Abstract
Gastroesophageal reflux disease (GERD) is a common condition that presents with symptoms of heartburn and regurgitation. Asthma is an equally common medical condition that often coexists with GERD. The clinical scenario of difficult-to-treat asthma in the setting of concomitant GERD leads to the possibility of GERD-induced asthma. However, asthma may also induce GERD, so confusion has developed about the role of GERD in patients with moderate to severe asthma. Acid-suppressive therapy may be initiated in patients with asthma, but controlled studies have recently questioned the role of such therapy and, thus, have caused further confusion in this field. Recent advancements in the field of esophageal physiologic testing in GERD have introduced the concept of impedance–pH monitoring, which suggests a possible role of nonacid reflux in those who continue to be symptomatic despite acid-suppressive therapy. However, recent data caution about the role of surgical fundoplication based solely on the results of impedance monitoring. This article reviews current knowledge in the fields of GERD and asthma and suggests a possible treatment option for this group of patients.
Keywords: Gastroesophageal reflux disease, asthma, impedance-pH, monitoring, surgical fundoplication, therapy
Gastroesophageal reflux disease (GERD) is a frequent presentation affecting more than 20% to 30% of the population in Western countries, and it is recognized as an important public health issue because of the considerable economic burden resulting from therapies, various medications, and loss of productivity.1 GERD is a chronic condition due to the reflux of stomach contents, which causes troublesome symptoms and/or complications such as esophagitis and Barrett esophagus.2 GERD may present typically, with heartburn and reflux, or atypically. An atypical presentation is now referred to as an extraesophageal syndrome according to the Montreal definition and classification of GERD (Figure 1).2 Common extraesophageal manifestations include dental erosion, laryngitis, laryngeal cancer, otitis media, postnasal drip syndrome, sinusitis, cough, hoarseness, chronic obstructive pulmonary disease, recurrent pneumonia, and asthma. Treating patients with extraesophageal reflux is often difficult and frequently results in the inappropriate and prolonged use of medications. The expenses of caring for this special group of patients continue to increase, with annual costs of more than $50 billion in the United States.3
Figure 1.
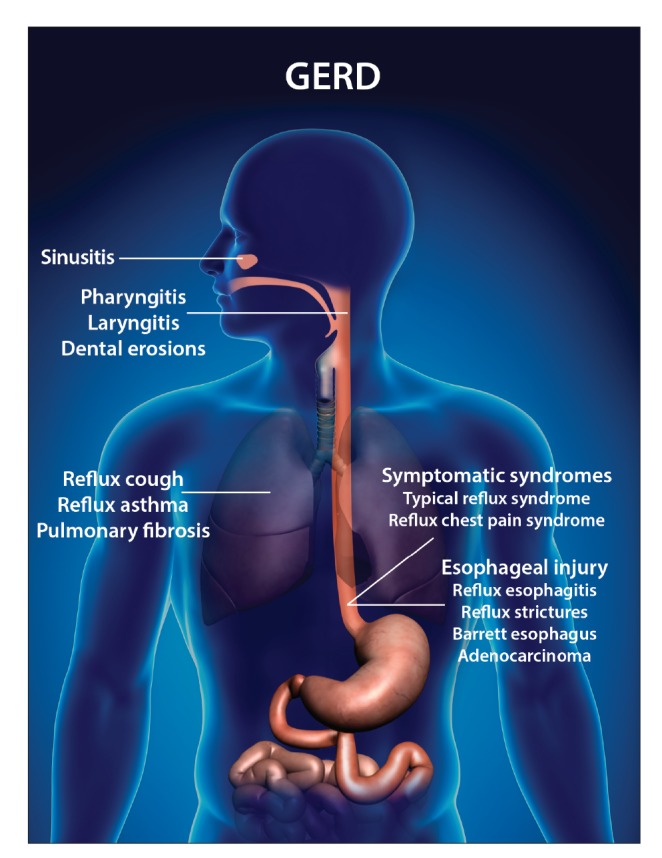
Esophageal and extraesophageal syndromes.
GERD, gastroesophageal reflux disease.
Asthma is characterized by chronic inflammation of the airways associated with airway hyperresponsiveness, which results in episodes of wheezing, chest tightness, shortness of breath, and cough, often at night or in the morning. Asthma is recognized as a highly prevalent health problem affecting an estimated 300 million people of all ages, ethnic groups, and geographic origins, and it is estimated that an additional 100 million people will be affected by 2025.4 The annual cost of asthma is estimated to be nearly $18 billion, with direct costs accounting for $10 billion and indirect costs of $8 billion.5
Gastroesophageal reflux and asthma are often encountered together, and complex interactions occur during which GERD may increase asthmatic symptoms or asthma may trigger or worsen GERD (Figure 2). The prevalence of GERD symptoms is often greater in patients with asthma than in the general population. In general, some studies have suggested that GERD symptoms such as heartburn and regurgitation are experienced by nearly 80% of patients with a diagnosis of asthma.6 A study of more than 100,000 veterans showed that patients with GERD were 1.15 times more likely to have asthma than were those without GERD.7 In addition, some studies employing pH monitoring have shown a prevalence of GERD of 30% to 65% among patients with asthma.8-12 This article examines insights into the relationship between GERD and asthma.
Figure 2.
