Abstract
Rab GTPases are highly conserved components of vesicle trafficking pathways. Rab5, as a master regulator of endocytic trafficking, has been shown to function in membrane tethering and docking. However, the function of Rab5 in meiosis has not been addressed. Here, we report elongated spindles and misaligned chromosomes, with kinetochore-microtubule misattachments, on specific depletion of Rab5a in mouse oocytes. Moreover, the localization and levels of centromere protein F (CENPF), a component of the nuclear matrix, are severely reduced at kinetochores in metaphase oocytes following Rab5a knockdown. Consistent with this finding, nuclear lamina disassembly in the transition from prophase arrest to meiosis I is also impaired in Rab5a-depleted oocytes. Notably, oocyte-specific ablation of CENPF phenocopies the meiotic defects resulting from Rab5a knockdown. In summary, our data support a model where Rab5a-positive vesicles, likely through interaction with nuclear lamina, modulate CENPF localization and levels at centromeres, consequently ensuring proper spindle length and kinetochore-microtubule attachment in meiotic oocytes.—Ma, R., Hou, X., Zhang, L., Sun, S.-C., Schedl, T., Moley, K., Wang, Q. Rab5a is required for spindle length control and kinetochore-microtubule attachment during meiosis in oocytes.
Keywords: CENPF, vesicles, chromosome
The oocyte is a central component of female fertility. Growth and maturation of the oocyte are highly coordinated processes involving both nuclear and cytoplasmic events (1). Accurate control of spindle assembly and chromosome organization is required for orderly meiosis during oocyte maturation, where errors in this process can lead to the generation of aneuploid eggs. Fertilization of aneuploid eggs in humans is a major cause of pregnancy loss, and if there is survival to term, it will result in developmental disabilities (2). Although various molecules have been proposed to contribute to spindle/chromosome defects in oocyte meiosis, the pathways and mechanisms that modulate the meiotic apparatus remain to be discovered.
Rab GTPases, an extended family of small GTPases, are highly conserved components of vesicle trafficking pathways. They function in determining vesicles identity and control vesicle budding, uncoating, motility, and fusion by recruiting a specific set of effector proteins (3, 4). In addition to their roles in vesicles, Rab GTPases, and their cofactors and adaptors, have been implicated in spindle-based cellular events. Epsin, an endocytic adaptor protein, is required for proper spindle morphogenesis, potentially through controlling membrane curvature (5). Rab6a regulates the dynamics of the dynein/dynactin complex at the kinetochores and, consequently, the inactivation of the Mad2-spindle checkpoint (6). Furthermore, elegant work of Holubcova et al. (7) revealed that vesicles positive for the Rab11a modulate an actin network for asymmetric spindle positioning in oocytes. Rab5, as the master regulator of the endocytic trafficking, has been well recognized to involve in membrane tethering and docking (8–11). Three isoforms of Rab5 (a, b, and c) share >90% of sequence identity yet can be functionally different (12). Of note, recent findings have suggested that Rab5 GTPase participates in chromosome congression in Drosophila and human mitotic cells (13, 14). However, the potential functional involvement of Rab5 in meiosis has not been addressed yet.
In this study, we set out to investigate the role of Rab5a during mouse oocyte meiosis. We discovered a novel function of Rab5a-containing vesicles: control of the spindle length and chromosome alignment through modulation of centromere protein F (CENPF) localization to the centromere, as reported below.
MATERIALS AND METHODS
All chemicals and culture media were purchased from Sigma (St. Louis, MO, USA) unless stated otherwise. ICR mice were used in this study. All experiments were approved by the Animal Care and Use Committee of Nanjing Medical University and were performed in accordance with institutional guidelines.
Antibodies
Rabbit polyclonal anti-Rab5a (cat no. ab18211), rabbit polyclonal anti-β-actin (ab5441), and rabbit polyclonal anti-CENPF (ab5) antibodies were purchased from Abcam (Cambridge, MA, USA); mouse monoclonal anti-α-tubulin-FITC antibody was purchased from Sigma (76074); human anti-centromere CREST antibody (09C-CS1058) was purchased from Fitzgerald Industries International (Concord, MA, USA); mouse monoclonal anti-NuMA antibody (610562) was purchased from BD Transduction Laboratories (Lexington, KY, USA); goat polyclonal anti-lamin A/C antibody (SC-6215) was obtained from Santa Cruz Biotechnology (San Jose, CA, USA); FITC-conjugated goat anti-rabbit IgG, FITC-conjugated donkey anti-goat IgG, and TRITC-conjugated goat anti-rabbit IgG were purchased from Thermo Fisher Scientific (Rockford, IL, USA); and Cy5-conjugated goat anti-human IgG and Cy5-conjugated goat anti-rabbit IgG were purchased from Jackson ImmunoResearch Laboratory (West Grove, PA, USA).
Oocyte collection and culture
Six- to 8-wk-old female mice were used for oocyte collection. To collect fully grown germinal vesicle (GV) oocytes, mice were superovulated with 5 IU pregnant mare serum gonadotropin (PMSG) by intraperitoneal injection, and 48 h later, cumulus-enclosed oocytes were obtained by manual rupturing of antral ovarian follicles. Cumulus cells were removed by repeatedly pipetting. For in vitro maturation, GV oocytes were cultured in M2 medium under mineral oil at 37°C in a 5% CO2 incubator.
Morpholino (MO) knockdown
Microinjection of MO, with a Narishige microinjector (Narishige Group, Tokyo, Japan), was used to knock down Rab5a and CENPF in mouse oocytes. Rab5a-MO 5′-TTGTTGCTCCTCGATTAGCCATGTC-3′ and CENPF-MO 5′-GGCCCAGCTCATCTTGTTTTATTTT-3′ (Gene Tools, Philomath, OR, USA) targeting initiation of translation were diluted with water to give a stock concentration of 1 mM, and then a 2.5 pl MO solution was injected into oocytes. A MO standard control was injected as control.
After injections, oocytes were arrested at the GV stage in M2 medium supplemented with 2.5 μM milrinone for 20 h to facilitate knockdown of mRNA translation, then washed 3 times in milrinone-free M2 medium, and cultured for different times.
