with PBS. Images were captured at 485 nm excitation and 535 nm emission wavelengths using a Leica DM2500 fluorescence microscope under 10× objective.
2.8. DNA isolation from treated and control cells
MDA cells were seeded in 25-cm2 cell culture flasks at 105 cells/mL in the presence of the above-mentioned concentrations of EA and cultured for 48 hours. A flask containing 105 cells/mL served as a negative control. At the end of treatment (48 hours), adherent cells were harvested by trypsinization and pooled with suspended dead cells. The cells were centrifuged and resuspended in PBS, and DNA was isolated using a NucleoSpin Blood Kit (Macheray-Nagel, Germany). DNA concentration was measured using Nanodrop spectrophotometer.
2.9. DNA fragmentation assay
DNA isolated from control and EA treated MDA cells was subjected to AGE on a 1.5% gel at 60 V for 90 minutes using 1× Tris-Borate-EDA buffer (TBE) in a Genei electrophoresis unit (Bengaluru, India). Gels were stained with ethidium bromide and DNA was visualized in a transilluminator as bright orange bands.
2.10. Data interpretation and statistical analysis
Results were expressed as mean ± standard deviation of experiments done in triplicate.
3. Results
Fig. 2, Fig. 3 respectively depict the dose-dependent effect of EA on MDA and normal cells. Fig. 2J shows the effect of 0.5 M EA on the viability of MDA-MB-231 breast cancer cells. It is clear from the figure that the impact of the strong aroma due to 0.5 M EA was sufficient for 100% cytotoxicity of MDA cells. EA vapor at 0.2 M also proved to be too toxic for MDA cells (Fig. 2I), while at 0.1 M, EA still had a strong cytotoxic effect (Fig. 2H). Normal kidney epithelial Vero cell line was more susceptible and sensitive to EA at 0.1 M as compared to MDA cells (Fig. 3H), while at 0.2 M EA, there was 100% cytotoxicity (Fig. 2I). At 0.05 M, EA was more cytotoxic to Vero as compared to MDA cells (Fig. 3G). However, EA at < 0.03 M concentrations, was found to be cytotoxic only to MDA cells. Vero cells, at these concentrations, remained more or less unaffected (Table 1, Table 2 and Fig. 3B–3D).
Fig. 2.
Phase contrast microscopy. (A) Controls showing untreated MDA-MB-231 human breast cancer cells. Cytotoxic activity of ethyl acetate vapor saturated flasks at (B) 0.02 M, (C) 0.026 M, (D) 0.03 M, (E) 0.034 M, (F) 0.04 M, (G) 0.05 M, (H) 0.1 M, (I) 0.2 M, and (J) 0.5 M on MDA-MB-231 cells after 48 hours (magnification 10×).
Fig. 3.
Phase contrast microscopy. (A) Controls showing untreated Vero normal kidney epithelial cells. Cytotoxic activity of ethyl acetate vapor saturated flasks at (B) 0.02 M, (C) 0.026 M, (D) 0.03 M, (E) 0.034 M, (F) 0.04 M, (G) 0.05 M, (H) 0.1 M, and (I) 0.2 M on Vero cells after 48 hours (magnification 10×).
Table 1.
Evaluation of effect of EA on MDA-MB-231 cells
| S. No. | EA (M) | No. of live cells |
% cytotoxicity | |
|---|---|---|---|---|
| Control | Treated | |||
| 1 | 0.5 | 1.06 × 106 ± 25.23 | 0 | 100 |
| 2 | 0.2 | 1.08 × 106 ± 32.61 | 3.0 × 104 ± 35.76 | 97 |
| 3 | 0.1 | 1.15 × 106 ± 19.67 | 1.5 × 105 ± 21.56 | 87 |
| 4 | 0.05 | 1.01 × 106 ± 18.43 | 2.8 × 105 ± 34.23 | 72 |
| 5 | 0.04 | 1.11 × 106 ± 22.23 | 5.8 × 105 ± 48.34 | 48 |
| 6 | 0.034 | 1.09 × 106 ± 12.98 | 6.5 × 105 ± 16.78 | 40 |
| 7 | 0.03 | 1.5 × 106 ± 21.13 | 9.0 × 105 ± 12.45 | 40 |
| 8 | 0.026 | 1.5 × 106 ± 17.15 | 9.4 × 105 ± 23.45 | 37 |
| 9 | 0.02 | 1.08 × 106 ± 16.45 | 7.5 × 105 ± 45.34 | 31 |
EA, ethyl acetate; S. No., serial number.
Table 2.
