Abstract
Cystathionine β-synthase-deficient homocystinuria (HCU) is a serious life-threatening inborn error of sulfur metabolism with poorly understood pathogenic mechanisms. We investigated the effect of HCU on hepatic cysteine oxidation in a transgenic mouse model of the disease. Cysteine dioxygenase (CDO) protein levels were 90% repressed without any change in mRNA levels. Cysteinesulfinic acid decarboxylase (CSAD) was induced at both the mRNA (8-fold) and protein (15-fold) levels. Cysteine supplementation normalized CDO protein levels without reversing the induction of CSAD. Regulatory changes in CDO and CSAD expression were proportional to homocysteine elevation, indicating a possible threshold effect. Hepatic and blood taurine levels in HCU animals were decreased by 21 and 35%, respectively, and normalized by cysteine supplementation. Expression of the cytoplasmic (GOT1) and mitochondrial (GOT2) isoforms of glutamic-oxaloacetic transaminase were repressed in HCU animals by 86 and 30%, respectively. HCU induced regulatory changes in CSAD, CDO, and GOT1 expression were normalized by taurine supplementation, indicating that cysteine is not the only sulfur compound that regulates hepatic cysteine oxidation. Collectively, our results indicate that HCU induces significant alterations of sulfur metabolism with the potential to contribute to pathogenesis and that cysteine and taurine have the potential to serve as adjunctive treatments in this disease.—Jiang, H., Stabler, S. P., Allen, R. H., Abman, S. H., Maclean, K. N. Altered hepatic sulfur metabolism in cystathionine β-synthase-deficient homocystinuria: regulatory role of taurine on competing cysteine oxidation pathways.
Keywords: glutamic-oxaloacetic transaminase, thiol metabolism, transsulfuration, cysteinesulfinate decarboxylase
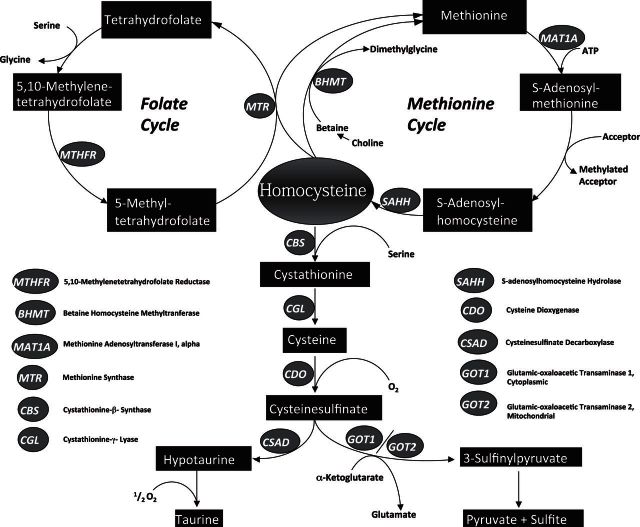
Cystathionine β-synthase [CBS; l-serine hydrolyase (adding homocysteine), EC 4.2.1.22] is localized at a key regulatory branch point in eukaryotic sulfur amino acid metabolism. CBS catalyzes a pyridoxal 5′-phosphate (PLP)-dependent β-replacement reaction that condenses homocysteine (Hcy) and serine to form cystathionine, which is subsequently converted to cysteine (Cys) by cystathionine γ-lyase (CGL; EC 4.4.1.1). The transsulfuration pathway thus serves as a catabolic mechanism for methionine (Met) and Hcy, and Cys serves as the vehicle for conversion of sulfur to the end products taurine or sulfate. Excess Cys is toxic, and this compound is catabolized by multiple alternative desulfuration reactions catalyzed by CBS and/or CGL that generate either hydrogen sulfide or sulfane sulfur, both of which are subsequently oxidized to sulfate. Alternatively, Cys can be catabolized by two alternate pathways that both require the initial oxidation of Cys to cysteinesulfinate in a reaction catalyzed by Cys dioxygenase (CDO; EC1.13.11.20). CDO thus serves as a critical regulator of tissue Cys levels and controls the relative flux between competing Cys catabolic pathways (1). The cysteinesulfinate generated by CDO is then either converted to hypotaurine in a reaction catalyzed by cysteinesulfinic acid decarboxylase (CSAD; EC4.1.1.29) or is transaminated into β-sulfinylpyruvate via the action of glutamic-oxaloacetic transaminase (GOT; EC 2.6.1.1). This latter enzyme exists in cytoplasmic (GOT1) and mitochondrial (GOT2) isoforms. Hypotaurine is subsequently converted to taurine, while β-sulfinylpyruvate subsequently dissociates to form sulfur dioxide and pyruvate. Sulfur dioxide is then hydrated to sulfite and subsequently oxidized to sulfate by sulfite oxidase. Figure 1 illustrates the metabolic relationships among Hcy, Cys, and related metabolites relevant to this report.
Figure 1.
Met and Cys metabolism in mammals. The transsulfuration-cysteine oxidation pathways and Met/folate cycle are shown.
Inactivating mutations in CBS result in classical homocystinuria (HCU), which, if untreated, typically results in cognitive impairment; a range of connective tissue disturbances, including Marfanoid skeletal abnormalities; osteoporosis; ectopia lentis; and a dramatically increased incidence of vascular disorders, including atherosclerosis and thromboembolic disease. Multiple reports have implicated oxidative stress as a major contributor to pathogenesis in HCU, and many treatment centers include dietary supplementation with Cys disulfide as an additional treatment for HCU (2–4).
To date, the vast majority of research on pathogenesis in HCU has focused primarily on the role of Hcy. With regard to metabolites distal to the block, HCU is accompanied by the complete abolition of cystathionine synthesis and significantly decreased levels of plasma and hepatic Cys and glutathione (5–7). All genetic forms of homocystinuria share a predisposition toward cardiovascular complications, but the occurrence of ectopia lentis is unique to HCU and is a common feature in sulfocysteinuria due to sulfite oxidase deficiency, which indicates a possible pathogenic contribution due to dysregulation of distal sulfur metabolism. We have recently shown that cystathionine confers significant protective effects against endoplasmic reticulum stress and Hcy induced hepatic and renal injury (8), but to date, the effect of HCU on Cys oxidation is uninvestigated. In light of the role of CBS in both Cys synthesis and desulfuration, it is clear that inactivation of this enzyme in HCU has the potential to significantly alter oxidative Cys catabolism and taurine synthesis in such a manner as to contribute to pathogenesis. In this paper, we investigate the effect of HCU in the presence and absence of Cys and taurine on the cysteinesulfinate–dependent Cys oxidation pathways. We observed that HCU results in significantly decreased hepatic and blood taurine levels and induces significant alterations in both pathways of Cys oxidation that are not solely due to decreased levels of Cys availability. In addition, we report that exogenous taurine exerts previously unsuspected regulatory effects on CDO, CSAD, and GOT1 expression levels in HCU. Collectively, our results indicate that HCU induces significant alterations of sulfur metabolism with the potential to contribute to pathogenesis and that Cys and taurine supplementation has the potential to serve as a useful adjunctive treatment in this disease.