Western blotting
A pool of ∼100 oocytes was lysed in Laemmli sample buffer containing protease inhibitor and then subjected to 10% SDS-PAGE. The separated proteins were transferred to a PVDF membrane. Membranes were blocked in TBS containing 0.1% Tween 20 and 5% low-fat dry milk for 1 h and then incubated with primary antibodies as follows: rabbit anti-Rab5a antibody (1:1000) or rabbit anti-CENPF antibody (1:1500). After multiple washes in TBS containing 0.1% Tween 20 and incubation with horseradish peroxidase-conjugated secondary antibodies, the protein bands were visualized using an ECL Plus Western Blotting Detection System (GE Healthcare, Piscataway, NJ, USA). The membrane was then washed and reblotted with anti-β-actin (1:5,000) or anti-tubulin antibody (1:3000) for loading control.
Immunofluorescence and confocal microscopy
Oocytes were fixed with 4% paraformaldehyde for 30 min and then permeabilized with 0.5% Triton X-100 for 20 min. Following blocking in 1% BSA-supplemented PBS for 1 h, samples were incubated overnight at 4°C with primary antibodies as follows: anti-Rab5a antibody, anti-lamin A/C antibody, anti-NuMA antibody, anti-CENPF antibody, and FITC-conjugated anti-tubulin antibody. To detect kinetochores, oocytes were labeled with CREST (1:500) according to the previous protocol (15). To examine the kinetochore-microtubule (K-MT) attachments, oocytes were briefly chilled at 4°C to induce depolymerization of nonkinetochore microtubules just before fixation. Chromosomes were stained with propidium iodide (PI; red) or Hoechst 33342 (blue) for 10 min. After washes with PBS, oocyte samples were mounted on antifade medium (Vectashield, Burlingame, CA, USA) and then examined under a laser-scanning confocal microscope (LSM 710; Zeiss, Oberkochen, Germany) equipped with the ×40 or ×63 oil objectives. To measure the fluorescent intensities of NuMA/CENPF, Z-stack images were used to generate a projection containing the sum of all the CREST/CENPF signals. Fluorescent intensity of NuMA/CENPF was quantified by placing a small circle around the signals. The average cytoplasmic fluorescence intensity was subtracted as background. ImageJ software (U.S. National Institutes of Health, Bethesda, MD, USA) was used to quantify the spindle length and fluorescence intensity as described previously (16, 17).
Chromosome spread
Chromosome preparations for metaphase II (MII) oocytes were as described previously (18). Oocytes were treated with 1% sodium citrate for 20 min, individually transferred to a glass slide, and then fixed with several drops of methanol/acetic acid (3:1). After air drying and nuclear staining, the chromosomes were observed by fluorescence microscopy.
Statistical analysis
Data are presented as means ± sd, unless otherwise indicated. Statistical comparisons were made with Student's t test and ANOVA when appropriate. Values of P < 0.05 were considered to be significant.
RESULTS
Cellular distribution of vesicular Rab5a during oocyte meiosis
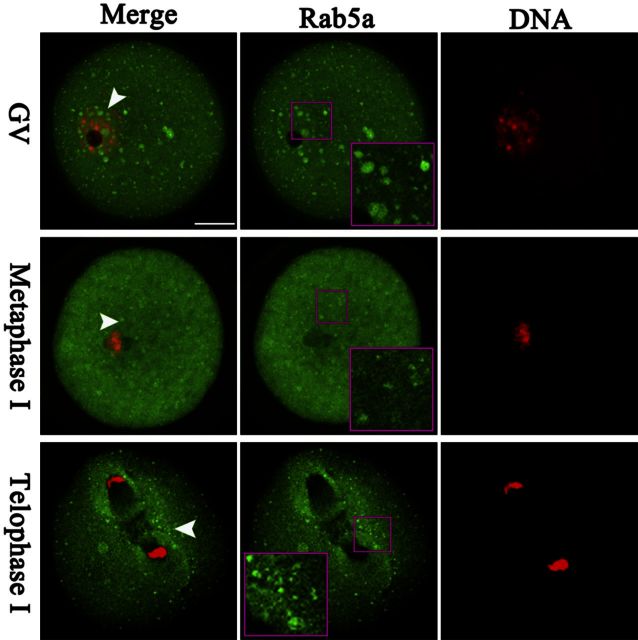
We first examined Rab5a distribution during mouse oocyte maturation. Immunostaining clearly showed the presence of Rab5a-containing vesicles in oocytes at different developmental stages (Fig. 1), which are termed Rab5a-positive vesicles in this study. These vesicles reside in the entire immature oocytes, and many of them appear to be accumulated in the GV (Fig. 1, arrowheads). As the oocytes enter into metaphase, the vesicles become uniformly localized in the cytoplasm. Of note, during anaphase and telophase, numerous vesicles become concentrated around the spindle/chromosome region (Fig. 1, inset). Such a dynamic distribution pattern suggests that vesicular Rab5a may have a function in the formation or stability of meiotic spindle/chromosomes or in regulation of meiotic progression.
Figure 1.
Cellular distribution of Rab5-positive vesicles during oocyte meiosis. Oocytes at GV, MI, and telophase I stages were immunolabeled with Rab5a antibody (green) for vesicles and counterstained with PI for DNA (red) and visualized by confocal microscopy. Arrowheads indicate examples of Rab5a-positive vesicles. Outlined regions are magnified in inset; 30 oocytes were analyzed for each group. Scale bar = 25 μm.
Depletion of Rab5a affects meiotic progression in oocytes
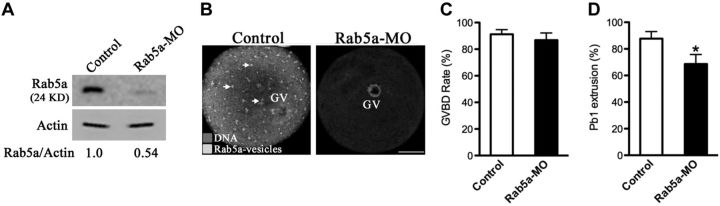
Next, to investigate the function of Rab5a in oocyte meiosis, we microinjected the Rab5a-targeting morpholino (Rab5a-MO) into fully grown oocytes. This led to a significant reduction of Rab5a protein and a corresponding loss of Rab5a-positive vesicles from oocytes, based on Western blot and immunostaining analysis (Fig. 2A, B). We then analyzed how the loss of Rab5a-positive vesicles affected oocyte maturation.
