Abstract
This study aimed to determine whether epigenetic malprogramming induced by high-fat diet (HFD) has an obesogenic effect on nonmated and mated female rats and their offspring. Further, it aimed to reprogram offspring's epigenetic malprogramming and phenotype by providing normal diet after weaning. Body weight (BW) was measured, and plasma and hypothalamic arcuate nuclei were collected for analysis of hormones, mRNA, and DNA CpG methylation of the promoter of Pomc, a key factor in control of food intake. In nonmated females, HFD decreased Pomc/leptin ratio by ∼38%. This finding was associated with Pomc promoter hypermethylation. While heavier during pregnancy, during lactation HFD dams showed sharper BW decrease (2.5-fold) and loss of Pomc promoter hypermethylation. Moreover, their weight loss was correlated with demethylation (r=−0.707) and with gadd45b mRNA expression levels (r=0.905). Even though offspring of HFD dams ate standard chow from weaning, they displayed increased BW, Pomc promoter hypermethylation, and vulnerability to HFD challenge (3-fold kilocalorie intake increase). These findings demonstrate a long-term effect of maternal HFD on CpG methylation of the Pomc promoter in the offspring, which was not reprogrammed by standard chow from weaning. Further, the results suggest a possible mechanism of demethylation of the Pomc promoter following pregnancy and lactation.—Marco, A., Kisliouk, T., Tabachnik, T., Meiri, N., Weller, A. Overweight and CpG methylation of the Pomc promoter in offspring of high-fat-diet-fed dams are not “reprogrammed” by regular chow diet in rats.
Keywords: obesity, leptin, epigenetics
The nutritional environment during early development has been shown to affect the expression of genes critical to regulation of energy intake and expenditure (1). Research has underscored the importance of this critical time in the development of neuroendocrine feedback loops regulating energy homeostasis in the hypothalamus (2). Infants of obese women are now recognized to have increased fat mass and insulin or leptin resistance in childhood (3). Since long-term consequences in the offspring may develop even independent of genetic background (4, 5), the perinatal environment has been considered as an important etiopathogenetic mechanism, interfering with programming and thus increasing health risks in the offspring as they grow and develop (4–6).
The existence of permanent overweight associated with hyperphagia in offspring of obese mothers might indicate that this phenotype results from a persisting dysregulation of food intake and body weight (BW; ref. 7). The regulation of energy balance is maintained by neuropeptides expressed by neurons within nuclei located in the mediobasal hypothalamus (8, 9). One of the major anorexigenic neuropeptides, α-melanocyte-stimulating hormone (α-MSH), the post-transcriptional cleavage product of proopiomelanocortin (Pomc), is expressed in the hypothalamic arcuate nucleus (Arc) and is axonally transported to the paraventricular nucleus, where it binds to the melanocortin 4 receptor (MC4-R) (9), decreasing food intake and BW. The hypothalamic expression of Pomc is regulated mainly by peripheral circulating leptin. Mediated by the hypothalamic leptin receptor, leptin binding to Pomc neurons activates a signal transduction mechanism that includes activation and binding of several transcription factors [e.g., specificity protein 1 (Sp1), nuclear factor κ-light-chain-enhancer of activated B cells (Nf-kB)] to the Pomc promoter (10–12). It was found that in common forms of obesity, both animals and humans become leptin resistant, a phenomenon associated with impaired regulation of energy homeostasis (8).
The expression level of proteins may be determined by an epigenetic code, based on both short- and long-term post-translational modifications of histones and DNA methylation (13, 14). In most cases, the extent of methylation of a promoter is inversely correlated with the activity of the respective gene (14, 15). Recent data have shown that environmental alterations, such as undernutrition, overfeeding, and high-fat diet (HFD) can induce hyperphagia, overweight, and consequent diabetogenic disturbances that might be mediated by epigenetic malprogramming (16–18). In fact, previous research on adult male rodents showed that hypermethylation on the promoter region of the Pomc gene interferes with transcription factor binding (11). This condition blocks the effect of high leptin levels and therefore may serve as one of the mechanisms of leptin resistance (11, 19).
Here we present a novel approach in which the effect of HFD was examined on nonmated females and lactating dams while focusing on CpG methylation of the promoter of the hypothalamic neuropeptide Pomc. We note that comparing nonmated to mated dams is not common in life-course studies, which traditionally focus on the offspring's health outcomes. In addition, we hypothesized that epigenetic malprogramming induced by maternal HFD will have a long-term effect on the offspring's vulnerability to develop obesity. Therefore, we tried to reprogram their obesogenic phenotype by providing a normal diet after weaning. The findings provide insight into the impact of maternal diet on central nervous system regulation of offspring metabolic function.
MATERIALS AND METHODS
Subjects
Wistar outbred female rats (supplied by Harlan Laboratories Ltd., Jerusalem, Israel) were raised as described previously (19) in the specific pathogen-free colony at Bar-Ilan University. Standard chow (C; 2018SCF; Teklad Global 6% Fat Rodent Diet; Harlan, Madison, WI, USA) or HFD (rodent diet with 60% fat; D12492; Research Diets, Inc., New Brunswick, NJ, USA) and water were freely available. The F0 females were allocated to the two groups randomly and were raised from postnatal day (PND) 22 to PND 80 on either HFD or C diet. At PND 80, half of each experimental group was euthanized for plasma and brain analyses (denoted as C or HFD nulliparous, n=10/group). The other halves were mated with a Wistar male (of average weight). Using vaginal smears, day of conception (estrus phase + sperm) was coded as “beginning of pregnancy,” trimester 0 (T0). During pregnancy, the dams were weighed at the end of each trimester (T1, T2, and T3). Litter size was adjusted to 8–10 offspring. At PND 22, the dams were weighed and euthanized (marked as C or HFD dams; n=5–6/group). All offspring (F1) were weaned at PND 22. A total of 22 female offspring were euthanized at weaning for hormonal, mRNA, and DNA methylation analyses (marked as C or HFD pups). An additional 36 offspring were raised on C diet till adulthood. BW and food intake were measured every fifth day. Chow intake was converted to kilocalories (1 g C diet=3.1 kcal), according to the manufacturer's data. At PND 80, 20 female offspring were euthanized (marked as C-C or HFD-C adults). The remaining rats received HFD challenge for 30 d (marked as C-C-HFc; n=5 or HFD-C-HFc; n=10). HFD consumption was measured every fifth day in grams and converted to kcal (1 g HFD diet=5.24 kcal), according to the manufacturer's data. The research protocol was approved by the Institutional Animal Care and Use Committee of Bar-Ilan University, and it adhered to the guidelines of the Society for Neuroscience. All efforts were made to minimize animal suffering and to reduce the number of animals used.