MATERIALS AND METHODS
Chemicals and reagents
Unless otherwise stated, all chemicals were obtained from Sigma-Aldrich (St Louis, MO, USA). Antibodies for CDO (ab53436) and CSAD (ab91016) were obtained from Abcam (Cambridge, MA, USA). GOT1 (AAS05482C) and GOT2 (AAS17435C) antibodies were obtained from Antibody Verify (Las Vegas, NV, USA). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH; A300-641A) antibody was obtained from Bethyl Laboratories (Montgomery, TX, USA).
Animal experiments, diet, and treatments
Male and Female HO mice (3–5 mo old) were generated as described previously (7). Male and female C57BL/6J mice (3–6 mo old) were used as wild-type (WT) controls and were bred in house. Mice were housed in individual cages on a 12 h light-dark cycle at a mean temperature of 22°C. All mice were maintained on standard chow (LabDietNIH5K67, PMI Nutrition International, Brentwood, MO, USA). Cys and taurine supplementation were performed by the addition of 1.5 and 20 mg/ml, respectively, of these compounds in drinking water, and treatment water was replenished 2×/wk. A paired-feeding design was used to ensure isocaloric intake among all experimental groups, and body weights were measured daily. No significant difference in body weight was found among mice in any of the experimental groups. All experiments were approved by the University of Colorado Health Sciences Center institutional animal care and use committee and were performed according to the U.S. National Institutes of Health (NIH) standards for animal care and use.
Genotype determinations
Mouse genotypes were determined initially by PCR analysis of genomic DNA obtained from tail snips as described previously (7). The genotypes of all animals used in this study were confirmed by determination of total Hcy (tHcy) levels in plasma samples obtained by nonlethal tail bleeding.
Measurement of CDO and CSAD mRNA
RNA was isolated using an RNeasy mini kit (Qiagen, Valencia, CA, USA) according to the manufacturer's standard protocol. Extracted RNA (200 ng) was treated with RNase-free DNase (Ambion, Grand Island, NY, USA,) and reverse-transcribed using random hexamers (Applied Biosystems, Carlsbad, CA, USA). Primers for CDO were (5′-3′) GATTCTGTGCTGGGGTGAA and (5′-3′) CAGTGGGAGTCCGTGTGAT. CSAD primer sequences were 5′-GGGACTTGGCACCGACAGT-3′ and 5′-GGGATCATCCTCCCTCTCTCA-3′. Real-time quantitative reverse transcriptase PCR (qRT-PCR) was performed using cDNA samples diluted 1:4, and 1 μl was used in each 20 μl qRT-PCR reaction and SYBR Green PCR Master Mix (Applied Biosystems). Transcript levels were analyzed on a Light Cycler 480 System II (Roche, Basel, Switzerland) over 40 cycles of 95°C for 10 s, 60°C for 10 s, and 72°C for 15 s, preceded by an initial 5 min step at 95°C. GAPDH was used as the normalizing endogenous control gene to standardize qRT-PCR data. All real-time qRT-PCR data were generated using RNA isolated from tissues of individual animals (n=8/group).
Measurement of thiols, taurine, and Met cycle metabolites in liver and plasma
Determination of plasma levels of tHcy and total Cys (tCys) was performed as described previously (9).
Taurine levels in liver extracts and whole blood were determined by HPLC using a Shodex Ionpak C-811 adsorption-distribution column (Showa-Denko America Inc., New York, NY, USA) combined with 3 mm perchloric acid as the mobile phase. The eluted taurine was derivatized with o-phthalaldehyde-2-mercaptoethanol reagent and measured at an absorbance of 350 nm as described previously (10).
Preparation of liver homogenates for Western blotting
Liver samples were homogenized in buffer containing 100 mM KPi, pH 7.4; 1 mM EDTA; and 1:50 (v/v) protease inhibitor cocktail from Sigma. The ratio of liver tissue to lysis buffer was 1 g of liver tissue to 5 ml of lysis buffer. The homogenate was subsequently centrifuged at 4°C at 20,000 g for 20 min, and the supernatant thus formed was used as a crude extract. The protein concentration of crude extracts was determined by the Bradford method, using bovine serum albumin as a standard (11).
SDS-PAGE and Western blotting analysis
Immunoblot analysis of total liver lysates was performed as described previously (12). CDO, CSAD, GOT1, and GOT2 antibodies were used at a dilution of 1:2000, while GAPDH was used at a 1:5000 dilution (v/v) as a loading control. Signals were detected using a Typhoon 9400 system (Amersham Pharmacia, Piscataway, NJ, USA) after incubation with appropriate fluorescein- or Texas red-conjugated secondary antibodies (Vector Laboratories, Burlingame, CA, USA) or Alexa Fluor 647-conjugated secondary antibody (Invitrogen, Grand Island, NY, USA) at 1:2500 (v/v). The relative intensities of protein bands were quantified by Quantity One 4.6.5 software (Bio-Rad, Hercules, CA, USA). Signal intensities from target protein bands were calculated relative to signal intensities GAPDH in liver homogenates.
Statistical analysis
All data are presented as means ± sd and were compared using the unpaired Student's t test. A value of P < 0.05 was considered statistically significant. Taurine and Cys supplementation data were analyzed by ANOVA.