Figure 2.
Effects of Rab5a depletion on oocyte maturation. Fully grown oocytes microinjected with Rab5a-MO were arrested at GV stage with milrinone for 20 h to block mRNA translation and then cultured in milrinone-free medium to evaluate the meiotic progression by phase-contrast microscopy. A sham MO standard was injected as control. A) Western blot showing partial knockdown of Rab5a after MO injection with actin as a loading control. Band intensity was calculated using ImageJ software, and the ratio of Rab5a/actin expression was normalized, and values are indicated. B) Immunostaining showing the loss of Rab5a-positive vesicles in oocytes with MO injection. Arrows indicate vesicles. Scale bar = 25 μm. C, D) Quantitative analysis of GVBD (C) and Pb1 (D) extrusion in control (n=150) and Rab5a-MO (n=120) oocytes. Bars represent means ± sd of results obtained in 3 independent experiments. *P < 0.05 vs. control.
Progression of oocyte maturation includes germinal vesicle breakdown (GVBD), microtubules organizing into the metaphase I (MI) spindle, chromosomes aligning at the MI metaphase plate, execution of the MI division, extruding the first polar body (Pb1), and then proceeding to arrest at MII until fertilization. Our results showed that Rab5a depletion appeared to affect neither the morphology nor the fraction of oocytes that underwent GVBD (86.8±5.4 vs. 91.2±3.5% control; Fig. 2C). However, Pb1 extrusion was decreased in Rab5a-MO oocytes compared with controls (68.6±7.2 vs. 87.7±5.3% control, P<0.05; Fig. 2D), indicative of the involvement of Rab5a in the meiotic process.
Proper spindle length and chromosome alignment in oocytes requires Rab5a
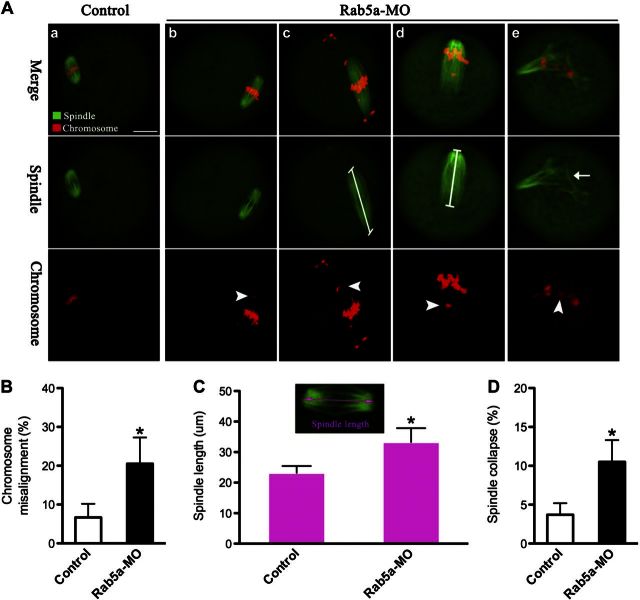
We then asked whether depletion of Rab5a also affects the meiotic apparatus in oocytes. To this end, oocytes were immunolabeled with anti-tubulin antibody to visualize the spindle and costained with PI for chromosomes. Confocal microscopy revealed that Rab5a-MO oocytes displayed a high frequency of chromosome misalignment (20.5±6.8 vs. 6.7±3.5% control, P<0.05; Fig. 3A, B, arrowheads) and obvious spindle morphology defects, remarkably including spindle elongation (Fig. 3Ac, d). Metaphase spindle lengths in Rab5a-MO oocytes were on average 40% longer than controls (Fig. 3A, C). An additional abnormality observed in Rab5a-MO oocytes was spindle collapse (10.5±2.8 vs. 3.7±1.5% control, P<0.05; Fig. 3Ae, D, arrow). These phenotypes contrasted sharply with metaphases in control oocytes, which presented a typical barrel-shape spindle and well-aligned chromosomes at the equator (Fig. 3Aa).
Figure 3.
Proper spindle morphology and chromosome alignment in oocytes requires Rab5a. A) Control and Rab5a-MO oocytes were stained with α-tubulin antibody to visualize the spindle (green) and counterstained with PI to visualize chromosomes (red): control MII oocytes present a typical barrel-shaped spindle and well-aligned chromosomes on the metaphase plate (a); aberrant spindle (arrows) and misaligned chromosomes (arrowheads) were readily observed in Rab5a-MO MII oocytes (b–e). Representative confocal sections are shown. Scale bar = 25 μm. B) Quantification of control and Rab5a-MO oocytes with chromosome misalignment. C) Quantitative analysis of spindle length in control and Rab5a-MO oocytes. D) Quantification of control and Rab5a-MO oocytes with spindle collapse. Data are expressed as mean ± sd percentage from 3 independent experiments in which ≥100 oocytes were analyzed. *P < 0.05 vs. controls.
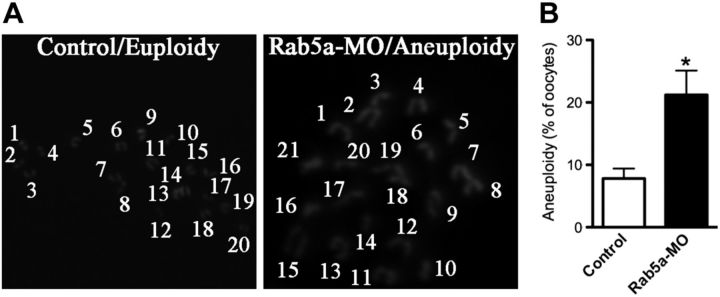
To determine whether the aberrant spindle and misaligned chromosomes in Rab5a-MO oocytes would act to generate aneuploid eggs, we analyzed the karyotype of matured oocytes. As postulated, loss of Rab5a resulted in ∼3-fold increase in incidence of aneuploidy eggs compared with controls (Fig. 4A, B). Altogether, these findings suggest that Rab5a-depleted oocytes are, in many cases, unable to properly assemble the meiotic spindle and align the meiotic chromosomes and thus prone to produce aneuploid eggs.