Tissues and plasma
Trunk blood for leptin analyses was collected in chilled vacuette tubes coated with EDTA. Plasma was stored at −80°C. Leptin levels were assessed using commercial ELISA kits (R&D Systems, Minneapolis, MN, USA), according to the manufacturer's instructions. Animals were euthanized by decapitation (between 1200 and 1400). Brains were removed and immediately frozen on dry ice and stored at −80°C. Coronal brain sections of the hypothalamus were cut using a cryostat (−2.3 to −4.5 mm from Bregma according to Paxinos and Watson; ref. 20), and the Arc was extracted from the brain, using a truncated needle. The punches from each rat were immediately immersed in RNALater (Ambion, Austin, TX, USA) and frozen on dry ice.
RNA isolation and real-time PCR
Total RNA was isolated using TriReagent (Molecular Research Center, Cincinnati, OH, USA) according to the manufacturer's instructions. Hypothalamic RNA (0.5 μg) was reverse-transcribed to single-stranded cDNA by SuperScript II Reverse Transcriptase and oligo (dT) plus random primers (Invitrogen, Carlsbad, CA, USA). Real-time PCR was performed, in duplicates, with 10 ng cDNA on a model 7000 sequence analysis system (Applied Biosystems, Foster City, CA, USA) with the Absolute Blue SYBR Green ROX Mix (ABgene, Epsom, UK) as described previously (21). Dissociation curves were analyzed following each real-time PCR to confirm the presence of only one product and the absence of primer dimer formation. The threshold cycle (Ct) for each tested gene (X) was used to quantify the relative abundance of that gene using the formula 2 − (Ct gene X − Ct standard). A recent article (22) suggested that hypoxanthine phosphoribosyltransferase 1 (Hprt1) and β-2 microglobulin (B2m) are the optimal reference genes for reverse-transcription quantitative PCR (RT-qPCR) in the rat hypothalamus for the study of obesity. This is because they did not change following HFD exposure. Therefore, the mRNA of all the genes was normalized to mRNA expression of Hprt1 and B2m. The following primers were used for real-time PCR: Pomc, forward GCTACGGCGGCTTCATGA and reverse CCTCACTGGCCCTTCTTGTG; growth arrest and DNA-damage-inducible, β (gadd45b), forward CTGCCTCCTGGTCACGAACT and reverse GGTTATTGCCTCTGCTCTCTTCA; Hprt1, forward GCGAAAGTGGAAAAGCCAAGT and reverse GCCACATCAACAGGACTCTTGTAG; B2m, forward TTCCACCCACCTCAGATAGAAAT and reverse TGTGAGCCAGGATGTAGAAAGAC.
DNA methylation analysis
Total DNA was isolated using TriReagent (Molecular Research Center) according to the manufacturer's instructions. The purified DNA was processed for bisulfite modification using the Imprint DNA Modification kit (Sigma, St. Louis, MO, USA) according to the manufacturer's instructions. The quality of the DNA after bisulfite reaction was evaluated using an agrose gel to verify that the DNA was intact and not nicked. The DNA methylation status of the Pomc promoter was evaluated as described previously (20); Pomc promoter area was amplified with the forward primer (−540/−515 bp upstream to the start of transcription) 5′-GTTTTGGGTTGTTATGATTTTTGAT-3′ and the reverse primer (−1/−23 bp upstream to the start of transcription) 5′-AATCCCTATCACTCTTCTCTCTT-3′. PCR amplification of the genomic fragment of Pomc promoter of 540 bp was performed using Biotaq DNA Polymerase (Bioline USA Inc, Taunton, MA, USA) at following conditions: an initial incubation at 95°C for 5 min, 40 cycles including denaturation at 95°C for 30 s, annealing at 58°C for 30 s, and elongation at 72°C for 45 s. The PCR products were separated on agarose gel (1.5%) followed by gel extraction with the QIAquick Gel Extraction Kit (Qiagen, Hilden, Germany) and cloning into PGEMT-easy vectors (Promega, Madison, WI, USA) according to the manufacturer's instructions. PGEMT-easy vectors were multiplied using JM109-competent Escherichia coli cells (Promega) and then purified from the bacteria with the QIAprep Spin Miniprep Kit (Qiagen). Pomc promoter sequence and its methylation status were analyzed by sequencing (Macrogene Inc., Seoul, Korea), and the percentage of 16 CpG sites (position −238 to −107) was measured from 6 to 11 positive clones per rat. All sequencing yielded a complete expected fragment of 540 bp. In each experimental group, between 13 and 20% of the sequences were completely converted; i.e., all C was converted to U, indicating that the bisulfite reaction was efficient.
Statistical analysis
Differences in weight gain and food intake were assessed by 2-way repeated measures ANOVA with 1 between-subjects factor (diet) and 1 within-subjects factor (age). Group differences on Pomc/leptin ratio were compared by 1-way ANOVA. Post hoc tests for simple effects with Bonferroni adjustment were used. Group differences in BW, mRNA expression levels (RT-qPCR), percentage total methylation, and leptin levels (ELISA) were analyzed by Student's t tests. Differences in methylation levels at specific sites were analyzed by χ2 analyses. Statistical analyses were performed with SPSS 20.0 software (IBM Corp., Armonk, NY, USA).
RESULTS
Chronic HFD feeding in dams had a long-term effect on BW of C-fed second-generation female offspring
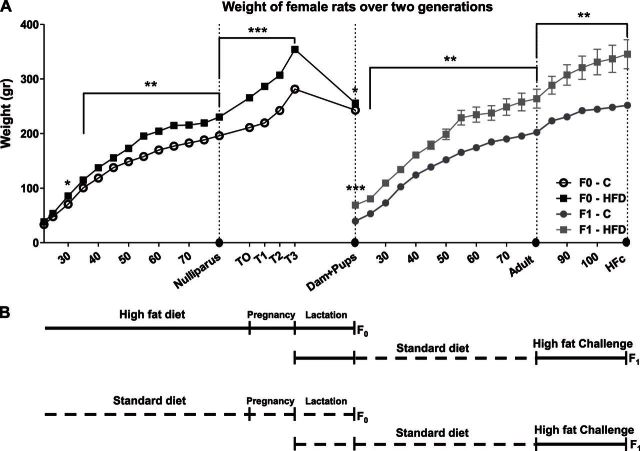
Wistar female rats (F0) that were raised from PND 22 on HFD presented a significantly higher BW compared to C-fed rats, starting from PND 30 until PND 90 (d 30, P<0.05; all other ages, P<0.01, Fig. 1A). After conception (T0), females fed HFD displayed significantly higher BW at the end of each trimester (T1, T2, and T3; all P<0.001; Fig. 1A). During the 3 wk of lactation, HFD-fed dams showed a sharper BW decrease [age×group interaction, ∼24% BW decrease, F (1,10)=46.4, P<0.001, Fig. 1A], compared to C-fed dams (∼9% BW decrease), but their BW at the end of lactation remained still higher (P<0.05, Figs. 1A and 3A). Offspring (F1) of the HFD dams presented higher BW than those of C dams at PND 22 (P<0.001, Figs. 1A and 5A). Although offspring of HFD dams fed C diet from weaning (HFD-C group), they consumed significantly more kilocalories per day (see Fig. 7A) and exhibited higher BW throughout their life, compared to the offspring of the C control group (C-C group; all P<0.01; Figs. 1A and 5D). During the 30 d of HFD challenge (PND 80 to PND 110), offspring of HFD dams were significantly heavier (all P<0.01; Fig. 1A).