RESULTS
Cys dioxygenase expression is repressed in HO HCU mice
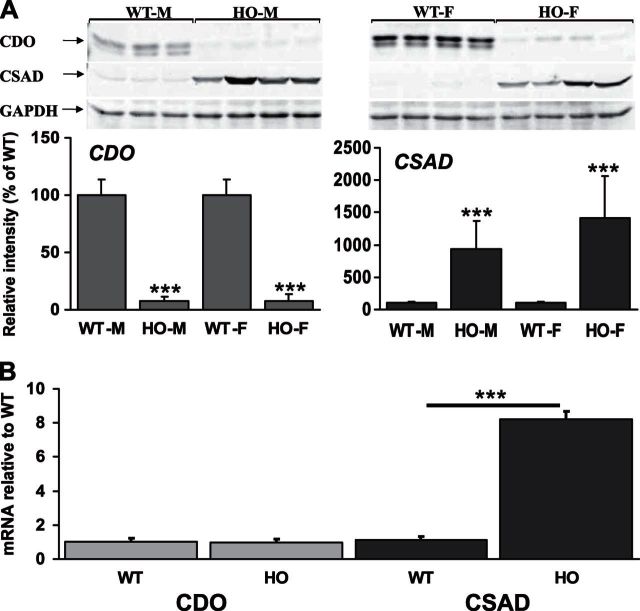
A critical determinant of the relative flux between desulfuration and oxidation of Cys is the initial oxidation of the Cys thiol by CDO to form cysteinesulfinate (also known as 3-sulfinoalanine; Fig. 1). This compound is then further processed by two alternative pathways to form either taurine or sulfate. Excess Cys is toxic, and previous work has shown that CDO is tightly regulated by multiple mechanisms to keep Cys levels within a relatively narrow range (1). To investigate the effects of HCU on CDO expression, we performed Western blotting analysis of hepatic CDO protein levels in WT control mice (n=16, tHcy 3.7 ± 0.5 μM, plasma tCys 222 ± 8 μM) and HO HCU (n=16, tHcy 338.4 ± 33.8 μM, tCys 89 ± 3 μM) mice of both genders (n=8/group). No significant difference in either tHcy or tCys was observed between male and female mice in either WT or HO mice. We observed a 90% decrease in hepatic CDO protein levels in both male and female HO HCU mice compared to WT controls (P<0.0001; Fig. 2A).
Figure 2.
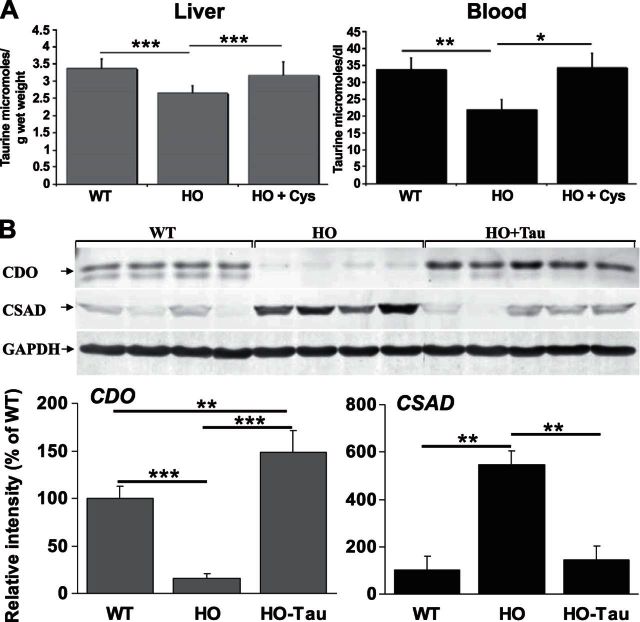
Hepatic expression of CDO is repressed and CSAD is induced in HO HCU mice. A) Western blotting analysis of hepatic CDO and CSAD expression levels in HO HCU mice. In this analysis and all Western blots shown in subsequent figures, the relative intensities of protein bands were quantified using Quantity One 4.6.5 software (Bio-Rad). Signal intensities from CDO and CSAD bands were calculated relative to GAPDH signal intensities. All blots shown are representative of ≥2 independent experiments, and blotting and immunostaining were performed as described in Materials and Methods. B) qRT-PCR analysis of hepatic CDO and CSAD mRNA levels in WT and HO mice. For each experimental sample, the relative abundance value was normalized to the value derived from the endogenous GAPDH control, and expression levels are shown as the relative fold-change compared to the WT control samples. Values are means ± sd of experiments performed in triplicate; 8 animals/group. ***P < 0.001.
An additional layer of post-translational regulation of CDO in response to intracellular Cys concentration occurs via substrate-turnover-dependent formation of a thioether cross-link between the sulfur of residue Cys93 and the aromatic side chain of residue Tyr157. The cross-linking of these two residues gives rise to an active-site protein derived cofactor that enhances the catalytic efficiency of CDO (13, 14). This mature activated form migrates faster than the non-cross-linked form and is responsible for the lower CDO band shown in Fig. 2. Although clearly visible in both male and female WT mice, this mature catalytically activated form of CDO was almost undetectable in both male and female HO mice.
Cysteinesulfinic acid decarboxylase is induced in HO HCU mice
Once cysteinesulfinate is formed by the action of CDO, the predominant mechanism for its catabolism is decarboxylation to hypotaurine by CSAD. The hypotaurine (2-aminoethanesulfinate) thus formed is subsequently oxidized to taurine (2-aminoethanesulfonate), although whether this latter step occurs spontaneously or is catalyzed is presently unknown. To further investigate the effect of HCU on oxidative Cys catabolism, we used Western blotting analysis of the mice described above to assess CSAD protein levels in WT and HO mice (Fig. 2A). We observed that HCU is accompanied by a 10- and 15-fold induction in hepatic CSAD expression in male and female HO mice, respectively (P=0.026 and 0.0069, respectively).
HCU-induced regulatory changes in CSAD but not CDO occur at the transcriptional level
Analysis of hepatic CDO mRNA levels by qRT-PCR indicated that the mechanism for repression of hepatic CDO expression was not transcriptional as the abundance of CDO mRNA in both male and female HO mice was indistinguishable from WT control mice (Fig. 2B). Analysis of CSAD mRNA levels by the same method detected an ∼8-fold induction of CSAD mRNA levels in both male and female HO mouse livers compared to WT controls (P<0.001, n=8/group; Fig. 2B).
Regulation of CDO and CSAD in HCU is not solely due to changes in Cys levels
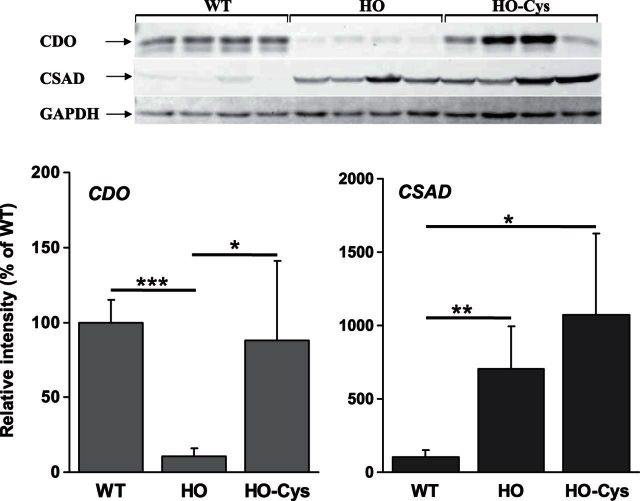
To date, all available evidence on the regulation of CDO indicates a primary role for Cys and HO mice with high levels of tHcy have previously been shown to exhibit significantly decreased levels of plasma and liver Cys (7). To investigate the role of decreased Cys on CDO and CSAD expression in HCU, we examined the expression levels of these enzymes in WT and HO mice in the presence and absence of 1 wk of Cys supplementation as described in Materials and Methods. In this analysis, CDO was again ∼90% repressed in untreated HO mice (Fig. 3). Cys supplementation restored CDO expression to a level that did not differ significantly from the WT controls. In the same animals, CSAD expression was again significantly induced in the untreated animals. Cys supplementation did not reverse this induction, and CSAD levels in HO mice thus treated were ∼10-fold higher than the WT controls.
