Abstract
Mesenchymal stem/stromal cells (MSCs) are emerging as important regulators of innate and adaptive immunity. In this context, both proinflammatory and anti-inflammatory effects have been described for MSCs. The mechanisms mediating this functional plasticity are poorly characterized at present. Here, we investigated the inflammatory responses of MSCs isolated from human nasal mucosa (nmMSCs) upon challenge with different Toll-like receptor (TLR) ligands. We found that TLR3 ligands induced the strongest release of both proinflammatory cytokines [interleukin (IL)-6 and IL-8] and type I interferon by nmMSCs compared with other TLR ligands. Notably, TLR3 ligands triggered a biphasic cytokine response, with an early peak of type I interferon at 4 h poststimulation and a late release of proinflammatory cytokines at 24 h poststimulation. While the early interferon response was subject to direct stimulation, the proinflammatory response was regulated by factors released during the early cytokine response, which subsequently enhanced sensitivity to TLR3 ligation and amplified the production of IL-6 and IL-8 but not that of interferon. Taken together, our findings indicate that TLR3 ligands polarize the inflammatory phenotype of MSCs in a time-dependent manner. Thus, our study proposes a novel model that helps to explain the strikingly dichotomous functionality of MSCs in inflammation and immunoregulation.—Dumitru, C. A., Hemeda, H., Jakob, M., Lang, S., Brandau, S. Stimulation of mesenchymal stromal cells (MSCs) via TLR3 reveals a novel mechanism of autocrine priming.
Keywords: proinflammatory cytokines, interferon type I, biphasic cytokine response, functional polarization, Poly I:C
Mesenchymal stem/stromal cells (MSCs) are multipotent adult stem cells that can differentiate into several lineages, including chondrocytes, osteoblasts, tendocytes, myocytes, endothelial cells, neural cells, and adipocytes. MSCs were originally identified in the bone marrow; however, they have now been isolated from many peripheral tissues, such as salivary glands, adipose tissue, connective tissue, tendon, pancreas, placenta, or muscle (1–3). Recently, our group successfully isolated and characterized human nasal mucosa-derived MSCs (nmMSCs) for the first time (4).
In addition to their stem/progenitor properties, MSCs also exhibit broad immunoregulatory abilities, as they can influence both the adaptive and innate immune cells. In this context, MSCs release a variety of factors with proinflammatory, immunosuppressive, or antiviral and anti-inflammatory effects (5). For instance, our group and others demonstrated that MSCs recruited and/or activated neutrophil granulocytes via release of interleukin (IL)-6 and IL-8, interferon β (IFNβ), granulocyte-macrophage colony-stimulating factor (GM-CSF), or macrophage migration inhibitory factor (MIF) (6, 7). Other studies found that MSCs released indolamine 2,3-dioxygenase (IDO), transforming growth factor β (TGFβ), prostaglandin E2 (PGE2), cyclooxygenase 2 (COX2), or human leukocyte antigen G5 (HLA-G5) to inhibit the effector T-cell immunity (8–12). This process was further shown to be mediated by MSCs either directly, by suppressing the proliferation of T cells, or by generating and expanding the regulatory T-cell population in a monocyte-dependent manner (13, 14). Thus, it appears that MSCs possess a remarkably complex functional plasticity in regard to inflammation and immunomodulation. The mechanisms mediating and guiding this plasticity of MSCs are, however, poorly characterized at present. A switch in the balance between high and low levels of IFNγ and tumor necrosis factor α (TNF-α) might polarize MSCs from an anti-inflammatory toward a proinflammatory phenotype (5). Recently, this polarization was also attributed to activation of MSCs by different Toll-like receptor (TLR) ligands (15).
The TLRs represent an evolutionary conserved family of receptors with the capacity to recognize “danger” signals, such as molecules expressed by pathogens or associated with tissue injury. TLRs are differentially expressed on leukocyte subsets, as well as on nonimmune cells, and regulate important aspects of innate and adaptive immune responses (16–18). Following ligation, TLRs trigger multiple intracellular signaling cascades that result in cellular activation and release of soluble mediators. TLR signaling involves activation of Toll/interleukin-1 receptor (TIR) domain-containing adapter molecules [myeloid differentiation primary response gene 88 (MyD88), TIR-domain-containing adaptor protein (TIRAP), TIR-domain-containing adapter inducing interferon-β (TRIF), or TRIF-related adaptor molecule (TRAM)] that, subsequently, activate various transcription factors (19). Depending on the type of TLR employed, nuclear factor κB (NF-κB) and/or interferon-regulatory factor (IRF) will be induced. Both NF-κB and IRF may be activated in response to TLRs, but the target genes induced by these factors are distinct. Specifically, NF-κB triggers mainly the release of proinflammatory cytokines, while IRF induces production of IFN type I (IFNα/β) (19). Among the TLRs, the TLR3 pathway is somewhat unique, because its signaling cascade is independent of MyD88 and commences with TRIF recruitment. TRIF, subsequently, phosphorylates IRF3 and results in the downstream expression of IFNβ (19).
In this study, we determined the TLR expression profile of nmMSCs and the consequences of TLR ligation regarding the release of cytokines by these cells. We found that stimulation with polyinosinic:polycytidylic acid (Poly I:C), a synthetic RNA compound and TLR3 ligand, induced the strongest cytokine response in nmMSCs. Specifically, Poly I:C stimulated nmMSCs to release high levels of both proinflammatory (IL-8 and IL-6) and antiviral/anti-inflammatory (IFN type I) cytokines, albeit with a strikingly different kinetics. Characterization of TLR3-induced molecular mechanisms in nmMSCs confirmed activation of both NF-κB and IRF3 signaling pathways and, additionally, demonstrated the involvement of p38-mitogen-activated protein kinase (MAPK) in the release of these cytokines by nmMSCs. Notably, we show for the first time that nmMSCs up-regulate TLR3 expression in an autocrine manner following stimulation with TLR3 ligands. The autocrine increase in TLR3 correlated with the sensitization of nmMSCs to a second TLR3 stimulation and, interestingly, with the polarization of nmMSCs toward a proinflammatory phenotype. These effects were also found in bone marrow MSCs (bmMSCs) on TLR3 stimulation. Thus, our findings uncover a novel time-dependent response of MSCs to TLR stimulation, which might explain their functional plasticity in inflammation and immunomodulation.
MATERIALS AND METHODS
Isolation and culture of MSCs
nmMSCs were isolated from the inferior nasal concha of healthy individuals (3 females, 2 males; 37–69 yr old) undergoing reduction of the nasal turbinate at the Department of Otorhinolaryngology, University Hospital Essen (Essen, Germany). bmMSCs were harvested from the iliac crest of healthy individuals (1 female, 2 male; 60–70 yr old) undergoing hip replacement at the Department of Orthopedics, University Hospital Essen. The isolation of nmMSCs was performed exactly as described previously (4). bmMSCs were kindly provided by Dr. M. Jäger and K. Mauel (Department of Orthopedics, University Hospital Essen) and expanded as described previously (20). The immunophenotype and the differentiation potential of the isolated cells were routinely tested as described previously (4). The MSCs were kept in culture for a maximum of 6 passages. For most experiments, MSCs in passages 3–5 were used. All studies were approved by the ethics committee of the Medical Faculty of the University of Duisburg-Essen, and informed written consent was obtained from each individual.
Stimuli, inhibitors, and antibodies
The following TLR ligands were used at the indicated concentrations: synthetic bacterial lipoprotein N-palmitoyl-S-[2,3-bis(palmitoyloxy)-propyl]-(R)-cysteinyl-(lysyl)3-lysine (PAM3CSK4; InvivoGen, Toulouse, France), Poly (I:C) (Sigma-Aldrich, Taufkirchen, Germany), lipopolysaccharide (LPS; kindly provided by Dr. K Brandenburg, Division of Biophysics, Research Center Borstel, Borstel, Germany), diacylated macrophage-activating lipopeptide 2 (MALP-2; Biomol, Hamburg, Germany), imiquimod (R837; InvivoGen, Toulouse, France), and synthetic cytosine-phosphate-guanine (CpG) oligonucleotides (pG-ODN2006; InvivoGen). Recombinant IFNβ was from Peprotech (Hamburg, Germany).