Figure 1.
Chronic HFD in the first generation had a long-term effect on second-generation offspring. A) Female Wistar rats (F0; n=20) were raised from PND 22 till PND 80 on either HFD or C diet. Offspring (F1) were weaned at PND 22 and were raised on C till adulthood. From PND 80, all groups received HFD challenge for 30 d. BW was measured every fifth day. Circles on the x axis indicate euthanasia points for both groups: F0 at PND 80 (nulliparous: n=10/group) and at the end of lactation (dams: C, n=7; HFD, n=6); F1 at PND 22 (pups: C or HFD), PND 80 (adults: C-C or HFD-C) and PND 110 (C-C-HFc or HFD-C-HFc). Adult offspring code reflects their dam's diet (C or HFD) and their own diet; HFc indicates HFD challenge. All data are presented as means ± sem. *P<0.05, **P<0.01, ***P<0.001 vs. C. B) Schematic representation of the experimental course. Broken line indicates C diet; solid line indicates HFD.
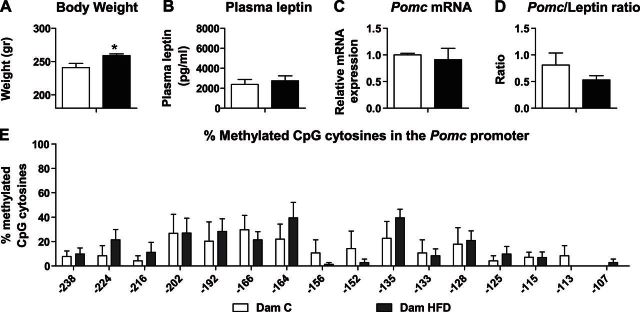
Figure 3.
At the end of lactation, HFD-fed dams demonstrated similar plasma leptin and Arc Pomc mRNA levels compared to C-fed dams. First-generation dams from both groups were euthanized at PND 22 (C dams: n=5; HFD dams: n=6). A–C) Average BW (A), plasma leptin levels (B), and Pomc mRNA levels (C). Hprt1 and B2m were used as standard genes. Relative gene expression in the C dams group was set to 1. D) Ratio between Pomc and leptin levels was calculated by dividing the raw data for each animal separately. E) Methylation analysis of the Pomc promoter −540 bp upstream to the ATG, in the hypothalamic Arc region of first-generation dams. Data are presented as mean ± sem percentage of methylation in each group. *P < 0.05 vs. C.
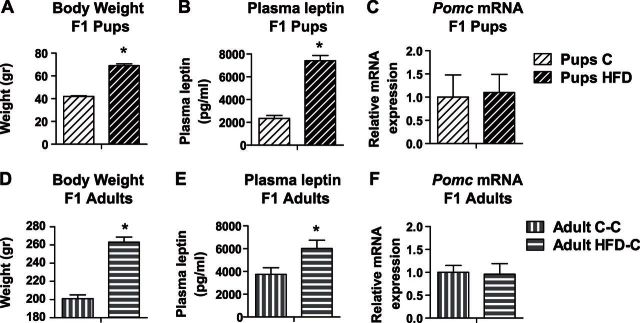
Figure 5.
Female offspring of HFD-treated dams presented significantly increased BW and leptin levels from weaning to adulthood. Second-generation offspring of either C- or HFD-fed dams were euthanized at weaning day (pups: C or HFD; A–C) or were raised till PND 80 (adults: C-C or HFD-C; D–F) on C diet. A, D) Average BW (n=10/group). B, E) Plasma leptin levels (pups: n=7–8/group; adults: n=6–8/group). C, F) mRNA levels of Pomc (pups: n=8–11/group; adults: n=5–6/group). Relative gene expression in the C group was set to 1. Data are presented as means ± sem. *P < 0.05 vs. C.
Figure 7.
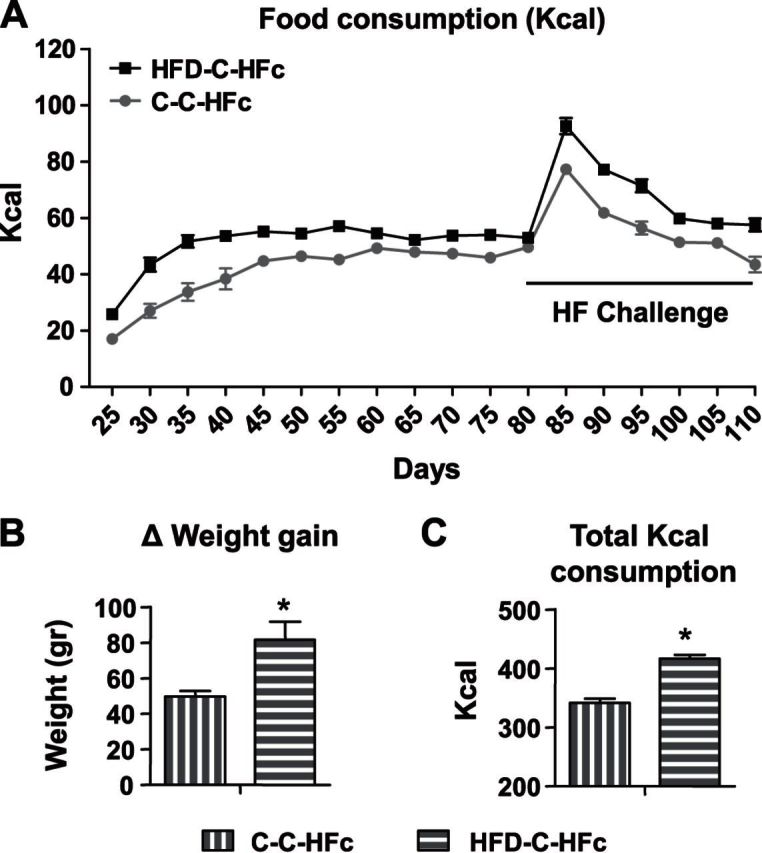
While providing HFD challenge, adult offspring of HFD-treated dams consumed significantly more kilocalories and gained significantly more weight, compared to offspring of control dams. Adult offspring of dams from both groups were given HFD challenge (HFc), from PND 80, for 30 d (C-C-HFc: n=5; HFD-C-HFc: n=10). A) Food intake was measured every fifth day, and energy intake was calculated according to the manufacturer's data (1 g C diet = 3.1 kcal; 1 g HFD = 5.24 kcal). B) Change in weight gain after 30 d of HFD challenge (PND 110 to PND 80). C) Total kilocalorie consumption in the HFD challenge. All data are presented as means ± sem. *P < 0.05 vs. C-C-HFc.