Figure 3.
Hepatic expression of CDO but not CSAD is normalized by Cys supplementation. Western blotting analysis of hepatic CDO and CSAD expression levels in HO HCU mice in the presence and absence of 1 wk of Cys treatment. Figure represents analysis for female mice, an identical regulatory pattern was also observed in male mice. *P < 0.05, **P < 0.01, ***P < 0.001.
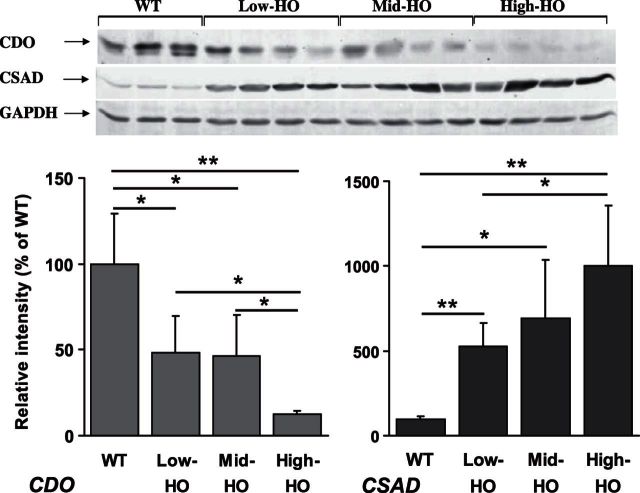
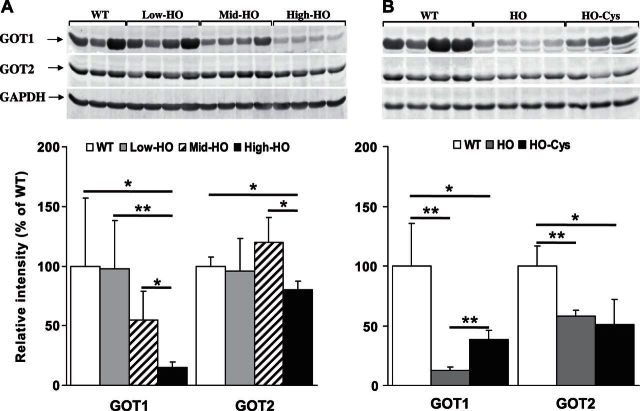
Previous work in our laboratory has identified HO mice with naturally occurring variance in the expression level of the human CBS transgene, resulting in different levels of elevated tHcy (12). These mice designated either low tHcy HO (tHcy 56.75 ± 27.6 μM, tCys 217.33 ± 14.5 μM, n=4/gender) or medium tHcy HO (tHcy 160 23 ± 7 μM, tCys 92.2 ± 15.4, n=4/gender) were assessed in combination with the mice described above (designated high tHcy HO) for CDO and CSAD expression levels (Fig. 4). In this analysis, we observed that the repression of CDO and induction of CSAD were proportional to the degree of tHcy elevation. Interestingly, in the low-tHcy HO mice where the plasma tHcy is 10-fold elevated but Cys levels do not differ significantly from the WT mice (P=0.4506), we still observed a statistically significant 50% repression of CDO expression (P=0.041) and 5-fold induction of CSAD expression (P=0.0032). Similarly, CDO expression drops off significantly in the high-tHcy HO mice compared to the medium-tHcy HO mice despite no significant difference in their respective plasma tCys levels (P=0.4712). Collectively, these observations are consistent with the possibility that both CDO and CSAD expression in HCU may be susceptible to the regulatory influence of sulfur compounds other than Cys.
Figure 4.
Degree of hepatic CDO repression and induction of CSAD is proportional to the level of tHcy. Western blotting analysis of hepatic CDO and CSAD expression levels in HO HCU mice with natural variance of Hcy. Low-tHcy HO: tHcy 56.75 ± 27.6 μM, tCys 217.33 ± 14.5 μM; medium-tHcy HO: tHcy 160 ± 23.7 μM, tCys 92.2 ± 15.4 μM; high-tHcy HO: tHcy 338.4 ± 33.8 μM, tCys 89.25 ± 3.8 μM. Figure represents analysis for female mice; an identical regulatory pattern was also observed in male mice. Values are means ± sd. *P < 0.05, **P < 0.01.
Hepatic and plasma taurine levels are decreased in HCU and are restored by Cys supplementation
Given the strong induction of CSAD in the HO HCU mice and the role of that enzyme in the endogenous synthesis of taurine, we determined the effect of HCU on whole blood and hepatic taurine levels in high-tHcy HO mice as described in Materials and Methods. As Cys supplementation restored normal levels of hepatic CDO expression in high-tHcy HO mice, we also investigated the effect of this treatment on liver and blood taurine levels. We observed an approximate 35% (P=0.0003, n=8) and 21% (P=0.0018, n=8) decrease in whole-blood and hepatic taurine levels, respectively, in high-tHcy HO mice compared to WT control animals (Fig. 5A). Taurine levels in the livers and whole blood of high-tHcy HO mice treated with Cys (n=8) did not differ significantly from those observed in the WT control animals (P>0.05 for both).
Figure 5.
A) Hepatic and blood levels of taurine are significantly decreased in HCU and restored by Cys supplementation. Hepatic and blood levels were determined in male WT and high-tHcy HO mice in the presence and absence of Cys supplementation as described in Materials and Methods. Values represent means ± sd derived from 8 animals/group. B) Taurine supplementation increases hepatic CDO protein levels and represses CSAD expression in HO HCU mice. Western blotting analysis of hepatic CDO and CSAD expression levels in high-tHcy HO HCU mice in the presence and absence of 1 wk of taurine treatment. Blot represents analysis for female mice; an identical regulatory pattern was also observed in male mice. *P < 0.05, **P < 0.01, ***P < 0.001.
Taurine supplementation induces hepatic CDO expression and represses hepatic CSAD
As blood and liver taurine levels are significantly decreased in our model of HCU, it is conceivable that the induction of CSAD represents an unsuccessful homeostatic mechanism attempting to restore taurine levels. To test this hypothesis, we treated 6 male high-tHcy HO mice (tHcy 318 ± 13.6 μM, tCys 103.5 ± 15.3 μM) with taurine for 1 wk as described in Materials and Methods and compared hepatic CDO and CSAD protein levels to a cohort of untreated male high-tHcy-HO mice (tHcy 294 ± 27.6 μM, tCys 111.6 ± 17.9 μM, n=6) and the WT control mice described above. We observed that taurine treatment induced a striking reversal of the repression of CDO expression and induced expression of this enzyme by 15-fold compared to untreated HO mice to a level that was significantly higher than that observed in the WT control group (Fig. 5B).