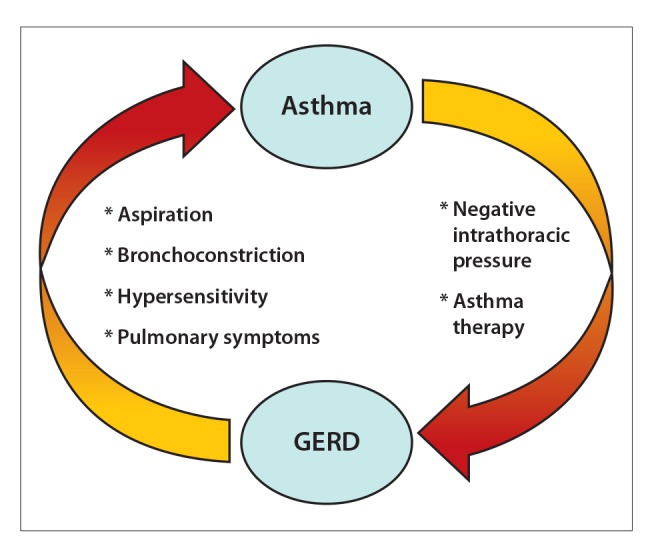
Asthma and GERD may exacerbate each other. GERD may induce bronchospasm, and asthma may induce GERD. Breaking the cycle by aggressively treating both conditions is the key to mitigating patients’ symptoms.
GERD, gastroesophageal reflux disease.
Effects of Gastroesophageal Reflux Disease on Asthma
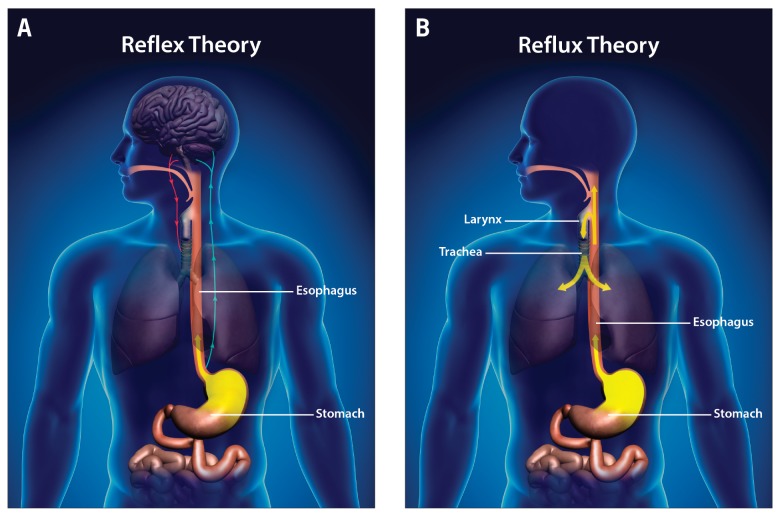
There are multiple mechanisms by which asthma and GERD can interact. Reflux may induce asthma either directly, by effects on the airway through an aspiration-induced response, or indirectly, via neurogenically induced inflammation. The esophagus and lungs have a common embryonic origin, so complex interactions are possible. Reflux of the gastroduodenal contents may induce bronchoconstriction through a vagus-mediated reflex, through neurally enhanced bronchial reactivity, or directly through microaspiration (Figure 3).
Figure 3.
Reflex (A) and reflux (B) pathophysiologic mechanisms in extraesophageal manifestations of gastroesophageal reflux disease.
Neurogenic inflammation may occur in the lungs as a consequence of vagus-mediated mechanisms or microaspiration. For example, animal studies have suggested an increased respiratory response after the instillation of acid into the esophagus,13,14 an effect that can be eliminated by bilateral vagotomy.15 In humans, esophageal exposure to acid may result in decreased peak expiratory flow, thus increasing airway resistance.16 These responses may be reduced by atropine therapy. However, other researchers suggest that acid reflux may actually be a precursor of more severe bronchospasm with future triggers. For example, Vincent and colleagues showed a correlation between the degree of acid reflux during pH monitoring and the dose of methacholine required to induce a response in the forced expiratory volume in 1 second (FEV1).17 The dose of methacholine required to induce a response was lower in patients with more significant reflux, suggesting an increase in airway hyperresponsiveness. Animal studies have also shown that intraesophageal acid perfusion stimulates the release of tachykinins into the airway, leading to cough or bronchospasm.18 Patterson and colleagues19 evaluated tachykinin levels and reflux parameters in a group of patients with chronic cough and mild asthma and in patients with no asthma. Their results showed increased tachykinin levels in those with reflux and a significant correlation between distal esophageal acid exposure and bronchial levels of substance P and neurokinin A, suggesting vagus-induced activation of airway sensory nerves.
In addition, the GERD-induced aspiration or microaspiration of gastroduodenal agents may increase airway resistance. Lopes and colleagues20 evaluated the effects of acid on airway inflammation and found that the esophageal infusion of hydrochloric acid did not alter airway resistance, whereas intratracheal exposure to acid caused a substantial increase in airway resistance. The authors concluded that tracheal microaspiration was the most likely mechanism to explain the effects of GERD on asthma. Animal data suggest that the infusion of acid into the esophagus may result in a cascade of inflammatory responses. For example, esophageal acid exposure may result in increased levels of substance P, leading to smooth-muscle contraction and increased vascular permeability.18 Additionally, acid reflux may increase lung resistance in a dose-dependent fashion mediated by the release of tachykinins from peripheral nerves.21 Lung epithelium may also sustain injury during direct contact with aspirated acid, resulting in a release of cytokines and increased inflammation.22
A causal relationship between GERD and asthma is difficult to establish because either condition can induce the other (Figure 1). However, one should suspect GERD-induced asthma in patients experiencing any of the following: asthma presenting initially in adulthood, poor control of asthma with medications, onset of heartburn or regurgitation before asthma events, and worsening of asthma events in association with the consumption of large meals or alcohol or with the supine position. Empiric proton pump inhibitor (PPI) therapy is often initiated in patients with asthma in order to assess their response to reflux therapy and determine whether GERD is a contributor to their asthma exacerbation.