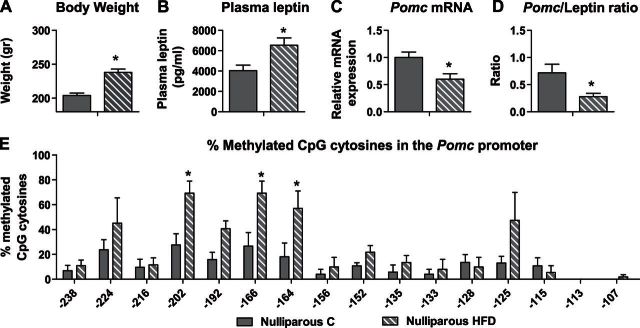
HFD induces overweight and hypermethylation of Pomc promoter in nulliparous female rats
After 2 mo of HFD feeding, nulliparous rats presented significantly reduced (∼40%) Pomc mRNA expression levels compared to nulliparous C-fed rats (n=6/group; P<0.05; Fig. 2C), despite significantly higher BW (nulliparous C: 204 g; HFD: 238 g; n=10/group; P<0.001; Fig. 2A) and plasma leptin levels (nulliparous C: 4041 pg/μl; HFD: 6539 pg/μl; n=6/group; P=0.01; Fig. 2B). The Pomc/leptin ratio was significantly lower in the HFD-fed nulliparous rats (n=6/group; P<0.05; Fig. 2D), compared to the C-fed nulliparous rats, which suggests that high levels of leptin did not affect Pomc expression adequately in those animals. We have demonstrated previously a correlation between BW and methylation status of the Pomc promoter in adult male rats (20). Therefore, it was of interest to analyze methylation profile of the Pomc promoter in age-matched HFD-fed female rats. We found high methylation levels at 3 specific sites on the Pomc promoter in the nulliparous HFD group compared to normal dieting counterparts (n=5/group; site −202: χ2=6.23, df=1, P+0.013; site −166: χ2=4.8, df=1, P=0.028; site −164: χ2=3.9, df=1, P=0.048; Fig. 2E).
Figure 2.
HFD induces overweight and hypermethylation of Pomc promoter in nulliparous female rats prior to pregnancy. First-generation nulliparous females from both groups were euthanized at PND 80. A) Average BW (g; n=10/group). B) Plasma leptin levels were assessed using a commercial ELISA kit (n=6/group). C) mRNA levels of Pomc. Arc nuclei were dissected and subjected to real-time PCR with Pomc specific primers. Hprt1 and B2m were used as standard genes. Relative gene expression in the nulliparous C group was set to 1 (n=6/group). D) Ratio between the Pomc and the leptin levels was calculated by dividing the raw data for each animal separately (n=6/group). E) Methylation analysis of the Pomc promoter −540 bp upstream to the ATG, in the hypothalamic Arc region of nulliparous rats (n=5/group). Data are presented as mean ± sem percentage of methylation in each group. *P < 0.05 vs. C.
At the end of lactation, HFD-fed dams demonstrated similar plasma leptin and Arc Pomc mRNA levels compared to C-fed dams
At the end of lactation, a decrease in BW difference was found between the HFD- and C-fed dams. Although HFD dams were still heavier compared to the C dams (241 and 256 g, respectively; n=5–6/group; P<0.05; Fig. 3A), they presented similar leptin levels and Pomc mRNA expression (n=5–6/group; Fig. 3B, C). In addition, Pomc/leptin ratio of HFD dams was similar to that of C dams (n=5–6/group; Fig. 3D). Moreover, comparison of methylation levels of the Pomc promoter between HFD and C dams did not reveal any significant differences (n=5–6/group; Fig. 3E). These methylation patterns in the HFD dams are in accordance with their normalized values of leptin, Pomc mRNA levels and the ratio between them.
HFD-fed dams presented increased gadd45b mRNA expression levels that were correlated with their weight loss
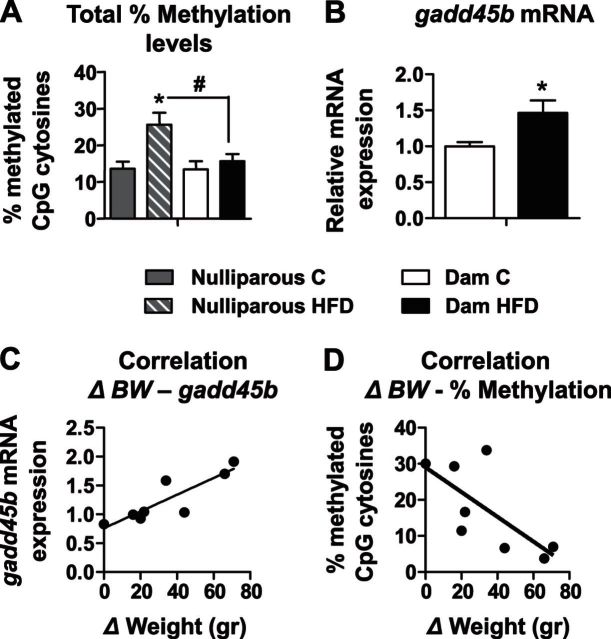
We compared the total percentage of methylation in the all of the F0 females. Results show that nulliparous HFD-fed rats presented significantly higher total percentage methylation on the Pomc promoter, compared to the nulliparous C-fed rats (26.6 and 13.6%, respectively; P<0.01; n=5–6/group; Fig. 4A). More important, the nulliparous HFD females presented significantly elevated total methylation levels (26.6%) compared to the HFD dams (15.7%; n=5–6/group; P<0.001; Fig. 4A). Since a loss of Pomc promoter hypermethylation in the HFD dams was found, we analyzed the levels of gadd45b, a protein recently suggested to have a potential role in active DNA demethylation (23). The HFD dams presented higher levels of gadd45b (1.56-fold) at the end of the lactation period, compared to nulliparous HFD rats (n=5–6/group; P<0.05; Fig. 4B). Further, we calculated the weight lost during lactation (BW at end of T3 − BW at end of weaning = ΔBW) for each dam and correlated it with gadd45b or the total percentage of methylation on the Pomc promoter. Remarkably, a significant positive correlation was found between ΔBW and gadd45b mRNA levels (n=5–6/group; r=0.905; P<0.005; Fig. 4C) and a negative correlation between ΔBW and total percentage of methylation on the Pomc promoter (n=5–6/group; r=−0.707; P<0.05; Fig. 4D).