In contrast to the effect of Cys, taurine supplementation completely normalized CSAD expression relative to the WT controls (Fig. 5B). The regulatory changes in CDO and CSAD expression induced in HO mice by taurine supplementation occurred without any significant alteration in plasma tCys levels between the two groups of HO HCU mice and further support the possibility that sulfur compounds other than Cys can play a regulatory role in the regulation of hepatic Cys oxidation in HCU.
Expression of cytoplasmic and mitochondrial isoforms of GOT are differentially repressed in HO HCU mice
In an alternative Cys oxidation pathway to that catalyzed by CSAD, cysteinesulfinate is transaminated with α-ketoglutarate to form the enzyme-bound keto acid β-sulfinylpyruvate. This compound dissociates to form sulfur dioxide and pyruvate. Sulfur dioxide is then hydrated to sulfite and subsequently oxidized to sulfate by sulfite oxidase (1). The transamination of cysteinesulfinate is catalyzed by the pyridoxal 5′-phosphate-dependent enzyme GOT, (also known as aspartate transaminase), which exists in cytoplasmic (GOT1) and mitochondrial (GOT2) isoforms that are encoded by separate genes (15). To our knowledge, no previous investigation has focused on the hepatic expression levels of GOT1 and GOT2 in HCU. To further investigate the influence of HCU on hepatic Cys oxidation, we assessed the relative hepatic expression levels of GOT1 and GOT2 in the WT control mice and the low-, medium-, and high-tHcy HO mice described above (Fig. 6A). We observed a significant 86% repression of GOT1 expression in the high-tHcy HO mice compared to WT controls (P=0.028). No significant decrease was found in hepatic GOT1 expression in either the medium- or low-tHcy HO mice compared to WT control mice, but a significant decrease was found between medium- and high-tHcy HO mice (P=0.018), which indicates a possible threshold effect. The hepatic expression levels of GOT2 were also only significantly repressed in the high-tHcy HO animals (P=0.016), but the degree of repression at ∼30% of the WT control was considerably more subtle than that observed for GOT1.
Figure 6.
A) Hepatic expression of both cytoplasmic and mitochondrial isoforms of glutamic-oxaloacetic transaminase are repressed in high-tHcy HO HCU mice. Western blotting analysis of hepatic GOT1 and GOT2 expression levels in HO HCU mice with natural variance of tHcy as described in Fig. 4. Figure represents analysis for female mice; an identical regulatory pattern was also observed in male mice. B) Cys supplementation alleviates the repression of GOT1 but not GOT2 in HO HCU mice. Western blotting analysis of hepatic GOT1 and GOT2 expression levels in high-tHcy HO HCU mice in the presence and absence of 1 wk of Cys treatment. Figure represents analysis for female mice; essentially identical results were observed in male mice. *P < 0.05, **P < 0.01.
Cys supplementation alleviates the repression of GOT1 but not GOT2 in high-tHcy HO HCU mice
The results described in the previous section are consistent with the possibility that expression levels of GOT1 and GOT2 show differential sensitivity to the effects of decreased Cys levels in HCU. To investigate this possibility, we analyzed hepatic GOT1 and GOT2 expression levels in the WT, high-tHcy HO, and high-tHcy HO mice supplemented with Cys described above. We observed that Cys supplementation resulted in a highly significant 3-fold increase in hepatic GOT1 expression in high-tHcy HO mice but did not induce any significant change in GOT2 protein levels (Fig. 6B). Although GOT1 expression was increased by Cys supplementation, the hepatic levels of this enzyme were still significantly lower than those observed in WT control animals. Collectively, these results indicate differential regulation of GOT1 and GOT2.
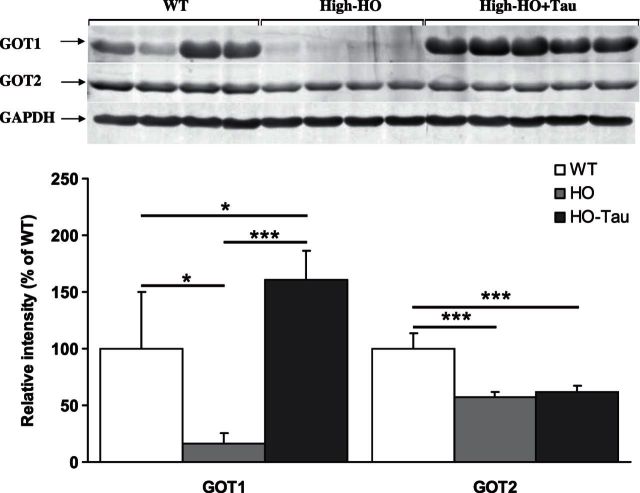
GOT1 but not GOT2 is significantly induced by taurine treatment
To date, no investigation has focused on the possible effects of taurine on GOT1 and GOT2 expression in HCU. The profound effects of taurine on CDO and CSAD hepatic expression described above raise the question as to whether these effects are specific to Cys oxidation directed toward taurine synthesis or represent a more general regulatory mechanism for Cys oxidation. To address this question, we assessed GOT1 and GOT2 hepatic expression levels in WT, high-tHcy HO, and high-tHcy HO mice supplemented with taurine as described above. Taurine supplementation resulted in an ∼10-fold induction in GOT1 expression levels compared to untreated high-tHcy HO mice (P<0.001) and were significantly higher than that observed in the untreated WT control mice (P<0.05) (Fig. 7). Expression levels of the mitochondrial isoform GOT2 were unaffected by taurine supplementation, and GOT2 expression remained significantly reduced in both groups (P<0.001 for both) of HO mice, adding further evidence that the expression of these two isoforms is independently regulated.
Figure 7.
GOT1 but not GOT2 is significantly induced by taurine treatment. Western blotting analysis of hepatic GOT1 and GOT2 expression levels in high-tHcy HO HCU mice in the presence and absence of 1 wk of taurine treatment. Figure represents analysis for female mice; an identical regulatory pattern was also observed in male mice. *P < 0.05, ***P < 0.001.
DISCUSSION
Decreased plasma tCys levels in patients with HCU have been known for some time (5–7, 16) and generally have been attributed to being a consequence of a block in the endogenous synthesis of this compound due to CBS inactivation. However, this mechanism is unlikely to be responsible for decreased tCys levels, as lowering tHcy in mouse models of HCU with either betaine treatment or Met restriction results in restoration of plasma tCys levels without restoring endogenous biosynthesis (7, 17, 18). Instead, it is more likely that decreased plasma tCys levels in HCU are a consequence of this compound forming mixed disulfides with Hcy and the subsequent excretion of these compounds in the urine. Cys disulfide supplementation is currently used as an additional treatment for HCU in many treatment centers (2–4). The ability of Cys supplementation to reverse much of the HCU-induced alterations of Cys metabolism reported in this paper indicate that Cys is a worthwhile adjuvential treatment for HCU.