Effects of Asthma on Gastroesophageal Reflux Disease
Pathophysiologically, asthma may predispose an individual to the reflux of gastroduodenal contents into the esophagus by a variety of mechanisms, including the following: increased intrathoracic pressure, vagus nerve dysfunction, altered diaphragmatic crural function, and decreased lower esophageal sphincter (LES) pressure due to medical therapies for asthma (Figure 1).23,24
Patients with asthma often have lung hyperinflation. Changes in the pressure gradient between the stomach and the esophagus may develop from the increased work of breathing and lung hyperinflation, which can then result in a herniation of the LES into the chest, impairing the barrier to reflux and potentially causing continued reflux due to decreased LES pressure. The complex problem of identifying the relationship between asthma and GERD is that, in a given patient with asthma, it is difficult to discern the inciting factor. Thus, in a given patient with difficult-to-control asthma, the response of the pulmonary symptoms to empiric GERD therapy is often used clinically to determine whether the 2 problems are causally linked.
Diagnosis of Gastroesophageal Reflux Disease–Related Asthma
The diagnosis of GERD-related asthma based on the presence of symptoms of heartburn or regurgitation is difficult because patients with asthma do not always have these classic symptoms of GERD. It is important to recognize that there are no diagnostic gold standards in this area. The roles of esophagogastroduodenoscopy (EGD), barium esophagography, and esophageal pH and impedance monitoring are still controversial. EGD and barium esophagography are limited by their inability to detect a temporal relationship between episodes of reflux and asthma or cough. The presence of esophagitis, which is not common at endoscopy in this group,25 suggests GERD but does not implicate reflux in primary symptoms of asthma. Leggett and colleagues9 used 24-hour ambulatory pH monitoring with dual probes (configured with a distal probe 5 cm above the LES and a proximal probe 15 cm above the LES) to assess GERD in patients with difficult-to-control asthma. The prevalence rates of reflux at the distal and proximal probes were 55% and 35%, respectively. However, the presence of reflux on pH monitoring does not necessarily suggest a causal link to asthma exacerbation.
Esophageal pH monitoring is among the most popular tests used in patients with suspected extraesophageal symptoms, such as asthma. The sensitivity of pH monitoring in diagnosing abnormal esophageal acid exposure in patients with GERD is 90%; however, in patients with asthma, pH monitoring has limited sensitivity and a specificity as low as 66%.26-28 Esophageal pH monitoring may correlate acid reflux events with cough or asthma spells; however, the use of symptom correlation may be problematic in asthma because it is often not directly and temporally related to reflux. Two commonly used indices, the symptom index (SI) and symptom association probability (SAP), attempt to correlate episodes of esophageal reflux with cough or asthma symptoms. However, a recent study by Slaughter and colleagues29 concluded that both the SI and SAP can be overinterpreted and are often prone to misinterpretation. The authors suggested that unless patients with GERD have high rates of esophageal acid exposure, both the SI and SAP are essentially chance occurrences at best. Furthermore, it was recently reported that up to 71% to 91% of patients do not accurately report their cough events when undergoing ambulatory acoustic monitoring, which further reduces enthusiasm for the use of symptom indices in pH monitoring.30 Thus, given the low predictive value of pH testing and the lack of reliability of symptom association indices, pH testing in patients with chronic cough or asthma may not be as useful as once thought.
Recent interest in assessing for nonacid reflux as the potential cause of persistent symptoms in patients with asthma has resulted in the use of ambulatory intraluminal impedance–pH monitoring. Unlike testing with traditional dual-channel pH probes, which reports only acidic changes in the esophagus, impedance monitoring can determine the presence of any remaining physiologic reflux regardless of pH.31 Furthermore, unlike other probe modalities, this device can detect the frequency, location, and direction of any gas or liquid refluxate along the esophagus as well as in the hypopharynx. Despite initial enthusiasm,32,33 outcome studies with this device are lacking, and the clinical relevance of impedance findings in patients with asthma who continue to have symptoms despite PPI therapy remains uncertain. The most recent uncontrolled surgical study found that on- or off-therapy impedance monitoring did not predict the response of symptoms to fundoplication.34 Interestingly, hiatal hernia, significant acid reflux at baseline, and regurgitation concomitant with laryngeal–pharyngeal reflux symptoms were important predictors of symptom response. Thus, the use of impedance monitoring in this group of patients whose symptoms often remain refractory despite PPI therapy continues to be uncertain.
