Abstract
Postprandial responses to food are complex, involving both genetic and environmental factors. We studied postprandial responses to a Big Mac meal challenge in monozygotic co-twins highly discordant for body weight. This unique design allows assessment of the contribution of obesity, independent of genetic liability. Comprehensive metabolic profiling using 3 analytical platforms was applied to fasting and postprandial serum samples from 16 healthy monozygotic twin pairs discordant for weight (body mass index difference >3 kg/m2). Nine concordant monozygotic pairs were examined as control pairs. Fecal samples were analyzed to assess diversity of the major bacterial groups by using 5 different validated bacterial group specific denaturing gradient gel electrophoresis methods. No differences in fecal bacterial diversity were detected when comparing co-twins discordant for weight (ANOVA, P<0.05). We found that within-pair similarity is a dominant factor in the metabolic postprandial response, independent of acquired obesity. Branched chain amino acids were increased in heavier as compared with leaner co-twins in the fasting state, but their levels converged postprandially (paired t tests, FDR q<0.05). We also found that specific bacterial groups were associated with postprandial changes of specific metabolites. Our findings underline important roles of genetic and early life factors in the regulation of postprandial metabolite levels.—Bondia-Pons, I., Maukonen, J., Mattila, I., Rissanen, A., Saarela, M., Kaprio, J., Hakkarainen, A., Lundbom, J., Lundbom, N., Hyötyläinen, T., Pietiläinen, K. H., Orešič, M. Metabolome and fecal microbiota in monozygotic twin pairs discordant for weight: a Big Mac challenge.
Keywords: obesity, metabolomics, bile acids
The human body functions in postprandial state most of the day, which might be one of the contributing factors to the increasing prevalence of obesity in Western societies (1). However, most of the studies aimed at deciphering the associations between the obesity and human metabolism, including those applying the metabolomics approach, are to date still limited to fasting conditions.
The applications of metabolomics to study the responses to different challenge tests so far implicate the involvement of energy metabolism, inflammation, and oxidation (2–7). The nutritional high-fat challenge to test the metabolic response capacity in humans was reported recently (8). However, the design of the earlier postprandial metabolomics studies did not allow for the differentiation of genetic and environmental factors behind the postprandial response in different phenotypes, such as in obese or lean subjects. In this context, the twin study design such as monozygotic (MZ) twin pairs discordant for weight is an ideal study setting. MZ twin pairs are genetically identical at the DNA sequence level; therefore, any phenotypic differences between the co-twins are acquired and thus attributed to environmental factors, including the diet (9, 10).
The correlations among gut microbiota, obesity, and insulin resistance have been widely studied (11, 12). Obesity has been associated with phylum-level changes in the microbiota, reduced bacterial diversity, and altered representation of bacterial genes and metabolic pathways in twins (13). The gut microbiota thus appears to function at the intersection between host genotype and diet modulating the host physiology and metabolism, ultimately affecting the host phenotype. It has been suggested that bile acids (BAs) are the key metabolic mediators between the gut microbiota and the host metabolism (14), including in response to a high-fat diet (15).
A Big Mac meal is a good example of a diet that combines a high proportion of fat and a high proportion of refined carbohydrates in the same meal. With the aim to elucidate the postprandial response to Western diet in obesity independent of genetic liability (i.e., acquired obesity), here we applied a standardized meal challenge, the Big Mac test (BMT), in MZ twins discordant for weight and studied fecal microbial composition together with fasting and postprandial serum metabolome.