Taurine is an organic osmolyte involved in cell volume regulation and provides a substrate for the formation of bile salts. It plays a role in the modulation of intracellular free calcium concentration, and although it is one of the few amino acids not incorporated into proteins, taurine is one of the most abundant amino acids in the brain, retina, muscle tissue, and organs throughout the body (19). Hence, our observation of decreased taurine levels in both blood and liver as a consequence of HCU has a number of possible implications for pathogenesis in this disease. The major dietary sources of taurine are seafood and meat, which, due to their high protein content, are restricted in the low-Met diet typically prescribed for patients with pyridoxine nonresponsive HCU. Consequently, the importance of endogenous taurine synthesis is likely to be of even greater physiological significance in this condition. In addition to serving as an organic osmolyte, antioxidant, and a conjugation substrate for bile acids (19), taurine has also been shown to act as an anti-inflammatory compound via its involvement in promoting the synthesis of taurine-chloramine (20, 21) in activated neutrophils and has been previously been shown to reduce production of TNF-α by macrophage (22, 23). Consequently, depletion of this compound has the potential to contribute to the oxidative stress and constitutive expression of proinflammatory cytokines that have previously been reported in HCU (12, 24). Similarly, a lack of taurine during neonatal development has been shown to result in problems with development of the central nervous system and could therefore contribute to the cognitive impairment that is a frequent complication of untreated or poorly controlled HCU (25).
Previous work has revealed that CDO plays a critical role in Cys homeostasis and is constitutively synthesized but degraded when Cys levels are low and that catalytic efficiency of the enzyme increases by up to 10-fold with increases in Cys availability. The rapid decrease in hepatic CDO protein levels during times of low Cys availability occurs almost exclusively via ubiquitination and subsequent proteasomal degradation (1). The qRT-PCR and Western blotting analyses of CDO expression in HO mice described above are thus consistent with previous studies in the Stipanuk laboratory (26–28), which have demonstrated that hepatic CDO protein, but not mRNA levels, change with increases in Cys availability.
Neither precursors nor products of Cys affect CDO concentration or activity, and no tested structural analogs of Cys or alternative thiols have been found to have any effect on the levels of CDO in vivo. Less dramatic changes in tHcy in the low-tHcy HO mice result in significant changes in the level of CDO protein without any apparent significant effect on tCys levels. Similarly taurine supplementation completely reversed the HCU-induced repression of CDO without any obvious effect on tCys levels. Collectively, these results are consistent with the possibility that Cys is not the only sulfur compound that can exert regulatory effects on CDO expression.
An alternative mechanism that could possibly contribute to the observed repression of CDO expression independent of decreased Cys levels comes from recent evidence linking HCU to a state of chronic inflammation and increased expression and production of inflammatory cytokines, such as TNF-α, in both mice and human patients (24). As well as contributing to cardiovascular complications and decreased bone density, TNF-α has previously been shown to repress CDO expression in a human medulloblastoma cell line (29, 30). In this context, note that taurine treatment completely normalizes plasma TNF-α levels in high-tHcy HO mice (unpublished results).
The observed HCU-induced repression of CDO and concomitant depletion of hepatic and blood levels of taurine raise the question as to whether this effect is limited to the liver or is also occurring in other tissues. Previous work using a liver-specific CDO-null mouse model reported that the abundance of CDO and the proportion of CDO existing as the mature, more active isoform increased in extrahepatic tissues and was accompanied with increased metabolism of Cys to hypotaurine by the CDO/CSAD pathway. This increase in extra hepatic taurine synthesis allowed the hepatic CDO-knockout mice to maintain normal hepatic levels of taurine. The apparent absence of this compensatory mechanism in HO mice indicates that the HCU-induced repression of CDO expression may not be limited to the liver and will be the subject of future investigation in our laboratory.
Currently, only limited data are available regarding the regulation of hepatic CSAD expression. Previous reports have indicated that CSAD expression is influenced by dietary protein content (31), but as all of the mice involved in the present study received identical levels of protein in their diet, this is not likely to be a factor in the observed induction of CSAD in HCU. Previous studies have shown dietary supplementation with Cys decreases both CSAD mRNA and enzyme activity in WT rats and raises the possibility that the higher levels of CSAD in the HO mice are a reflection of lower levels of Cys in HCU (28). However, while the repression of CDO is clearly reversed by Cys supplementation, the increased expression of CSAD is still maintained in the presence of Cys supplementation, indicating that this regulatory change is unlikely to be due to decreased Cys availability. It is conceivable that CSAD induction in HCU is a homeostatic response designed to maintain optimal taurine levels in the liver. This possibility would be consistent with a previous report where kittens were fed a taurine-depleted diet resulting in a 5-fold induction of CSAD activity without any discernible effect on CDO expression levels (32). The observed depletion of hepatic taurine in HCU animals indicates that such a response is ultimately futile, presumably due to the absence of the required cysteinesulfinate substrate as a consequence of the concomitant repression of CDO.
It has previously been reported that brain CSAD activity is activated by PKC-mediated phosphorylation and inhibited by dephosphorylation (33, 34). In our analysis of liver CSAD expression, we only observed one molecular form of the enzyme, indicating that phosphorylation-mediated regulation of this enzyme may be specific for neural tissues rather than a general mechanism. Alternatively, recent work has indicated that hepatic CSAD expression is regulated by bile acids in a process that involves the nuclear receptors farnesoid X receptor and small heterodimer partner (35). The idea that HCU might induce altered bile acid metabolism is intriguing as it would offer a possible explanation for the observed hepatopathy that is sometimes observed in this disease and also has the potential to contribute to other pathogenic features such as vascular dysfunction. Future investigations in our laboratory will focus on the influence of HCU on bile acid metabolism.