Effects of the Treatment of Asthma on Gastroesophageal Reflux Disease
GERD can also be exacerbated by the variety of medications taken by patients with asthma, including β-adrenergic agonists, theophylline, and high doses of oral corticosteroids.35,36 These medications may reduce LES pressure, resulting in the reflux of gastroduodenal contents into the esophagus. In a prospective, single-blinded, placebo-controlled, crossover study, Lazenby and colleagues37 showed that treatment with prednisone resulted in a significant increase in acid contact time in both the distal and proximal esophagus. GERD symptoms may be increased by 170% in patients with asthma who take theophylline compared with those who take placebo.36 Causal relationships have been shown between β-adrenergic agonists and LES tone, with β-adrenergic agonists producing a reduction in LES tone in dose-dependent fashion.35 Thus, increasing therapies for asthma may have an adverse outcome by increasing GERD, which may, in turn, exacerbate more asthma attacks.
Effects of the Treatment of Gastroesophageal Reflux Disease on Asthma
Initial studies in patients with asthma who underwent treatment for GERD showed mixed results but overall suggested a decrease in symptoms.38-47 These findings led to recommendations in the National Asthma Education and Prevention Program Expert Panel Report 3 that “patients who have asthma and complain of frequent heartburn or pyrosis, particularly those who have frequent nighttime asthma symptoms, may benefit from gastroesophageal reflux treatment. Consider evaluation in patients with poorly controlled asthma for GERD, even in the absence of suggestive GERD symptoms.”48 However, subsequent studies have shown variable and inconsistent results regarding changes in lung function, asthma symptoms, and asthma-related quality of life after acid-suppressive therapy.49-52 The variability in study results is likely due to limitations such as small numbers of participants (<60), use of histamine-2 blockers rather than PPIs, and short duration of treatment (4-12 weeks).
Subsequently, a Cochrane systematic review of the data regarding the role of acid-suppressive therapy in patients with asthma concluded that reflux therapy cannot be recommended for those with uncontrolled asthma because the “treatment of GERD with the goal of improving asthma control was not supported by the literature.”49 Based on this review of the data, a large randomized, controlled trial with a parallel group design and a longer duration of treatment was suggested. However, subsequent trials, consistent with these recommendations, again demonstrated inconsistent results in terms of asthma outcomes.50-52 In a study by Sontag and colleagues50 that compared the therapeutic benefit of surgical fundoplication with that of ranitidine and that of placebo in patients who had asthma, 75% of those who underwent fundoplication experienced a decrease in nocturnal asthma symptoms, compared with 9.2% of those treated with histamine-2 receptor antagonists alone and 4.2% of control patients. A subsequent placebo-controlled study by Littner and colleagues51 of 207 patients with moderate to severe asthma did not show any decrease in asthma symptoms after twice-daily PPI therapy compared with placebo. However, after acid-suppressive therapy, the patients experienced improvement in asthma-related quality of life. A later study, by Kiljander and colleagues,52 assessed the therapeutic benefit of 40 mg of esomeprazole twice daily vs placebo for 16 weeks in 770 patients with moderate to severe asthma. The authors found no improvements in daily peak expiratory flow rate and asthma-related quality of life and no decreases in asthma symptoms or exacerbations in those treated with esomeprazole. However, they did observe a statistically significant improvement in morning and evening peak expiratory flow among the PPI-treated subjects in the subgroup with both GERD and nocturnal respiratory symptoms.
Kiljander and colleagues53 also studied the benefit of once- or twice-daily esomeprazole in improving asthma control. The evaluation included 961 patients with moderate to severe asthma and symptomatic GERD. After 26 weeks of therapy, the patients showed no improvement in mean morning peak expiratory flow in comparison with baseline values. In addition, there were no changes in rescue bronchodilator use, percentage of days without asthma symptoms, or frequency of severe asthma exacerbations. However, the authors did show that treatment with both PPI dosages was associated with a statistically significant improvement in FEV1 and asthma-related quality of life. The improvement in FEV1 was small, with increases of 0.091 L (95% CI, 0.03-0.15; P=.0039) and 0.121 L (95% CI, 0.06-0.18; P<.0001) for once- and twice-daily dosing, respectively. Notably, the authors found that patients treated with any dosage of PPI therapy experienced significant improvement in asthma-related quality of life compared with those treated with placebo. Therefore, based on the preceding studies, there may be a benefit of improving asthma-related quality of life associated with treating reflux; however, this benefit may be limited to those with concomitant symptomatic GERD. Thus, the role of GERD therapy in improving more objective asthma-related measures continues to be controversial.
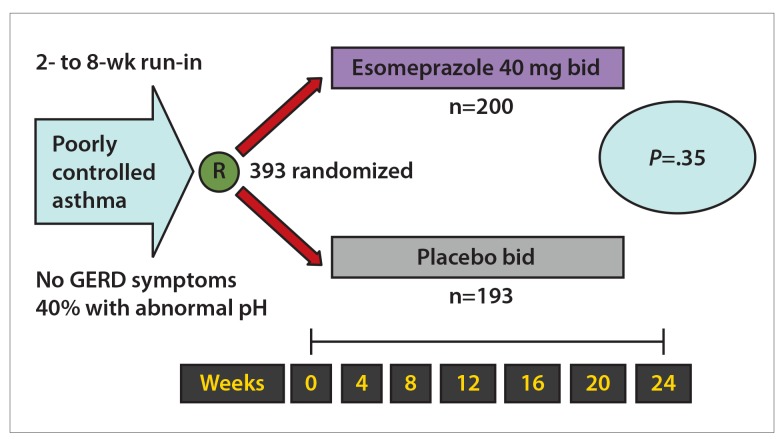
The American Lung Association Asthma Clinical Research Centers conducted a trial addressing the important clinical issue of GERD therapy in patients who do not have concomitant reflux symptoms.8 Patients with asthma and no GERD symptoms underwent pH testing and then were randomized to 40 mg of esomeprazole twice daily or to placebo and were followed for 6 months. Overall, the study did not show any benefit of PPI therapy with respect to the rate of asthma attacks, asthma symptoms, nocturnal awakening, quality of life, or lung function (Figure 4), even though nearly half of the patients had abnormal acid reflux on pH testing at baseline. Thus, the results suggested that PPI therapy was of no benefit in patients with asthma without concomitant typical GERD symptoms and that pH monitoring did not reliably identify a subgroup of patients who were likely to benefit from GERD treatment. Based on the results of this trial, it was recommended that clinicians treating patients with poorly controlled asthma should focus their attention on other potential triggers of asthma symptoms.
