Abstract
Context
Multiple endocrine neoplasia type 2B (MEN2B) is a rare autosomal-dominant cancer syndrome characterized in part by metastatic medullary thyroid cancer (MTC) and pheochromocytoma. Cushing disease is a rare cause of endogenous hypercortisolism in children.
Case Description
We describe a 21-year-old African-American male who was diagnosed at age 10 with an ACTH-secreting pituitary microadenoma. At age 16 he developed medullary thyroid cancer and was found to have multiple endocrine neoplasia type 2B with the characteristic M918T mutation of the RET proto-oncogene. Following thyroidectomy, he was initiated on Vandetanib, a tyrosine kinase inhibitor, and has since had stable disease over the last 5 years.
Conclusions
Our patient is the first individual with MEN2B to be described with Cushing disease. The RET oncogene may play a role in pituitary tumorigenesis; alternatively, the coexistence of these two entities may represent an extremely rare coincidence.
Key terms: Hypercortisolemia, neuroendocrine tumor, genetic syndrome, RET
Background
Multiple endocrine neoplasia type 2B (MEN2B) is caused by a mutation of the RET oncogene and is characterized by the presence of medullary thyroid cancer (MTC) in all affected individuals and variable presentations of pheochromocytoma, marfanoid body habitus, mucosal and eyelid neuromas, and ganglioneuromatosis of the gastrointestinal tract. The prevalence of MEN 2B is estimated between 1 in 600,000 to 1 in 4 million [1].
The overall incidence of endogenous Cushing syndrome is approximately 2 to 5 new cases per million people per year. Only approximately 10% of the new cases each year occur in children [2]. Cushing disease caused by an ACTH-secreting pituitary microadenoma in a patient with MEN2B has not previously been described.
Case Report and Methods
The patient was diagnosed with Cushing disease in 2008 at age 13, when he presented with a three-year history of progressive weight gain and decreased linear growth. In addition, he demonstrated striae, facial swelling, abdominal adiposity, hyperpigmentation over the neck, dorsocervical fat pad, and proximal leg weakness. Laboratory values and therapeutic interventions are presented in Table 1. The patient underwent two transsphenoidal surgeries at the age of 13 years without biochemical remission. The initial MRI showed only a pars intermedia cyst. This reading was confirmed on a second, high resolution MRI scan with thin cuts through the pituitary, obtained 2 weeks later. No tumor or positivity for ACTH was seen in the first 2 surgeries. At age 14, he underwent inferior petrosal sinus sampling that localized the lesion to the left side. A left hemihypophysectomy was performed at the age of 14. Histopathological examination was consistent with a pituitary adenoma with strong positive immunohistochemical staining for ACTH (Figure 1). Following his 3rd TSS he achieved biochemical remission of Cushing’s disease, but subsequently developed hypopituitarism with growth hormone deficiency, hypogonadism, hypothyroidism, and adrenal insufficiency. The patient was started on medical management with growth hormone, hydrocortisone, levothyroxine, and later, testosterone. At the age of 16, he presented with diffuse anterior cervical chain lymphadenopathy, a neck mass and frequent diarrhea. A thyroid ultrasound showed calcifications in the left thyroid lobe and calcitonin was elevated at 30,344 pg/ml (reference range <16 pg/ml). The patient underwent a lymph node biopsy and left thyroid lobe resection. Intraoperative regional metastatic disease and mediastinal extension was appreciated. Histopathological examination confirmed medullary thyroid carcinoma (MTC) (Figure 1). MRI of his neck and chest identified pulmonary nodules suspicious for metastasis. Subsequent staging with neck and chest CT showed metastatic lesions on the thyroid bed, bilaterally in the neck area, as well as a right humeral sclerotic lesion. He was diagnosed with metastatic MTC and growth hormone therapy was discontinued. Closer inspection revealed coarse facial features, mucosal neuromas, as well as a marfanoid body habitus. Subsequently he underwent genetic testing, which was positive for the RET M918T mutation, confirming the clinical diagnosis of MEN2B. He was started on Vandetanib as part of a clinical trial at the National Institutes of Health at the age of 16 years and 10 months; since that point his disease course has been stable [3]. Of note, there was no family history of parathyroid or thyroid cancer, pituitary tumors, pheochromocytoma, or pancreatic tumors.
Table 1.

