Abstract
Bone cells exposed to real microgravity display alterations of their cytoskeleton and focal adhesions, two major mechanosensitive structures. These structures are controlled by small GTPases of the Ras homology (Rho) family. We investigated the effects of RhoA, Rac1, and Cdc42 modulation of osteoblastic cells under microgravity conditions. Human MG-63 osteoblast-like cells silenced for RhoGTPases were cultured in the automated Biobox bioreactor (European Space Agency) aboard the Foton M3 satellite and compared to replicate ground-based controls. The cells were fixed after 69 h of microgravity exposure for postflight analysis of focal contacts, F-actin polymerization, vascular endothelial growth factor (VEGF) expression, and matrix targeting. We found that RhoA silencing did not affect sensitivity to microgravity but that Rac1 and, to a lesser extent, Cdc42 abrogation was particularly efficient in counteracting the spaceflight-related reduction of the number of focal contacts [−50% in silenced, scrambled (SiScr) controls vs. −15% for SiRac1], the number of F-actin fibers (−60% in SiScr controls vs. −10% for SiRac1), and the depletion of matrix-bound VEGF (−40% in SiScr controls vs. −8% for SiRac1). Collectively, these data point out the role of the VEGF/Rho GTPase axis in mechanosensing and validate Rac1-mediated signaling pathways as potential targets for counteracting microgravity effects.—Guignandon, A., Faure, C., Neutelings, T., Rattner, A., Mineur, P., Linossier, M.-T., Laroche, N., Lambert, C., Deroanne, C., Nusgens, B., Demets, R., Colige, A., Vico, L. Rac1 GTPase silencing counteracts microgravity-induced effects on osteoblastic cells.
Keywords: VEGF, spaceflight, MG-63, RhoA, Cdc42
Most astronauts who have experienced space-related conditions eventually have significant bone loss (1). In vitro, the differentiation of bone-forming cells is impaired in microgravity (2). The causal relationship between these two observations is presently unclear and remains speculative. It has been consistently observed, however, that bone cells exposed to real microgravity display major alterations of their cytoskeleton integrity (3–5). Cytoskeleton integrity is essential for a variety of cell processes, such as proliferation, migration, signal transduction, apoptosis (6, 7), fibrillogenesis, and differentiation (8). The cytoskeleton is responsive to changes in the mechanical environment because it is physically connected to the extracellular matrix through focal adhesions (FAs). FAs are connecting and signaling complexes composed of transmembrane integrins linked to a variety of structural and signaling molecules, such as vinculin, zyxin, FAK, and Src (9).
FA assembly and disassembly, as well as maturation, are regulated by external mechanical forces, and the size and shape of individual FAs is related to the amount of tension applied to them (10). Donald Ingber's theory of tensegrity implies that, in a dynamic and reciprocal way, the external mechanical forces acting on a cell are balanced by the generation of an intracellular tension through the actin/myosin machinery (11). Fibronectin fibrillogenesis is dependent on internal tension and contractility (12) and supports osteoblast differentiation (13) after priming of the α5β1 integrin. We and others have reported a reduction in the number, distribution, and size of the FAs (4, 5, 14, 15) in cells exposed to microgravity. Modification of cellular tension during microgravity-related conditions may trigger significant changes in FA functionality and consequently alterations of cell phenotype, which would explain the impairment of osteoblasts' cytoskeleton integrity and differentiation in microgravity.
Cytoskeleton, FA dynamics, and fibronectin fibrillogenesis are regulated by small GTPases of the Ras homology (Rho) family, comprising ∼20 members, among which the archetypes RhoA, Rac1, and Cdc42 are the most extensively studied (16). Each of them elicits distinct effects on actin cytoskeleton dynamics. Since RhoA is responsible for internal cellular tension, its activity is associated with stable, large FAs, in contrast to Rac1 and Cdc42, which are responsible for peripheral actin polymerization, initiation of migration, and formation of small peripheral focal complexes that may be stabilized into focal contacts as tension increases (10, 17). There is extensive evidence that integrins control actin polymerization via members of the Rho family of small GTPases (18).
In this context, microgravity effects on bone-forming cells could be depicted as a down-regulation of RhoA (few large contacts, loss of actin stress fibers, reduced fibronectin fibrillogenesis, and impaired osteoblast differentiation); or an overstimulation of Rac1/Cdc42, explaining why actively remodeled peripheral contacts do not mature into focal contacts and support fibronectin fibrillogenesis; or both. We wanted to decipher the role of RhoA, Rac1, and Cdc42 in the sensitivity of osteoblastic cells to microgravity. To do so, we used modulation of these molecules with targeted siRNA (19–22) during exposure to conditions of microgravity.
Further to their structural and adhesive functions, FAs also serve as operational centers for many signal transduction pathways initiated by integrins and other transmembrane receptors, such as vascular endothelial growth factor receptors (VEGFRs; ref. 23). Several models have described altered growth factor signaling in microgravity (24–26). Since we have reported that alternative splicing of VEGFA was regulated by the mechanical environment (27) and since we and others have demonstrated that VEGF, in particular its matrix-bound isoforms, may exert an antiapoptotic activity (28), we speculate that cells exposed to microgravity present a dysregulated expression of VEGF or its isoforms, or downstream signaling linked to altered Rho GTPase activities, or both. In fact, VEGF is known to stimulate RhoA in many cell types, particularly in endothelial cells (29, 30). Furthermore, VEGFA is recognized for its ability to promote fibronectin fibrillogenesis (29), leading us to regard it as a significant mediator of cellular response in microgravity, as already mentioned by van Oostveldt and colleagues (31) in a profiling study.
Human MG-63 osteoblast-like cells silenced for Rho GTPases were cultured in the European Space Agency (ESA) Biobox automated bioreactor aboard the Foton-M3 satellite and compared to replicate ground-based controls. The cells were fixed during the mission for postflight analysis of focal contacts, F-actin polymerization, VEGF expression, and matrix-targeting isoforms. We observed that RhoA silencing did not affect sensitivity to microgravity but that Rac1, and to a lesser extent Cdc42, abrogation was particularly efficient when it came to counteracting a reduction of the number of focal contacts, actin polymerization, and matrix-bound VEGF (mVEGF) depletion. Collectively, these data point out the role of the VEGF/Rho GTPase axis in mechanosensing and validate Rac1-mediated signaling pathways as potential targets in microgravity.
MATERIALS AND METHODS
Cell culture
The human osteoblastic osteosarcoma cell line MG-63 (American Type Culture Collection, Manassas, VA, USA) was cultured with Dulbecco's modified Eagle's medium [DMEM; with 25 mM hydroxyethyl piperazine ethane sulfonic acid (HEPES) and sodium bicarbonate] supplemented with 10% fetal calf serum (FCS; Lonza, Petit-Rechain, Belgium), 2 mM l-glutamine, and antibiotics (50 U/ml penicillin and 50 μg/ml streptomycin) in a humidified atmosphere of 5% CO2 at 37°C and amplified by trypsinization with 1× trypsin-EDTA.