Evaluation of effect of EA on Vero cells
| S. No. | EA (M) | No. of live cells |
% cytotoxicity | |
|---|---|---|---|---|
| Control | Treated | |||
| 1. | 0.5 | 1.15 × 106 ± 38.54 | 0 | 100 |
| 2. | 0.2 | 1.08 × 106 ± 37.64 | 0 | 100 |
| 3. | 0.1 | 1.08 × 106 ± 37.64 | 2.0 × 104 ± 11.12 | 98 |
| 4. | 0.05 | 5.4 × 105 ± 12.34 | 4.0 × 105 ± 8.67 | 26 |
| 5. | 0.04 | 3.2 × 105 ± 34.23 | 2.4 × 105 ± 14.78 | 25 |
| 6. | 0.034 | 6.0 × 105 ± 23.56 | 5.6 × 105 ± 13.67 | 7 |
| 7. | 0.03 | 6.0 × 105 ± 12.34 | 5.8 × 105 ± 12.56 | 3 |
| 8. | 0.026 | 5.4 × 105 ± 13.23 | 6.4 × 105 ± 23.12 | 0 |
| 9. | 0.02 | 3.2 × 105 ± 8.23 | 4.9 × 105 ± 8.67 | 0 |
EA, ethyl acetate; S. No., serial number.
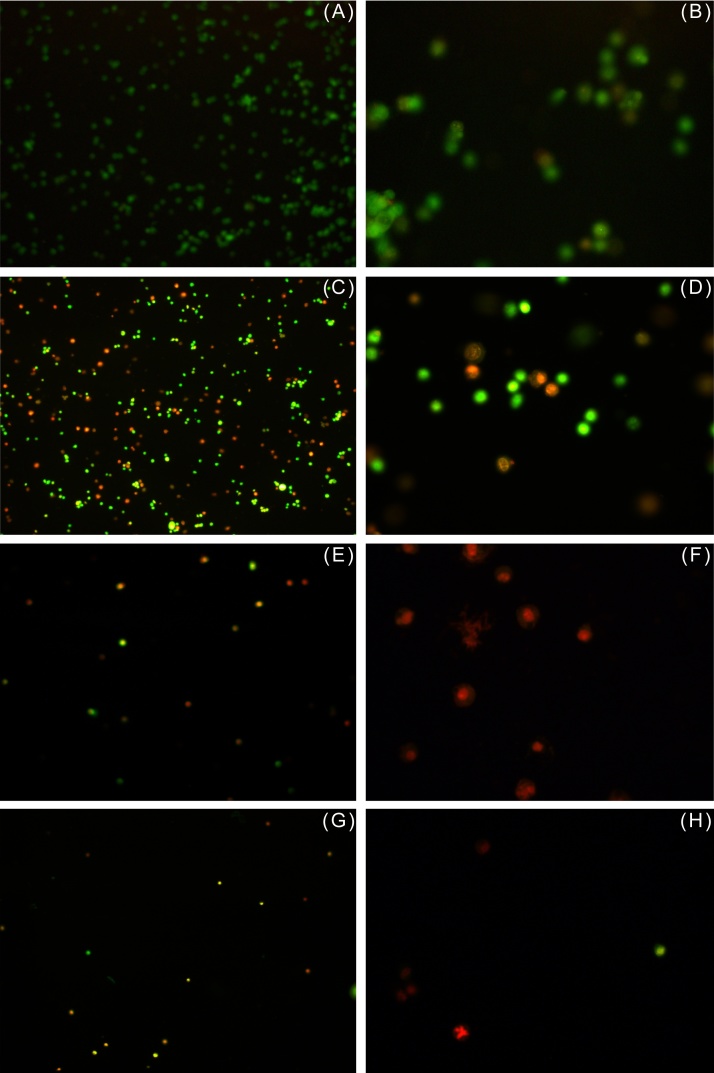
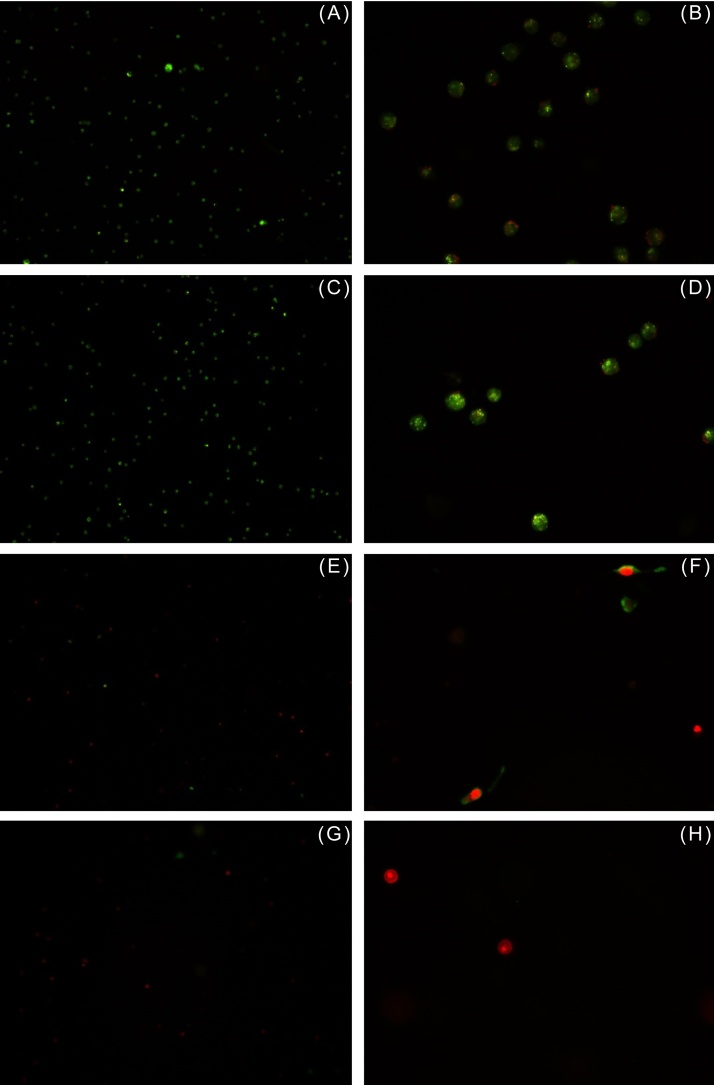
In order to distinguish, whether the cytotoxic effect of EA vapor was due to apoptosis or necrosis, EA-treated MDA and Vero cells were subjected to acridine orange–ethidium bromide assay as described above. Cells were stained with a mixture of acridine orange and ethidium bromide and observed under a fluorescent microscope. Acridine orange can permeate cells and stain the nuclei green (indicating live cells), while ethidium bromide, which stains the nuclei orange–red, can be taken up only by cells that have lost cytoplasmic membrane integrity, and hence indicate dead cells.14 As shown in Fig. 4A and 5A , untreated MDA and Vero