SB202190 (p38-MAPK inhibitor), U0126 [MEK1/extracellular signal-regulated kinase (ERK) inhibitor], and Bay 11-7082 (NF-κB inhibitor) were from Calbiochem (Darmstadt, Germany). Wortmannin [phosphatidylinositol-3 kinase (PI3K) inhibitor] was kindly provided by Dr. M. Palmada (Department of Molecular Biology, University of Duisburg-Essen, Essen, Germany). Bafilomycin (vacuolar-type H+-ATPase inhibitor) was from Sigma-Aldrich. The protease inhibitor cocktail sets I and III were from Merck (Darmstadt, Germany). PhosStop was from Roche (Mannheim, Germany).
Neutralizing anti-IFNβ antibodies were from Peprotech. Rabbit anti-p38 (Thr180/Tyr182), rabbit anti-ERK1/2 (Thr202/Tyr204), and rabbit anti-protein kinase B (Akt; Ser473) antibodies were from Cell Signaling Technology (Danvers, MA, USA). Rabbit anti-NF-κB (p65 subunit) and goat anti-IRF3 antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). All secondary antibodies (DyLight 488-conjugated donkey anti-rabbit, alkaline phosphatase-conjugated goat anti-rabbit, and Alexa Fluor 488-conjugated donkey anti-goat) were from Jackson Immunoresearch (West Grove, PA, USA).
Quantificaton of cytokines in supernatants (SNs)
Quantification of IL-6 and IL-8 was performed using commercially available enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Wiesbaden, Germany) as instructed by the manufacturer. Detection of bioactive IFN type I in SNs was performed using human embryonic kidney (HEK)-Blue IFN-α/β sensor cells (Invitrogen, Toulouse, France) according to the manufacturer's protocol. A Synergy 2 microplate reader (BioTek, Bad Friedrichshall, Germany) was used to determine sample absorbance at 450 nm (for IL-6/IL-8) or 620 nm (for IFN I). Multiplex screening assays were performed using human cytokine group I 17-plex and group II 5-plex (Bio-Rad Life Sciences, München, Germany), according to the protocol provided by the manufacturer. Data were analyzed using the Bio-Plex Manager 4.1.1 software (Bio-Rad).
RNA isolation and RT-PCR
Total RNA was purified from MSCs using the NucleoSpin RNA II kit (Macherey-Nagel, Düren, Germany) according to the manufacturer's instructions and quantified by an average optical density (OD) OD260nm/OD280nm using a Synergy HT Multi-Mode Microplate reader (BioTek). Approximately 0.4 μg of total RNA was reverse transcribed using SuperScript II RNase H− Reverse Transcriptase kit with Hexamer random primers (both from Invitrogen), according to the manufacturer's protocol. Amplification was performed in a thermocycler (Bio-Rad). Amplified DNA fragments were separated on a 2% agarose gel and photographed after ethidium bromide staining.
Quantitative RT-PCR was performed on diluted cDNA samples from nmMSCs using DyNAmo Capillary SYBR Green qPCR kit (Thermo Scientific, Bonn, Germany) and Roche LightCycler instrument (Roche). The relative expression level of the reference gene β-actin was used to normalize gene expression in each sample. The sequences and annealing temperatures for each primer are shown in Table 1.
Table 1.
Primers and sequences
| Primer | Sequences, 5′–3′ | Annealing temperature (°C) | Amplicon (bp) |
|---|---|---|---|
| Hu_beta-actin | F: AGCGGGAAATCGTGCGTG | 55–60 | 307 |
| R: GGGTACATGGTGGTGCCG | |||
| Hu_CXCR1 | F: CGCCAGGCTTACCATCCA | 62 | 127 |
| R: CAAACAGCGGCACGATGA | |||
| Hu_CXCR2 | F: GAGGTGTCCTACAGGTGAAAAGC | 62 | 151 |
| R: GGGGGCAGGGTAGAGCTGTA | |||
| Hu_IFNA4 | F: GAAGAAATACAGCCCTTGTGC | 60 | 114 |
| R: TGAACCAGGTTTCAATCCTTCC | |||
| Hu_IFNAR1 | F: CGCCTGTGATCCAGGATTATCC | 60 | 156 |
| R: TGGTGTGTGCTCTGGCTTTCAC | |||
| Hu_IFNAR2 | F: ACCGTCCTAGAAGGATTCAGCG | 60 | 107 |
| R: CCAACAATCTCAAACTCTGGTGG | |||
| Hu_IFNB1 | F: ATGACCAACAAGTGTCTCCTCCAAA | 60 | 375 |
| R: TTCTTCCAGGACTGTCTTCA | |||
| Hu_IL-6 | F: ATGTAGCCGCCCCACACAGA | 60 | 190 |
| R: CATCCATCTTTTTCAGCCAT | |||
| Hu_IL-6R | F: TCACTGTGTCATCCACGACG | 63 | 132 |
| R: CTGGATTCTGTCCAAGGCGT | |||
| Hu_IL-8 | F: CATGACTTCCAAGCTGGCCGTG | 61 | 246 |
| R: TCCTTGGGGTCCAGACAGAGC | |||
| Hu_IRF-3 | F: ACCAGCCGTGGACCAAGAG | 65 | 65 |
| R: TACCAAGGCCCTGAGGCAC | |||
| Hu_IRF-7 | F: TGGTCCTGGTGAAGCTGGAA | 65 | 134 |
| R: GATGTCGTCATAGAGGCTGTTGG | |||
| Hu_MyD88 | F: CGCCGGATGGTGGTGGTTGT | 60 | 181 |
| R: TGTAGTCGCAGACAGTGATGAACC | |||
| Hu_NFKBIA | F: GTCAAGGAGCTGCAGGAGAT | 60 | 111 |
| R: GATGGCCAAGTGCAGGAA | |||
| Hu_TLR1 | F: GCCCAAGGAAAAGAGCAAAC | 60 | 134 |
| R: AAGCAGCAATATCAACAGGAG | |||
| Hu_TLR2 | F: TCTCCCATTTCCGTCTTTTT | 60 | 125 |
| R: GGTCTTGGTGTTCATTATCTTC | |||
| Hu_TLR3 | F: TAAACTGAACCATGCACTCT | 60 | 101 |
| R: TATGACGAAAGGCACCTATC | |||
| Hu_TLR4 | F: GAAGCTGGTGGCTGTGGA | 60 | 212 |
| R: GATGTAGAACCCGCAAG | |||
| Hu_TLR5 | F: TTGCTCAAACACCTGGACAC | 60 | 149 |
| R: CTGCTCACAAGACAAACGAT | |||
| Hu_TLR6 | F: GTGCCATTACGAACTCTA | 60 | 108 |
| R: TTGTTGGGAATGCTGTT | |||
| Hu_TLR7 | F: CTGACCACTGTCCCTGAG | 60 | 264 |
| R: AACCCACCAGACAAACCA | |||
| Hu_TLR8 | F: CCTCCTGGGCTATTGGC | 60 | 117 |
| R: GTGACTTCATCTTGCTAGCA | |||
| Hu_TLR9 | F: CGCCAACGCCCTCAAGACA | 60 | 121 |
| R: GAAGTCCATAAAGGCCGCCCC | |||
| Hu_TLR10 | F: CTCCCAACTTTGTCCAGAAT | 60 | 131 |
| R: GGTGGGAATGCAATAGAAT | |||
| Hu_TRIF | F: ACCTTCTGCGAGGATTTCCAGG | 62 | 113 |
| R: CGACAGTCGAAGTTGGAGGTGA |
F, forward; R, reverse.