Preparation of siRNA-transfected cells
The chemically synthesized, desalted, deprotected, and PAGE-purified 21-nt siRNAs were from Eurogentec (Liège, Belgium). The sequences of the siRNA-targeting RhoA, Rac1, and Cdc42, as well as an inactive scrambled sequence used as the control, have been described previously (21, 22) and are detailed in Table 1. Each pair of oligoribonucleotides was annealed at a concentration of 20 μM in 50 mM NaCl, 1 mM EDTA, and 10 mM Tris-HCl (pH 7.5). siRNA transfection was performed as described elsewhere (32). Briefly, calcium phosphate–mediated transfection was performed overnight (14–16 h) on subconfluent MG-63 cells at a final concentration of 20 nM siRNA. The cells were washed twice with PBS and once with complete medium. This last step was defined as time 0. The silencing was effective for at least 7 d in proliferating cells. Silenced cells could be frozen and thawed without any loss of viability. Validation of silencing was performed by Western blot, as shown in Supplemental Fig. S1.
Table 1.
siRNA sequences
| Target | Oligonucleotide sequence siRNA, 5′–3′ |
|---|---|
| RhoA | F: GAAGUCAAGCAUUUCUGUCTT |
| R: GACAGAAAUGCUUGACUUCTT | |
| Rac1 | F: UCUCGAUCGUGUCUUUAUCTT |
| R: GAUAAAGACACGAUCGAGATT | |
| Cdc42 | F: GAUAACUCACCACUGUCCATT |
| R: UGGACAGUGGUGAGUUAUCTT | |
| Scrambled | F: UUGCAUACAGGACUCGUUATT |
| R: UAACGAGUCCUGUAUGCAATT |
F, forward; R, reverse.
Hardware description and spaceflight experiment schedule
The Biobox bioreactor was equipped with 2 programmable incubators containing, among other biological experiments, our Obadis experiment involving Rho GTPase-silenced MG-63 cells. The MG-63 cells were seeded on collagen-coated glass coverslips at a density of 5000 cells/cm2 and cultured in HEPES-DMEM, as described above. The seeded coverslips were contained in automated culturing devices developed by the Center for Concepts in Mechatronics (CCM; Nuenen, The Netherlands). Each device consisted of 2 culture chambers of 1.8 ml, each connected to 3 reservoirs filled with medium or fixative. Medium and fixative were transferred from the reservoirs into the culture chambers by the action of springs, released according to a preprogrammed time line. In this way, the Biobox was able to complete the entire sequence of the experiment robotically.
The MG-63 cultures in the Biobox were exposed to 69 h of microgravity before fixation. Fixation was achieved by means of 1% paraformaldehyde (PFA) for one of the culture chambers and with RNAlater (Ambion-Life Technologies, Austin, TX, USA) for the other.
After landing, the culture units were opened, and coverslips with cells fixed in PFA were cut into 2 pieces: one for VEGF and the other for fibronectin immunohistochemistry and quantification. Conditioned medium collected during spaceflight was processed for VEGF quantification. For technical reasons (cross contamination with PFA), we were not able to determine the VEGF concentration in any of the samples.
Supernatants and cells of chambers that received RNAlater were collected, and RNA extraction was performed as described below.
Fluorescence immunostaining
Vinculin, F-actin, and DAPI
The cells were rinsed with PBS and treated with 10 mM ethanolamine in PBS (or 0.1 M glycine in PBS) for 5 min. They were permeabilized with 0.1% Triton-X100 in PBS for 1 min, incubated with mouse anti-human vinculin (hVIN1; Sigma, Diegem, Belgium; 1:100) for 2 h at 37°C, and then incubated with goat anti-mouse AlexaFluor 488 (Life Technologies, Ghent, Belgium; 1:200) for 1 h at 37°C for visualization of FA. For visualization of actin stress fibers, the cells were incubated in rhodamine-phalloidin (Molecular Probes, Eugene OR, USA) diluted 1:100 in PBS for 15 min. Before the application of mounting medium, the cells were incubated with 1 μg/ml DAPI for 10 min, to label the nuclei. Quantitative measurements were obtained by means of ImageJ software (U.S. National Institutes of Health, Bethesda, MD, USA; http://rsb.info.nih.gov.gate2.inist.fr/ij).
Fibronectin
The cells were incubated with rabbit anti-human fibronectin (Sigma-Aldrich; F3648, 1:200) for 2 h at 37°C, followed by an incubation with goat anti-rabbit AlexaFluor 546 (1:250) for 1 h at 37°C. Before application of mounting medium, the cells were incubated with 1 μg/ml DAPI for 10 min, to label the nuclei. The fibronectin area was subsequently analyzed with ImageJ software.
VEGF
After a rinse in PBS, the samples were incubated for 60 min at 37°C with mouse primary antibodies directed against human VEGF (Millipore, Livingston, UK; MAB3734) diluted 1:50 in PBS with 10% FCS. Samples were rinsed with PBS and incubated for 60 min at 37°C with goat anti-mouse AlexaFluor 488 (1:200). Before application of mounting medium, the cells were incubated with 1 μg/ml DAPI for 10 min, to label the nuclei and check for absence of cells in the selected fields. The VEGF-positive area representative of VEGF entrapment in the matrix was subsequently analyzed with ImageJ software.
Image analyses
Image analyses were performed with ImageJ software. Procedures for the quantification of focal contacts and actin stress fibers have been described previously (14). Briefly, all analyses were performed after background removal and thresholding of the image. The cells were manually delineated, and each positive vinculin contact was counted in the cells as well as in the relative area of vinculin (vinculin area expressed as a percentage of the cell area). A minimum of 50 cells/condition were analyzed. A similar approach was used for F-actin quantification. Briefly, F-actin images were processed using the FeatureJ Hessian filter in ImageJ. Thresholded images were analyzed, and the number of fibers per field of view was determined. A minimum of 20 fields/condition were analyzed. To determine the percentage of VEGF and fibronectin-positive area, a minimum of 30 images (fields)/condition were quantified. The cells were examined with a fluorescence-equipped microscope (DMRB; Leica, Bensheim, Germany) and imaged with a ×40 or ×63 objective at a resolution of 650 × 515 pixels.
RNA extraction and reverse transcription (RT)
Total RNA was isolated using the RNeasy Mini Kit (Qiagen, Inc., Valencia, CA, USA). Complementary DNA (cDNA) was synthesized from 2 μg of total RNA using an RNA polymerase chain reaction (PCR) kit (First-Strand cDNA Synthesis Kit for RT-PCR; Roche, Indianapolis, IN, USA).
Real-time PCR
The cDNA mixtures were diluted 1:20 in water, and 8 μl was subjected to real-time PCR with SYBR Green I dye (LightCycler Faststart DNA Master SYBR green I; Roche). The reactions were performed in a 20 μl PCR mixture containing 4 μl Master Mix 5× concentrate (a dNTP mixture, with dUTP instead of dTTP, MgCl2, SYBR Green I dye, TaqDNA polymerase, and reaction buffer), 2 μl 10 μM primers (sense and antisense), and 6 μl PCR-grade water.
The 5′-AGCTGTTTGCAGACAAAGTT-3′ and 5′-CCAAAGACCACATGCTTGCC-3′ oligoribonucleotides were used for forward and reverse primers for human cyclophilin, respectively; 5′-GGACATCTTCCAGGAGTACC-3′ and 5′-GGCTTGTCACATTTTTCTTG-3′ for human VEGF121; 5′-CAAGAAAATCCCTGTGGGCC-3′ and 5′-CTCCTGCCCGGCTCACCGCC-3′ for human VEGF165; and 5′-CCTGGAGCGTTCCCTGTGGG-3′ and 5′-CTCCTGCCCGGCTCACCGCC-3′ for human VEGF189.