|
|
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variables (normal range) | 13 y 5 m | 14 y | 14 y 2 m | 14 y 5 m | 14 y 7 m | 16 y 7 m | 16 y 9 m | 16 y 10 m | 18 y 8 m | 21 y |
| Cortisol a.m. (5.0 – 25.0 mcg/dL) | 26 | 15 | 1 | <1 | ||||||
| Cortisol p.m. (<4.4 mcg/dL) | 20 | 22 | <1 | |||||||
| 24-Hour free urine Cortisol (3.5 – 45.0 mcg/24h) | 170 | 71 | 1.4 | |||||||
| ACTH level (5 – 46 pg/mL) | 33 | 55 | 5.1 | |||||||
| Calcitonin (<16 pg/mL) | 30,344 | 56,507 | 5,137 | 1,734 | 1,020 | |||||
| CEA (0.8–3.4 ng/mL) | 61 | 45 | 22 | 27 | ||||||
Abbreviations: TSS, transsphenoidal surgery; y, year; m, month; ACTH, adrenocorticotrophic hormone; CEA, carcinoembryonic antigen.
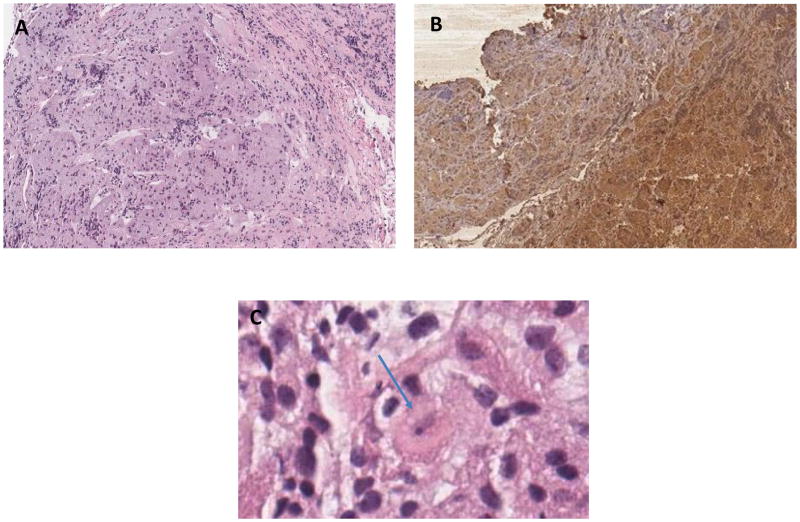
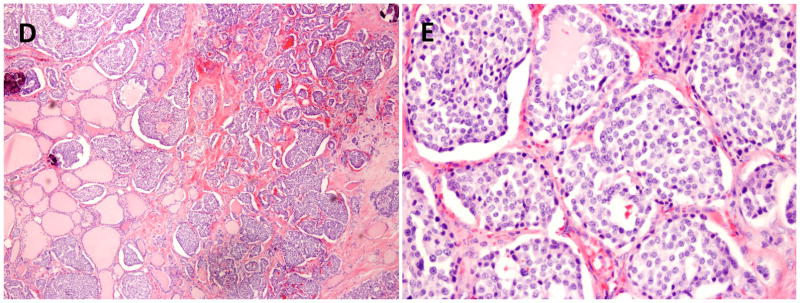
Figure 1.
Histological examination of the pituitary gland demonstrates areas of architectural distortion and acinar expansion with displaced reticulin network, staining positively for ACTH, with adjacent normal pituitary tissue. On Panel C, a few cells showed Crooke’s changes with replacement of cytoplasmic granules of basophil cells by homogeneous hyaline material (see arrow).
A. Pituitary Adenoma hematoxylin and eosin (H&E) 4×
B. Pituitary Adenoma with Immunostaining for ACTH 4×
C. Pituitary Adenoma H&E 40 x,
Histological examination of the thyroid gland demonstrates medullary thyroid carcinoma with characteristic nests of spindle shaped cells and amyloid deposits.