The ability of taurine to restore normal expression levels of GOT1 reveals a previously unsuspected regulatory crosstalk between the taurine biosynthetic pathway and the GOT1 sulfate pathway. In addition to the transamination of cysteinesulfinate, GOT1 and GOT2 function as aspartate transaminases catalyzing the reversible transfer of an α-amino group between aspartate and glutamate. GOT1 and GOT2 can also act on l-tyrosine, l-phenylalanine, and l-tryptophan and have an important role in multiple aspects of amino acid metabolism and in the urea and tricarboxylic acid cycles. The repression of GOT1 expression in HCU thus has the potential to exert effects on the neuroactive compounds glutamate and aspartate in the CNS and could conceivably contribute to cognitive impairment in HCU. The end product of the GOT1/2 pathway is inorganic sulfate, which is the fourth most abundant anion in mammalian blood, and the repression of these enzymes could conceivably lead to decreased blood and tissue levels of this compound. Sulfite oxidase is involved in the same pathway and inactivation of this enzyme would likely cause a similar decrease in sulfate production. Interestingly, both Sulfite oxidase deficiency and HCU incur ectopia lentis via an as yet unknown mechanism (36). Inorganic sulfate is essential for hepatic sulfonation, a crucial mechanism for decreasing the toxicity of secondary bile acids, such as lithocholate. Disruption of this process in HCU could create intrahepatic choleostatic conditions and subsequent liver injury. Future work is required to see whether HCU induces decreased plasma and tissue sulfate levels and disrupted bile acid metabolism.
To summarize, three major conclusions can be drawn from the studies described here. First, we show that HCU induces significant regulatory changes in both of the major pathways of Cys oxidation with the potential to contribute to pathogenesis via multiple mechanisms. Second, HCU results in significant depletion of liver and blood taurine, and this compound exerts previously unsuspected regulatory effects on both Cys oxidation pathways. Finally, this work indicates that that both Cys and taurine supplementation offer significant therapeutic potential for HCU and could be used in addition to conventional strategies designed to lower tHcy levels.
Acknowledgments
K.N.M. gratefully acknowledges financial support from the William R. Hummel Homocystinuria Research Fund and Hayley's Heroes research fund.
The authors thank Professor Martha Stipanuk (Cornell University, Ithaca, NY, USA) for useful discussions and Dr. Joe Hurt (University of Colorado) for critical reading of the manuscript.
Footnotes
- CDO
- cysteine dioxygenase
- CSAD
- cysteinesulfinate decarboxylase
- CBS
- cystathionine β-synthase
- CGL
- cystathionine γ-lyase
- Cys
- cysteine
- GAPDH
- glyceraldehyde 3-phosphate dehydrogenase
- GOT
- glutamic-oxaloacetic transaminase
- HCU
- homocystinuria
- Hcy
- homocysteine
- Met
- methionine
- qRT-PCR
- quantitative reverse transcriptase PCR
- tHcy
- total homocysteine
- tCys
- total cysteine
- WT
- wild type
REFERENCES
- 1. Stipanuk M. H., Ueki I. (2011) Dealing with methionine/homocysteine sulfur: cysteine metabolism to taurine and inorganic sulfur. J. Inherit. Metab. Dis. 34, 17–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lee P. J., Briddon A. (2007) A rationale for cystine supplementation in severe homocystinuria. J. Inherit. Metab. Dis. 30, 35–38 [DOI] [PubMed] [Google Scholar]
- 3. Adam S., Almeida M. F., Carbasius Weber E., Champion H., Chan H., Daly A., Dixon M., Dokoupil K., Egli D., Evans S., Eyskens F., Faria A., Ferguson C., Hallam P., Heddrich-Ellerbrok M., Jacobs J., Jankowski C., Lachmann R., Lilje R., Link R., Lowry S., Luyten K., MacDonald A., Maritz C., Martins E., Meyer U., Muller E., Murphy E., Robertson L. V., Rocha J. C., Saruggia I., Schick P., Stafford J., Stoelen L., Terry A., Thom R., van den Hurk T., van Rijn M., van Teefelen-Heithoff A., Webster D., White F. J., Wildgoose J., Zweers H. (2013) Dietary practices in pyridoxine non-responsive homocystinuria: a European survey. Mol. Genet. Metab. 110, 454–459 [DOI] [PubMed] [Google Scholar]
- 4. Schiff M., Blom H. J. (2012) Treatment of inherited homocystinurias. Neuropediatrics 43, 295–304 [DOI] [PubMed] [Google Scholar]
- 5. Maclean K. N., Gaustadnes M., Oliveriusova J., Janosik M., Kraus E., Kozich V., Kery V., Skovby F., Rudiger N., Ingerslev J., Stabler S. P., Allen R. H., Kraus J. P. (2002) High homocysteine and thrombosis without connective tissue disorders are associated with a novel class of cystathionine beta-synthase (CBS) mutations. Hum. Mutat. 19, 641–655 [DOI] [PubMed] [Google Scholar]
- 6. Maclean K. N., Sikora J., Kozich V., Jiang H., Greiner L. S., Kraus E., Krijt J., Crnic L. S., Allen R. H., Stabler S. P., Elleder M., Kraus J. P. (2010) Cystathionine beta-synthase null homocystinuric mice fail to exhibit altered hemostasis or lowering of plasma homocysteine in response to betaine treatment. Mol. Genet. Metab. 101, 163–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Maclean K. N., Sikora J., Kozich V., Jiang H., Greiner L. S., Kraus E., Krijt J., Overdier K. H., Collard R., Brodsky G. L., Meltesen L., Crnic L. S., Allen R. H., Stabler S. P., Elleder M., Rozen R., Patterson D., Kraus J. P. (2010) A novel transgenic mouse model of CBS-deficient homocystinuria does not incur hepatic steatosis or fibrosis and exhibits a hypercoagulative phenotype that is ameliorated by betaine treatment. Mol. Genet. Metab. 101, 153–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Maclean K. N., Greiner L. S., Evans J. R., Sood S. K., Lhotak S., Markham N. E., Stabler S. P., Allen R. H., Austin R. C., Balasubramaniam V., Jiang H. (2012) Cystathionine protects against endoplasmic reticulum stress-induced lipid accumulation, tissue injury, and apoptotic cell death. J. Biol. Chem. 287, 31994–32005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stabler S. P., Lindenbaum J., Savage D. G., Allen R. H. (1993) Elevation of serum cystathionine levels in patients with cobalamin and folate deficiency. Blood 81, 3404–3413 [PubMed] [Google Scholar]