Real-time PCR was performed on the LightCycler 3.0 (Roche). A typical protocol included a denaturation step at 95°C for 480 s followed by 40 cycles of 95°C for 15 s, Tm (°C) annealing for 10 s, and a 72°C extension for 20 s. Standard curves (1:20–1:320 dilution) were used to determine the 100% efficiency of PCR for each gene. The fluorescence levels of the samples were collected using this reference line. The obtained values were normalized by their respective cyclophilin controls. In the graphs, the genes are expressed as the percentage of silenced, scrambled (SiScr) controls at 1 g (100%).
Statistical analysis
Statistical analysis was performed with Statistica6 software (StatSoft Inc., Tulsa, OK, USA). All data were subjected to 1-way analysis of variance (ANOVA). When F values for a given variable were found to be significant, comparisons among individual samples were assessed by the post hoc method of Scheffé. Results were considered to be significantly different at P < 0.05. All data are presented as means ± sd of a minimum of 30 determinations in any condition.
RESULTS
Microgravity-induced morphological changes in Rho GTPase-modulated osteoblastic cells
To decipher the role of a particular Rho GTPase in gravisensing, we used cells transfected with siRNA sequences capable of efficiently and specifically down-regulating RhoA, Cdc42, and Rac1, as described previously (refs. 21, 23; see Materials and Methods) and shown in Supplemental Fig. S1A for our cells. We found that silencing of MG-63 cells was effective as early as d 1 and lasted ≥3 additional days. In this context, the duration of the exposure to microgravity (69 h) was selected to guarantee an optimal silencing of Rho GTPases. The silenced cells were compared with cells transfected with a scrambled inactive sequence (SiScr control). None of the siRNAs significantly modified the cell proliferation rate or apoptosis.
To evaluate the morphologic changes after spaceflight, we fixed the cells with a very low PFA concentration and processed them for triple detection of vinculin, F-actin, and DAPI (not shown), enabling an appreciation of FA formation, cytoskeletal tension, and nuclear morphology. After 69 h of microgravity exposure, the SiScr control cells presented a reduced number of vinculin-positive focal contacts (Fig. 1A) and a reduced FA area (focal area/cell area; Fig. 1C). Consistent with the reduced number of FAs, a reduced number of stress fibers (Fig. 1B) and a smaller relative stress fiber area (Fig. 1C) indicated a significantly reduced actin polymerization.
Figure 1.
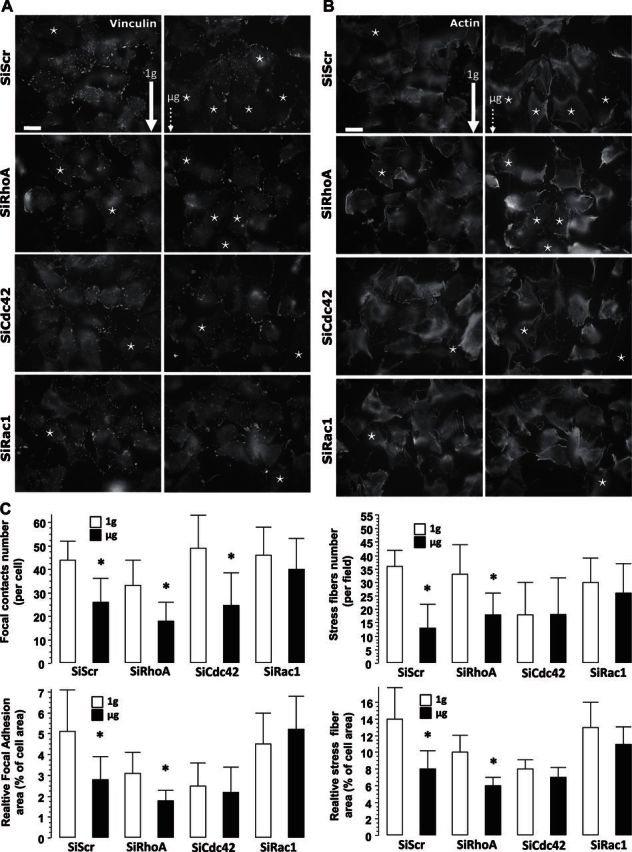
Morphological description of MG-63 cells after exposure to microgravity. A, B) Left panels: 1 g conditions (ground-based control; solid arrow). Right panels: microgravity (μg) conditions (dotted arrow). Cells were processed for detection of FAs by using vinculin antibody (A) and of stress fibers by using rhodamine phalloidin (B) staining. SiScr corresponds to cells transfected with scrambled sequences of SiRNA (SiRhoA, Rac1, and Cdc42) of their respective specific siRNA. Images clearly show that with SiScr and SiRhoA, the cells presented an absence of large focal contacts when compared to the 1 g control cells. It is noteworthy that SiRac1 and SiCdc42 cells exposed to microgravity presented an adhesive profile similar to that of the controls. Stars indicate major cell detachment. Scale bars = 10 μm. C) A minimum of 30 cells extracted from a minimum of 10 fields were used for quantification of focal contact morphometry, as well as the number of stress fibers and their area. Open bars, ground-based control cells; solid bars, microgravity-exposed cells. Error bars represent sd (n = 30). *P < 0.01.
A similar trend in morphology was observed for SiRhoA cells, as compared to SiScr cells, and some cells were not properly spread in comparison with ground-based cells exposed to 1 g (Fig. 1A, B). Concerning quantitative morphometry, we found reductions in the number of focal contacts and relative adhesion area, as well as a reduced number of stress fibers per cell and relative stress fiber area (Fig. 1C) for the SiCdc42 and SiRhoA cells exposed to microgravity, as compared to the 1 g controls. By contrast, SiRac1 exposed to microgravity did not present major cell detachment (Fig. 1B) and 1 g cells displayed a well-spread phenotype with numerous contacts and well-developed actin stress fibers (Fig. 1B).
Quantitative results confirmed that microgravity did not significantly reduce the number and area of focal contacts or the number and relative area of stress fibers in SiRac1 (Fig. 1C). The analysis of the Cdc42 cells was more complex, as these cells showed a significant reduction in focal contacts (Fig. 1A, C), but were unaffected by microgravity conditions regarding other morphometric parameters (Fig. 1C). These results can be explained by the fact that values found for SiCdc42 parameters were already lowered by the silencing condition, the lowest levels of the studied conditions.
Microgravity-induced reduction of fibronectin fibrillogenesis
As cell tension may affect fibronectin fibrillogenesis, we performed immunostaining of fibronectin (Fig. 2A) and quantified the percentage of field area positive for fibronectin (Fig. 2B). Fibronectin was deposited equally by SiRac1, SiCdc42, and SiScr control cells under 1 g conditions, whereas a tendency (although statistically insignificant) toward a decreased fibronectin area was observed for SiRhoA cells (Fig. 2B). After exposure to microgravity, a notable reduction in fibronectin fibrillogenesis of up to 50% was measured for all cell types (Fig. 2B) except for the SiRac1 cells, in which it was not significantly reduced (−20%).
Figure 2.