Figure 4.
Increased aneuploidy in oocytes depleted of Rab5a. A) Chromosome spread of control and Rab5a-MO MII oocytes. Representative fluorescence images of euploid and aneuploid oocytes are shown. B) Quantification of aneuploidy in control and Rab5a-MO oocytes. Data are expressed as mean ± sd percentage; 30 oocytes/group were analyzed. *P < 0.05 vs. controls.
Disrupted K-MT attachments in Rab5a-depleted oocytes
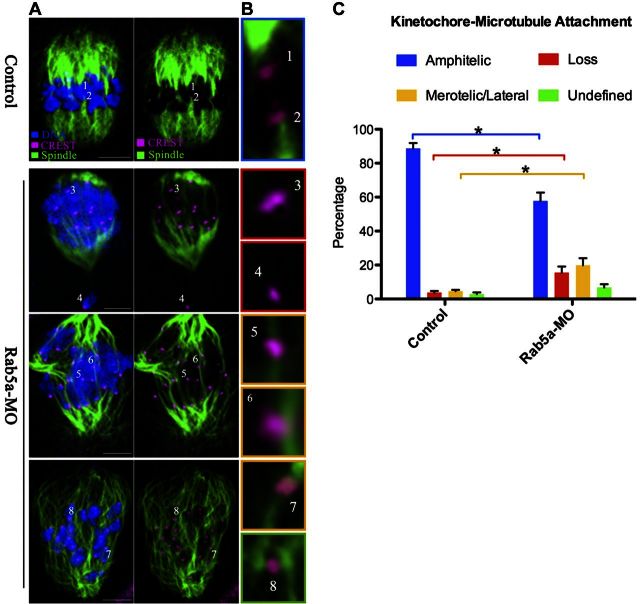
Chromosome movement during meiosis involves a dynamic interaction between spindle microtubules and kinetochores, a macromolecular complex that localizes at the centromere of chromosomes. This interaction is required for bipolar attachment and tension between K-MT and subsequent alignment of chromosomes to the metaphase plate (19). The frequently observed spindle defects and chromosome misalignment in Rab5a-MO oocytes suggested that K-MT attachments are defective. To directly analyze K-MT attachment status, we immunostained kinetochores with CREST and microtubules with anti-tubulin antibody following destabilizing dynamic nonkinetochore microtubules through a brief cold treatment (Fig. 5). Four types of K-MT attachments were detected by confocal scanning and categorized as amphitelic attachment (the predominant form in normal cells; each kinetochore attached to one of the poles; Fig. 5B, chromosomes 1 and 2), loss attachment (kinetochores unattached to either pole; Fig. 5B, chromosomes 3 and 4), merotelic/lateral attachment (1 kinetochore attached to both poles; Fig. 5B, chromosomes 5–7), as well as undefined attachment (Fig. 5B, chromosome 8), indicated by different color. Merotelic and loss attachments are regarded as errors that must be corrected because they would cause chromosome missegregation if they persisted until anaphase (20). Quantitative analysis (Fig. 5C) demonstrated that the loss/merotelic attachment in Rab5a-MO oocytes was greatly increased in frequency relative to control cells (loss: 15.5±3.6 vs. 3.8±0.9% control, P<0.05; merotelic/lateral: 19.9±4.6 vs. 4.6±0.8% control, P<0.05), whereas the proportion of amphitelic attachment was accordingly decreased (57.8±4.9 vs. 88.7±3.2% control, P<0.05). These K-MT misattachments, if uncorrected, would lead to imbalanced force on the kinetochore and, consequently, establish unstable chromosome biorientation, which could contribute to the chromosome alignment failure observed in Rab5a-depleted oocytes.
Figure 5.
Correct K-MT attachments depend on Rab5a. A) Control and Rab5a-MO MI oocytes were stained with anti-tubulin (microtubules, green), CREST (kinetochore, purple), and Hoechst 33342 (chromosomes, blue). Representative confocal sections are shown. B) Magnified views for the K-MT attachments in the oocytes shown in A. Kinetochore-microtubule attachments are classified into 4 categories: amphitelic (chromosomes 1 and 2), loss (chromosome 3 and 4), merotelic/lateral (chromosome 5–7), and undefined (chromosome 8) attachment. Image border colors correspond to the categories. C) Quantitative analysis of K-MT attachments in oocytes as indicated. Attachment of kinetochores to microtubules was assessed through examination of the full series of Z-axis focal planes. Kinetochores in regions where fibers were not easily visualized were not included in the analysis; 10 control oocytes and 8 Rab5a-MO oocytes were analyzed. Scale bars = 10 μm. *P < 0.05 vs. controls.
Depletion of Rab5 impairs oocyte nuclear lamina disassembly
We then searched for a potential mechanism that would explain the requirement of Rab5a for maintaining spindle morphology and chromosome alignment in oocytes. To date, a battery of Rab5 effector proteins mediating diverse cellular events have been identified in different tissue and cell types (13, 14, 21, 22). Of note, recent findings in Caenorhabditis elegans and Drosophila suggest that Rab5 also promotes the disassembly of the nuclear lamina structure (13, 14). Lamin is the major component of the nuclear lamina that is dispersed on mitotic entry. Among all the Rab5 targets, Mud (NuMA-like protein in Drosophila) and CENPF are of particular interest to us because they have been implicated to associate with nuclear matrix (23, 24) and affect spindle poles and kinetochore function in mitotic cells (25, 26). Based on these data, we proposed that depletion of Rab5a might impair nuclear envelope/matrix disassembly, which in turn perturbs the functions of NuMA and/or CENPF, resulting in the defects of spindle morphology and chromosome alignment in oocytes.