cells (controls) displayed uniform green-colored nuclei indicating live cells, while cells treated with increasing concentrations of EA were progressively stained orange–red (Fig. 4C, 4 E, and 4 G). Few bright green, early apoptotic cells with nuclear condensation as well as orange–red, late apoptotic cells with fragmented chromatin and apoptotic bodies were detected in the acridine orange–ethidium bromide assay. As discussed earlier, Vero cells showed greater susceptibility to 0.1 M EA as compared to MDA cells (Fig. 5E).
Fig. 4.
EA induced necrosis of human breast cancer cell line MDA-MB-231, visualized with acridine orange–ethidium bromide assay. (A) Cells from control flask; cells in presence of (C) 0.026 M (E) 0.1 M, and (G) 0.2 M EA after 48 hours (10× magnification) (B, D, F, H) same at 40× magnification, respectively. Uniformly stained green-colored nuclei indicate live cells; whereas uniformly stained orange–red-colored cells are indicative of necrosis.
EA, ethyl acetate.
Fig. 5.
EA induced necrosis of normal kidney epithelial cell line Vero. (A) Cells from control flask; cells in presence of (C) 0.026 M, (E) 0.1 M, and (G) 0.2 M EA after 48 hours (10× magnification) (B, D, F, H) same at 40× magnification, respectively. Uniformly stained green-colored nuclei indicate live cells; whereas uniformly stained orange–red-colored cells are indicative of necrosis.
EA, ethyl acetate.