MATERIALS AND METHODS
Subjects and the twin study design
Twin pairs included in the current study were recruited from 2 population-based longitudinal studies, FinnTwin16 (FT16) and FinnTwin12 (FT12), each consisting of 5 consecutive birth cohorts of Finnish twins (16). Description of the screening process of this study has been characterized in more detail earlier (17). In short, of the >5000 twin pairs, of which 1/3 are MZ, we were able to find 16 MZ pairs discordant for weight [body mass index (BMI) difference=>3 kg/m2] (10) aged 22–32 yr in 2008–2009. The discordant heavier (DH) co-twins had a mean ± sd BMI = 30.6 ± 5.1 kg/m2, while the discordant leaner (DL) co-twins had a BMI = 24.8 ± 3.9 kg/m2. Nine concordant MZ pairs (BMI difference <3 kg/m2) were examined as concordant control (C) pairs. Two of these pairs were overweight-normal weight pairs (BMI=25.3±1.3 kg/m2), 4 pairs were overweight-overweight (BMI=27.1±1.1 kg/m2), and 3 pairs were obese-obese (BMI=32.3±2.4 kg/m2). The cutoff used for overweight was 25 kg/m2, and for obesity 30 kg/m2. The 6 nonobese control pairs, i.e., with BMI < 30 kg/m2, were designated concordant leaner (CL). All subjects (n=50; 32 women and 18 men) were Caucasian and healthy (based on medical history, clinical examination, and structured psychiatric interview), normotensive, and did not use any medications except oral contraceptives. Their weight had been stable for ≥3 mo before study, and they had not used antibiotics in the prior 3 mo period. Monozygosity was confirmed by the genotyping of 10 informative genetic markers (18). Subjects provided written informed consent. The protocol was designed and performed according to the principles of the Helsinki Declaration and was approved by the Ethical Committee of the Helsinki University Central Hospital.
Nutritional challenge
The meal test with a standardized McDonald's Big Mac Meal (Big Mac hamburger, 100 g French fries, and 400 g sucrose-sweetened Coca-Cola) contained 979 kcal (123 g of carbohydrates, 40 g of fat, and 32 g of protein; http://www.mcdonalds.fi). It was eaten in 10–15 min. Venous blood samples were collected at 4 time points: 0 min (fasting condition, after a 10- to 12-h overnight fast), 30, 60, and 120 min after the postprandial meal intake. Plasma was separated by centrifugation (3,200 rpm; 15 min; +4°C), frozen immediately at −80°C, and stored until analysis.
Body composition
Body composition was measured by dual-energy X-ray absorptiometry (DEXA; version 8.8; Lunar Prodigy, Madison, WI, USA). Subcutaneous and intra- abdominal fat were determined by magnetic resonance imaging of 16 transaxial scans reaching from 8 cm above to 8 cm below the 4th and 5th lumbar interspace and liver fat by magnetic resonance spectroscopy as described previously (17).
Clinical chemistry
Plasma glucose was measured using the spectrophotometric hexokinase and glucose-6-phosphate dehydrogenase assay (Roche Diagnostics, Indianapolis, IN, USA) and serum insulin with time-resolved immunofluorometric assay (Perkin Elmer, Wellesley, MA, USA) at all time points (0, 30, 60 and 120 min). The Matsuda index (MI) was used to estimate insulin sensitivity (19). In addition, fasting serum high-sensitivity CRP was measured by Cobas CRP(Latex)HS (Roche Diagnostics), and serum total cholesterol, HDL cholesterol, and triglyceride concentrations with respective enzymatic kits (Roche Diagnostics). LDL cholesterol concentrations were calculated using the formula by Friedewald et al. (20).
Metabolomics
Plasma samples were analyzed by two global profiling analytical platforms and a targeted profiling platform. Two-dimensional gas chromatography coupled to time-of-flight mass spectrometry (GC×GC-TOFMS) was applied to measure small polar metabolites as described previously (21). A global lipidomics platform using ultraperformance liquid chromatography coupled to quadrupole-TOFMS (UPLC-QTOFMS) was applied to measure molecular lipids (22). In addition, a targeted platform based in UPLC coupled to triple-quadrupole MS (UPLC-QqQMS) was applied to quantify BAs for both unconjugated and conjugated forms as described previously (14).