D. Medullary carcinoma of the thyroid 10×
E. Medullary carcinoma of the thyroid 20×
Discussion
MEN2B is an autosomal dominant cancer syndrome characterized by variable penetrance of pheochromocytoma, MTC, mucosal and eyelid neuromas, marfanoid body habitus, and ganglioneuromatosis of the gastrointestinal tract. It is caused by a gain-of-function mutation of the RET proto-oncogene, and is characterized by the early development of an aggressive form of MTC in all affected individuals. The differential diagnosis of this patient’s ACTH-dependent Cushing Syndrome included paraneoplastic ectopic ACTH or CRH production from the MTC, pituitary metastasis of MTC, vs. Cushing disease. This case could not be explained by pituitary metastasis from MTC, as the histopathology from the patient’s pituitary tumor was consistent with CD [4]. Although MTCs are well known to secrete corticotropin releasing hormone (CRH) or adrenocorticotrophic hormone (ACTH) leading to ectopic Cushing Syndrome (CS),[5] this case could not be explained by ectopic tumor as the adenoma resection proved curative, ACTH levels decreased to below normal, and pathology was consistent with CD. To the best of our knowledge, this is the first reported case of Cushing disease in a patient with MEN2B. Evidence linking this patients ACTH-secreting pituitary microadenoma directly with the RET mutation comes from other studies linking pituitary adenomas to syndromes causing pheochromocytoma/paraganglioma [6]. The RET proto-oncogene plays an important role in neuronal organogenesis [7]. RET is an in-vivo interaction partner of AIP (Aryl Hydrocarbon Receptor-Interacting Protein) in the pituitary gland. AIP is a tumor-suppressor protein found mutated in pituitary adenomas. [8]. One case of a growth hormone secreting adenoma in a patient with MEN2A and the RET C634F mutation has been reported [9]. One case of a patient with MEN2A and Cushing disease has been reported who was found to have the RET C634A mutation[10]. In 1968, Steiner et al described an individual with MTC, pheochromocytoma, and Cushing disease, however the RET mutation was not identified as it was not discovered for another 25 years[11]. In summary, we report a 21-year-old African American man with history of Cushing disease status-post three transsphenoidal surgeries and residual anterior hypopituitarism with MEN2B complicated by metastatic medullary thyroid cancer. Coexistence of the two diseases is likely due to a common pathogenic mechanism.
Acknowledgments
Funding information: National Institutes of Health (NIH)
This work was supported by the intramural programs of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the Laboratory of Pathology, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD
Footnotes
Disclosure Statement: The authors have nothing to disclose
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Moline J, Eng C. Multiple endocrine neoplasia type 2: an overview. Genet Med. 2011;13(9):755–64. doi: 10.1097/GIM.0b013e318216cc6d. [DOI] [PubMed] [Google Scholar]
- 2.Stratakis CA. Cushing syndrome in pediatrics. Endocrinol Metab Clin North Am. 2012;41(4):793–803. doi: 10.1016/j.ecl.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fox E, et al. Vandetanib in children and adolescents with multiple endocrine neoplasia type 2B associated medullary thyroid carcinoma. Clin Cancer Res. 2013;19(15):4239–48. doi: 10.1158/1078-0432.CCR-13-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhatoe HS, et al. Pituitary metastasis from medullary carcinoma of thyroid: case report and review of literature. J Neurooncol. 2008;89(1):63–7. doi: 10.1007/s11060-008-9586-5. [DOI] [PubMed] [Google Scholar]
- 5.Barbosa SL, et al. Ectopic adrenocorticotropic hormone-syndrome in medullary carcinoma of the thyroid: a retrospective analysis and review of the literature. Thyroid. 2005;15(6):618–23. doi: 10.1089/thy.2005.15.618. [DOI] [PubMed] [Google Scholar]
- 6.Denes J, et al. Heterogeneous genetic background of the association of pheochromocytoma/paraganglioma and pituitary adenoma: results from a large patient cohort. J Clin Endocrinol Metab. 2015;100(3):E531–41. doi: 10.1210/jc.2014-3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murakumo Y, et al. RET and neuroendocrine tumors. Pituitary. 2006;9(3):179–92. doi: 10.1007/s11102-006-0263-4. [DOI] [PubMed] [Google Scholar]
- 8.Vargiolu M, et al. The tyrosine kinase receptor RET interacts in vivo with aryl hydrocarbon receptor-interacting protein to alter survivin availability. J Clin Endocrinol Metab. 2009;94(7):2571–8. doi: 10.1210/jc.2008-1980. [DOI] [PubMed] [Google Scholar]
- 9.Saito T, et al. Coincidence of multiple endocrine neoplasia type 2A with acromegaly. Am J Med Sci. 2010;340(4):329–31. doi: 10.1097/MAJ.0b013e3181e73fba. [DOI] [PubMed] [Google Scholar]
- 10.Naziat A, et al. Confusing genes: a patient with MEN2A and Cushing’s disease. Clin Endocrinol (Oxf) 2013;78(6):966–8. doi: 10.1111/cen.12072. [DOI] [PubMed] [Google Scholar]
- 11.Steiner AL, Goodman AD, Powers SR. Study of a kindred with pheochromocytoma, medullary thyroid carcinoma, hyperparathyroidism and Cushing’s disease: multiple endocrine neoplasia, type 2. Medicine (Baltimore) 1968;47(5):371–409. doi: 10.1097/00005792-196809000-00001. [DOI] [PubMed] [Google Scholar]