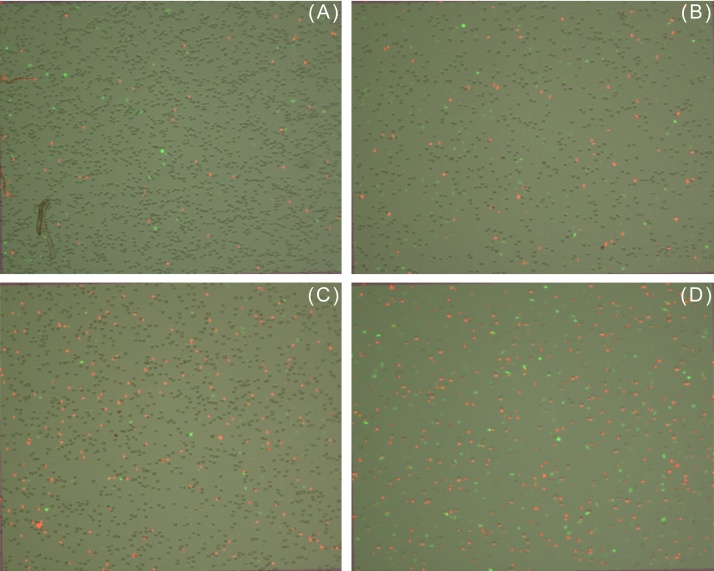
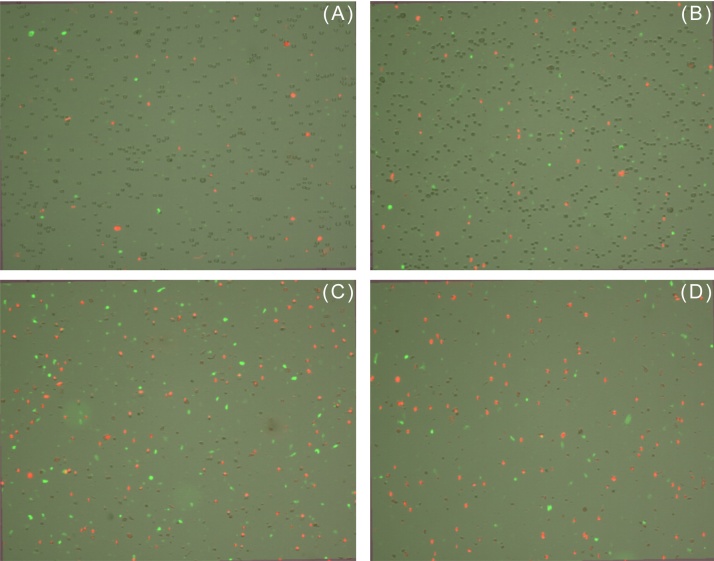
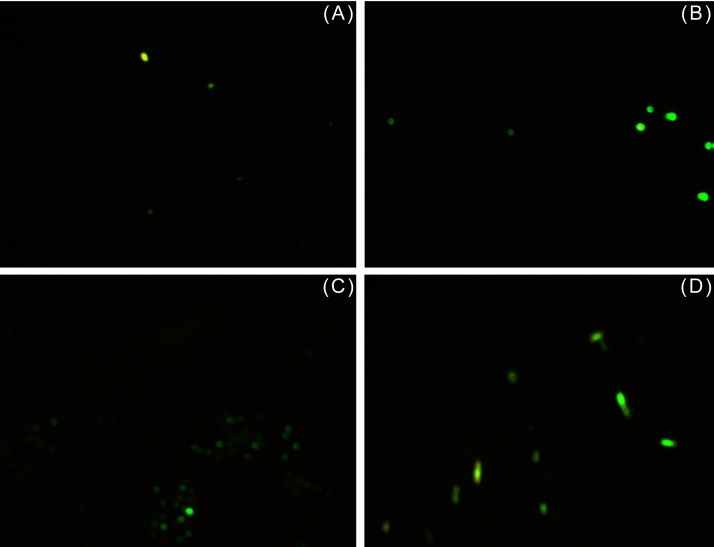
EA-treated MDA and Vero cells were also subjected to AnnexinV/PI staining. Annexin V, a protein with high affinity for phosphatidylserine is commonly used as a marker for cells undergoing apoptosis in conjugation with a green fluorescent dye such as Alexa Fluor 488. As is evident from Fig. 6, Fig. 7 and Table 3, EA induced predominantly necrosis in the studied cells. The percentage of cells undergoing apoptosis was found to be negligible in MDA (0–2%) and Vero (0–1%) cells. Since ROS are markers of oxidative stress and are commonly implicated in induction of necrosis, their generation was assessed at intervals of 1 hour and 24 hours after treatment of MDA and Vero cells with EA. No ROS were detected after 1 hour in both EA-treated cell lines but after 24 hours, a small elevation in ROS levels was detected in both EA-treated cells lines as compared to their respective controls (Fig. 8, Fig. 9). Only a slight increase in fluorescent intensity was observed in MDA cells as EA concentration increased. This suggests that ROS generation is not the main trigger for induction of EA-mediated necrosis and other species might be involved. This warrants further investigation.
Fig. 6.
Apoptosis assay using Annexin V/PI to determine cell viability and health. EA induced necrosis of human breast cancer cell line MDA-MB-231. (A) Cells from control flask; cells in presence of (B) 0.026 M, (C) 0.1 M, and (D) 0.2 M EA after 48 hours. Green fluorescent cells were labeled with Annexin and designated apoptotic; PI-labeled cells fluoresced red and were dead cells; yellow fluorescent cells were labeled with both Annexin and PI and were also counted as dead; whereas unstained cells were counted as live.