Figure 4.
Dams' weight loss was correlated with demethylation and with gadd45b mRNA expression levels. First-generation dams from both groups were euthanized at PND 22 (C dams: n=5; HFD dams: n=6). A) Total percentage of methylation in F0 females was analyzed and compared between groups (shaded bar, nulliparous C; striped bar, nulliparous HFD; open bar, C dams; solid bar, HFD dams). B) mRNA levels of gadd45b from Arc were measured by real-time PCR with gadd45b specific primers. Hprt1 and B2m were used as standard genes. Relative gene expression in the C dam group was set to 1 (open bar) and compared to the HFD dam group (solid bar). C, D) Pearson product-moment correlations were performed between dam's weight loss during lactation and gadd45b mRNA levels (C) or total percentage of methylation on the Pomc promoter (D). *P < 0.05 vs. C; #P < 0.001 vs. nulliparous HFD.
C-fed female offspring of HFD-fed dams presented significantly increased BW and leptin levels
Offspring of the HFD-treated dams presented high mean BW (C pups: 42 g; HFD pups: 69 g; n=10/group; P<0.001; Fig. 5A) and leptin levels (C pups: 2350 pg/ml; HFD pups: 7149 pg/ml; n=7–8/group; P<0.001; Fig. 5B) at weaning day. However, no group differences were discovered in Pomc mRNA expression (n=8–11/group; Fig. 5C). Interestingly, although pups from both of the groups were weaned to standard rodent chow, HFD-C offspring failed to maintain lean energy balance throughout their life, so that at PND 80 they still presented increased BW (adult C-C: 201 g; adult HFD-C: 216 g; n=10/group; P<0.005; Fig. 5D), leptin levels (adult C-C: 3748 pg/ml; adult HFD-C: 6017 pg/ml; n=6–8/group; P=0.01; Fig. 5E) and no differences in the Pomc mRNA levels (n=5–6/group; Fig. 5F) compared to C-C. Both pups and adult offspring of HFD dams presented low Pomc/leptin ratios (means±sem: 0.16±0.025, n=7, and 0.47±0.009, n=5, respectively) compared to control offspring (0.52±0.1, n=5, and 0.88±0.13, n=5, respectively; P < 0.005), indicating impaired leptin-Pomc signaling.
Hypermethylation of the Pomc promoter, observed in offspring of HFD-fed dams, was maintained till adulthood
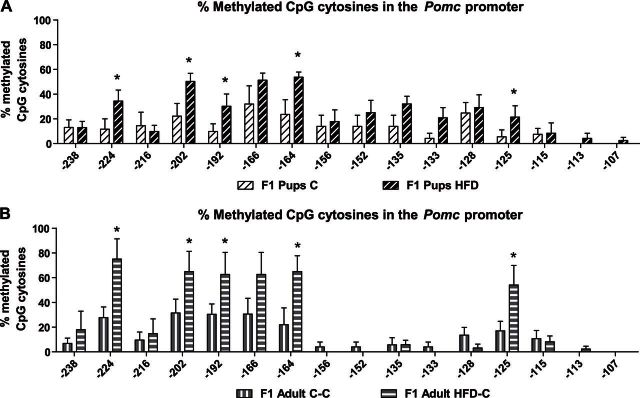
In accordance with their obesity phenotype, HFD pups presented hypermethylation across the entire Pomc promoter at PND 22 (C pups: n=6; HFD pups: n=8; site −224: χ2=3.96, df=1, P=0.047; site −202: χ2=4.16, df=1, P=0.041; site −192: χ2=4.89, df=1, P=0.027; site −164: χ2=5.03, df=1, P=0.025; site −125: χ2=4.37, df=1, P=0.036; Fig. 6A). To evaluate whether the methylation pattern of the Pomc promoter is conserved in the offspring of HFD-fed dams from weaning to adulthood, we analyzed these data after the pups consumed the C diet for 2 mo. As depicted in Fig. 6B, the hypermethylation pattern of HFD-C offspring was maintained to adulthood, across the entire Pomc promoter (adults: n=5/group; site −224: χ2=9.7, df=1, P=0.002; site −202: χ2=5.98, df=1, P=0.0014; site −192: χ2=4.97, df=1, P=0.026; site −164: χ2=9.53, df=1, P=0.002; site −125: χ2=6.768, df=1, P=0.0009; Fig. 6B). These results suggest that hypermethylation formed during critical time periods is stable and has a long-term effect on energy balance.
Figure 6.
Hypermethylation on the Pomc promoter, observed on PND 22 in the offspring of the HFD-treated dams, was maintained till adulthood. Methylation analysis of the Pomc promoter −540 bp upstream to the ATG, in the hypothalamic Arc region in offspring of HFD-treated dams or control dams at weaning day (PND 22; n=6–8/group; A) and adulthood (PND 80; n=5/group; B) in C-fed offspring. Data are presented as mean ± sem percentage of methylation in each group. *P < 0.05 vs. C.
In an HFD challenge, hypermethylated offspring of HFD-fed dams consumed significantly more kilocalories and gained significantly more weight compared to controls
To test the response of the adult offspring to a high-energy food environment, adult offspring were subjected to a 30 d HFD challenge from PND 80 (C-C: n=5; HFD-C: n=10). At 5 d into the challenge, both groups presented large increases of HFD intake (C-C: 155%; HFD-C: 174%). Nevertheless, HFD-C offspring presented a higher neophilia toward the HFD, compared to the control group [age×group interaction, F (1,9)=30.9, P<0.001]. After the initial 5 d, a continuous reduction in kilocalorie consumption was observed until PND 110 (Fig. 7A). Furthermore, when we analyzed the total kilocalorie consumption, HFD-C offspring consumed significantly more kilocalories over the 30 d of HFD challenge (C-C: 341 kcal; HFD-C: 416 kcal; P<0.001; Fig. 7C) and presented a significantly greater weight gain from PND 80 to PND 110, compared to the C-C group (C-C: 49.6 g; HFD-C: 81.5 g; P<0.05; Fig. 7B). These results suggest that, despite initial enthusiasm toward the new diet, C-C animals could compensate, reducing intake of a high-energy diet, thus maintaining normal BW increase. However, HFD-C rats failed to compensate for the HFD and therefore gained more weight, on top of their greater initial BW.