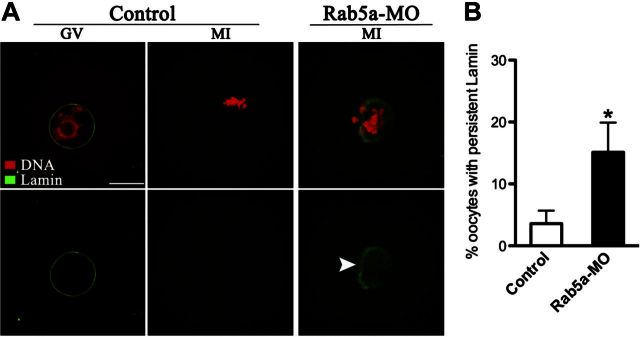
To test this hypothesis, we first examined whether the nuclear envelope disassembly was disrupted during Rab5a-MO oocyte maturation. Immunostaining with anti-lamin A/C antibody showed the presence of intact nuclear lamina in control GV oocytes. With the resumption of meiosis, nuclear lamina was completely dispersed in most of prometaphase/metaphase of control oocytes (Fig. 6A). In contrast, persistent lamin around the region of meiotic chromosomes, albeit not as intact as controls, was observed in >15% of Rab5a-MO metaphase oocytes (Fig. 6A, B). The results suggest that Rab5a is essential for the properly disassembly of nuclear lamina during oocyte maturation.
Figure 6.
Depletion of Rab5a impairs nuclear lamina disassembly in oocytes. A) Control and Rab5a-MO oocytes were labeled with anti-lamin A/C antibody (green) and counterstained with PI to visualize chromosomes (red). Representative images of GV and MI oocytes are shown. Arrowhead in Rab5a-MO MI oocyte shows residual nuclear lamina as judged by lamin A/C staining. Scale bar = 25 μm. B) Quantification of control and Rab5a-MO oocytes showing persistent lamin at metaphase stage. At least 50 oocytes were counted for each group. Bars represent means ± sd. *P < 0.05 vs. controls.
Loss of Rab5a does not affect NuMA localization or levels in oocytes
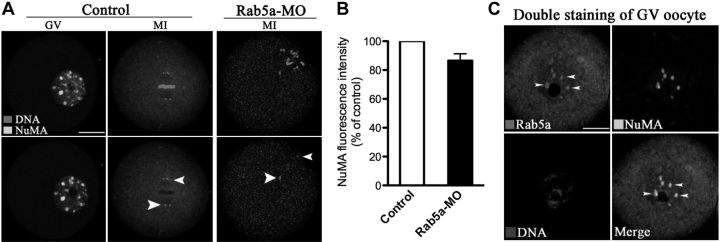
NuMA has been implicated in spindle assembly and chromosome congression in mitotic cells (25, 27). Kolano et al. (28) recently showed that without functional NuMA in mouse oocytes, microtubules lose connection to spindle poles, resulting in highly disorganized early spindle assembly and long spindles with hyperfocused poles, phenotypes that are highly similar to what we observed following Rab5a knockdown. Thus, we assess the affect of Rab5a depletion on NuMA localization and/or levels in oocytes. We observed nuclear NuMA puncta in GV oocytes and then concentrated at the spindle poles at metaphase in control oocytes (Fig. 7A), as previously reported (29). Double staining further revealed colocalization of NuMA and Rab5a vesicles inside the GV (Fig. 7C). Confocal microscopy and quantitative analysis, however, demonstrated that Rab5a depletion in oocytes had no significant effects on NuMA localization or levels (Fig. 7A, B), suggesting that NuMA is unlikely to be an effector protein for Rab5a in oocytes.
Figure 7.
Loss of Rab5a in oocytes has no effect on NuMA localization and levels. A) Control and Rab5a-MO oocytes were labeled with anti-NuMA antibody (green) and counterstained for chromosomes (red). Arrowheads indicate NuMA localization. Representative images of GV and MI oocytes are shown. Scale bar = 25 μm. B) Quantification of NuMA staining in control and Rab5a-MO MI oocytes. At least 50 oocytes were analyzed for each experiment. C) Double staining of GV oocytes with anti-NuMA and anti-Rab5a antibodies. Error bars indicate ± sd.
Pronounced CENPF reduction in oocytes depleted of Rab5a
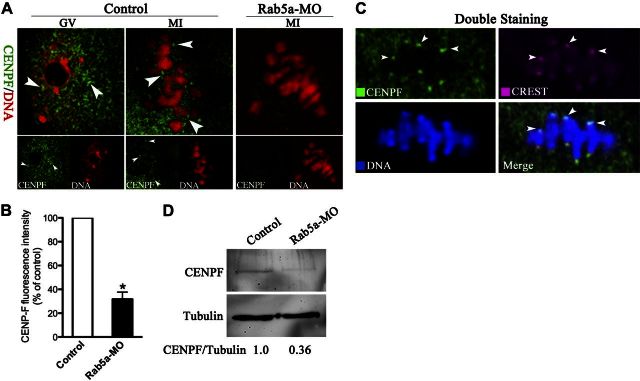
CENPF is a component of the nuclear matrix in G2 phase and has been reported to be required for chromosome alignment and stable microtubule attachments at kinetochores in mitotic cells (23, 26, 30). Nonetheless, its function during meiosis remains unknown. To test whether the effect of Rab5a depletion on spindles and chromosomes may be mediated through CENPF, we first examined the localization/levels of endogenous CENPF by immunofluorescence. As indicated in Fig. 8A (arrowheads), CENPF puncta were uniformly distributed in GV and then predominantly translocated to the kinetochores in metaphase of control oocytes, consistent with a function for CENPF in chromosome movement. Double staining was also performed in metaphase oocytes and confirmed that CENPF puncta colocalize with kinetochores, indicated by arrowheads (Fig. 8C). Notably, following Rab5a knockdown, localization of CENPF to kinetochores was severely reduced and total CENPF was significantly decreased, based on immunostaining and Western blotting respectively (Fig. 8A, B, D). These data suggested that CENPF localization/levels at kinetochores during oocyte meiosis depend on Rab5a.
Figure 8.