EA, ethyl acetate; PI, propidium iodide.
Fig. 7.
Apoptosis assay using Annexin V/PI to determine cell viability and health. EA induced necrosis of normal kidney epithelial cell line Vero. (A) Cells from control flask; cells in presence of (B) 0.026 M, (C) 0.1 M, and (D) 0.2 M EA after 48 hours. Green fluorescent cells were labeled with Annexin and designated apoptotic; PI-labeled cells fluoresced red and were dead; yellow fluorescent cells were labeled with both Annexin and PI and were also counted as dead; whereas unstained cells were counted as live.
EA, ethyl acetate; PI, propidium iodide.
Table 3.
Live, dead, and apoptotic populations in apoptosis assay of EA-treated MDA-MB-231 and Vero cells
| S. No. | Control/treated cells | Total cell no. (cells/mL) | Live (cells/mL) | % | Dead (cells/mL) | % | Apoptotic (cells/mL) | % |
|---|---|---|---|---|---|---|---|---|
| MDA-MB-231 cells | ||||||||
| 1. | Control | 1.04 × 106 | 1.01 × 106 | 97 | 2.1 × 104 | 2 | 0.05 × 104 | 0 |
| 2. | 0.026M EA | 1.04 × 106 | 6.81 × 105 | 65 | 3.37 × 105 | 32 | 2.38 × 104 | 2 |
| 3. | 0.1 MEA | 1.01 × 106 | 1.21 × 105 | 12 | 8.79 × 105 | 87 | 1.0 × 104 | 1 |
| 4. | 0.2 MEA | 0.90 × 106 | 2.70 × 104 | 3 | 8.71 × 105 | 97 | 0.02 × 104 | 0 |
| Vero cells | ||||||||
| 1. | Control | 2.86 × 106 | 2.71 × 106 | 95 | 1.36 × 105 | 5 | 1.05 × 104 | 0 |
| 2. | 0.026M EA | 2.97 × 106 | 2.72 × 106 | 92 | 2.31 × 105 | 8 | 1.76 × 104 | 1 |
| 3. | 0.1M EA | 3.43 × 106 | 3.44 × 105 | 10 | 3.07 × 106 | 89 | 2.57 × 104 | 1 |
| 4. | 0.2M EA | 3.08 × 106 | 2.33 × 105 | 8 | 2.83 × 106 | 92 | 2.24 × 104 | 1 |
EA, ethyl acetate; S. No., serial number.
Fig. 8.
ROS generation in MDA-MB-231 cells following 24 hours EA treatment and staining with 2,7-dichlorofluorescein diacetate. (A) Cells from control flask; cells in presence of (B) 0.026 M, (C) 0.1 M, and (D) 0.2 M EA (magnification 10×).
Fig. 9.
ROS generation in Vero cells following 24 hours EA treatment and staining with 2,7-dichlorofluorescein diacetate. (A) Cells from control flask; cells in presence of (B) 0.026 M, (C) 0.1 M, and (D) 0.2 M EA (magnification 10×).
EA, ethyl acetate.
Further confirmation of induction of necrosis by EA was done by DNA fragmentation assay, which revealed solid DNA bands with no sign of DNA ladder formation, which is a hallmark property of apoptosis (Fig. 10). Moreover, DNA fragmentation in necrosis is a postlytic event that takes place after complete lysis of cells, as compared to apoptosis, which is a prelytic event. These results corroborated the fact that EA vapor induced predominantly necrosis and not apoptosis in cancer cells. All the experiments were performed three times independently.
Fig. 10.
Gel electrophoresis of DNA isolated from live MDA-MB-231 cells in control and EA treated flasks. Lane 1: DNA from Flask 1 (Control); Lane 2: DNA from Flask 2 (0.02 M EA); Lane 3: DNA from Flask 3 (0.026 M EA); Lane 4: DNA from Flask 4 (0.03 M EA); Lane 5: DNA from Flask 5 (0.034 M EA); Lane 6: DNA from Flask 6 (0.04 M EA); Lane 7: DNA from Flask 7 (0.05 M EA); Lane 8: DNA from Flask 8 (0.1 M EA); Lane 9: DNA from Flask 9 (0.2 M EA); and Lane 10: DNA from Flask 10 (0.5 M EA). EA did not induce DNA ladder formation, a hallmark property of apoptosis. The data are representative of three independent experiments.
EA, ethyl acetate.