DISCUSSION
Environmental influences can affect the set point of gene expression by epigenetic programming (6, 24, 25). Here we demonstrated that chronic HFD feeding of nonmated female rats significantly increased BW and plasma leptin levels and attenuated Pomc mRNA expression, which were associated with hypermethylation of the Pomc promoter. Nevertheless, in the mated HFD dams, this effect was completely abolished after the lactation period, and the weight loss during lactation was correlated with demethylation. Moreover, the offspring of HFD-fed dams showed a long-term effect on Pomc CpG methylation programming that was not reprogrammed by providing standard chow after weaning.
In this study, we differentiate among the epigenetic effects occurring during early developmental, gestational, and postpartum periods and those taking place from weaning till adulthood. We found that nulliparous female rats fed an HFD for a long period presented relatively low Pomc mRNA expression, despite significantly higher BW and plasma leptin levels. Attenuated Pomc mRNA expression was associated with hypermethylation of the Pomc promoter. Similar findings were obtained in our previous study using male rats (20). These data suggest that hypermethylation on the promoter region of the Pomc gene can be modified at postlactation periods, thus blocking leptin's effects on Pomc mRNA transcription. Although most previous studies of epigenetic programming focused on perinatal periods (18, 26, 27), our data support the notion that hypothalamic plasticity at postweaning/adult developmental periods in response to different types of diets is not exclusive to the early development period.
Here we present a novel approach that examined the effect of HFD on the methylation pattern in the dams themselves, following the energetically demanding periods of pregnancy and lactation (26). Although dams fed an HFD kept displaying significantly higher BW during pregnancy, after 3 wk of lactation, HFD-fed dams showed a sharper BW decrease. In contrast to the HFD-fed nulliparous group, at the end of lactation, HFD-treated dams presented normal Pomc/leptin ratio and methylation levels on the Pomc promoter. Pregnancy and lactation are energy-consuming events, which naturally induce females to increase food intake and accumulate fat, yet the phenomenon of a sharp BW loss in obese dams during lactation has been described previously (27). One reasonable explanation is that obese dams are challenged by the demands of rapidly growing preobese pups, using their energy stores for nursing. Interestingly, beyond the groups, weight loss was negatively correlated with demethylation on the Pomc promoter and positively with gadd45b mRNA expression levels, which were recently suggested to have a potential role in active DNA demethylation (23). Thus, the mechanism of demethylation of the Pomc promoter, in response to extreme energetic challenges like lactation, cannot be ruled out. Several other enzymes, such as the 10–11 translocation (Tet) family have also been proposed as responsible for the demethylation mechanism (23, 28), but much more research is needed.
Early developmental temporary windows of heightened brain plasticity, called sensitive periods, affect neural circuits. It is assumed that the period of perinatal development is one of the most critical windows during which adverse conditions and exposures can influence the growth and development of the fetus, as well as the offspring's postnatal health and behavior (4, 6, 29). It has been suggested that epigenetic mechanisms, including methylation of DNA sequences, could represent the molecular mechanism underlying perinatal programming in those periods. Different studies showed that environmental alterations, such as maternal stress (4, 30), undernutrition (31), smoking (32), and fetal alcohol exposure (33) during sensitive periods affect gene expression in the offspring via altering DNA methylation patterns. Of special relevance, the nutritional environment during the perinatal period has been shown to alter the expression of genes critical to regulation of energy balance. Here we found that offspring exposure to maternal HFD resulted in an obesity/preobesity phenotype already at PND 22, including significantly increased BW, increased leptin levels, and hypermethylation on the Pomc promoter. These results are consistent with the known physiological responses to unbalanced nutrition during pregnancy and lactation, since overeating (16), obesity, or even undernutrition (18, 31) were found to be high-risk factors, both to the dam and her offspring. Alarmingly, approximately 25% of pregnant women in Europe and the United States are overweight or obese (3, 34). Offspring of obese mothers are overweight at birth and have an increased risk of being overweight and diabetic during childhood and in adult life (35, 36). Thus, epigenetic malprogramming during the perinatal period may be one of the factors affecting the continuous increase in childhood obesity.
It was previously suggested that epigenetic modifications that occur in critical time periods can have a long-term effect on the offspring (4, 33). Moreover, it has been shown that exposure of the parental generation to environmental stimuli such as smell (37) can be transmitted in rodents across generations via epigenetic mechanisms (6, 38). Therefore, one of the main goals of this study was to investigate whether offspring of HFD-fed dams will retain the obese phenotype and will not be reprogrammed by consuming C diet after weaning. Phenotype reprogramming has been reported following intervention during sensitive periods by adding high levels of multivitamins (39) or folate to the pup's diet (40, 41). In addition, postnatal leptin treatment may program methylation of an appetite-related gene in the hypothalamus of animals fed an HFD (42). However, in our study, 2 mo of postweaning normal nutrition did not alter the obese phenotype of the rats raised by HFD-fed dams. These mature offspring retained significantly high BW and leptin levels as well as hypermethylation on the Pomc promoter, and they even failed to regulate caloric intake successfully after HFD challenge.
The prevailing dogma assumes that hypermethylation on CpG dinucleotides within gene promoters leads to blockage of transcription mechanisms and thus decreases mRNA expression (14, 15). In the offspring of HFD-treated dams, despite hypermethylation of the Pomc promoter found at PND 22 and PND 80, no accompanying change in Pomc mRNA levels was observed. The exceptionally increased levels of leptin seen in these offspring should have up-regulated the expression of Pomc, leading eventually to increased energy expenditure and reduced BW (2). However, the results show that the offspring in these groups failed to reduce their BW, indicating that high levels of leptin did not affect Pomc expression adequately. These results are reinforced in light of the fact that the ratio between leptin and Pomc was particularly low in these groups, suggesting a blunted response of the Pomc neurons due to the hypermethylation on its promoter. Yet, it is still not clear why only nonmated females showed decreased levels of Pomc mRNA expression. It is possible that this phenomenon depends on the time and duration of exposure to HFD, in addition to other deficits in the regulatory mechanisms of the leptin-Pomc pathway.