Figure 4.
Randomization of patients with poorly controlled asthma to proton pump inhibitor therapy or placebo showed no benefit of acid-suppressive therapy. The primary outcome was a decrease of 30% or more in peak expiratory flow for 2 days.
GERD, gastroesophageal reflux disease.
It would seem intuitive that patients with asthma who have symptomatic reflux would be more likely to respond to acid-suppressive therapy, but controlled data do not suggest this to be true. It is possible that it is not the presence of GERD, but rather the degree and possibly the extent of GERD, that may be important in this group. Therefore, future controlled trials in this area must focus on these and potentially other possible predictors of the response of asthma to PPI therapy. In one uncontrolled study, dual esophageal pH monitoring demonstrated that patients with both proximal and distal esophageal acid exposure had a significantly higher incidence of nocturnal cough than did those with distal esophageal acid exposure alone.54 In the American Lung Association Study of Acid Reflux and Asthma,55 a subgroup (n=242) of participants underwent dual esophageal pH monitoring, with slightly more than one-third of the subjects showing proximal esophageal acid exposure. Patients who had proximal esophageal acid exposure showed worse asthma-related quality of life; however, the concordance between proximal and distal esophageal acid exposure was poor. Importantly, there were no differences in asthma-related clinical outcomes or measures in the subgroup with proximal esophageal acid exposure in comparison with other groups.
Conclusion
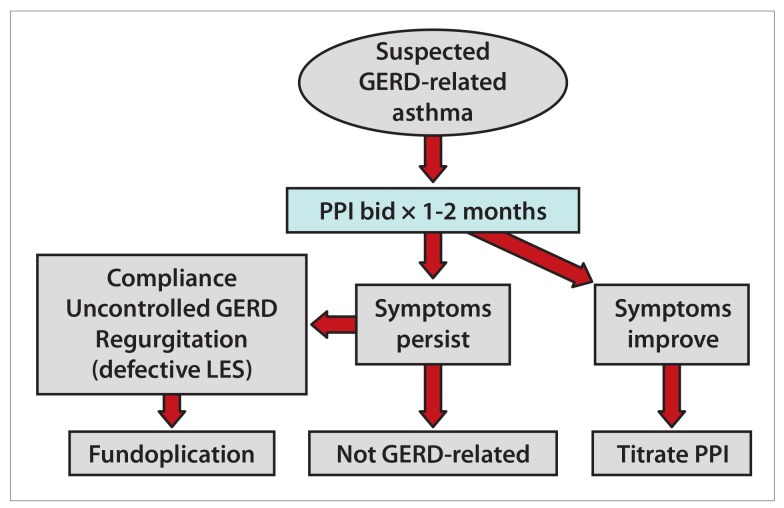
GERD is a common clinical condition that often occurs together with asthma. The 2 conditions may or may not be related in a given individual who has both diagnoses. There are plausible pathogenic explanations for the role of reflux in asthma through both direct aspiration and neurogenic mechanisms. Because the 2 conditions often coexist, the current guidelines suggest that patients with both asthma and symptomatic GERD can be treated with acid-suppressive medications (Figure 5). For a patient suspected of having GERD-induced asthma and no warning symptoms (dysphagia, anemia, or chest pain), we recommend initial empiric therapy with a PPI twice daily for 1 to 2 months. If the asthma symptoms decrease, then continued therapy for an additional 2 months may be necessary to achieve moderate relief. However, if the symptoms persist and the patient does not have concomitant heartburn and/or regurgitation, then GERD as the cause of asthma is less likely. In order to measure the degree of acid reflux in this group, we recommend off-PPI pH testing. If there is moderate to severe reflux at baseline and evidence of a defective LES and hiatal hernia, then surgical fundoplication may be considered. The role of surgical fundoplication in patients who do not respond to PPI therapy and do not have regurgitation is, as of yet, unknown and awaits future trials. Meanwhile, in a symptomatic patient with asthma, we must focus on optimizing asthma control as well as other factors, including patient compliance, proper inhaler technique, and the control of other significant comorbid conditions.
Figure 5.
A suggested algorithm for the diagnosis and treatment of suspected GERD-related asthma.
GERD, gastroesophageal reflux disease; LES, lower esophageal sphincter; PPI, proton pump inhibitor.
Footnotes
The authors have no relevant conflicts of interest to disclose.