As is evident from Table 4 and Fig. 10, DNA concentration was at a maximum in cells from the control flask 1 and a minimum in cells from the treatment flask 10 (0.5 M EA). As EA concentration increased, cell death increased with fewer live adherent cells.
Table 4.
DNA estimation using Nanodrop
| Sample code | EA (M) | Amount of DNA (ng/μL) |
|---|---|---|
| 1 | Nil | 20.7 |
| 2 | 0.02 | 16.3 |
| 3 | 0.026 | 19.8 |
| 4 | 0.03 | 18.5 |
| 5 | 0.034 | 14.4 |
| 6 | 0.04 | 14.5 |
| 7 | 0.05 | 12.2 |
| 8 | 0.1 | 7.9 |
| 9 | 0.2 | 3.0 |
| 10 | 0.5 | 1.9 |
EA, ethyl acetate.
4. Discussion
In this paper, we have described the unique anticancer property of aroma/vapor of EA on human breast cancer and normal Vero cell lines. A number of esters are responsible for many of the pleasant smells of fruits such as apples.16 It is often said “that having an apple a day keeps the doctor at bay”. It is possible that esters might be responsible for the protective action of fruits such as apples against diseases like cancer. This requires further investigation.
Complementary medicine is a term used to describe treatment modalities other than additional forms of treatment that may be prescribed along with allopathic medicine.17 In the past, complementary medicine had claims of magical cures and remedies for cancer; many of which have now been proved to be false and ineffective. However, a strategic combination of traditional and complementary medicine in recent years has garnered increasing interest in this area.17 This approach is currently being adopted at leading cancer hospitals and self-help groups. A major role in palliative care is being played by such beneficial therapies.
Complementary medicine is sometimes erroneously referred to as alternative therapy or alternative medicine, and it is necessary to understand the difference between the two. While complementary medicine is recognized as a proven therapy by many healthcare professionals, alternative therapy is not.17 Complementary medicine is an adjunct therapy, given alongside with chemotherapy, whereas alternative medicine replaces chemotherapy altogether and includes non-recognized and untested treatments that can be harmful.
One form of complementary medicine therapy is sensory therapy that takes into account the five senses of smell, site, taste, sound, and touch, as well as the total energy of the body.17 Aromatherapy is a type of sensory therapy that is based on the sense of smell or aroma. Research on the effectiveness of aromatherapy is limited. Frankincense essential oil, steam-distilled from Boswellia sacra tree, has been tested against several cell lines and has been found to be effective against all cell lines, with no harm to healthy, normal cells.18 Recently, frankincense essential oil has also been found to be effective against breast cancer cells and ovarian cancer, thereby leading to the possibility that the essential oil may be effective to target late-stage breast and ovarian cancers.19, 20 It has also been reported recently that the vapor of volatile oils distilled from Litsea cubeba seeds induces apoptosis and causes cell cycle arrest in lung cancer cells.21
For therapeutic purposes, an ideal anticancer agent should be one having little or no side effects on normal cells.21 Such agents have considerable potential to be studied and developed further as prospective anticancer agents in drug-discovery programs.22 Hence, the present study also entailed a study of the effect of EA on the survival and viability of normal cells. It was found that that EA has little cytotoxicity against non-cancerous cells.
There are two major mechanisms of cell death: necrosis or apoptosis. For the longest time, apoptosis was believed to be the standard mode of cell death during development processes, homeostasis, infection, and pathogenesis.23, 24 Apoptosis or programmed cell death, is a kind of cell death that is generally triggered by normal, healthy processes in the body.25 By contrast, necrosis is mostly considered as premature death of cells and living tissue due to cellular injury. However recent genetic research26, 27, 28 on inhibition of necrosis by chemical agents26, 29, 30 has revealed that like apoptosis, necrosis can be a regulated process. Novel regulatory pathways of necrosis have been elucidated and accordingly, necrosis is currently classified into various subtypes depending upon the pathway as necroptosis, parthanatos, oxytosis, ferroptosis, ETosis, NETosis, pyronecrosis, and pyroptosis.31
Each pathway of regulated necrosis comprises four levels in a molecular cascade, namely, triggering by physicochemical stimuli, initiation of mediators leading to signal propagation and amplification (mediation), and response to the above stimuli resulting in necrotic cell death (execution). Level 1 is triggered by agents such as hypothermia, hypoxia, UV radiation, ROS,32, 33 Ca2+, ischemia–reperfusion injury, phorbol-12-myristate-13-acetate, lipopolysaccharide, and N–methyl-N′–nitro–N–nitrosoguanidine, which are known to cause damage to the plasma membrane.31
The present study demonstrated that EA induced necrosis in cancer cells via a pathway other than generation of ROS. Although apoptosis was also detected in small amounts (Fig. 6, Fig. 7 and Table 3), necrosis remained as the predominant mechanism of cell death. It would be our endeavor in future to unravel the pathway underlying necrosis induction and determine changes in total protein expression of cells exposed to EA as compared to unexposed cancer cells.