Immunofluorescence assays
nmMSCs were seeded on sterile coverslips overnight. The cells were stimulated as indicated and fixed/permeabilized with BD Cytofix/Cytoperm (BD Biosciences, Heidelberg, Germany) for 45 min at room temperature. Samples were stained with the primary antibodies for 1 h at room temperature. Secondary reactions were performed with fluorescently-coupled antibodies for 30 min at room temperature. The samples were mounted in Fluoprep (bioMerieux, Marcy l'Etoile, France) and analyzed with a Zeiss Axioscope 2 microscope (Zeiss, Jena, Germany).
SDS-PAGE and Western blot analysis
nmMSCs were stimulated as indicated and were then lysed with a buffer containing 25 mM HEPES (pH 7.3), 0.1% SDS, 1% Triton X-100, 10 mM EDTA, 10 mM sodium pyrophosphate, 10 mM NaF, 125 mM NaCl, 1% protease inhibitor cocktail I, 1% protease inhibitor cocktail III, and 10% PhosStop. Cell debris was removed by centrifugation, and the lysates were incubated with SDS sample buffer (final concentrations 50 mM Tris, pH 6.8.;4% glycerin; 0.8% SDS; 1.6% β-mercaptoethanol; and 0.04% bromphenol blue). Samples were further processed by SDS-PAGE and immunoblotting, respectively, as described previously (21).
Statistical analysis
Where applicable, data are presented as means ± sd, and statistical analysis was performed with the 2-tailed paired Student's t test. The level of significance was set at P ≤ 0.05 in all studies.
RESULTS
TLR expression and functions in nmMSCs
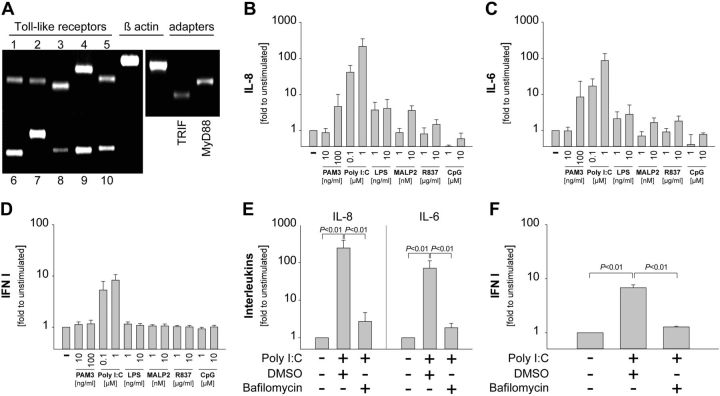
In initial studies, we determined the expression pattern of TLRs in nmMSCs. To this end, we used specific primers to detect the levels of human TLR1-10 from total RNA. The results showed that nmMSCs moderately expressed TLR1, TLR2, TLR5, TLR8, and TLR10, and strongly expressed TLR3, TLR4, TLR6, TLR7, and TLR9 (Fig. 1A). Furthermore, PCR analysis confirmed that nmMSCs expressed the major TLR adapter proteins TRIF and MyD88 (Fig. 1A). Next, we stimulated nmMSCs with different TLR agonists for 24 h and determined the release of IL-6, IL-8, and IFN I, since these cytokines are known to be produced upon TLR ligation. We found that Poly I:C (TLR3 agonist) was the strongest inducer of IL-8 (Fig. 1B), IL-6 (Fig. 1C), and IFN I (Fig. 1D) release by nmMSCs. Multiplex analysis further revealed that nmMSCs responded to Poly I:C stimulation with the release of additional cytokines. However, most of these cytokines (with the exception of IFNγ, TNF-α, and VCAM-1) were released at low levels, which did not exceed 500 pg/ml (Supplemental Fig. S1A, B). To confirm that Poly I:C-induced cytokine release was indeed mediated via TLR3 (an endosomal receptor), we inhibited endosomal acidification in nmMSCs by bafilomycin. This procedure almost completely inhibited the release of IL-8, IL-6, and IFN type I by nmMSCs upon Poly I:C stimulation (Fig. 1E, F). Taken together, these findings indicate that nmMSCs express TLR3 and are strongly modulated to release various cytokines upon TLR3 ligation.
Figure 1.
nmMSCs express TLR3 and respond strongly to TLR3 stimulation. A) Total RNA was isolated from unstimulated nmMSCs and the expression levels of TLRs, TRIF, and MyD88 were analyzed by TaqDNA polymerase PCR. Samples from 3 independent experiments and donors were pooled. B–D) nmMSCs were stimulated with the following TLR ligands: PAM3 (TLR1/2), Poly I:C (TLR3), LPS (TLR4), MALP2 (TLR2/6), R837 (TLR7), and CpG (TLR9). SNs were collected after 24 h. Levels of IL-8 (B) and IL-6 (C) in the SNs were determined by ELISA; levels of IFN type I (D) were assessed using HEK-Blue IFN-α/β sensor cells. E, F) nmMSCs were stimulated for 24 h with 1 μM Poly I:C in the presence or absence of 100 nM bafilomycin or DMSO control. Levels of IL-8 and IL-6 (E) or IFN type I (F) in the SNs were determined as above. TLR-induced cytokine production is depicted as fold change to unstimulated nmMSCs. Data are means ± sd of 3 independent donors. Where indicated, statistical analysis was performed with the Student's t test, and the level of significance was set at P ≤ 0.05.
TLR3 ligands activate distinct signaling pathways in nmMSCs
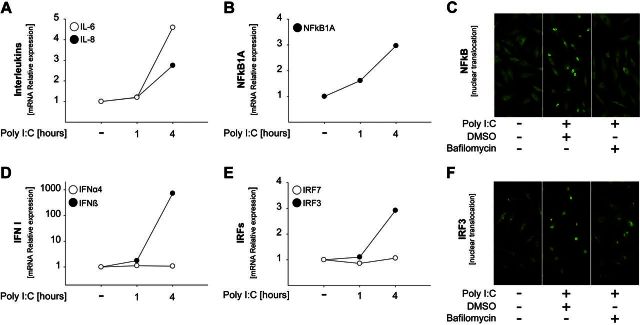
In the next set of studies, we analyzed the molecular mechanisms of cytokine production induced by Poly I:C stimulation in nmMSCs. We observed that Poly I:C induced gene expression of IL-8 and IL-6 already at 4 h poststimulation (Fig. 2A). This correlated with an increase in NF-κB1A gene expression (Fig. 2B). TLR3-induced NF-κB signaling was further confirmed by the nuclear translocation of NF-κB upon Poly I:C stimulation, which was abrogated in the presence of bafilomycin (Fig. 2C). In addition, treatment of nmMSCs with the NF-κB pathway inhibitor Bay 11–7082 blocked Poly I:C-induced nuclear translocation of NF-κB and the release of IL-8, respectively (Supplemental Fig. S2A, B). Gene expression analysis of type I IFN showed that Poly I:C induced production of IFNβ but not IFNα4 (Fig. 2D). In agreement with these results, we observed the up-regulation of IRF3 (IFNβ activator) but not IRF7 (IFNα activator) upon Poly I:C stimulation (Fig. 2E). Furthermore, the activation of IRF3 was confirmed by immunofluorescence studies, which showed that IRF3 translocated to the nucleus in Poly I:C-stimulated nmMSCs but failed to translocate in bafilomycin-treated cells (Fig. 3F).
Figure 2.