References
- 1.Spechler SJ, Jain SK, Tendler DA, Parker RA. Racial differences in the frequency of symptoms and complications of gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2002;16(10):1795–1800. doi: 10.1046/j.1365-2036.2002.01351.x. [DOI] [PubMed] [Google Scholar]
- 2.Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R. Global Consensus Group. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. 2006;101(8):1900–1920. doi: 10.1111/j.1572-0241.2006.00630.x. quiz 1943. [DOI] [PubMed] [Google Scholar]
- 3.Francis DO, Rymer JA, Slaughter JC, et al. High economic burden of caring for patients with suspected extraesophageal reflux. Am J Gastroenterol. 2013;108(6):905–911. doi: 10.1038/ajg.2013.69. [DOI] [PubMed] [Google Scholar]
- 4.Masoli M, Fabian D, Holt S, Beasley R. Global Initiative for Asthma (GINA) Program. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy. 2004;59(5):469–478. doi: 10.1111/j.1398-9995.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 5.Asthma and Allergy Foundation of America. Asthma facts and figures. [Accessed October 5, 2014]. http://www.aafa.org/display.cfm?id=8&sub=42
- 6.Field SK, Underwood M, Brant R, Cowie RL. Prevalence of gastroesophageal reflux symptoms in asthma. Chest. 1996;109(2):316–322. doi: 10.1378/chest.109.2.316. [DOI] [PubMed] [Google Scholar]
- 7.El-Serag HB, Sonnenberg A. Comorbid occurrence of laryngeal or pulmonary disease with esophagitis in United States military veterans. Gastroenterology. 1997;113(3):755–760. doi: 10.1016/s0016-5085(97)70168-9. [DOI] [PubMed] [Google Scholar]
- 8.Mastronarde JG, Anthonisen NR, Castro M, et al. American Lung Association Asthma Clinical Research Centers. Efficacy of esomeprazole for treatment of poorly controlled asthma. N Engl J Med. 2009;360(15):1487–1499. doi: 10.1056/NEJMoa0806290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leggett JJ, Johnston BT, Mills M, Gamble J, Heaney LG. Prevalence of gastroesophageal reflux in difficult asthma: relationship to asthma outcome. Chest. 2005;127(4):1227–1231. doi: 10.1378/chest.127.4.1227. [DOI] [PubMed] [Google Scholar]
- 10.Kiljander TO, Laitinen JO. The prevalence of gastroesophageal reflux disease in adult asthmatics. Chest. 2004;126(5):1490–1494. doi: 10.1378/chest.126.5.1490. [DOI] [PubMed] [Google Scholar]
- 11.Harding SM, Guzzo MR, Richter JE. 24-h esophageal pH testing in asthmatics: respiratory symptom correlation with esophageal acid events. Chest. 1999;115(3):654–659. doi: 10.1378/chest.115.3.654. [DOI] [PubMed] [Google Scholar]
- 12.Sontag SJ, O’Connell S, Khandelwal S, et al. Most asthmatics have gastroesophageal reflux with or without bronchodilator therapy. Gastroenterology. 1990;99(3):613–620. doi: 10.1016/0016-5085(90)90945-w. [DOI] [PubMed] [Google Scholar]
- 13.Ekström T, Tibbling L. Esophageal acid perfusion, airway function, and symptoms in asthmatic patients with marked bronchial hyperreactivity. Chest. 1989;96(5):995–998. doi: 10.1378/chest.96.5.995. [DOI] [PubMed] [Google Scholar]
- 14.Wu DN, Tanifuji Y, Kobayashi H, et al. Effects of esophageal acid perfusion on airway hyperresponsiveness in patients with bronchial asthma. Chest. 2000;118(6):1553–1556. doi: 10.1378/chest.118.6.1553. [DOI] [PubMed] [Google Scholar]
- 15.Mansfield LE, Hameister HH, Spaulding HS, Smith NJ, Glab N. The role of the vague nerve in airway narrowing caused by intraesophageal hydrochloric acid provocation and esophageal distention. Ann Allergy. 1981;47(6):431–434. [PubMed] [Google Scholar]
- 16.Harding SM, Schan CA, Guzzo MR, Alexander RW, Bradley LA, Richter JE. Gastroesophageal reflux-induced bronchoconstriction. Is microaspiration a factor? Chest. 1995;108(5):1220–1227. doi: 10.1378/chest.108.5.1220. [DOI] [PubMed] [Google Scholar]
- 17.Vincent D, Cohen-Jonathan AM, Leport J, et al. Gastro-oesophageal reflux prevalence and relationship with bronchial reactivity in asthma. Eur Respir J. 1997;10(10):2255–2259. doi: 10.1183/09031936.97.10102255. [DOI] [PubMed] [Google Scholar]
- 18.Hamamoto J, Kohrogi H, Kawano O, et al. Esophageal stimulation by hydrochloric acid causes neurogenic inflammation in the airways in guinea pigs. J Appl Physiol. 1997;82(3):738–745. doi: 10.1152/jappl.1997.82.3.738. [DOI] [PubMed] [Google Scholar]
- 19.Patterson RN, Johnston BT, Ardill JE, Heaney LG, McGarvey LP. Increased tachykinin levels in induced sputum from asthmatic and cough patients with acid reflux. Thorax. 2007;62(6):491–495. doi: 10.1136/thx.2006.063982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopes FD, Alvarenga GS, Quiles R, et al. Pulmonary responses to tracheal or esophageal acidification in guinea pigs with airway inflammation. J Appl Physiol. 2002;93(3):842–847. doi: 10.1152/japplphysiol.00013.2002. [DOI] [PubMed] [Google Scholar]