In conclusion, this study showed the cytotoxic effect of vapor/aroma on MDA-MB-231 human breast cancer cells and normal Vero kidney epithelial cells in vitro. It was found that EA at a concentration of 0.026 M caused significant cytotoxicity in MDA cells (without even coming in contact with cells), and at the same time showed no effect on the normal cell line Vero. The study also demonstrated that EA induced necrosis in cancer cells. This study is believed to be the first of its kind and warrants further investigation before it can be considered as a prospective adjunct/complementary therapy along with mainstream treatment for cancer. In future, the effect of EA will be studied on a panel of cancer and normal cell lines and on animal models of cancer, and changes in protein profile and expression of cancer cells in the presence of EA will be studied and compared to those in untreated cancer cells.
Conflicts of interest
The authors declare that they have no competing interests.
References
- 1.Boehm K., Büssing A., Ostermann T. Aromatherapy as an adjuvant treatment in cancer care– a descriptive systematic review. Afr J Trad Complement Altern Med. 2012;9:503–518. doi: 10.4314/ajtcam.v9i4.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Cancer Society. What are complementary and alternative methods? www.cancer.org/treatment/treatmentsandsideeffects/complementaryandalternativemedicine/complementaryandalternativemethodsandcancer/complementary-andalternative-methods-and-cancer-what-are-cam. Published March 31, 2015. Accessed January 18, 2016.
- 3.Walter G. The use of aromatherapy in cancer treatment. Kaplan University Complementary and Alternative Medicine. www.healthandwellness.kaplan.edu/articles/cam/The%20Use%20of%20Aromatherapy%20in%20Cancer%20Treatment. Published November 30, 2011. Accessed January 18, 2016.
- 4.National Cancer Institute. Aromatherapy and Essential Oils. www.cancer.gov/about-cancer/treatment/cam/patient/aromatherapy-pdq. Published December 17, 2014. Accessed January 19, 2016.
- 5.Fassa P. Cancer industry trying to co-opt most recent potential natural cure-frankincense. www.naturalnews.com/035296 frankincense cancer natural cure.html. Published March 17, 2012. Accessed January 19, 2016.
- 6.Cotton S. Ethyl Acetate. University of Bristol School of Chemistry Website. www.chm.bris.ac.uk/motm/ethylacetate/ethylv.htm. Accessed January 25, 2016.
- 7.Indoor Air Quality UK. IAQUK Resources- Ethyl Acetate. www.iaquk.org.uk/ResourcesEthylAcetate.html. Accessed January 31, 2016.
- 8.Ethyl Acetate. www.hazard.com/msds/mf/baker/baker/files/e2850.htm. Published August 8, 1996. Accessed February 2, 2016.
- 9.Okabe S., Kodama Y., Cao H., Johannessen H., Zhao C.M., Wang T.C. Topical application of acetic acid in cytoreduction of gastric cancer. A technical report using mouse model. J Gastroenterol Hepatol. 2012;27:40–48. doi: 10.1111/j.1440-1746.2012.07070.x. [DOI] [PubMed] [Google Scholar]
- 10.Bibby M.C., Double J.A., Phillips R.M., Loadman P.M. Factors involved in the anti-cancer activity of the investigational agents LM985 (flavone acetic acid ester) and LM975 (flavone acetic acid) Br J Cancer. 1987;55:159–163. doi: 10.1038/bjc.1987.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cummings J., Smyth J.F. Flavone 8-acetic acid: our current understanding of its mechanism of action in solid tumors. Cancer Chemother Pharmacol. 1989;24:269–272. doi: 10.1007/BF00304756. [DOI] [PubMed] [Google Scholar]
- 12.Khajah M.A., Almorhi I., Mathew P.M., Lugmani Y.A. Extracellular alkaline pH leads to increased metastatic potential of estrogen receptor silenced endocrine resistant breast cancer cells. PLOS One. 2013;8:e76327. doi: 10.1371/journal.pone.0076327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khan M.A., Ahmad R., Srivastava A.N. Effect of methyl butyrate aroma on the survival and viability of human breast cancer cells in vitro. J Egypt Natl Canc Inst. 2016;28:81–88. doi: 10.1016/j.jnci.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 14.Maliyakkal N., Udupa N., Pai K.S.R., Rangarajan A. Cytotoxic and apoptotic activities of extracts of Withania somnifera and Tinospora cordifolia in human breast cancer cells. Int J Appl Res Nat Prod. 2003;6:1–10. [Google Scholar]
- 15.Rizvi S.H.M., Parveen A., Verma A.K., Ahmad I., Arshad M., Mahdi A.M. Aluminium induced endoplasmic reticulum stress mediated cell death in SH-SY5Y neuroblastoma cell line is independent of p53. PLOS One. 2014;9:e98409. doi: 10.1371/journal.pone.0098409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burdock G.A., editor. 6th ed. CRC Press; London, UK: 2009. Fenaroli's handbook of flavor ingredients. [Google Scholar]
- 17.Cleveland Clinic. What is complementary medicine. www.chemocare.com/complementary-medicine.aspx. Accessed February 2, 2016.