Rab5a Depletion reduces the CENPF localization/levels on kinetochores in oocytes. A) Control and Rab5a-MO oocytes were labeled with anti-CENPF antibody (green) and counterstained for chromosomes (red). Representative images of GV and MI oocytes are shown; arrowheads indicate CENPF localization. B) Quantification of CENPF staining in control and Rab5a-MO oocytes; 30 oocytes/group were analyzed. C) Double staining of metaphase oocytes with CREST antibody (red) and CENPF antibody (green), and counterstaining of chromosome with Hoechst 33342 (blue), confirming the CENPF puncta colocalization with kinetochores (arrowheads). D) Western blot showing the dramatically reduced CENPF protein levels in oocytes following Rab5a knockdown, with tubulin as a loading control. Bars represent means ± sd. *P < 0.05 vs. controls.
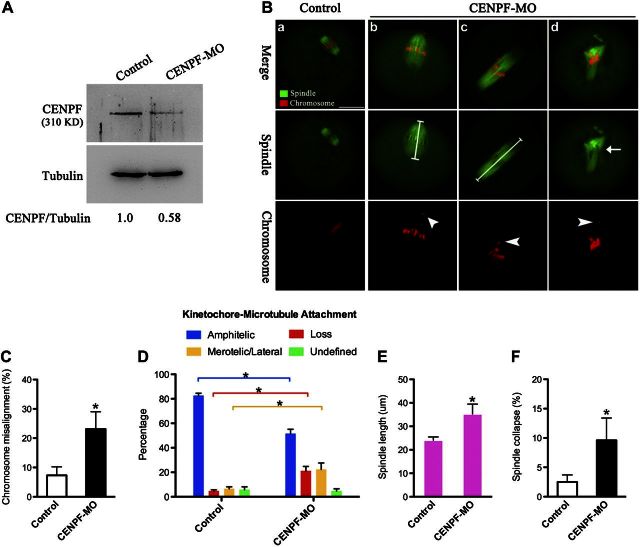
CENPF knockdown phenocopies the spindle/chromosome defects resulting from Rab5a depletion
Given the drastic effects of Rab5a depletion on CENPF localization/levels, we reasoned that functional ablation of CENPF may recapitulate at least some of the meiotic defects observed in Rab5a-MO oocytes. To investigate this possibility, fully grown oocytes were microinjected with CENPF-targeting morpholino (CENPF-MO) and then stained with anti-tubulin antibody to evaluate spindle and counterstained with PI for chromosomes. Endogenous levels of CENPF were partially reduced as evidenced by Western blot (Fig. 9A). Consistent with the hypothesis, CENPF-MO oocytes displayed similar phenotypes (Fig. 9B–F). Chromosome misalignment was readily observed in oocytes depleted of CENPF (23.1±5.9 vs. 7.3±2.9% control, P<0.05; arrowheads). CENPF knockdown elevated the proportion of K-MT misattachments in oocytes, and the frequency of elongated spindles and disorganized spindles was also markedly increased, in comparison to control oocytes (length: 35.3±4.2 vs. 24.1±1.4 μm control, P< 0.05; collapse: 9.6±3.8 vs. 2.5±1.2% control, P<0.05). In addition, to test whether Rab5a and CENPF might function in a parallel or in a linear pathway, simultaneous depletion of Rab5a and CENPF in oocytes was conducted. We found that the aberrant meiotic phenotypes of the single knockdowns were not exacerbated in the double knockdown oocytes (data not shown), consistent with Rab5a and CENPF operating in the same pathway. Together, these observations suggested that CENPF is a major, if not unique, target mediating the action of Rab5a on meiotic apparatus during oocyte maturation.
Figure 9.
CENPF knockdown phenocopies the spindle/chromosome defects resulting from Rab5a depletion. A) Reduced CENPF levels after CENPF-MO injection were confirmed by Western blot. Tubulin served as a loading control. Band intensity was calculated using ImageJ; ratio of CENPF/tubulin expression was normalized, and values are indicated. B) Control and CENPF-MO oocytes were stained with α-tubulin antibody to visualize the spindle (green) and counterstained with PI to observe chromosomes (red). Representative examples of spindle and chromosomes in control MII oocytes (a) and CENPF-MO oocytes (b–d). Arrow indicates the abnormal spindle; arrowheads indicate misaligned chromosomes. Scale bar = 25 μm. C) Quantification of control (n=110) and CENPF-MO (n=100) oocytes with chromosome misalignment. D) Quantitative analysis of K-MT attachments in oocytes as indicated (10 control oocytes and 10 CENPF-MO oocytes). E) Quantitative analysis of spindle length in control (n=45) and CENPF-MO (n=50) oocytes. F) Quantification of control (n=110) and CENPF-MO (n=100) oocytes with spindle collapse. Data are expressed as mean ± sd percentage. *P < 0.05 vs. controls.
DISCUSSION
The cytoplasm of a typical eukaryotic cell is populated with a variety of membranous organelles, and a vast array of molecules traffic between these organelles by vesicular transport. Rab GTPases are key regulatory factors that impinge on vesicle identity, coating, motility, and fusion, etc. (3, 31). In the present study, we showed that MO-mediated Rab5a ablation led to the pronounced loss of Rab5a-positive vesicles in mouse oocytes (Fig. 2). A high frequency of chromosome misalignment was detected in these oocytes depleted of Rab5a-positive vesicles (Fig. 3), as described in mitotic cells (13, 14). Furthermore, we provide evidence that such meiotic defects could dramatically increase aneuploidy in eggs (Fig. 4). Attachment between spindle microtubules and chromosomes depends on the kinetochore. The kinetochore is a hierarchical protein assembly of nearly 100 proteins that links centromeric DNA to spindle microtubules and thereby couples forces generated by microtubule dynamics to power chromosome movement. The interplay between kinetochores and microtubules leads to the establishment of biorientation (32, 33). Defects in chromosome alignment may therefore arise from impairment of chromosome attachment to kinetochores. In support of this notion, the K-MT attachment errors were readily observed in metaphase oocytes depleted of Rab5a (Fig. 5). These observations collectively suggest that loss of Rab5a-positive vesicles could disrupt the K-MT attachment, and thereby contribute to the chromosome congress failure in oocytes. So far, the mechanisms by which spindle length is restrained in mammalian oocytes remain poorly understood (34). Here we identified that, in addition to the chromosome misalignment, oocytes depleted of either Rab5a or CENPF presented a novel phenotype: spindle elongation (Fig. 3). This finding indicates that Rab5a and CENPF act in a process that functions in spindle length control in oocytes. Also noteworthy is that Rab11a-positive vesicles have recently been identified as a cytoskeletal regulator to affect the asymmetric spindle positioning during mouse oocyte maturation (7). All these findings support the concept that vesicle-based pathways play important roles in modulating the meiotic apparatus of oocytes, which deserve further study.