TLR3 ligands activate the NF-κB and IRF3 pathways in nmMSCs. A, B, D, E) nmMSCs were stimulated with 1 μM Poly I:C for the indicated times. RNA was isolated and subjected to quantitative RT-PCR analysis. mRNA expression levels for IL-6 and IL-8 (A), NF-κB1A (B), IFN type 1 (D), and IRF3 and IRF7 (E) were normalized to β-actin, and the levels in the unstimulated sample were set as 1. C, F) nmMSCs were seeded on coverslips and stimulated for 2 h with 1 μM Poly I:C in the presence or absence of 100 nM bafilomycin or DMSO control. The subcellular localization of NF-κB (C) and IRF3 (F) was determined by fluorescence microscopy. Shown are representative results from 1 of 3 independent donors at 400-fold magnification.
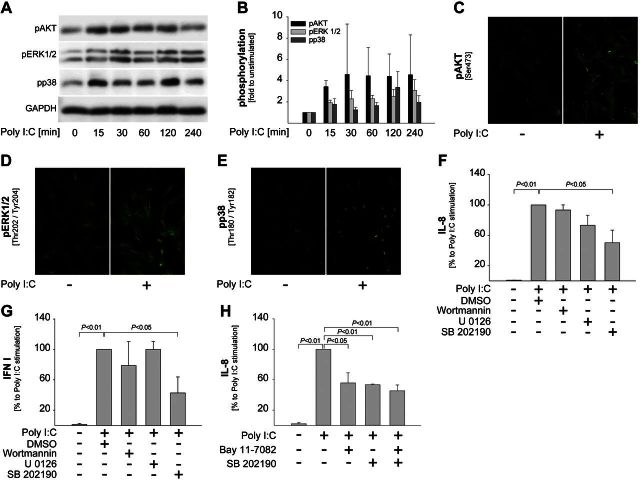
Figure 3.
TLR3 ligands activate p38-MAPK to trigger cytokine release in nmMSCs. A, B) nmMSCs were stimulated with 1 μM Poly I:C for the indicated times. The cells were lysed and subjected to Western blot analysis to assess the levels of phosphorylated AKT, ERK1/2, and p38-MAPK. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as control for the quality and quantity of cell lysates. A) Representative results from 1 of 3 independent donors. B) Densitometric analysis of the Western blots using the ImageJ software. Phosphorylation levels in the unstimulated samples were set as 1. Data are means ± sd of 3 independent donors. C–E) nmMSCs were seeded on coverslips and stimulated with Poly I:C for 2 h. Phosphorylation of AKT (C), ERK1/2 (D), and p38-MAPK (E) was assessed by fluorescence microscopy. F, G) nmMSCs were incubated with Wortmannin (1 μM), U0126 (10 μM), SB202190 (10 μM), or DMSO control for 1 h. Inhibitors were washed away, and cells were stimulated with 1 μM Poly I:C. SNs were collected after 24 h. F) Levels of IL-8 were determined by ELISA. G) Levels of IFN type I were assessed using HEK-Blue IFN-α/β sensor cells. H) nmMSCs were stimulated with 1 μM Poly I:C in the presence or absence of Bay 11–7082 (1 μM) and SB202190 (10 μM) taken alone or in combination. SNs were collected 24 h later, and levels of IL-8 were assessed by ELISA. Cytokine levels induced by Poly I:C in the absence of inhibitors were considered as 100%. Data are means ± sd of 3 independent donors. Statistical analysis was performed with the Student's t test, and the level of significance was set at P ≤ 0.05.
While the involvement of NF-κB and IRF in the cytokine response to TLR stimulation is relatively well characterized, the role of protein kinases in this process is less clear. We, therefore, performed Western blot and immunofluorescence studies to determine whether Poly I:C might activate major protein kinase signaling pathways, such as phosphatidylinositide 3-kinase (PI3K)/Akt or MAPK. The results showed that Poly I:C rapidly phosphorylated Akt (Fig. 3A, C), ERK1/2 (Fig. 3A, D), and p38-MAPK (Fig. 3A, E). The densitometric analysis of the Western blots from all nmMSCs donors is presented in Fig. 3B. To determine the functional relevance of these kinases in Poly I:C-induced cytokine production, we stimulated nmMSCs with Poly I:C in the presence of specific pharmacological inhibitors. Inhibition of PI3K/Akt by wortmannin or of MEK/ERK by U0126 had no significant effect on Poly I:C-induced IL-8 and IFN type I production by nmMSCs (Fig. 3F, G). In contrast, inhibition of p38-MAPK by SB202190 significantly blocked the release of both cytokines upon Poly I:C stimulation (Fig. 3F, G). In addition, we found that cotreatment of nmMSCs with SB202190 and Bay 11–7082 had no additive effect on IL-8 production compared with the individual inhibitors (Fig. 3H), which suggests that p38-MAPK operates within the same signaling cascade as NF-κB. Thus, our findings indicate that stimulation of nmMSCs via TLR3 activates p38-MAPK, NF-κB, and IRF3 to induce cytokine release by these cells.
TLR3 ligands stimulate MSCs to release IFNβ, which up-regulates TLR3 in an autocrine manner
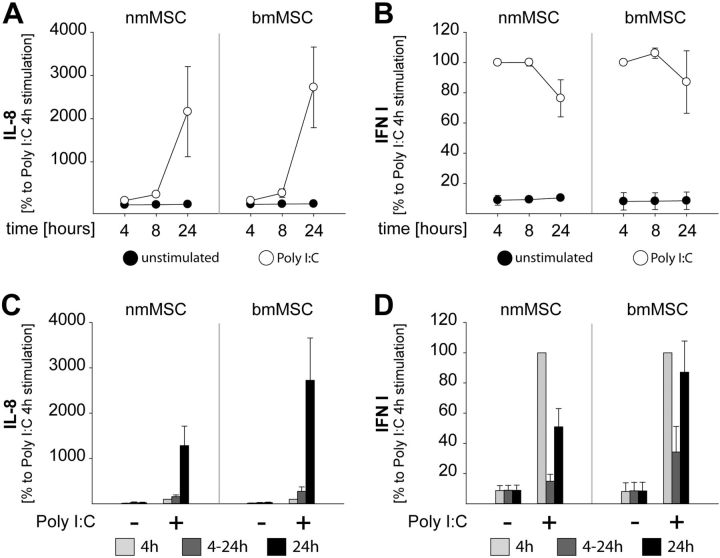
Unlike many mobile myeloid immune cells, which leave the site of infection or injury on encountering TLR ligands, tissue-resident stromal cells, including MSCs, are considerably more sessile. As a consequence, MSCs are likely to be exposed to TLR ligands for prolonged time periods and might undergo autocrine/paracrine activation loops. Here, we investigated whether Poly I:C-induced production of proinflammatory cytokines (IL-8/IL-6) by MSCs is regulated in an autocrine manner. These studies were also prompted by our observations that the release pattern of IL-8 significantly differed from that of IFN type I. Specifically, we found that IL-8 was continuously induced by Poly I:C and dramatically increased at 24 h poststimulation (Fig. 4A, C). In addition, Fig. 4C indicates that high amounts of IL-8 were only produced if Poly I:C was present during the entire 24 h stimulation period. In contrast, IFN type I was released only in the first 4 h of stimulation and its levels were even slightly reduced at 24 h poststimulation (Fig. 4B, D). Similar results were obtained on stimulation of bmMSCs with Poly I:C (Fig. 4), which indicates that this response is a common feature of tissue-specific and bmMSCs.
Figure 4.
TLR3 ligands induce an early IFN type I release and a late increase in IL-8 levels. A, B) MSCs (nmMSCs or bmMSCs) were stimulated with 1 μM Poly I:C and SNs were collected at 4 h, 8 h and 24 h poststimulation. Levels of IL-8 (A) and IFN type I (B) were determined as above. C, D) MSCs were continuously stimulated with 1 μM Poly I:C for 4 or 24 h. In addition, Poly I:C was washed away after 4 h, fresh medium was added, and the cells were allowed to release cytokines for another 20 h (4–24 h). Levels of IL-8 (C) and IFN type I (D) in the SNs were assessed as above. Cytokine levels induced by Poly I:C at 4 h poststimulation were considered as 100%. Data are means ± sd of 3 independent donors.