- 10. Hirai T., Ohyama H., Kido R. (1987) A direct determination of taurine in perchloric acid-deproteinized biological samples. Anal. Biochem. 163, 339–342 [DOI] [PubMed] [Google Scholar]
- 11. Bradford M. M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 [DOI] [PubMed] [Google Scholar]
- 12. Jiang H., Stabler S. P., Allen R. H., Maclean K. N. (2012) Altered expression of apoA-I, apoA-IV and PON-1 activity in CBS deficient homocystinuria in the presence and absence of treatment: Possible implications for cardiovascular outcomes. Mol. Genet. Metab. 107, 55–65 [DOI] [PubMed] [Google Scholar]
- 13. McCoy J. G., Bailey L. J., Bitto E., Bingman C. A., Aceti D. J., Fox B. G., Phillips G. N., Jr. (2006) Structure and mechanism of mouse cysteine dioxygenase. Proc. Natl. Acad. Sci. U. S. A. 103, 3084–3089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ye S., Wu X., Wei L., Tang D., Sun P., Bartlam M., Rao Z. (2007) An insight into the mechanism of human cysteine dioxygenase. Key roles of the thioether-bonded tyrosine-cysteine cofactor. J. Biol. Chem. 282, 3391–3402 [DOI] [PubMed] [Google Scholar]
- 15. Panteghini M. (1990) Aspartate aminotransferase isoenzymes. Clin. Biochem. 23, 311–319 [DOI] [PubMed] [Google Scholar]
- 16. Mudd S. H., Levy H. L., Kraus J. P. (2001) Disorders of transsulfuration. In The Metabolic and Molecular Bases of Inherited Disease (Scriver C. R., Beaudet A. L., Sly W. S., Valle D., Childs B., Kinzler K., Vogelstein B., eds) pp. 2007–2056, McGraw-Hill, New York [Google Scholar]
- 17. Maclean K. N., Jiang H., Greiner L. S., Allen R. H., Stabler S. P. (2012) Long-term betaine therapy in a murine model of cystathionine beta-synthase deficient homocystinuria: decreased efficacy over time reveals a significant threshold effect between elevated homocysteine and thrombotic risk. Mol. Genet. Metab. 105, 395–403 [DOI] [PubMed] [Google Scholar]
- 18. Gupta S., Melnyk S. B., Kruger W. D. (2014) Cystathionine beta-synthase-deficient mice thrive on a low-methionine diet. FASEB J. 28, 781–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Huxtable R. J. (1992) Physiological actions of taurine. Physiol. Rev. 72, 101–163 [DOI] [PubMed] [Google Scholar]
- 20. Wojtecka-Lukasik E., Czuprynska K., Maslinska D., Gajewski M., Gujski M., Maslinski S. (2006) Taurine-chloramine is a potent antiinflammatory substance. Inflamm. Res. 55(Suppl. 1), S17–S18 [DOI] [PubMed] [Google Scholar]
- 21. Wojtecka-Lukasik E., Gujski M., Roguska K., Maslinska D., Maslinski S. (2005) Taurine chloramine modifies adjuvant arthritis in rats. Inflamm. Res. 54(Suppl. 1), S21–S22 [DOI] [PubMed] [Google Scholar]
- 22. Weiss S. J., Klein R., Slivka A., Wei M. (1982) Chlorination of taurine by human neutrophils. Evidence for hypochlorous acid generation. J. Clin. Invest. 70, 598–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Marcinkiewicz J., Grabowska A., Bereta J., Stelmaszynska T. (1995) Taurine chloramine, a product of activated neutrophils, inhibits in vitro the generation of nitric oxide and other macrophage inflammatory mediators. J. Leukoc. Biol. 58, 667–674 [DOI] [PubMed] [Google Scholar]
- 24. Keating A. K., Freehauf C., Jiang H., Brodsky G. L., Stabler S. P., Allen R. H., Graham D. K., Thomas J. A., Van Hove J. L., Maclean K. N. (2011) Constitutive induction of pro-inflammatory and chemotactic cytokines in cystathionine beta-synthase deficient homocystinuria. Mol. Genet. Metab. 103, 330–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sturman J. A. (1993) Taurine in development. Physiol. Rev. 73, 119–147 [DOI] [PubMed] [Google Scholar]
- 26. Lee J. I., Londono M., Hirschberger L. L., Stipanuk M. H. (2004) Regulation of cysteine dioxygenase and gamma-glutamylcysteine synthetase is associated with hepatic cysteine level. J. Nutr. Biochem. 15, 112–122 [DOI] [PubMed] [Google Scholar]
- 27. Bella D. L., Hirschberger L. L., Hosokawa Y., Stipanuk M. H. (1999) Mechanisms involved in the regulation of key enzymes of cysteine metabolism in rat liver in vivo. Am. J. Physiol. 276, E326–E335 [DOI] [PubMed] [Google Scholar]
- 28. Bella D. L., Hahn C., Stipanuk M. H. (1999) Effects of nonsulfur and sulfur amino acids on the regulation of hepatic enzymes of cysteine metabolism. Am. J. Physiol. 277, E144–E153 [DOI] [PubMed] [Google Scholar]
- 29. Tsuboyama-Kasaoka N., Shozawa C., Sano K., Kamei Y., Kasaoka S., Hosokawa Y., Ezaki O. (2006) Taurine (2-aminoethanesulfonic acid) deficiency creates a vicious circle promoting obesity. Endocrinology 147, 3276–3284 [DOI] [PubMed] [Google Scholar]
- 30. Qusti S., Parsons R. B., Abouglila K. D., Waring R. H., Williams A. C., Ramsden D. B. (2000) Development of an in vitro model for cysteine dioxygenase expression in the brain. Cell Biol. Toxicol. 16, 243–255 [DOI] [PubMed] [Google Scholar]
- 31. Jerkins A. A., Jones D. D., Kohlhepp E. A. (1998) Cysteine sulfinic acid decarboxylase mRNA abundance decreases in rats fed a high-protein diet. J. Nutr. 128, 1890–1895 [DOI] [PubMed] [Google Scholar]
- 32. Rentschler L. A., Hirschberger L. L., Stipanuk M. H. (1986) Response of the kitten to dietary taurine depletion: effects on renal reabsorption, bile acid conjugation and activities of enzymes involved in taurine synthesis. Comp. Biochem. Physiol. B. 84, 319–325 [DOI] [PubMed] [Google Scholar]
- 33. Tang X. W., Hsu C. C., Schloss J. V., Faiman M. D., Wu E., Yang C. Y., Wu J. Y. (1997) Protein phosphorylation and taurine biosynthesis in vivo and in vitro. J. Neurosci. 17, 6947–6951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wu J. Y., Tang X. W., Schloss J. V., Faiman M. D. (1998) Regulation of taurine biosynthesis and its physiological significance in the brain. Adv. Exp. Med. Biol. 442, 339–345 [DOI] [PubMed] [Google Scholar]
- 35. Kerr T. A., Matsumoto Y., Matsumoto H., Xie Y., Hirschberger L. L., Stipanuk M. H., Anakk S., Moore D. D., Watanabe M., Kennedy S., Davidson N. O. (2013) Cysteine sulfinic acid decarboxylase regulation: a role for farnesoid X receptor and small heterodimer partner in murine hepatic taurine metabolism. [E-pub ahead of print] Hepatol. Res. 10.1111/hepr.12230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sadiq M. A., Vanderveen D. (2013) Genetics of ectopia lentis. Semin. Ophthalmol. 28, 313–320 [DOI] [PubMed] [Google Scholar]