Based on the literature (13, 14, 23–28), we reasoned that NuMA and/or CENPF are likely to be major targets mediating the effects of Rab5a-positive vesicles on spindle/chromosomes in oocytes. However, Rab5a depletion had little effects on NuMA distribution/levels (Fig. 7). Instead, we discovered that loss of Rab5a caused considerable reduction of CENPF localization/levels at kinetochores in meiotic oocytes (Fig. 8). Previous work has shown that CENPF is a large coiled-coil protein that transiently localizes to the outer kinetochore (32) and is required for stable microtubule capture at kinetochores in mitotic cells (26, 30, 35). However, its function in meiosis was unknown. Here we showed that CENPF knockdown results in spindle/chromosome defects and K-MT misattachments in oocytes, similar to the phenotypes of Rab5a knockdown oocytes (Fig. 9), suggesting that CENPF is a critical target through which Rab5a-positive vesicles exert their effects on oocyte meiosis. We cannot rule out that other Rab5a targets also may mediate this process. As reported in other systems (13, 14, 36), we also found that Rab5a-positive vesicles influence nuclear lamina disassembly during oocyte maturation. Since CENPF is known to be a nuclear matrix protein, impairment of lamina disassembly during oocyte meiosis perhaps interferes with the release and subsequent translocation of CENPF to kinetochores or leads to destabilization of CENPF. Due to the limitation of oocyte number and technical reason, we have not yet been able to directly analyze the dynamic interaction between nuclear envelope disassembly and CENPF release in mouse oocytes. In summary, our data support a model where Rab5a-positive vesicles, likely through interaction with nuclear lamina, promote CENPF localization/levels at kinetochores, consequently ensuring proper spindle morphology and K-MT attachment in meiotic oocytes.
Oocyte quality is a critical element dictating the fertility of a female. This study reveals Rab5a-positive vesicles as cytoskeletal regulators that are required for meiotic spindle morphology and meiotic chromosome alignment in oocytes. As any errors in this process result in pregnancy loss and infertility, this vesicle-based pathway is not only of fundamental scientific interest but also vital for mammalian reproduction.
Acknowledgments
The authors thank Dong Zhang (Nanjing Medical University) for technical assistance.
This work was supported by National Key Scientific Research Projects (2014CB943200), National Natural Science Foundation (31301181), and Natural Science Foundation of the Jiangsu Higher Education Institutions (13KJA310001) of China.
The authors declare no conflicts of interest.
Footnotes
- CENPF
- centromere protein F
- GV
- germinal vesicle
- GVBD
- germinal vesicle breakdown
- K-MT
- kinetochore-microtubule
- MI
- metaphase I
- MII
- metaphase II
- MO
- morpholino
- Pb1
- first polar body
- PI
- propidium iodide
- PMSG
- pregnant mare serum gonadotropin
REFERENCES
- 1. Krisher R. L. (2004) The effect of oocyte quality on development. J. Animal Sci. 82(E-Suppl.), E14–E23 [DOI] [PubMed] [Google Scholar]
- 2. Hassold T., Hunt P. (2001) To err (meiotically) is human: the genesis of human aneuploidy. Nat. Rev. Genet. 2, 280–291 [DOI] [PubMed] [Google Scholar]
- 3. Barr F. A. (2013) Review series: Rab GTPases and membrane identity: causal or inconsequential? J. Cell Biol. 202, 191–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stenmark H. (2009) Rab GTPases as coordinators of vesicle traffic. Nat. Rev. Mol. Cell Biol. 10, 513–525 [DOI] [PubMed] [Google Scholar]
- 5. Liu Z., Zheng Y. (2009) A requirement for epsin in mitotic membrane and spindle organization. J. Cell Biol. 186, 473–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Miserey-Lenkei S., Couedel-Courteille A., Del Nery E., Bardin S., Piel M., Racine V., Sibarita J. B., Perez F., Bornens M., Goud B. (2006) A role for the Rab6A' GTPase in the inactivation of the Mad2-spindle checkpoint. EMBO J. 25, 278–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Holubcova Z., Howard G., Schuh M. (2013) Vesicles modulate an actin network for asymmetric spindle positioning. Nat. Cell Biol. 15, 937–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bucci C., Lutcke A., Steele-Mortimer O., Olkkonen V. M., Dupree P., Chiariello M., Bruni C. B., Simons K., Zerial M. (1995) Co-operative regulation of endocytosis by three Rab5 isoforms. FEBS Lett. 366, 65–71 [DOI] [PubMed] [Google Scholar]
- 9. Horiuchi H., Lippe R., McBride H. M., Rubino M., Woodman P., Stenmark H., Rybin V., Wilm M., Ashman K., Mann M., Zerial M. (1997) A novel Rab5 GDP/GTP exchange factor complexed to Rabaptin-5 links nucleotide exchange to effector recruitment and function. Cell 90, 1149–1159 [DOI] [PubMed] [Google Scholar]
- 10. Christoforidis S., McBride H. M., Burgoyne R. D., Zerial M. (1999) The Rab5 effector EEA1 is a core component of endosome docking. Nature 397, 621–625 [DOI] [PubMed] [Google Scholar]
- 11. Simonsen A., Gaullier J. M., D'Arrigo A., Stenmark H. (1999) The Rab5 effector EEA1 interacts directly with syntaxin-6. J. Biol. Chem. 274, 28857–28860 [DOI] [PubMed] [Google Scholar]