Additional research is needed to understand the mechanisms and whether reprogramming and subsequent maintenance of reduced BW is possible as a secondary prevention. Some relevance of these findings applies to humans, since recently published data show that epigenetic changes in the brain, specifically CpG methylation of the gene promoter, may be reflected in other cells, such as white blood cells, which therefore may be used as biomarkers for early detection and prevention of obesity. For example, an identical pattern of Pomc methylation was found between postmortem dissected Pomc neurons and peripheral blood cells from the same patient (43). Another study presented an association of weight regain with specific methylation levels in the NPY and Pomc promoters in leukocytes of obese men (44). However, one of the limitations of this study lies in the fact that the use of chronic diet containing 60% fat is not relevant to humans. Since humans usually feed on a more balanced or less exaggerated diet, it can be assumed that the physiological effects will be moderate.
In summary, in this study, we observed a clear correspondence between the methylation levels of the Pomc promoter and weight gain. In postpartum dams at weaning, the BW decrease from gestation was accompanied by demethylation and increased gadd45b mRNA expression levels. Perinatal exposure to maternal diet-induced obesity led to a long-term acquired alteration in DNA methylation patterns of the Pomc promoter in the offspring, which was not reprogrammed by providing standard chow after weaning. Moreover, methylation pattern of the Pomc promoter may potentially be a prognostic biomarker for early detection and prevention of obesity.
Acknowledgments
The authors thank Yael Lavi, Lilach Eliyahu, and Tomer Cramer for help in performing part of the experiments.
This research was supported by the Israel Science Foundation (grant 1532/12).
Footnotes
- Arc
- arcuate nucleus
- B2m
- β-2 microglobulin
- BW
- body weight
- C
- normal chow
- gadd45b
- growth arrest and DNA-damage-inducible, β
- HFD
- high-fat diet
- Hprt1
- hypoxanthine phosphoribosyltransferase 1
- MC4-R
- melanocortin 4 receptor
- Nf-kB
- nuclear factor κ-light-chain-enhancer of activated B cells
- PND
- postnatal day
- Pomc
- proopiomelanocortin
- RT-qPCR
- reverse-transcription quantitative PCR
- Sp1
- specificity protein 1
- T0–3
- trimester 0–3
REFERENCES
- 1. McMillen I. C., Robinson J. S. (2005) Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol. Rev. 85, 571–633 [DOI] [PubMed] [Google Scholar]
- 2. Bouret S. G., Simerly R. B. (2006) Developmental programming of hypothalamic feeding circuits. Clin. Genet. 70, 295–301 [DOI] [PubMed] [Google Scholar]
- 3. Catalano P. M. (2010) Obesity, insulin resistance, and pregnancy outcome. Reproduction 140, 365–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Champagne F. A., Meaney M. J. (2006) Stress during gestation alters postpartum maternal care and the development of the offspring in a rodent model. Biol. Psychiatry 59, 1227–1235 [DOI] [PubMed] [Google Scholar]
- 5. Desai M., Beall M., Ross M. G. (2013) Developmental origins of obesity: programmed adipogenesis. Curr. Diab. Rep. 13, 27–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Roth T. L. (2012) Epigenetics of neurobiology and behavior during development and adulthood. Dev. Psychobiol. 54, 590–597 [DOI] [PubMed] [Google Scholar]
- 7. Bouret S. G. (2013) Organizational actions of metabolic hormones. Front. Neuroendocrinol. 34, 18–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pan H., Guo J., Su Z. (2014) Advances in understanding the interrelations between leptin resistance and obesity. Physiol. Behav. 130C, 157–169 [DOI] [PubMed] [Google Scholar]
- 9. Schneeberger M., Gomis R., Claret M. (2014) Hypothalamic and brainstem neuronal circuits controlling homeostatic energy balance. J. Endocrinol. 220, T25–46 [DOI] [PubMed] [Google Scholar]
- 10. Fan C., Liu X., Shen W., Deckelbaum R. J., Qi K. (2011) The regulation of leptin, leptin receptor and pro-opiomelanocortin expression by N-3 PUFas in diet-induced obese mice is not related to the methylation of their promoters. Nutr. Metab. (Lond.) 8, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shi X., Wang X., Li Q., Su M., Chew E., Wong E. T., Lacza Z., Radda G. K., Tergaonkar V., Han W. (2013) Nuclear factor kappaB (NF-kappaB) suppresses food intake and energy expenditure in mice by directly activating the Pomc promoter. Diabetologia 56, 925–936 [DOI] [PubMed] [Google Scholar]
- 12. Yang G., Lim C. Y., Li C., Xiao X., Radda G. K., Li C., Cao X., Han W. (2009) FoxO1 inhibits leptin regulation of pro-opiomelanocortin promoter activity by blocking STAT3 interaction with specificity protein 1. J. Biol. Chem. 284, 3719–3727 [DOI] [PubMed] [Google Scholar]
- 13. Kouzarides T. (2007) Chromatin modifications and their function. Cell 128, 693–705 [DOI] [PubMed] [Google Scholar]
- 14. Miranda T. B., Jones P. A. (2007) DNA methylation: the nuts and bolts of repression. J. Cell. Physiol. 213, 384–390 [DOI] [PubMed] [Google Scholar]
- 15. Haberman R. P., Quigley C. K., Gallagher M. (2012) Characterization of CpG island DNA methylation of impairment-related genes in a rat model of cognitive aging. Epigenetics 7, 1008–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Plagemann A., Harder T., Brunn M., Harder A., Roepke K., Wittrock-Staar M., Ziska T., Schellong K., Rodekamp E., Melchior K., Dudenhausen J. W. (2009) Hypothalamic proopiomelanocortin promoter methylation becomes altered by early overfeeding: an epigenetic model of obesity and the metabolic syndrome. J. Physiol. 587, 4963–4976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Waterland R. A., Jirtle R. L. (2003) Transposable elements: targets for early nutritional effects on epigenetic gene regulation. Mol. Cell. Biol. 23, 5293–5300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stevens A., Begum G., Cook A., Connor K., Rumball C., Oliver M., Challis J., Bloomfield F., White A. (2010) Epigenetic changes in the hypothalamic proopiomelanocortin and glucocorticoid receptor genes in the ovine fetus after periconceptional undernutrition. Endocrinology 151, 3652–3664 [DOI] [PubMed] [Google Scholar]