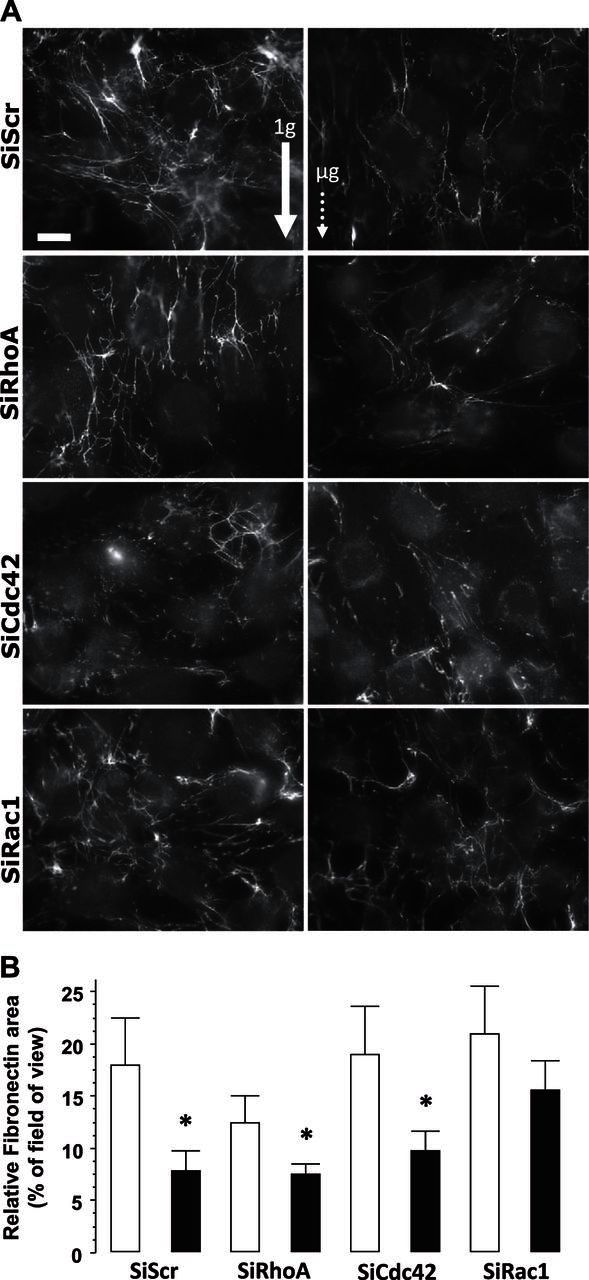
A) Images of fibronectin deposited by MG-63 cells after exposure to microgravity. Left panels: 1 g conditions (ground-based control; solid arrow). Right panels: microgravity (μg) conditions (dotted arrow). Cells were processed for detection of fibronectin by using a specific antibody. SiScr corresponds to cells transfected with scrambled sequences of SiRNA (SiRhoA, Rac1, and Cdc42) of their respective specific SiRNA. Scale bar = 10 μm. B) A minimum of 15 fields were used for the quantification of the fibronectin area. Open bars, ground-based control cells; solid bars: microgravity-exposed cells. Error bars represent sd (n = 30). *P < 0.01.
Microgravity-induced VEGFA mRNA splicing modulation
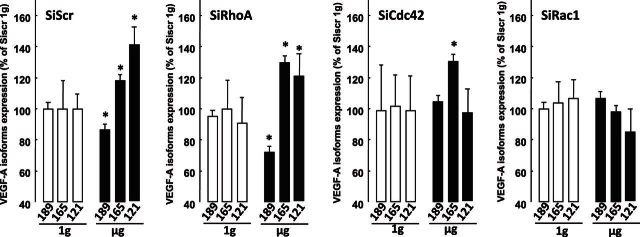
As VEGFA (so called VEGF) may up-regulate fibronectin, and since its signaling depends on its alternative splicing into various isoforms with different domains, as well as its ability to bind a heparin-associated matrix, we investigated the effect of microgravity-related conditions on VEGF isoform expressions. We evaluated VEGF121; a diffusible VEGF isoform, VEGF189, which strongly binds to matrix; and VEGF165, a splice variant with intermediate binding properties. Under 1 g conditions, the 3 VEGF isoforms were similarly expressed by the control and the Rho GTPase-silenced cells (Fig. 3). By contrast, SiScr and SiRhoA cells exposed to microgravity conditions expressed a significantly increased level of the fully and intermediately diffusible isoforms VEGF121 and VEGF165 (+40 and +20%, respectively) and a modest but significantly decreased level of mVEGF189 (−15%) (Fig. 3). In the SiCdc42 cells, only the VEGF165 isoform was up-regulated under microgravity conditions (+35%); the other two VEGF isoform levels remained unchanged (Fig. 3).
Figure 3.
mRNA expression of VEGF variants under 1 g and microgravity (μg) conditions. Values are expressed as percentages of VEGF165 in SiScr cells (100%). Open bars, ground-based control cells; solid bars, microgravity-exposed cells. Error bars represent sd (n = 6). *P < 0.01.
Notably, silencing of Cdc42 seemed to block the microgravity-induced expression of soluble VEGF121, as well as the down-regulation of mVEGF189, but had no apparent effect on VEGF165 induction (Fig. 3). As for SiRac1 cells, there was no difference in VEGF isoform expression in the microgravity-exposed cells (Fig. 3).
Microgravity-induced modulation of VEGF entrapment in the matrix
We previously demonstrated that MG-63 cells express mVEGF preferentially when exposed to mechanical stretching (27). As this VEGF isoform is particularly important in limiting apoptosis, we wanted to know whether microgravity effects could be explained, at least partly, by restricting VEGF entrapment in the extracellular matrix. We performed immunostaining of VEGF in nonpermeabilized cultures to detect mainly mVEGF. mVEGF was visualized as fibrillar-positive staining with a very limited cell-associated background staining (Fig. 4A). mVEGF immunostaining was reduced in the SiScr cells exposed to microgravity (Fig. 4A) and changed significantly (−25%) in quantitative measurements (Fig. 4B). SiRhoA and SiCdc42 cells presented a similar reduction, up to 50%, under microgravity conditions. By contrast, entrapment of VEGF in the matrix was unaffected by microgravity in SiRac1 cells (Fig. 4). Altogether, these results indicate that silencing of Rac1 cells blocks the reduction of fibrillogenesis and maintains VEGF targeting to the matrix, probably via a sustained VEGF189 expression. Of note, silencing of Cdc42 and Rac1 tended to increase VEGF entrapment in the matrix but not significantly, when compared to the SiScr control.
Figure 4.