In total, 924 metabolites were detected in ≥80% of the subjects in all time points of the study. A total of 359 small polar metabolites were detected by GC×GC-TOFMS platform, of which 117 were identified; 551 molecular lipids were detected by UPLC-QTOFMS, of which 263 were identified; and 14 BAs were quantified by UPLC-QqQMS. For further data processing and data analysis, only identified metabolites (n=394) were considered.
Fecal microbiota characterization
Fecal samples were collected at baseline. Most of the twins provided the fecal samples during the visit in the metabolic laboratory. Those who did not performed the sampling at home and froze the sample “as such” in a sterile 50 ml tube in their home freezer (−20°C). The frozen samples were transported to the laboratory by express mail delivery. The samples were transferred to −80°C in the laboratory until further analysis. Analyses were performed using 5 different bacterial denaturing gradient gel electrophoresis (DGGE) methods developed and validated by Valtion Teknillinen Tutkimuskeskus (VTT) Technical Research Center of Finland. Predominant bacterial universal PCR-DGGE (Univ-DGGE) was performed as described previously (23) using primers that target 16S rRNA variable region V6–V8 (24). Bacterial group-specific DGGE methods for Eubacterium rectale (Erec; Lachnospiraceae; group-specific primers target 16S rRNA variable region V3–V4; ref. 25), Clostridium leptum (Clept; Ruminococcaceae; group-specific primers target 16S rRNA variable region V5–V6; ref. 26), Bacteroides spp. (Bfra; group-specific primers target 16S rRNA variable region V3–V5; ref. 26), and Bifidobacterium spp. (Bif; group-specific primers target 16S rRNA variable region V1–V3; ref. 27) were performed as described previously to assess the diversity and intratwin pair similarity of the major bacterial groups of the human fecal microbiota. PCR products were separated in polyacrylamide gels with a denaturing gradient of 38–60% (Univ-DGGE and Erec-DGGE), 45–55% (Bif-DGGE), or 30–60% (Clept-DGGE and Bfra-DGGE), where 100% is 7 M urea and 40% (v/v) deionized formamide. The methods were previously validated using clone libraries, which have proven that we are able to detect those bacteria that comprise >1% of the studied bacterial populations (25, 26). In case of Erec, Clept, and Bfra, this equals a detection limit of 0.1% of the total bacterial population. When group-specific Bif-DGGE was applied, resolution was even as low as 0.01% of the total population. The comparison of the profiles was performed by calculating a similarity percentage using Bionumerics 5.1 software (Applied Maths NV, Sint-Martens-Latem, Belgium). Clustering was performed with Pearson correlation and the unweighted-pair group method with arithmetic mean (UPGMA). Amplicons with the total surface area of ≥1% were included in the similarity analysis. The intrapair similarities were divided into intervals for each bacterial group analyzed. Mean differences between groups were evaluated by ANOVA (P<0.05).
Statistical analyses
Differences in the clinical characteristics between the co-twins were compared by paired Wilcoxon's signed rank test. Male and female pairs were combined because MZ co-twins were inherently matched for sex.
Metabolomics data were normalized by using log2-transformed values and scaled into zero mean and unit variance to obtain metabolite profiles comparable to each other for clustering.
Bayesian model-based clustering was applied on the scaled data to group metabolites (small polar metabolites, lipids, and BAs) into clusters. The analyses were performed using the mclust2 method (28) implemented in R.
Linear mixed models were applied to the twin-pair data using the R package nlme (R Project for Statistical Computing; http://www.r-project.org/). The mean concentration value of each cluster or BA was considered as the quantitative outcome of each model. The fixed effect variables were group (DH vs. DL vs. CL) and time point (0 vs. 30 vs. 60 vs. 120 min); and the random effect variables were twin (subject number) and twin-pair (family number). Age and gender were added as covariates to the model. The overall group differences were tested using the F statistic, and the post hoc analyses were done using Tukey's all-pair comparisons.