- 12. Chen P. I., Kong C., Su X., Stahl P. D. (2009) Rab5 isoforms differentially regulate the trafficking and degradation of epidermal growth factor receptors. J. Biol. Chem. 284, 30328–30338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Capalbo L., D'Avino P. P., Archambault V., Glover D. M. (2011) Rab5 GTPase controls chromosome alignment through lamin disassembly and relocation of the NuMA-like protein Mud to the poles during mitosis. Proc. Natl. Acad. Sci. U. S. A. 108, 17343–17348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Serio G., Margaria V., Jensen S., Oldani A., Bartek J., Bussolino F., Lanzetti L. (2011) Small GTPase Rab5 participates in chromosome congression and regulates localization of the centromere-associated protein CENP-F to kinetochores. Proc. Natl. Acad. Sci. U. S. A. 108, 17337–17342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang D., Ma W., Li Y. H., Hou Y., Li S. W., Meng X. Q., Sun X. F., Sun Q. Y., Wang W. H. (2004) Intra-oocyte localization of MAD2 and its relationship with kinetochores, microtubules, and chromosomes in rat oocytes during meiosis. Biol. Reprod. 71, 740–748 [DOI] [PubMed] [Google Scholar]
- 16. Wang Q., Chi M. M., Moley K. H. (2012) Live imaging reveals the link between decreased glucose uptake in ovarian cumulus cells and impaired oocyte quality in female diabetic mice. Endocrinology 153, 1984–1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fu W., Chen H., Wang G., Luo J., Deng Z., Xin G., Xu N., Guo X., Lei J., Jiang Q., Zhang C. (2013) Self-assembly and sorting of acentrosomal microtubules by TACC3 facilitate kinetochore capture during the mitotic spindle assembly. Proc. Natl. Acad. Sci. U. S. A. 110, 15295–15300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hunt P. A., Koehler K. E., Susiarjo M., Hodges C. A., Ilagan A., Voigt R. C., Thomas S., Thomas B. F., Hassold T. J. (2003) Bisphenol a exposure causes meiotic aneuploidy in the female mouse. Curr. Biol. 13, 546–553 [DOI] [PubMed] [Google Scholar]
- 19. Tanaka T. U., Clayton L., Natsume T. (2013) Three wise centromere functions: see no error, hear no break, speak no delay. EMBO Rep. 14, 1073–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gregan J., Polakova S., Zhang L., Tolic-Norrelykke I. M., Cimini D. (2011) Merotelic kinetochore attachment: causes and effects. Trends Cell Biol. 21, 374–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Galvez T., Gilleron J., Zerial M., O'Sullivan G. A. (2012) SnapShot: mammalian Rab proteins in endocytic trafficking. Cell 151, 234–234 e232 [DOI] [PubMed] [Google Scholar]
- 22. Kawasaki M., Nakayama K., Wakatsuki S. (2005) Membrane recruitment of effector proteins by Arf and Rab GTPases. Curr. Opin. Struct. Biol. 15, 681–689 [DOI] [PubMed] [Google Scholar]
- 23. Liao H., Winkfein R. J., Mack G., Rattner J. B., Yen T. J. (1995) CENP-F is a protein of the nuclear matrix that assembles onto kinetochores at late G2 and is rapidly degraded after mitosis. J. Cell Biol. 130, 507–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Radulescu A. E., Cleveland D. W. (2010) NuMA after 30 years: the matrix revisited. Trends Cell Biol. 20, 214–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Silk A. D., Holland A. J., Cleveland D. W. (2009) Requirements for NuMA in maintenance and establishment of mammalian spindle poles. J. Cell Biol. 184, 677–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Holt S. V., Vergnolle M. A., Hussein D., Wozniak M. J., Allan V. J., Taylor S. S. (2005) Silencing Cenp-F weakens centromeric cohesion, prevents chromosome alignment and activates the spindle checkpoint. J. Cell Sci. 118, 4889–4900 [DOI] [PubMed] [Google Scholar]
- 27. Haren L., Gnadt N., Wright M., Merdes A. (2009) NuMA is required for proper spindle assembly and chromosome alignment in prometaphase. BMC Res. Notes 2, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kolano A., Brunet S., Silk A. D., Cleveland D. W., Verlhac M. H. (2012) Error-prone mammalian female meiosis from silencing the spindle assembly checkpoint without normal interkinetochore tension. Proc. Natl. Acad. Sci. U. S. A. 109, E1858–E1867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yu L. Z., Xiong B., Gao W. X., Wang C. M., Zhong Z. S., Huo L. J., Wang Q., Hou Y., Liu K., Liu X. J., Schatten H., Chen D. Y., Sun Q. Y. (2007) MEK1/2 regulates microtubule organization, spindle pole tethering and asymmetric division during mouse oocyte meiotic maturation. Cell Cycle 6, 330–338 [DOI] [PubMed] [Google Scholar]
- 30. Bomont P., Maddox P., Shah J. V., Desai A. B., Cleveland D. W. (2005) Unstable microtubule capture at kinetochores depleted of the centromere-associated protein CENP-F. EMBO J. 24, 3927–3939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hutagalung A. H., Novick P. J. (2011) Role of Rab GTPases in membrane traffic and cell physiology. Physiol. Rev. 91, 119–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tanaka K. (2013) Regulatory mechanisms of kinetochore-microtubule interaction in mitosis. Cell. Mol. Life Sci. 70, 559–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Foley E. A., Kapoor T. M. (2013) Microtubule attachment and spindle assembly checkpoint signalling at the kinetochore. Nat. Rev. Mol. Cell Biol. 14, 25–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Howe K., FitzHarris G. (2013) Recent insights into spindle function in mammalian oocytes and early embryos. Biol. Reprod. 89, 71. [DOI] [PubMed] [Google Scholar]
- 35. Yang Z., Guo J., Chen Q., Ding C., Du J., Zhu X. (2005) Silencing mitosin induces misaligned chromosomes, premature chromosome decondensation before anaphase onset, and mitotic cell death. Mol. Cell. Biol. 25, 4062–4074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Audhya A., Desai A., Oegema K. (2007) A role for Rab5 in structuring the endoplasmic reticulum. J. Cell Biol. 178, 43–56 [DOI] [PMC free article] [PubMed] [Google Scholar]