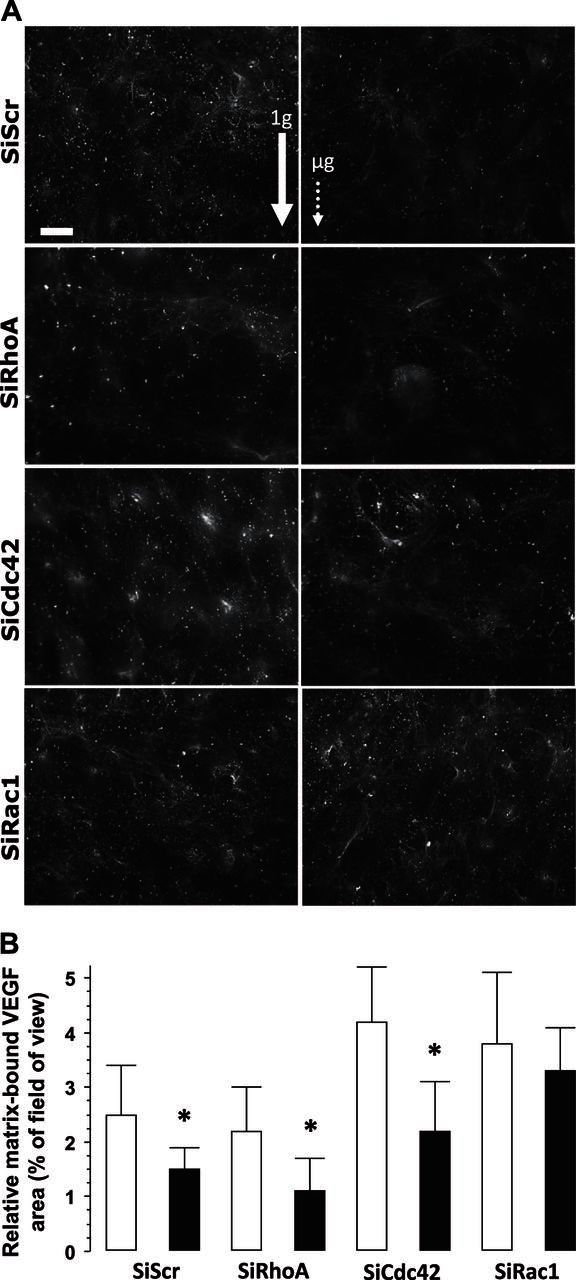
A) Images of VEGF deposition by MG-63 cells after exposure to microgravity. Left panels: 1 g conditions (ground-based control; solid arrow). Right panels: microgravity (μg) conditions (dotted arrow). Cells were processed for detection of fibronectin by using specific antibody. SiScr corresponds to cells transfected with scrambled sequences of SiRNA (RhoA, Rac1, and Cdc42) of their respective specific siRNA. Scale bar = 10 μm. B) A minimum of 15 fields were used for quantification of the mVEGF area. Open bars, ground-based control cells; solid bars, microgravity-exposed cells. Error bars represent sd (n = 30). *P < 0.01.
DISCUSSION
This study confirms that exposure of osteoblasts to microgravity impairs their cytoskeleton stability and reduces cellular tension, as well as FA formation or stability (4). Harrison and coworkers (5) recently reported similar results and also that, of the contacts established during spaceflight in osteoblasts, zyxin-positive contacts were almost absent, thus confirming that FAs in microgravity were less mature than those formed under 1 g conditions. As expected, the diminished focal contacts were associated with a reduced presence of stress fibers within cells exposed to microgravity. We also confirmed that these cytoskeletal alterations impaired matrix deposition by decreasing fibronectin fibrillogenesis.
Hughes-Fulford and Gilbertson (33) reported that fibronectin expression and deposition were altered early after microgravity exposure but were rapidly restored to control levels. This apparent discrepancy may be related to the type of cells studied but, more important, to the confluence of cultures, because fibrillogenesis is highly dependent on cell density. In fact, for quantitative imaging in our conditions, we selected only subconfluent areas, where fibrillogenesis may be more limited. A reduction of stress fibers and thus cell tension decreases fibrillogenesis initiation, and it is therefore conceivable that an alteration of stress fibers blocks or delays fibronectin fibrillogenesis, as observed in our cells.
A general picture clearly emerges: in microgravity-related conditions, osteoblasts form focal complexes (small, clustered structures, located at the cell edge) that cannot be stabilized into mature focal contacts by internal tension (34, 35). These contacts may present an important turnover due to the reduced cell tension, which would explain their inability to mature into focal/fibrillar ones and to support fibrillogenesis/matrix deposition. Furthermore, matrix degradation by MMP, known to be up-regulated in bone exposed to microgravity (36), may account for the inability of osteoblasts to adhere and differentiate in microgravity (37), and MMP induction associated with cytoskeleton alteration (21) during spaceflight may have a profound effect on the immobilization and release of ECM-sequestrated components, such as growth factors.
We report in this article that the fraction of VEGF entrapped in the matrix was significantly reduced in microgravity. Despite the fact that mRNA expression of mVEGF165 and mVEGF189 isoforms was reduced, we cannot exclude that VEGF was also degraded. mVEGF presents specific signaling as compared to soluble isoforms. It has been found that it mediates a sustained level of VEGFR2 phosphorylation and clustering, as compared to its soluble counterpart (38, 39). mVEGF does not have to be internalized to be active on VEGFR2 phosphorylation (40) but requires cooperation with integrins. Of interest, mVEGF also promotes reciprocal responses on β1-integrin by inducing its association with Fas, a response that is absent on exposure to soluble VEGF (38).
In conjunction with personal observations that mVEGF is more efficient in protecting MG-63 cells from apoptotic signals (41), it is tempting to propose that cells exposed to challenges that limit mVEGF (such as microgravity) are more sensitive to apoptotic signals. The reduced entrapment observed in microgravity may be supported by regulation of alternative splicing of VEGF. We report that cells exposed to microgravity modestly but significantly down-regulate VEGF189 and up-regulate the VEGF165 and VEGF121 isoforms. In the absence of a matrix to trap VEGF165 (as presented in this article regarding fibronectin), it may be speculated that VEGF165 represents a soluble source of VEGF with VEGF121. Unfortunately, for technical reasons (protein conservation and cross-contamination with fixative), we were unable to measure the soluble VEGF concentration in our samples. The modified splicing of VEGF in the absence of mechanical stimulation mirrored the modification of splicing found under mechanical stimulation, where MG-63 cells up-regulated mVEGF isoforms (27). An alternative splicing of VEGF may be sensitive to mechanical challenges and thus provide an interesting means of establishing cell mechanosensitivity.
The main original results presented in this study concern Rho GTPases. Other studies have already put forward that RhoA becomes altered by simulated microgravity conditions (42–44). We were surprised that silencing of RhoA did not reinforce the sensitivity of the cell to microgravity conditions or worsen adhesion or fibrillogenesis alteration. It is noteworthy that, in 1 g control conditions, the SiRhoA cells did not form smaller contacts, nor was the number of stress fibers reduced. Only fibronectin fibrillogenesis was affected. This result suggests that, in the absence of mechanical challenge, Rho GTPase signaling pathways can bypass the absence of RhoA. Since RhoA silencing in ground-based conditions does not mimic all the microgravity-induced effects and the normal sensitivity of SiRhoA cells to microgravity, these data strongly support the argument that microgravity-related effects are not primarily mediated by RhoA-dependent pathways. Nevertheless, we cannot exclude that microgravity conditions also depress other RhoA-related GTPases, such as RhoB and RhoC. Silencing of these 3 Rho GTPases is necessary for the complete disorganization of F-actin fibers observed in spaceflight (21).
Rac1 silencing suppressed microgravity-induced alterations of the cytoskeleton, fibronectin fibrillogenesis, and alternative splicing and entrapment of VEGF in the matrix. These observations lead us to propose that Rac1-dependent pathways are involved in the cellular response to microgravity, which would explain the insensitivity of SiRac1 cells to microgravity-related conditions. Rac1 is involved in many cellular functions, such as actin polymerization, vesicular traffic, β-catenin nuclear translocation (45), reactive oxygen species (ROS) formation, and autophagy (46). By temporarily silencing Rac1, we can expect to block the dynamic remodeling of the cell structures (in particular the adhesive ones), thus preventing Rac1-silenced cells from migration, detachment, and anoikis. In the context of bone biology, Rac1 is of central importance, as it is responsible for the nuclear translocation of β-catenin involved in Wnt signaling and osteoblastogenesis (45). In this context, Rac1 may be presented as a countermeasure of microgravity challenges in a differentiated cell line, but cannot be proposed as a permanent treatment of multipotent cells, as it may reduce osteoblastogenesis (8) by limiting Wnt signaling. Taking advantage of the novel and selective Rac1 inhibitor (NSC23766), an intermittent treatment (as proposed for PTH) by using this inhibitor could be particularly appropriate.