Paired t tests (P<0.05) were performed for each metabolite to determine differences in metabolite relative concentrations between discordant co-twins at baseline and end point of the study. A false discovery rate (FDR) with a significance threshold set at q < 0.05 was calculated to correct for multiple comparisons.
The pairwise Spearman rank correlation between the metabolite concentrations for each time point was used as a measure of the within-twin similarity change in discordant co-twins along the postprandial response. All analyses were conducted with statistical software SPSS 20.1 (IBM, Armonk, NY, USA) and R language version 3.00. (R Project for Statistical Computing).
Partial correlation network analysis
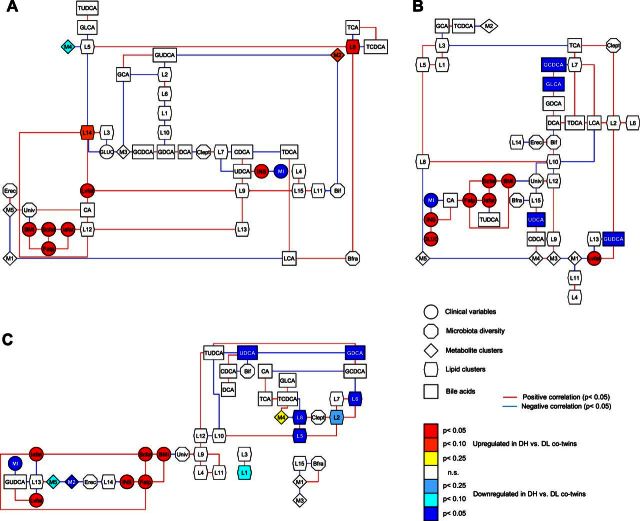
Data were twin normalized by using the within-pair difference between the heavier and the leaner co-twins for clusters, and by using the log2 of the ratio between the heavier and the leaner co-twins for variables. Dependency network analysis using the Genenet package (29) was performed on metabolite and lipid clusters, BA concentrations, fecal microbiota diversity, glucose (Gluc), insulin (Ins), MI, BMI, fat percentage (Fatp), subcutaneous fat (Scfat), intra-abdominal fat (Iafat), and liver fat (Lvfat), separately for twin pairs at baseline, and end point of the study. An additional network showing the changes between end point and baseline was also built. For BMI, MI, fat depots, and microbiota diversity, only baseline values were measured. In these networks, nonmissing edges denote nonzero partial correlations (P<0.05) between pairs of variables and thus imply direct interactions. Node color corresponds to significance and direction of regulation comparing co-twins. The yED graphical editor (30) was used for network visualization.
RESULTS
Clinical characteristics of the MZ twin pairs
Physical and biochemical characteristics of the 25 MZ twin pairs are shown in Table 1. As expected, the DH co-twins from discordant pairs had more body fat, intra-abdominal, subcutaneous, and liver fat, as compared with their DL co-twins. The insulin levels of DH co-twins were significantly higher than in DL co-twins. No differences were observed in serum total cholesterol or LDL cholesterol levels, while HDL cholesterol was significantly higher in DL as compared with their DH co-twins. None of these variables differed between the C co-twins, when ordered by their minor weight difference.
Table 1.