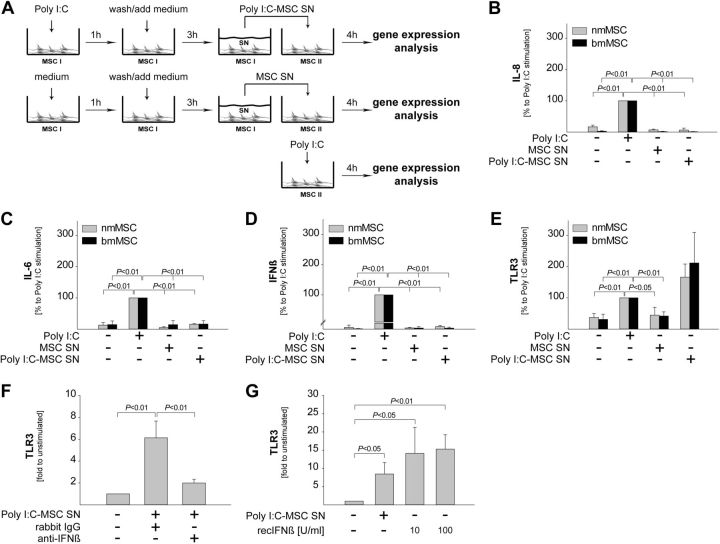
To address the potential autocrine effects of Poly I:C on MSCs, we used the experimental design outlined in Fig. 5A. We stimulated MSCs (both nmMSCs and bmMSCs) with Poly I:C for 1 h, thoroughly washed the stimulus away, and allowed the cells to release cytokines for another 3 h. The resulting SNs (Poly I:C-MSC SNs) were added over naive MSCs from the same donors, and gene expression was assessed 4 h later. SNs from unstimulated MSCs (MSC SNs) were used as control. The effect of Poly I:C-MSC SNs on the expression of various genes was compared with the effect of direct stimulation with Poly I:C (Fig. 5A). The results showed that, in contrast to Poly I:C direct stimulation, Poly I:C-MSC SNs did not up-regulate the expression of IL-6, IL-8 or IFN type I (Fig. 5B–D). However, Poly I:C-MSC SNs up-regulated the expression of TLR3, and this effect was even stronger than in the samples stimulated directly with Poly I:C (Fig. 5E). The expression levels of TRIF (TLR3 adapter protein), IL-6 receptor (IL-6R), CXCR1/2 (IL-8 receptors), and IFNAR1/2 (IFN type I receptors) were not affected by stimulation with Poly I:C-MSC SNs (data not shown).
Figure 5.
TLR3 ligands up-regulate the expression of TLR3 in an autocrine manner via IFNβ. A) MSCs were stimulated with 1 μM Poly I:C for 1 h. Poly I:C was washed away, and the cells were allowed to release cytokines for 3 h. SNs (Poly I:C-MSC SNs) were collected, added over naive MSCs from the same donor, and changes in mRNA levels were determined after 4 h. As controls, SNs from unstimulated MSCs (MSC SNs) were used. In addition, MSCs were stimulated directly with Poly I:C for 4 h. B–E) Expression levels of IL-8 (B), IL-6 (C), IFNβ (D), and TLR3 (E) are presented as percentage of the direct stimulation with Poly I:C. F, G) nmMSCs were stimulated with Poly I:C-MSC SNs in the presence or absence of 0.1 μg/ml anti-IFNβ neutralizing antibodies or isotype control (F) and with recombinant IFNβ at the indicated concentrations (G). mRNA levels of TLR3 were determined at 4 h poststimulation and are presented as fold increase to unstimulated samples. Data are means ± sd of 3 independent donors. Statistical analysis was performed with the Student's t test, and the level of significance was set at P ≤ 0.05.
Next, we sought to identify the autocrine factors responsible for the up-regulation of TLR3 in MSCs. To this end, we stimulated nmMSCs with Poly I:C-MSC SNs in the presence or absence of neutralizing antibodies against IL-6, IL-8, and IFNβ. We found that inhibition of IFNβ (Fig. 5F), but not of IL-8 or IL-6 (data not shown), prevented TLR3 up-regulation by Poly I:C-MSC SNs. Furthermore, recombinant IFNβ up-regulated the expression of TLR3 in nmMSCs (Fig. 5G). Taken together, these data indicate that IFNβ released by nmMSCs on the initial Poly I:C stimulation up-regulates TLR3 in an autocrine manner.
TLR3 ligands polarize MSCs toward a proinflammatory phenotype
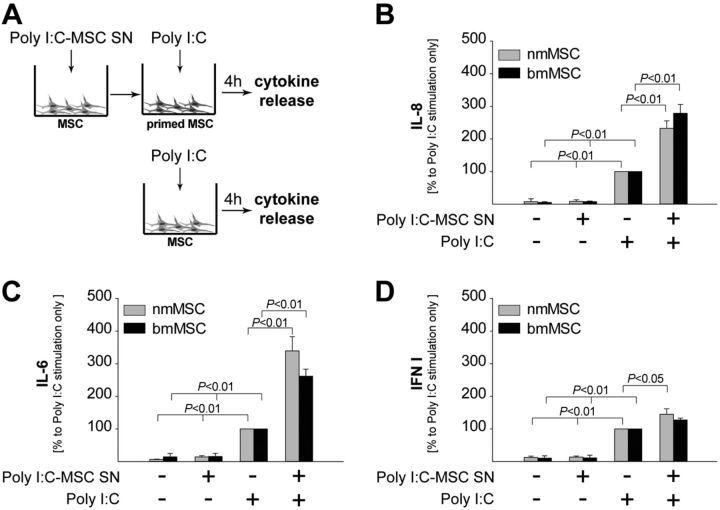
The observation that TLR3 was induced by Poly I:C via autocrine activation prompted us to hypothesize that Poly I:C-stimulated MSCs might be rendered more sensitive to a second challenge with Poly I:C. To test this hypothesis, nmMSCs or bmMSCs were primed with Poly I:C-MSC SNs and subsequently stimulated with Poly I:C. Unprimed MSCs were used as control (Fig. 6A). The release of IL-8, IL-6, and IFN type I was assessed 4 h later and showed that all cytokines were produced at higher levels by primed MSCs on Poly I:C stimulation (Fig. 6B–D). However, while the release of IL-8 and IL-6 by the primed MSCs was strongly enhanced compared with their unprimed counterparts (Fig. 6B, C), the release of IFN type I was only slightly higher in primed MSCs (Fig. 6D).
Figure 6.
Autocrine activation preferentially amplifies the release of inflammatory cytokines. A) Poly I:C-MSC SNs were obtained as described in Fig. 5A and were used to prime naive MSCs from the same donors. Unprimed MSCs were used as control. B–D) Both primed and unprimed MSCs were stimulated with 1 μM Poly I:C for 4 h, and levels of IL-8 (B), IL-6 (C), and IFN type I (D) were assessed in the SNs. Cytokine levels released by unprimed MSCs on Poly I:C stimulation were considered as 100%. Data are means ± sd of 3 independent donors. Statistical analysis was performed with the Students' t test, and the level of significance was set at P ≤ 0.05.