- 21.Ricciardolo FL, Gaston B, Hunt J. Acid stress in the pathology of asthma. J Allergy Clin Immunol. 2004;113(4):610–619. doi: 10.1016/j.jaci.2003.12.034. [DOI] [PubMed] [Google Scholar]
- 22.Stein MR. Advances in the approach to gastroesophageal reflux (GER) and asthma. J Asthma. 1999;36(4):309–314. doi: 10.3109/02770909909068223. [DOI] [PubMed] [Google Scholar]
- 23.Zerbib F, Guisset O, Lamouliatte H, Quinton A, Galmiche JP, Tunon-De-Lara JM. Effects of bronchial obstruction on lower esophageal sphincter motility and gastroesophageal reflux in patients with asthma. Am J Respir Crit Care Med. 2002;166(9):1206–1211. doi: 10.1164/rccm.200110-033OC. [DOI] [PubMed] [Google Scholar]
- 24.Choy D, Leung R. Gastro-oesophageal reflux disease and asthma. Respirology. 1997;2(3):163–168. doi: 10.1111/j.1440-1843.1997.tb00073.x. [DOI] [PubMed] [Google Scholar]
- 25.Kavitt RT, Yuksel ES, Slaughter JC, et al. The role of impedance monitoring in patients with extraesophageal symptoms. Laryngoscope. 2013;123(10):2463–2468. doi: 10.1002/lary.23734. [DOI] [PubMed] [Google Scholar]
- 26.Irwin RS. Chronic cough due to gastroesophageal reflux disease: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(1 suppl):80S–94S. doi: 10.1378/chest.129.1_suppl.80S. [DOI] [PubMed] [Google Scholar]
- 27.Irwin RS, Curley FJ, French CL. Chronic cough. The spectrum and frequency of causes, key components of the diagnostic evaluation, and outcome of specific therapy. Am Rev Respir Dis. 1990;141(3):640–647. doi: 10.1164/ajrccm/141.3.640. [DOI] [PubMed] [Google Scholar]
- 28.Chandra KM, Harding SM. Therapy insight: treatment of gastroesophageal reflux in adults with chronic cough. Nat Clin Pract Gastroenterol Hepatol. 2007;4(11):604–613. doi: 10.1038/ncpgasthep0955. [DOI] [PubMed] [Google Scholar]
- 29.Slaughter JC, Goutte M, Rymer JA, et al. Caution about overinterpretation of symptom indexes in reflux monitoring for refractory gastroesophageal reflux disease. Clin Gastroenterol Hepatol. 2011;9(10):868–874. doi: 10.1016/j.cgh.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 30.Kavitt RT, Higginbotham T, Slaughter JC, et al. Symptom reports are not reliable during ambulatory reflux monitoring. Am J Gastroenterol. 2012;107(12):1826–1832. doi: 10.1038/ajg.2012.342. [DOI] [PubMed] [Google Scholar]
- 31.Kahrilas PJ, Sifrim D. High-resolution manometry and impedance-pH/ manometry: valuable tools in clinical and investigational esophagology. Gastroenterology. 2008;135(3):756–769. doi: 10.1053/j.gastro.2008.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mainie I, Tutuian R, Agrawal A, Adams D, Castell DO. Combined multichannel intraluminal impedance-pH monitoring to select patients with persistent gastro-oesophageal reflux for laparoscopic Nissen fundoplication. BrJ Surg. 2006;93(12):1483–1487. doi: 10.1002/bjs.5493. [DOI] [PubMed] [Google Scholar]
- 33.Mainie I, Tutuian R, Shay S, et al. Acid and non-acid reflux in patients with persistent symptoms despite acid suppressive therapy: a multicentre study using combined ambulatory impedance-pH monitoring. Gut. 2006;55(10):1398–1402. doi: 10.1136/gut.2005.087668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Francis DO, Goutte M, Slaughter JC, et al. Traditional reflux parameters and not impedance monitoring predict outcome after fundoplication in extraesophageal reflux. Laryngoscope. 2011;121(9):1902–1909. doi: 10.1002/lary.21897. [DOI] [PubMed] [Google Scholar]
- 35.Crowell MD, Zayat EN, Lacy BE, Schettler-Duncan A, Liu MC. The effects of an inhaled beta(2)-adrenergic agonist on lower esophageal function: a dose-response study. Chest. 2001;120(4):1184–1189. doi: 10.1378/chest.120.4.1184. [DOI] [PubMed] [Google Scholar]
- 36.Ekström T, Tibbling L. Influence of theophylline on gastro-oesophageal reflux and asthma. Eur J Clin Pharmacol. 1988;35(4):353–356. doi: 10.1007/BF00561363. [DOI] [PubMed] [Google Scholar]
- 37.Lazenby JP, Guzzo MR, Harding SM, Patterson PE, Johnson LF, Bradley LA. Oral corticosteroids increase esophageal acid contact times in patients with stable asthma. Chest. 2002;121(2):625–634. doi: 10.1378/chest.121.2.625. [DOI] [PubMed] [Google Scholar]
- 38.Kiljander TO, Salomaa ER, Hietanen EK, Terho EO. Gastroesophageal reflux in asthmatics: a double-blind, placebo-controlled crossover study with omeprazole. Chest. 1999;116(5):1257–1264. doi: 10.1378/chest.116.5.1257. [DOI] [PubMed] [Google Scholar]