Another significant function of Rac1 concerns the fact that it is the regulatory subunit of NADPH oxydases, thus controlling an important source of ROS apart from mitochondria. Since microgravity conditions and relaxation of endogenous tension, as observed in retracting collagen gels (47), can trigger ROS production (48, 49), the silencing of Rac1 may limit ROS production, thereby protecting cells from ROS-induced damage and apoptosis. Interestingly, Rho GTPases are regulated by ROS-dependent pathways, and this regulation may explain the antagonist regulation of RhoA and Rac1 (50).
FA-dependent production of ROS after response to growth factors (a Rac1-dependent process) is limited by an αVβ3/RhoA stimulation after adhesion on fibronectin (51). In line with these regulations, we can speculate that the reduction of FA along with the diminution of fibronectin deposition (as presented in this report) is associated with reduced αVβ3/RhoA signaling. The ROS production controlled by Rac1 activity is thus no longer limited.
Despite the originality of this work in its field, it has certain limitations. It would have been interesting to monitor or measure Rac1 activation in our cells, to establish whether cells exposed to microgravity activate Rac1/Cdc42-dependent pathways. It is noteworthy that, in ground-based experiments on the same cells, the silencing of Rac1 was unable to induce a sustained RhoA activation (data not shown), thus reinforcing the idea that protection of SiRac1 cells from microgravity effects cannot be explained by subsequent RhoA activation. To monitor the activation of Rho GTPases in spaceflight, we propose to stably transfect cells with Rho-specific biosensors [proposal of the FLUOLIVE consortium (project: Live Cell Imaging of the Cytoskeleton Dynamics and Its Regulation in Microgravity)]. In that case, the limiting technical condition is to be able to monitor Förster resonance energy transfer (FRET) online aboard the International Space Station. Another important limitation is that the data obtained from cell lines have to be confirmed with primary osteoblasts.
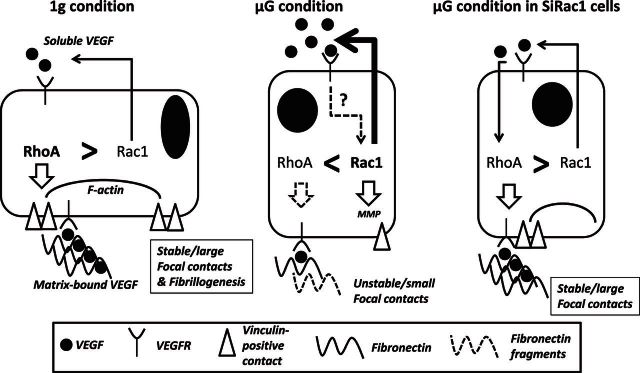
In summary, as shown in Fig. 5 and mainly on the basis of results collected in SiRac1 cells, we propose that, under 1 g conditions, RhoA activity promotes stabilization of contacts and fibrillogenesis. Under such circumstances, the VEGF produced can be immobilized in the matrix and, in cooperation with integrins, sustain RhoA and limit Rac1 activity. Since we have established that the silencing of Rac1 blocks the increased production of soluble VEGF induced under microgravity conditions, we may link the Rac1 activity to soluble VEGF secretion. Interestingly, an increase in Rac1 activity has already been associated with excessive angiogenesis (52). In ground-based controls, RhoA activity may prevail over that of Rac1, as cells cultivated on stiff substratum are known to possess high RhoA activity. In microgravity, we believe that a reduced VEGF entrapment limits cooperation of VEGFR with integrins, and thus the inhibitory action of focal contacts on Rac1 activity. In this condition, Rac1 may prevail over RhoA (Fig. 5). Although many actors, apart from VEGF, may be involved in regulation of RhoA/Rac1 activities, the specificity of soluble vs. mVEGF for Rho GTPase signaling, if any, could be of primary importance. Further investigations are needed (especially in spaceflight) to establish the complex interplay between VEGF signaling and Rho GTPase regulation, with special attention to redox status modifications of cells after exposure to mechanical challenges.
Figure 5.
Graphic summary. Under ground-based control (1 g) conditions, RhoA activity is permissive for stabilization of contacts and fibrillogenesis. In this condition, the produced VEGF can be immobilized in the matrix (Fbn), and it can be speculated that the RhoA activity is able to limit that of Rac1 when in cooperation with integrins. Since we have established that the silencing of Rac1 blocked the increased production of soluble VEGF induced in microgravity (μg) conditions, we can link the Rac1 activity with VEGF production. In 1 g controls, RhoA activity may prevail over that of Rac1, as cells cultivated on stiff substratum are known to possess high RhoA activity. In microgravity, we propose that the reduction of VEGF entrapment by limitation of Fbn deposition and increase in soluble isoform of VEGF limits the inhibitory effect of focal contacts on Rac1 activity. An imbalanced increase in Rac1 activity in microgravity may explain why Rac1 prevails over RhoA. In SiRac1 cells, we believe that the inability of silenced cells to increase Rac1 protects them from microgravity-induced effects.
CONCLUSIONS
By means of targeted silencing on osteoblastic cells, we observed that RhoA is not the primary target of microgravity in spaceflight conditions. We confirmed that osteoblast cytoskeleton integrity is impaired in spaceflight and is associated with modulation of alternative splicing of an important mechanosensitive target (VEGF), leading to a dramatic reduction in VEGF. We established that inhibition of Rac1/Cdc42 pathways protects the cells against deleterious effects, thus providing new routes of investigation to countermeasure microgravity effects.
Supplementary Material
Acknowledgments
The spaceflight opportunity on Foton-M3 was provided by the ESA. The authors acknowledge the support of the ESA and the Center for Concepts in Mechatronics (CCM; Nuenen, The Netherlands) for providing help during the preparation and integration of the Obadis experiment in the Biobox.
This study received financial support from the ESA, European Research in Space and Terrestrial Osteoporosis (ERISTO; contract 14232/NL/SH; CCN3), the Microgravity Application Program (MAP; AO-99–122, contract 14426), and the Centre National d'Etudes Spatiales (CNES; the French space agency). Moreover, this work was supported in part by the Institut National de la Santé et de la Recherche Médicale (INSERM), the University Jean Monnet, and the Institute for Science and Engineering (IFRESIS; St-Etienne, France).