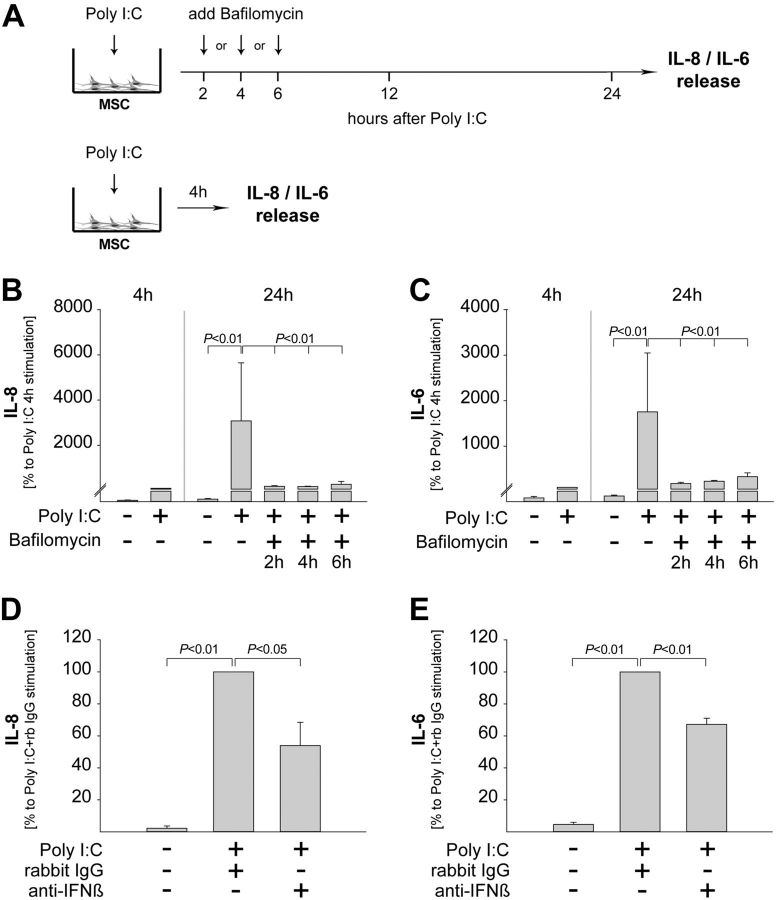
In the final set of studies, we blocked TLR3 signaling subsequently to Poly I:C stimulation by adding bafilomycin at 2, 4, or 6 h poststimulation. The levels of proinflammatory cytokines were determined at 24 h poststimulation (Fig. 7A). For comparison, the levels of proinflammatory cytokines were determined after stimulation with Poly I:C for 4 h (Fig. 7A). The results showed that the late inhibition of TLR3 signaling diminished the release of both IL-8 and IL-6 to levels similar to those observed after 4 h of stimulation with Poly I:C (Fig. 7B, C). To strengthen these findings, we prevented the autocrine up-regulation of TLR3 by adding anti-IFNβ neutralizing antibodies (see Fig. 5F). The results showed that Poly-I:C-induced release of IL-8 and IL-6 was significantly inhibited in the absence of TLR3 up-regulation. (Fig. 7D, E). Thus, these data further substantiate a model in which the strong release of IL-6/IL-8 beyond 4 h of stimulation requires continuous stimulation together with amplification of inflammatory TLR3 signaling by autocrine priming (Fig. 8).
Figure 7.
MSCs require TLR3 up-regulation and continuous ligation to release high levels of proinflammatory cytokines. A) nmMSCs were stimulated with 1 μM Poly I:C for 24 h. Bafilomycin (100 nM) was added at 2, 4, or 6 h after Poly I:C stimulation. In addition, nmMSCs were stimulated with Poly I:C for 4 and 24 h without addition of bafilomycin. B, C) Levels of IL-8 (B) and IL-6 (C) in all SNs were assessed by ELISA. nmMSCs were stimulated with Poly I:C in the presence or absence of anti-IFNβ neutralizing antibodies or isotype control. D, E) Levels of IL-8 (D) and IL-6 (E) were assessed by ELISA at 24 h poststimulation. Data are means ± sd of 3 independent donors. Statistical analysis was performed with the Student's t test, and the level of significance was set at P ≤ 0.05.
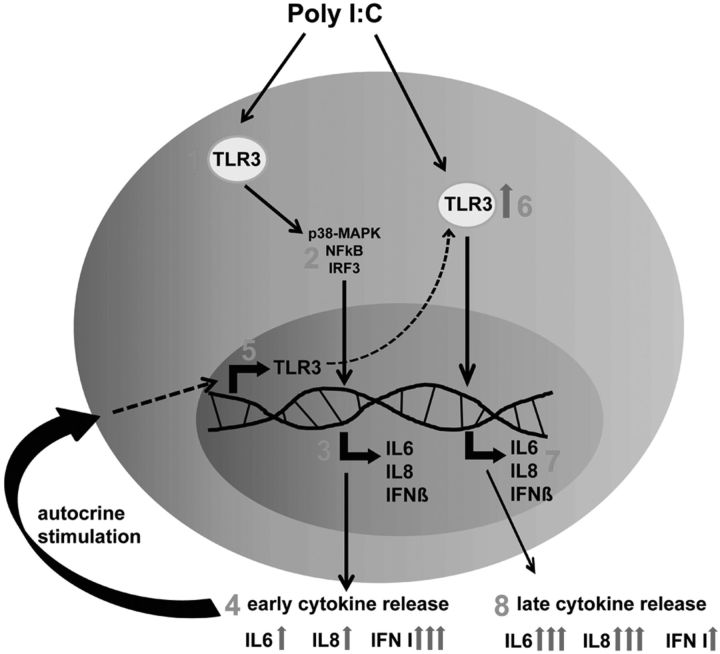
Figure 8.
Proposed model of TLR3-induced cytokine response in MSCs. Stimulation of MSCs via TLR3 (1) activates p38-MAPK, NF-κB and IRF3 (2) to induce release of IL-6, IL-8 and IFN type I (3). The initial stimulation with Poly I:C results in a strong release of IFN type I and a weak release of IL-6 and IL-8 (4). Subsequently, MSC-derived IFNβ up-regulates the expression of TLR3 in an autocrine manner (5), which enhances the sensitivity of MSCs to secondary stimulation with Poly I:C (6). The sensitized MSCs release high levels of IL-6 and IL-8 but only low levels of IFN type I (7, 8). Thus, time-dependent polarization of MSCs operates through autocrine amplification of TLR3-induced inflammatory cytokine production.
DISCUSSION
In this study, we investigated the cytokine response of nmMSCs following TLR ligation. Our results showed that nmMSCs expressed, to various degrees, all 10 known human TLRs. However, nmMSCs responded strongest to stimulation with TLR3 ligands by releasing significant amounts of both proinflammatory (IL-6 and IL-8) and antiviral/anti-inflammatory (IFN type I) cytokines. These findings indicate that nmMSCs might play important roles in inflammation-associated conditions (such as infections or tissue injury) and confirm the immunomodulatory plasticity of MSCs proposed by recent studies (5).
Consequently, we focused on elucidating the molecular mechanisms triggered by TLR3 ligation in nmMSCs and found that both NF-κB and IRF3 pathways were rapidly activated in these cells. However, while activation of the IRF3/IFN type I pathway has been previously reported for human MSCs following stimulation with TLR3 ligands, the activation of the NF-κB/IL pathway has been mainly linked to stimulation by TLR4 ligands in this cell type (7, 15, 22). Furthermore, our study identified p38-MAPK as an important regulator of TLR3-induced cytokine release by nmMSCs. At present, it is not clear how p38-MAPK controls cytokine production in nmMSCs; nevertheless, our findings indicate that p38-MAPK is involved in the same signaling pathway as NF-κB. Exploratory studies from our group suggest that p38-MAPK operates downstream of NF-κB-dependent gene regulation, since p38-MAPK inhibition by SB202190 did not prevent the nuclear translocation of NF-κB (data not shown). Interestingly, p38-MAPK was proposed to be a major stabilizer of cytokine mRNA. Both the NF-κB targets IL-6 and IL-8 have AU-rich elements (AREs) in the 3′-untranslated regions (UTRs) that render them prone to instability, and several studies showed that p38-MAPK prevented their mRNA degradation via the downstream effector molecule MK2 (23–25). Similarly, p38-MAPK did not affect nuclear translocation of IRF3 (data not shown) and, thus, might be involved in the stabilization of IFNB mRNA, which also contains a 3′UTR ARE (26). However, in the absence of specific inhibitors for IRF3, additional proof is needed to determine whether p38-MAPK and IRF3 are involved in the same signaling cascade to induce IFN type I in nmMSCs.