- 18.Ananda E. Research update: frankincense essential oil causes death of breast cancer cells. www.anandaapothecary.com/weblog/2012/05/reaserch-update-frankincense-essential-oil-causes-death-of-breast-cancer-cells. Published May 31, 2012. Accessed March 1, 2016.
- 19.Suhail M.M., Wu W., Cao A., Mondalek F.G., Fung K.M., Shih P.T. Boswellia sacra essential oil induces tumor cell specific apoptosis and suppresses tumor aggressiveness in cultured human breast cancer cells. BMC Complement Altern Med. 2011;11:129. doi: 10.1186/1472-6882-11-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zielinski E. Can frankincense oil kill cancer and boost immunity? www.drericz.com/frankincense-oil-cancer-immunity/. Accessed March 1, 2016.
- 21.Seal S., Chatterjee P., Bhattacharya S., Pal D., Dasgupta S., Kundu R. Vapor of volatile oils from Litsea cubeba seed induces apoptosis and causes cell cycle arrest in lung cancer cells. PLoS One. 2012;7:e47014. doi: 10.1371/journal.pone.0047014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buolamwini J.K. Novel anticancer drug discovery. Curr Opin Chem Biol. 1999;3:500–509. doi: 10.1016/S1367-5931(99)80073-8. [DOI] [PubMed] [Google Scholar]
- 23.Suzanne M., Steller H. Shaping organisms with apoptosis. Cell Death Differ. 2003;20:669–675. doi: 10.1038/cdd.2013.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taylor R., Cullen S., Martin S. Apoptosis: controlled demolition at the cellular level. Nat Rev Mol Cell Biol. 2008;9:231–241. doi: 10.1038/nrm2312. [DOI] [PubMed] [Google Scholar]
- 25.Cotter T.G. Apoptosis and cancer: the genesis of a research field. Nat Rev Cancer. 2009;9:501–507. doi: 10.1038/nrc2663. [DOI] [PubMed] [Google Scholar]
- 26.Sun L., Wang H., Wang Z., He S., Chen S., Liao D. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell. 2012;148:213–227. doi: 10.1016/j.cell.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 27.Vandenabeele P., Galluzzi L., Vanden Berghe T., Kroemer G. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol. 2010;11:700–714. doi: 10.1038/nrm2970. [DOI] [PubMed] [Google Scholar]
- 28.Zhang D.W., Shao J., Lin J., Zhang N., Lu B.J., Lin S.C. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 2009;325:332–336. doi: 10.1126/science.1172308. [DOI] [PubMed] [Google Scholar]
- 29.Teng X., Degterev A., Jagtap P., Xing X., Choi S., Denu R. Structure–activity relationship study of novel necroptosis inhibitors. Bioorg Med Chem Lett. 2005;15:5039–5044. doi: 10.1016/j.bmcl.2005.07.077. [DOI] [PubMed] [Google Scholar]
- 30.Degterev A., Huang Z., Boyce M., Li Y., Jagtap P., Mizushima N. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1:112–119. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- 31.Vanden B.T., Linkermann A., Jouan-Lanhouet S., Walczak H., Vandenabeele P. Regulated necrosis: the expanding network of non-apoptotic cell death pathways. Nat Rev Mol Cell Biol. 2014;15:135–147. doi: 10.1038/nrm3737. [DOI] [PubMed] [Google Scholar]
- 32.Foyer C.H., Descourvieres P., Kunert K.J. Protection against oxygen radicals: an important defence mechanism studied in transgenic plants. Plant Cell Environ. 1994;17:507–523. [Google Scholar]
- 33.Foyer C.H., Lopez-Delgado H., Dat J.F., Scott I.M. Hydrogen peroxide and glutathione-associated mechanisms of acclimatory stress tolerance and signaling. Physiol Plantarum. 1997;100:241–254. [Google Scholar]