The authors declare no conflicts of interest.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- cDNA
- complementary DNA
- ESA
- European Space Agency
- FA
- focal adhesion
- FCS
- fetal calf serum
- HEPES
- hydroxyethyl piperazine ethane sulfonic acid
- mVEGF
- matrix-bound vascular endothelial growth factor
- PCR
- polymerase chain reaction
- PFA
- paraformaldehyde
- Rho
- Ras homology
- ROS
- reactive oxygen species
- RT
- reverse transcription
- SiScr
- silenced, scrambled
- VEGF
- vascular endothelial growth factor
- VEGR
- vascular endothelial growth factor receptor
REFERENCES
- 1. Vico L., Collet P., Guignandon A., Lafage-Proust M. H., Thomas T., Rehaillia M., Alexandre C. (2000) Effects of long-term microgravity exposure on cancellous and cortical weight-bearing bones of cosmonauts. Lancet 355, 1607–1611 [DOI] [PubMed] [Google Scholar]
- 2. Carmeliet G., Bouillon R. (1999) The effect of microgravity on morphology and gene expression of osteoblasts in vitro. FASEB J. 13(Suppl.), S129–S134 [DOI] [PubMed] [Google Scholar]
- 3. Burger E. H., Klein-Nulend J. (1998) Microgravity and bone cell mechanosensitivity. Bone 22, 127S–130S [DOI] [PubMed] [Google Scholar]
- 4. Hughes-Fulford M. (2003) Function of the cytoskeleton in gravisensing during spaceflight. Adv. Space Res. 32, 1585–1593 [DOI] [PubMed] [Google Scholar]
- 5. Nabavi N., Khandani A., Camirand A., Harrison R. E. (2011) Effects of microgravity on osteoclast bone resorption and osteoblast cytoskeletal organization and adhesion. Bone 49, 965–974 [DOI] [PubMed] [Google Scholar]
- 6. Buravkova L. B., Gershovich P. M., Gershovich J. G., Grigor'ev A. I. (2010) Mechanisms of gravitational sensitivity of osteogenic precursor cells. Acta Nat. 2, 28–36 [PMC free article] [PubMed] [Google Scholar]
- 7. Capulli M., Rufo A., Teti A., Rucci N. (2009) Global transcriptome analysis in mouse calvarial osteoblasts highlights sets of genes regulated by modeled microgravity and identifies a “mechanoresponsive osteoblast gene signature”. J. Cell. Biochem. 107, 240–252 [DOI] [PubMed] [Google Scholar]
- 8. McBeath R., Pirone D. M., Nelson C. M., Bhadriraju K., Chen C. S. (2004) Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev. Cell 6, 483–495 [DOI] [PubMed] [Google Scholar]
- 9. Schiller H. B., Fassler R. (2013) Mechanosensitivity and compositional dynamics of cell-matrix adhesions. EMBO Rep. 14, 509–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Balaban N. Q., Schwarz U. S., Riveline D., Goichberg P., Tzur G., Sabanay I., Mahalu D., Safran S., Bershadsky A., Addadi L., Geiger B. (2001) Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nat. Cell Biol. 3, 466–472 [DOI] [PubMed] [Google Scholar]
- 11. Ingber D. E. (2008) Tensegrity and mechanotransduction. J. Bodyw. Mov. Ther. 12, 198–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cukierman E., Pankov R., Stevens D. R., Yamada K. M. (2001) Taking cell-matrix adhesions to the third dimension. Science 294, 1708–1712 [DOI] [PubMed] [Google Scholar]
- 13. Hamidouche Z., Fromigue O., Ringe J., Haupl T., Vaudin P., Pages J. C., Srouji S., Livne E., Marie P. J. (2009) Priming integrin alpha5 promotes human mesenchymal stromal cell osteoblast differentiation and osteogenesis. Proc. Natl. Acad. Sci. U. S. A. 106, 18587–18591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guignandon A., Lafage-Proust M. H., Usson Y., Laroche N., Caillot-Augusseau A., Alexandre C., Vico L. (2001) Cell cycling determines integrin-mediated adhesion in osteoblastic ROS 17/2.8 cells exposed to space-related conditions. FASEB J. 15, 2036–2038 [DOI] [PubMed] [Google Scholar]
- 15. Guignandon A., Vico L., Alexandre C., Lafage-Proust M. H. (1995) Shape changes of osteoblastic cells under gravitational variations during parabolic flight–relationship with PGE2 synthesis. Cell Struct. Funct. 20, 369–375 [DOI] [PubMed] [Google Scholar]
- 16. Ridley A. J. (2006) Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol. 16, 522–529 [DOI] [PubMed] [Google Scholar]
- 17. Schwarz U. S., Balaban N. Q., Riveline D., Bershadsky A., Geiger B., Safran S. A. (2002) Calculation of forces at focal adhesions from elastic substrate data: the effect of localized force and the need for regularization. Biophys. J. 83, 1380–1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hall A. (2012) Rho family GTPases. Biochem. Soc. Trans. 40, 1378–1382 [DOI] [PubMed] [Google Scholar]
- 19. Elbashir S. M., Harborth J., Lendeckel W., Yalcin A., Weber K., Tuschl T. (2001) Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 411, 494–498 [DOI] [PubMed] [Google Scholar]
- 20. Giang Ho T. T., Stultiens A., Dubail J., Lapiere C. M., Nusgens B. V., Colige A. C., Deroanne C. F. (2011) RhoGDIalpha-dependent balance between RhoA and RhoC is a key regulator of cancer cell tumorigenesis. Mol. Biol. Cell 22, 3263–3275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Deroanne C. F., Hamelryckx D., Ho T. T., Lambert C. A., Catroux P., Lapiere C. M., Nusgens B. V. (2005) Cdc42 downregulates MMP-1 expression by inhibiting the ERK1/2 pathway. J. Cell Sci. 118, 1173–1183 [DOI] [PubMed] [Google Scholar]
- 22. Ho T. T., Merajver S. D., Lapiere C. M., Nusgens B. V., Deroanne C. F. (2008) RhoA-GDP regulates RhoB protein stability: potential involvement of RhoGDIalpha. J. Biol. Chem. 283, 21588–21598 [DOI] [PubMed] [Google Scholar]
- 23. Goel H. L., Mercurio A. M. (2012) Enhancing integrin function by VEGF/neuropilin signaling: implications for tumor biology. Cell Adh. Migr. 6, 554–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kumei Y., Akiyama H., Hirano M., Shimokawa H., Morita S., Mukai C., Nagaoka S. (1999) Space flight modulates signal transduction pathway of growth factor receptors in rat osteoblasts. Biol. Sci. Space 13, 142–143 [PubMed] [Google Scholar]
- 25. de Groot R. P., Rijken P. J., den Hertog J., Boonstra J., Verkleij A. J., de Laat S. W., Kruijer W. (1991) Nuclear responses to protein kinase C signal transduction are sensitive to gravity changes. Exp. Cell Res. 197, 87–90 [DOI] [PubMed] [Google Scholar]
- 26. Rijken P. J., Boonstra J., Verkleij A. J., de Laat S. W. (1994) Effects of gravity on the cellular response to epidermal growth factor. Adv. Space Biol. Med. 4, 159–188 [DOI] [PubMed] [Google Scholar]
- 27. Faure C., Linossier M. T., Malaval L., Lafage-Proust M. H., Peyroche S., Vico L., Guignandon A. (2008) Mechanical signals modulated vascular endothelial growth factor-A (VEGF-A) alternative splicing in osteoblastic cells through actin polymerisation. Bone 42, 1092–1101 [DOI] [PubMed] [Google Scholar]