Of particular interest were our findings that stimulation with TLR3 ligands up-regulated the expression of TLR3 in an autocrine manner. While enhancement of TLR3 expression has been previously linked to stimulation by Poly I:C (22), we are the first to demonstrate that this process is a consequence of autocrine factors, namely IFNβ, - rather than of direct Poly I:C stimulation in MSCs. Subsequent to the autocrine up-regulation of TLR3, nmMSCs, and also bmMSCs, were sensitized to a second challenge with Poly I:C. Interestingly, however, sensitization of MSCs resulted in a strong release of IL-8 and IL-6 but only in a slightly enhanced release of IFN type I. These results, together with our findings that IFN type I is only released at early stages and does not accumulate during the course of 24 h stimulation (Fig. 4C, D), are rather surprising, since IFN type I is known to induce its own production via a positive feedback loop (27). Thus, it seems that the IFN type I pathway is somehow blocked in MSCs after the initial TLR3 ligation. The inhibition of IFN type I signaling can occur at multiple levels. Nevertheless, suppressor of cytokine signaling (SOCS) family members, which block JAK activity, were shown to play major roles in this process (28) and might also be responsible for hindering the autocrine IFN type I induction in Poly I:C-stimulated MSCs. This possibility is supported by recent studies from Tomchuck et al. (29), who demonstrated that stimulation of human bmMSCs with Poly I:C resulted in up-regulation of SOCS1 and SOCS3. These findings were also confirmed in our experimental system, where we found that Poly I:C induced a 5.08 ± 1.06-fold increase of SOCS3 mRNA and a 28.46 ± 3.90-fold increase of SOCS1 mRNA in nmMSCs at 4 h poststimulation (Supplemental Fig. S3A, B).
The suppression of IFN type I, together with the strongly enhanced release of IL-8/IL-6 observed at 24 h poststimulation (which often exceeded 100 ng/ml/24 h/106 cells), might indicate that MSCs switch toward a proinflammatory phenotype during the course of stimulation with TLR3 ligands. While polarization of human MSCs into MSC1 and MSC2 has been recently proposed by Waterman et al. (15), this phenomenon was attributed to stimulation via different TLRs (i.e., proinflammatory for TLR4 ligation and immunosuppressive for TLR3 ligation). Our study shows that continuous stimulation of MSCs with TLR3 ligands induces even larger amounts of proinflammatory cytokines than stimulation with TLR4 ligands (Fig. 1B, C). These findings are also in line with recent studies from Cassatella et al. (7), who demonstrated that Poly I:C-stimulated bmMSCs released more IL-6 than their LPS-stimulated counterparts and were more potent activators of neutrophil granulocytes. A significant difference between the first and the latter two studies was the duration of stimulation with Poly I:C. Specifically, Waterman et al. (15) stimulated their MSCs with Poly I:C for only 1 h and washed it away before analyzing cytokine release at 24 h. In contrast, MSCs were exposed to Poly I:C for the entire 24 h period before cytokine analysis in both our study and the study of Cassatella et al. (7). Here, we clearly show that MSCs do not release large amounts of proinflammatory cytokines upon short exposure to TLR3 ligands (Fig. 4C). The opposite, however, occurs upon long-term continuous stimulation with Poly I:C (Figs. 4A, C and 7B, C). Thus, our data provide an explanation for the apparent discrepancy regarding TLR-induced cytokine profile of MSCs and show that TLR3 alone can fine-tune the release of immunomodulatory mediators by these cells in a time-dependent manner.
In summary, different hypotheses are currently being explored to explain and characterize the cellular and molecular mechanisms that guide this striking functional plasticity of MSCs. On one hand, the polarization of MSCs can be regulated by different TLR ligands (15). On the other hand, the expression levels of inflammatory cytokines such as IFN-γ and TNF-α might determine the functional phenotype of MSCs, as indicated by the licensing model (30). Third, MSCs may indirectly polarize an immune response by modulating the functional phenotype of accessory immune cells, as recently shown in macrophages (13, 31). Our study proposes a fourth model of time-dependent MSCs functional plasticity that is controlled by autocrine priming. Thus, our findings elucidate novel mechanisms that regulate the biology of MSCs and, ultimately, contribute to a better understanding of the role of MSCs in inflammation and immunomodulation.
Supplementary Material
Acknowledgments
The authors thank Kirsten Bruderek and Sebastian Vollmer (Department of Otorhinolaryngology, University Hospital Essen) for excellent technical support. The authors are grateful to Dr. Karl S. Lang (Department of Immunology, University Hospital Essen), as well as to Dr. Ulf Dittmer and Dr. Katrin Gibbert (Department of Virology, University Hospital Essen) for the critical reading and helpful comments on our manuscript.
This study was supported by a grant from the Deutsche Forschungsgemeinschaft (DFG) to S.B.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- AKT/PKB
- protein kinase B
- bmMSC
- bone marrow mesenchymal stem/stromal cell
- CpG
- cytosine-phosphate-guanine
- ELISA
- enzyme-linked immunosorbent assay
- ERK
- extracellular signal-regulated kinase
- HEK
- human embryonic kidney
- IFN
- interferon
- IL
- interleukin
- IRF
- interferon-regulatory factor
- LPS
- lipopolysaccharide
- MALP-2
- macrophage-activating lipopeptide 2
- MAPK
- mitogen-activated protein kinase
- MSC
- mesenchymal stem/stromal cell
- MyD88
- myeloid differentiation primary response gene 88
- NF-κB
- nuclear factor κB
- nmMSC
- nasal mucosa-derived mesenchymal stem/stromal cell
- PAM3CSK4
- N-palmitoyl-S-[2,3-bis(palmitoyloxy)-propyl]-(R)-cysteinyl-(lysyl)3-lysine
- PI3K
- phosphatidylinositide 3-kinase
- Poly I:C
- polyinosinic:polycytidylic acid
- SN
- supernatant
- SOCS
- suppressor of cytokine signaling
- TIR
- Toll/interleukin-1 receptor
- TLR
- Toll-like receptor
- TNF-α
- tumor necrosis factor α
- TRIF
- TIR-domain-containing adapter inducing interferon-β
REFERENCES
- 1. Miao Z., Jin J., Chen L., Zhu J., Huang W., Zhao J., Qian H., Zhang X. (2006) Isolation of mesenchymal stem cells from human placenta: comparison with human bone marrow mesenchymal stem cells. Cell Biol. Int. 30, 681–687 [DOI] [PubMed] [Google Scholar]
- 2. Nathan S., Das De S., Thambyah A., Fen C., Goh J., Lee E. H. (2003) Cell-based therapy in the repair of osteochondral defects: a novel use for adipose tissue. Tissue Eng. 9, 733–744 [DOI] [PubMed] [Google Scholar]
- 3. Prockop D. J., Gregory C. A., Spees J. L. (2003) One strategy for cell and gene therapy: harnessing the power of adult stem cells to repair tissues. Proc. Natl. Acad. Sci. U. S. A. 100(Suppl. 1), 11917–11923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jakob M., Hemeda H., Janeschik S., Bootz F., Rotter N., Lang S., Brandau S. (2010) Human nasal mucosa contains tissue-resident immunologically responsive mesenchymal stromal cells. Stem Cells Dev. 19, 635–644 [DOI] [PubMed] [Google Scholar]