Physical and biochemical characteristics of the 25 monozygotic twin pairs at baseline
| Parameter | Pairs discordant for weight, n = 16 |
Pairs concordant for weight, n = 9 |
|||
|---|---|---|---|---|---|
| DH co-twin | DL co-twin | P | Nonobese pairs, n = 6 | Obese pairs, n = 3 | |
| Sex | 4 male/12 female pairs | 3 male/3 female | 2 male/1 female | ||
| Age [mean (min and max) yr] | 25.0 (24.4, 32.1) | 30.5 (29.6, 31.1) | 31.9 (25.6, 32.8) | ||
| Use of oral contraceptive medication | 5/6 | 6/6 | 1/3 | 1/1 | |
| BMI (kg/m2) | 30.2 (26.4, 34.1) | 24.0 (21.9, 27.4) | <0.001 | 26.6 (25.6, 27.5) | 32.7 (29.8, 34.5) |
| Intra-abdominal fat (cm3) | 1047 (656, 1775) | 571 (327, 800) | <0.001 | 926 (232, 1261) | 1445 (948, 1630) |
| Subcutaneous fat (cm3) | 5947 (4364, 7878) | 3717 (2622, 4850) | <0.001 | 2644 (2476, 4409) | 4628 (3296, 6124) |
| Body fat (%) | 43.3 (34.5, 47.6) | 35.2 (28.5, 40.4) | <0.001 | 29.7 (22.7, 39.3) | 37.1 (27.9, 41.4) |
| Liver fat (%) | 0.9 (0.6, 4.9) | 0.6 (0.4, 0.9) | <0.01 | 1.1 (0.3, 1.7) | 1.2 (0.5, 4.9) |
| Serum total cholesterol (mM) | 4.3 (3.9, 4.9) | 4.6 (3.9, 4.8) | 0.75 | 4.3 (3.3,5.1) | 4.4 (3.7, 5.4) |
| Serum HDL cholesterol (mM) | 1.3 (1.1, 1.5) | 1.7 (1.3, 2.0) | <0.01 | 1.4 (1.0,1.5) | 1.2 (1.0,1.3) |
| Serum LDL cholesterol (mM) | 2.7 (2.4, 3.0) | 2.5 (2.1, 2.9) | 0.14 | 2.7 (1.8, 3.7) | 2.8 (2.2, 3.1) |
| Serum triglycerides (mM) | 1.1 (0.8, 1.3) | 1.0 (0.8, 1.2) | 0.37 | 1.0 (0.6,1.1) | 1.8 (0.5, 2.8) |
| Serum glucose (mU/L) | 5.5 (5.2, 5.8) | 5.3 (5.2, 5.4) | 0.15 | 5.3 (5.0, 5.4) | 6.0 (5.7, 6.2) |
| Serum insulin (mU/L) | 8.7 (5.2, 13.3) | 6.5 (4.7, 8.3) | 0.03 | 5.5 (4.3, 7.2) | 6.8 (5.7, 13.3) |
| Serum high-sensitivity CRP (mg/L) | 1.3 (0.6, 5.8) | 1.1 (0.5, 4.1) | 0.43 | 0.6 (0.3, 1.7) | 1.4 (1.0, 2.1) |
| Matsuda index | 4.6 (3.5, 7.5) | 6.6 (4.4, 9.0) | 0.13 | 10.3 (4.9, 14.7) | 5.2 (2.4, 8.5) |
Data are presented as medians with interquartile range in parentheses. P values are for DH vs. DL co-twins for weight; paired Wilcoxon's test.
Serum metabolome in acquired obesity
Three analytical platforms were applied to characterize the metabolome in response to BMT challenge: UPLC-MS platform for global profiling of molecular lipids (22), GC×GC-TOFMS platform for global profiling of polar metabolites (21), and UPLC-MS/MS platform for the analysis of BAs (14). A complete dataset included 263 lipids, 117 polar metabolites, and 14 BAs.