- 19. Marco A., Kisliouk T., Weller A., Meiri N. (2013) High fat diet induces hypermethylation of the hypothalamic Pomc promoter and obesity in post-weaning rats. Psychoneuroendocrinology 38, 2844–2853 [DOI] [PubMed] [Google Scholar]
- 20. Paxinos G., Watson C. (1998) The Rat Brain in Stereotaxic Coordinates, 4th Ed., Academic Press, San Diego, CA, USA [Google Scholar]
- 21. Katz A., Meiri N. (2006) Brain-derived neurotrophic factor is critically involved in thermal-experience-dependent developmental plasticity. J. Neurosci. 26, 3899–3907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li B., Matter E. K., Hoppert H. T., Grayson B. E., Seeley R. J., Sandoval D. A. (2014) Identification of optimal reference genes for RT-qPCR in the rat hypothalamus and intestine for the study of obesity. Int. J. Obes. (Lond.) 38, 192–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sultan F. A., Wang J., Tront J., Liebermann D. A., Sweatt J. D. (2012) Genetic deletion of Gadd45b, a regulator of active DNA demethylation, enhances long-term memory and synaptic plasticity. J. Neurosci. 32, 17059–17066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gomes M. V., Pelosi G. G. (2013) Epigenetic vulnerability and the environmental influence on health. Exp. Biol. Med. (Maywood) 238, 859–865 [DOI] [PubMed] [Google Scholar]
- 25. Szyf M., Bick J. (2013) DNA methylation: a mechanism for embedding early life experiences in the genome. Child Dev. 84, 49–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tups A. (2009) Physiological models of leptin resistance. J. Neuroendocrinol. 21, 961–971 [DOI] [PubMed] [Google Scholar]
- 27. Zagoory-Sharon O., Schroeder M., Levine A., Moran T. H., Weller A. (2008) Adaptation to lactation in OLETF rats lacking CCK-1 receptors: body weight, fat tissues, leptin and oxytocin. Int. J. Obes. (Lond.) 32, 1211–1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rudenko A., Dawlaty M. M., Seo J., Cheng A. W., Meng J., Le T., Faull K. F., Jaenisch R., Tsai L. H. (2013) Tet1 is critical for neuronal activity-regulated gene expression and memory extinction. Neuron 79, 1109–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Symonds M. E., Sebert S. P., Hyatt M. A., Budge H. (2009) Nutritional programming of the metabolic syndrome. Nat. Rev. Endocrinol. 5, 604–610 [DOI] [PubMed] [Google Scholar]
- 30. Blaze J., Roth T. L. (2013) Exposure to caregiver maltreatment alters expression levels of epigenetic regulators in the medial prefrontal cortex. Int. J. Dev. Neurosci. 31, 804–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Begum G., Davies A., Stevens A., Oliver M., Jaquiery A., Challis J., Harding J., Bloomfield F., White A. (2013) Maternal undernutrition programs tissue-specific epigenetic changes in the glucocorticoid receptor in adult offspring. Endocrinology 154, 4560–4569 [DOI] [PubMed] [Google Scholar]
- 32. Novakovic B., Ryan J., Pereira N., Boughton B., Craig J. M., Saffery R. (2013) Postnatal stability and tissue- and time-specific effects of methylation change in response to maternal smoking throughout pregnancy. Epigenetics 9, 377–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Laufer B. I., Mantha K., Kleiber M. L., Diehl E. J., Addison S. M., Singh S. M. (2013) Long-lasting alterations to DNA methylation and ncRNAs could underlie the effects of fetal alcohol exposure in mice. Dis. Model Mech. 6, 977–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Galtier-Dereure F., Boegner C., Bringer J. (2000) Obesity and pregnancy: complications and cost. Am. J. Clin. Nutr. 71, 1242S–1248S [DOI] [PubMed] [Google Scholar]
- 35. Clausen T. D., Mathiesen E. R., Hansen T., Pedersen O., Jensen D. M., Lauenborg J., Damm P. (2008) High prevalence of type 2 diabetes and pre-diabetes in adult offspring of women with gestational diabetes mellitus or type 1 diabetes: the role of intrauterine hyperglycemia. Diabetes Care 31, 340–346 [DOI] [PubMed] [Google Scholar]
- 36. Wright C. S., Rifas-Shiman S. L., Rich-Edwards J. W., Taveras E. M., Gillman M. W., Oken E. (2009) Intrauterine exposure to gestational diabetes, child adiposity, and blood pressure. Am. J. Hypertens. 22, 215–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dias B. G., Ressler K. J. (2013) Parental olfactory experience influences behavior and neural structure in subsequent generations. Nat. Neurosci. 17, 89–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Szyf M. (2013) Lamarck revisited: epigenetic inheritance of ancestral odor fear conditioning. Nat. Neurosci. 17, 2–4 [DOI] [PubMed] [Google Scholar]
- 39. Cho C. E., Sanchez-Hernandez D., Reza-Lopez S. A., Huot P. S., Kim Y. I., Anderson G. H. (2013) High folate gestational and post-weaning diets alter hypothalamic feeding pathways by DNA methylation in Wistar rat offspring. Epigenetics 8, 710–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cho C. E., Sanchez-Hernandez D., Reza-Lopez S. A., Huot P. S., Kim Y. I., Anderson G. H. (2013) Obesogenic phenotype of offspring of dams fed a high multivitamin diet is prevented by a post-weaning high multivitamin or high folate diet. Int. J. Obes. (Lond.) 37, 1177–1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Langie S. A., Achterfeldt S., Gorniak J. P., Halley-Hogg K. J., Oxley D., van Schooten F. J., Godschalk R. W., McKay J. A., Mathers J. C. (2013) Maternal folate depletion and high-fat feeding from weaning affects DNA methylation and DNA repair in brain of adult offspring. FASEB J. 27, 3323–3334 [DOI] [PubMed] [Google Scholar]
- 42. Palou M., Pico C., McKay J. A., Sanchez J., Priego T., Mathers J. C., Palou A. (2011) Protective effects of leptin during the suckling period against later obesity may be associated with changes in promoter methylation of the hypothalamic pro-opiomelanocortin gene. Br. J. Nutr. 106, 769–778 [DOI] [PubMed] [Google Scholar]
- 43. Kuehnen P., Mischke M., Wiegand S., Sers C., Horsthemke B., Lau S., Keil T., Lee Y. A., Grueters A., Krude H. (2012) An Alu element-associated hypermethylation variant of the POMC gene is associated with childhood obesity. PLoS Genet. 8, e1002543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Crujeiras A. B., Campion J., Diaz-Lagares A., Milagro F. I., Goyenechea E., Abete I., Casanueva F. F., Martinez J. A. (2013) Association of weight regain with specific methylation levels in the NPY and POMC promoters in leukocytes of obese men: a translational study. Regul. Pept. 186, 1–6 [DOI] [PubMed] [Google Scholar]