- 5. Bernardo M. E., Fibbe W. E. (2013) Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell 13, 392–402 [DOI] [PubMed] [Google Scholar]
- 6. Brandau S., Jakob M., Hemeda H., Bruderek K., Janeschik S., Bootz F., Lang S. (2010) Tissue-resident mesenchymal stem cells attract peripheral blood neutrophils and enhance their inflammatory activity in response to microbial challenge. J. Leukoc. Biol. 88, 1005–1015 [DOI] [PubMed] [Google Scholar]
- 7. Cassatella M. A., Mosna F., Micheletti A., Lisi V., Tamassia N., Cont C., Calzetti F., Pelletier M., Pizzolo G., Krampera M. (2011) Toll-like receptor-3-activated human mesenchymal stromal cells significantly prolong the survival and function of neutrophils. Stem Cells 29, 1001–1011 [DOI] [PubMed] [Google Scholar]
- 8. English K., Ryan J. M., Tobin L., Murphy M. J., Barry F. P., Mahon B. P. (2009) Cell contact, prostaglandin E(2) and transforming growth factor beta 1 play non-redundant roles in human mesenchymal stem cell induction of CD4+CD25(High) forkhead box P3+ regulatory T cells. Clin. Exp. Immunol. 156, 149–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Francois M., Romieu-Mourez R., Li M., Galipeau J. (2012) Human MSC suppression correlates with cytokine induction of indoleamine 2,3-dioxygenase and bystander M2 macrophage differentiation. Mol. Ther. 20, 187–195 [DOI] [PubMed] [Google Scholar]
- 10. Maccario R., Podesta M., Moretta A., Cometa A., Comoli P., Montagna D., Daudt L., Ibatici A., Piaggio G., Pozzi S., Frassoni F., Locatelli F. (2005) Interaction of human mesenchymal stem cells with cells involved in alloantigen-specific immune response favors the differentiation of CD4+ T-cell subsets expressing a regulatory/suppressive phenotype. Haematologica 90, 516–525 [PubMed] [Google Scholar]
- 11. Nemeth K., Leelahavanichkul A., Yuen P. S., Mayer B., Parmelee A., Doi K., Robey P. G., Leelahavanichkul K., Koller B. H., Brown J. M., Hu X., Jelinek I., Star R. A., Mezey E. (2009) Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat. Med. 15, 42–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Selmani Z., Naji A., Zidi I., Favier B., Gaiffe E., Obert L., Borg C., Saas P., Tiberghien P., Rouas-Freiss N., Carosella E. D., Deschaseaux F. (2008) Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells 26, 212–222 [DOI] [PubMed] [Google Scholar]
- 13. Melief S. M., Schrama E., Brugman M. H., Tiemessen M. M., Hoogduijn M. J., Fibbe W. E., Roelofs H. (2013) Multipotent stromal cells induce human regulatory T cells through a novel pathway involving skewing of monocytes toward anti-inflammatory macrophages. Stem Cells 31, 1980–1991 [DOI] [PubMed] [Google Scholar]
- 14. Stagg J., Galipeau J. (2013) Mechanisms of immune modulation by mesenchymal stromal cells and clinical translation. Curr. Mol. Med. 13, 856–867 [DOI] [PubMed] [Google Scholar]
- 15. Waterman R. S., Tomchuck S. L., Henkle S. L., Betancourt A. M. (2010) A new mesenchymal stem cell (MSC) paradigm: polarization into a pro-inflammatory MSC1 or an Immunosuppressive MSC2 phenotype. PLoS One 5, e10088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hwa Cho H., Bae Y. C., Jung J. S. (2006) Role of toll-like receptors on human adipose-derived stromal cells. Stem Cells 24, 2744–2752 [DOI] [PubMed] [Google Scholar]
- 17. Nagai Y., Garrett K. P., Ohta S., Bahrun U., Kouro T., Akira S., Takatsu K., Kincade P. W. (2006) Toll-like receptors on hematopoietic progenitor cells stimulate innate immune system replenishment. Immunity 24, 801–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pevsner-Fischer M., Morad V., Cohen-Sfady M., Rousso-Noori L., Zanin-Zhorov A., Cohen S., Cohen I. R., Zipori D. (2007) Toll-like receptors and their ligands control mesenchymal stem cell functions. Blood 109, 1422–1432 [DOI] [PubMed] [Google Scholar]
- 19. Zhu J., Mohan C. (2010) Toll-like receptor signaling pathways–therapeutic opportunities. Mediators Inflamm. 2010, 781235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hemeda H., Jakob M., Ludwig A. K., Giebel B., Lang S., Brandau S. (2010) Interferon-gamma and tumor necrosis factor-alpha differentially affect cytokine expression and migration properties of mesenchymal stem cells. Stem Cells Dev. 19, 693–706 [DOI] [PubMed] [Google Scholar]
- 21. Dumitru C. A., Fechner M. K., Hoffmann T. K., Lang S., Brandau S. (2012) A novel p38-MAPK signaling axis modulates neutrophil biology in head and neck cancer. J. Leukoc. Biol. 91, 591–598 [DOI] [PubMed] [Google Scholar]
- 22. Opitz C. A., Litzenburger U. M., Lutz C., Lanz T. V., Tritschler I., Koppel A., Tolosa E., Hoberg M., Anderl J., Aicher W. K., Weller M., Wick W., Platten M. (2009) Toll-like receptor engagement enhances the immunosuppressive properties of human bone marrow-derived mesenchymal stem cells by inducing indoleamine-2,3-dioxygenase-1 via interferon-beta and protein kinase R. Stem Cells 27, 909–919 [DOI] [PubMed] [Google Scholar]
- 23. McCormick C., Ganem D. (2005) The kaposin B protein of KSHV activates the p38/MK2 pathway and stabilizes cytokine mRNAs. Science 307, 739–741 [DOI] [PubMed] [Google Scholar]
- 24. Patil C., Zhu X., Rossa C., Jr., Kim Y. J., Kirkwood K. L. (2004) p38 MAPK regulates IL-1beta induced IL-6 expression through mRNA stability in osteoblasts. Immunol. Invest. 33, 213–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Winzen R., Kracht M., Ritter B., Wilhelm A., Chen C. Y., Shyu A. B., Muller M., Gaestel M., Resch K., Holtmann H. (1999) The p38 MAP kinase pathway signals for cytokine-induced mRNA stabilization via MAP kinase-activated protein kinase 2 and an AU-rich region-targeted mechanism. EMBO J. 18, 4969–4980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Khabar K. S., Young H. A. (2007) Post-transcriptional control of the interferon system. Biochimie (Paris) 89, 761–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Honda K., Taniguchi T. (2006) IRFs: master regulators of signalling by Toll-like receptors and cytosolic pattern-recognition receptors. Nat. Rev. Immunol. 6, 644–658 [DOI] [PubMed] [Google Scholar]
- 28. Baker B. J., Akhtar L. N., Benveniste E. N. (2009) SOCS1 and SOCS3 in the control of CNS immunity. Trends Immunol. 30, 392–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tomchuck S. L., Henkle S. L., Coffelt S. B., Betancourt A. M. (2012) Toll-like receptor 3 and suppressor of cytokine signaling proteins regulate CXCR4 and CXCR7 expression in bone marrow-derived human multipotent stromal cells. PLoS One 7, e39592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Krampera M. (2011) Mesenchymal stromal cell ‘licensing’: a multistep process. Leukemia 25, 1408–1414 [DOI] [PubMed] [Google Scholar]
- 31. Melief S. M., Geutskens S. B., Fibbe W. E., Roelofs H. (2013) Multipotent stromal cells skew monocytes towards an anti-inflammatory interleukin-10-producing phenotype by production of interleukin-6. Haematologica 98, 888–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.