The complete datasets from the 2 global metabolomics platforms were first surveyed by applying bayesian model-based clustering. Polar metabolite profiles were decomposed into 5 and molecular lipids into 15 clusters (Table 2). As expected, the division of clusters to a large extent follows different metabolite functional or structural groups. Different fasting levels and postprandial (30, 60, and 120 min after Big Mac meal) response patterns across the study groups were observed in several clusters (Fig. 1A). Mean metabolite levels in cluster M2, which mainly included carbohydrates (e.g., d-galactose, d-arabinose, and 4-ketoglucose) and amino acids including branched chain amino acids (BCAAs) isoleucine and valine, showed a converging pattern after the BMT within the discordant twin pairs, with significantly higher fasting levels in DH as compared with DL co-twins. In contrast, fatty acids clustered in M4 had a converging pattern in the opposite direction after the BMT, with significantly higher fasting levels in DL as compared with their DH co-twins. Mean metabolite levels in the other 3 clusters (M1, M3, and M5) did not show any significant difference between DH and DL co-twins. Nine of 15 lipid clusters comprised of triglycerides (TGs), with major compositional differences between the clusters being the carbon number and double bond content. Six of the TG clusters showed a significant increase in their levels at the 120 min time point, but none were significantly different between DH and DL co-twins.
Table 2.
Description of clusters in the metabolomics and lipidomics platforms
| Name | Size | Description | Examples of metabolites |
P |
|
|---|---|---|---|---|---|
| Groupa | TPb | ||||
| M1 | 59 | Sugars, sugar alcohols, microbiota-derived organic acids | d-glucose, d-fructose, galactilol, acetic acid, 3-hydroxybenzoic acid, malic acid | 0.01c | <0.001 |
| M2 | 27 | Sugars, sugar acids, indole and derivatives, amino acids | Galactose, arabinose, myo-inositol, allonic acid, indole, 1H-indole-2-acetic acid, isoleucine | 0.23 | <0.001 |
| M3 | 18 | Amino acids and organic acids | Tryptophan, tyrosine, propanoic acid, butanedioic acid | 0.03d | <0.01 |
| M4 | 8 | Fatty acids | Lauric acid, myristic acid, palmitic acid | 0.22 | <0.001 |
| M5 | 6 | Amino acids and TCA-metabolites | Methionine, serine, citric acid, α-ketoglutaric acid | 0.33 | 0.01 |
| L1 | 19 | LPCs | LPC(14:0), LPC(18:1), LPC(20:5), LPC(22:6) | 0.71 | <0.001 |
| L2 | 102 | SMs, major phospholipids | SM(d18:1/18:0), PC(34:2), PE(34:2) | 0.26 | <0.001 |
| L3 | 24 | PEes, DHA-containing ceramides, MGs | PE(34:3e), PE(36:6e), Cer(d18:1/22:6), MG(18:2) | 0.63 | 0.001 |
| L4 | 25 | Medium-chain TGs | TG(18:1/18:1/18:1), TG(18:1/18:2/18:1), TG(52:4), TG(54:6) | 0.75 | <0.001 |
| L5 | 5 | PEs with 36 carbons | PE(36:2), PE(36:2e), PE(36:3e), PE(36:4e) | 0.33 | <0.001 |
| L6 | 12 | PLs with 38 or 40 carbons with 3–4 double bonds | PC(38:2), PC(38:4), PC(40:3), PC(40:4), PE(40:3), PE(40:4) | 0.20 | <0.001 |
| L7 | 13 | Long-chain TGs, PUFA-containing TGs | TG(55:6), TG(56:7), TG(58:10), TG(59:5) | 0.65 | <0.001 |
| L8 | 10 | PEs and PEes with mainly 36 and 38 carbons | PE(38:4e), PE(38:5e), PE(38:6e) | 0.21 | <0.001 |
| L9 | 15 | SFA and MUFA-containing TGs | TG(14:0/16:0/16:0), TG(16:0/18:0/18:1) | 0.31 | <0.001 |
| L10 | 4 | SFA and MUFA-containing TGs with 50 carbons | TG(16:0/18:1/16:0), TG(16:0/18:1/18:1) | 0.80 | <0.001 |
| L11 | 8 | Long-chain TGs with 2–4 double bounds | TG(56:2), TG(58:2), TG(60:3), TG(60:4) | 0.45 | <0.001 |
| L12 | 4 | Short-chain SFA- and MUFA-containing TGs | TG(38:0), TG(40:0), TG(40:1), TG(42:1) | 0.34 | 0.71 |
| L13 | 5 | Medium-chain SFA- and MUFA-containing TGs | TG(42:0), TG(44:0), TG(44:1), TG(46:2) | 0.30 | 0.02 |
| L14 | 8 | Medium-chain SFA- and MUFA-containing odd-chain TGs | TG(45:0), TG(47:1), TG(49:0), TG(53:0), TG(55:1) | 0.49 | <0.01 |
| L15 | 9 | Medium-chain TGs with 3–5 double bounds | TG(46:3), TG(48:4), TG(50:5), TG(52:4) | 0.70 | <0.001 |
| BA | 14 | Unconjugated and conjugated primary and secondary BAs | CA, TCA, GCA, DCA, TDCA, GDCA | 0.29 | <0.001 |
M, metabolite cluster; L, lipid cluster.