- 28. Infanger M., Kossmehl P., Shakibaei M., Baatout S., Witzing A., Grosse J., Bauer J., Cogoli A., Faramarzi S., Derradji H., Neefs M., Paul M., Grimm D. (2006) Induction of three-dimensional assembly and increase in apoptosis of human endothelial cells by simulated microgravity: impact of vascular endothelial growth factor. Apoptosis 11, 749–764 [DOI] [PubMed] [Google Scholar]
- 29. Van Nieuw Amerongen G. P., Koolwijk P., Versteilen A., van Hinsbergh V. W. (2003) Involvement of RhoA/Rho kinase signaling in VEGF-induced endothelial cell migration and angiogenesis in vitro. Arterioscler. Thromb. Vasc. Biol. 23, 211–217 [DOI] [PubMed] [Google Scholar]
- 30. Bryan B. A., Dennstedt E., Mitchell D. C., Walshe T. E., Noma K., Loureiro R., Saint-Geniez M., Campaigniac J. P., Liao J. K., D'Amore P. A. (2010) RhoA/ROCK signaling is essential for multiple aspects of VEGF-mediated angiogenesis. FASEB J. 24, 3186–3195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dieriks B., De Vos W. H., Moreels M., Ghardi M., Hennekam R., Broers J. L., Baatout S., van Oostveldt P. (2011) Multiplexed profiling of secreted proteins for the detection of potential space biomarkers. Mol. Med. Rep. 4, 17–23 [DOI] [PubMed] [Google Scholar]
- 32. Deroanne C., Vouret-Craviari V., Wang B., Pouyssegur J. (2003) EphrinA1 inactivates integrin-mediated vascular smooth muscle cell spreading via the Rac/PAK pathway. J. Cell Sci. 116, 1367–1376 [DOI] [PubMed] [Google Scholar]
- 33. Hughes-Fulford M., Gilbertson V. (1999) Osteoblast fibronectin mRNA, protein synthesis, and matrix are unchanged after exposure to microgravity. FASEB J. 13(Suppl.), S121–S127 [DOI] [PubMed] [Google Scholar]
- 34. Danen E. H., Sonneveld P., Brakebusch C., Fassler R., Sonnenberg A. (2002) The fibronectin-binding integrins alpha5beta1 and alphavbeta3 differentially modulate RhoA-GTP loading, organization of cell matrix adhesions, and fibronectin fibrillogenesis. J. Cell Biol. 159, 1071–1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zaidel-Bar R., Milo R., Kam Z., Geiger B. (2007) A paxillin tyrosine phosphorylation switch regulates the assembly and form of cell-matrix adhesions. J. Cell Sci. 120, 137–148 [DOI] [PubMed] [Google Scholar]
- 36. Blaber E. A., Dvorochkin N., Lee C., Alwood J. S., Yousuf R., Pianetta P., Globus R. K., Burns B. P., Almeida E. A. (2013) Microgravity induces pelvic bone loss through osteoclastic activity, osteocytic osteolysis, and osteoblastic cell cycle inhibition by CDKN1a/p21. PloS One 8, e61372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Carmeliet G., Nys G., Bouillon R. (1997) Microgravity reduces the differentiation of human osteoblastic MG-63 cells. J. Bone Miner. Res. 12, 786–794 [DOI] [PubMed] [Google Scholar]
- 38. Chen T. T., Luque A., Lee S., Anderson S. M., Segura T., Iruela-Arispe M. L. (2010) Anchorage of VEGF to the extracellular matrix conveys differential signaling responses to endothelial cells. J. Cell Biol. 188, 595–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Anderson S. M., Chen T. T., Iruela-Arispe M. L., Segura T. (2009) The phosphorylation of vascular endothelial growth factor receptor-2 (VEGFR-2) by engineered surfaces with electrostatically or covalently immobilized VEGF. Biomaterials 30, 4618–4628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Anderson S. M., Shergill B., Barry Z. T., Manousiouthakis E., Chen T. T., Botvinick E., Platt M. O., Iruela-Arispe M. L., Segura T. (2011) VEGF internalization is not required for VEGFR-2 phosphorylation in bioengineered surfaces with covalently linked VEGF. Integr. Biol. 3, 887–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Faure C., Vico L., Tracqui P., Laroche N., Vanden-Bossche A., Linossier M. T., Rattner A., Guignandon A. (2010) Functionalization of matrices by cyclically stretched osteoblasts through matrix targeting of VEGF. Biomaterials 31, 6477–6484 [DOI] [PubMed] [Google Scholar]
- 42. Kumei Y., Shimokawa H., Ohya K., Katano H., Akiyama H., Hirano M., Morita S. (2007) Small GTPase Ras and Rho expression in rat osteoblasts during spaceflight. Ann. N. Y. Acad. Sci. 1095, 292–299 [DOI] [PubMed] [Google Scholar]
- 43. Higashibata A., Imamizo-Sato M., Seki M., Yamazaki T., Ishioka N. (2006) Influence of simulated microgravity on the activation of the small GTPase Rho involved in cytoskeletal formation–molecular cloning and sequencing of bovine leukemia-associated guanine nucleotide exchange factor. BMC Biochem. 7, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Meyers V. E., Zayzafoon M., Douglas J. T., McDonald J. M. (2005) RhoA and cytoskeletal disruption mediate reduced osteoblastogenesis and enhanced adipogenesis of human mesenchymal stem cells in modeled microgravity. J. Bone Miner. Res. 20, 1858–1866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wu X., Tu X., Joeng K. S., Hilton M. J., Williams D. A., Long F. (2008) Rac1 activation controls nuclear localization of beta-catenin during canonical Wnt signaling. Cell 133, 340–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Carroll B., Mohd-Naim N., Maximiano F., Frasa M. A., McCormack J., Finelli M., Thoresen S. B., Perdios L., Daigaku R., Francis R. E., Futter C., Dikic I., Braga V. M. (2013) The TBC/RabGAP Armus coordinates Rac1 and Rab7 functions during autophagy. Dev. Cell 25, 15–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kheradmand F., Werner E., Tremble P., Symons M., Werb Z. (1998) Role of Rac1 and oxygen radicals in collagenase-1 expression induced by cell shape change. Science 280, 898–902 [DOI] [PubMed] [Google Scholar]
- 48. Sun Y., Shuang F., Chen D. M., Zhou R. B. (2013) Treatment of hydrogen molecule abates oxidative stress and alleviates bone loss induced by modeled microgravity in rats. Osteoporosis Int. 24, 969–978 [DOI] [PubMed] [Google Scholar]
- 49. Wang J., Zhang J., Bai S., Wang G., Mu L., Sun B., Wang D., Kong Q., Liu Y., Yao X., Xu Y., Li H. (2009) Simulated microgravity promotes cellular senescence via oxidant stress in rat PC12 cells. Neurochem. Int. 55, 710–716 [DOI] [PubMed] [Google Scholar]
- 50. Mitchell L., Hobbs G. A., Aghajanian A., Campbell S. L. (2013) Redox regulation of Ras and Rho GTPases: mechanism and function. Antioxid. Redox Signal. 18, 250–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lin L. J., Grimme J. M., Sun J., Lu S., Gai L., Cropek D. M., Wang Y. (2013) The antagonistic roles of PDGF and integrin alphavbeta3 in regulating ROS production at focal adhesions. Biomaterials 34, 3807–3815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hoang M. V., Nagy J. A., Senger D. R. (2011) Active Rac1 improves pathologic VEGF neovessel architecture and reduces vascular leak: mechanistic similarities with angiopoietin-1. Blood 117, 1751–1760 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.