- 39.Boeree MJ, Peters FT, Postma DS, Kleibeuker JH. No effects of high-dose omeprazole in patients with severe airway hyperresponsiveness and (a)symptomatic gastro-oesophageal reflux. Eur Respir J. 1998;11(5):1070–1074. doi: 10.1183/09031936.98.11051070. [DOI] [PubMed] [Google Scholar]
- 40.Levin TR, Sperling RM, McQuaid KR. Omeprazole improves peak expiratory flow rate and quality of life in asthmatics with gastroesophageal reflux. Am J Gastroenterol. 1998;93(7):1060–1063. doi: 10.1111/j.1572-0241.1998.329_q.x. [DOI] [PubMed] [Google Scholar]
- 41.Teichtahl H, Kronborg IJ, Yeomans ND, Robinson P. Adult asthma and gastrooesophageal reflux: the effects of omeprazole therapy on asthma. Aust N Z J Med. 1996;26(5):671–676. doi: 10.1111/j.1445-5994.1996.tb02938.x. [DOI] [PubMed] [Google Scholar]
- 42.Ford GA, Oliver PS, Prior JS, Butland RJ, Wilkinson SP. Omeprazole in the treatment of asthmatics with nocturnal symptoms and gastro-oesophageal reflux: a placebo-controlled cross-over study. Postgrad Med J. 1994;70(823):350–354. doi: 10.1136/pgmj.70.823.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gustafsson PM, Kjellman NI, Tibbling L. A trial of ranitidine in asthmatic children and adolescents with or without pathological gastro-oesophageal reflux. Eur Respir J. 1992;5(2):201–206. [PubMed] [Google Scholar]
- 44.Larrain A, Carrasco E, Galleguillos F, Sepulveda R, Pope CE II. Medical and surgical treatment of nonallergic asthma associated with gastroesophageal reflux. Chest. 1991;99(6):1330–1335. doi: 10.1378/chest.99.6.1330. [DOI] [PubMed] [Google Scholar]
- 45.Ekström T, Lindgren BR, Tibbling L. Effects of ranitidine treatment on patients with asthma and a history of gastro-oesophageal reflux: a double blind crossover study. Thorax. 1989;44(1):19–23. doi: 10.1136/thx.44.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nagel RA, Brown P, Perks WH, Wilson RS, Kerr GD. Ambulatory pH monitoring of gastro-oesophageal reflux in “morning dipper” asthmatics. BMJ. 1988;297(6660):1371–1373. doi: 10.1136/bmj.297.6660.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kjellén G, Tibbling L, Wranne B. Effect of conservative treatment of oesophageal dysfunction on bronchial asthma. Eur J Respir Dis. 1981;62(3):190–197. [PubMed] [Google Scholar]
- 48.Busse WW, Lemanske RF Jr. Expert Panel Report 3: moving forward to improve asthma care. J Allergy Clin Immunol. 2007;120(5):1012–1014. doi: 10.1016/j.jaci.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 49.Gibson PG, Henry RL, Coughlan JL. Gastro-oesophageal reflux treatment for asthma in adults and children. Cochrane Database Syst Rev. 2000;(2):CD001496. doi: 10.1002/14651858.CD001496. [DOI] [PubMed] [Google Scholar]
- 50.Sontag SJ, O’Connell S, Khandelwal S, et al. Asthmatics with gastroesophageal reflux: long term results of a randomized trial of medical and surgical antireflux therapies. Am J Gastroenterol. 2003;98(5):987–999. doi: 10.1111/j.1572-0241.2003.07503.x. [DOI] [PubMed] [Google Scholar]
- 51.Littner MR, Leung FW, Ballard ED II, Huang B, Samra NK. Lansoprazole Asthma Study Group. Effects of 24 weeks of lansoprazole therapy on asthma symptoms, exacerbations, quality of life, and pulmonary function in adult asthmatic patients with acid reflux symptoms. Chest. 2005;128(3):1128–1135. doi: 10.1378/chest.128.3.1128. [DOI] [PubMed] [Google Scholar]
- 52.Kiljander TO, Harding SM, Field SK, et al. Effects of esomeprazole 40 mg twice daily on asthma: a randomized placebo-controlled trial. Am J Respir Crit Care Med. 2006;173(10):1091–1097. doi: 10.1164/rccm.200507-1167OC. [DOI] [PubMed] [Google Scholar]
- 53.Kiljander TO, Junghard O, Beckman O, Lind T. Effect of esomeprazole 40 mg once or twice daily on asthma: a randomized, placebo-controlled study. Am J Respir Crit Care Med. 2010;181(10):1042–1048. doi: 10.1164/rccm.200910-1537OC. [DOI] [PubMed] [Google Scholar]
- 54.Tomonaga T, Awad ZT, Filipi CJ, et al. Symptom predictability of refluxinduced respiratory disease. Dig Dis Sci. 2002;47(1):9–14. doi: 10.1023/a:1013290715062. [DOI] [PubMed] [Google Scholar]
- 55.DiMango E, Holbrook JT, Simpson E, et al. American Lung Association Asthma Clinical Research Centers. Effects of asymptomatic proximal and distal gastroesophageal reflux on asthma severity. Am J Respir Crit Care Med. 2009;180(9):809–816. doi: 10.1164/rccm.200904-0625OC. [DOI] [PMC free article] [PubMed] [Google Scholar]