Group comparison (DH, DL, C).
Time point comparison (0, 30, 60, and 120 min).
Tukey's significant pair comparison: P = 0.002 (DL vs. C).
Tukey's significant pair comparison: P = 0.03 (DH vs. C), P = 0.01 (DL vs. C).
Figure 1.
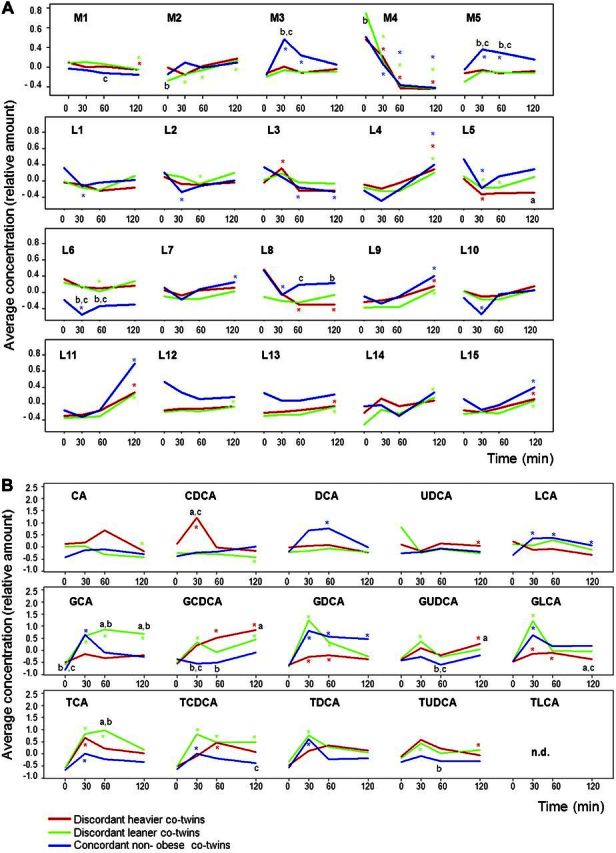
A) Mean average concentration (in relative amount) of metabolite (M) and lipid (L) clusters for twin groups [DH co-twins (red), DL co-twins (green), and concordant nonobese CL co-twins (blue)] over time (0, 30, 60, 120 min after the BMT). B) Mean average concentration (in relative amount) of unconjugated and conjugated BAs for twin groups over time. For visualization purposes, BA concentrations were scaled to show the mean average concentration of BAs in the same units used for metabolite and lipid clusters in A. G, glycine-conjugated BAs; T, taurine-conjugated BAs. Significant differences between groups for each time point are indicated by letters; asterisks indicate differences between time point and baseline for each group. aP < 0.05 vs. CL; bP < 0.05 vs. DH; cP < 0.05 vs. DL; *P < 0.05 vs. baseline for DH (red), DL (green), CL (blue).
When surveying the data at the individual metabolite level, the time-dependent metabolite response profiles showed that both fasting as well as postprandial metabolic profiles tended to be similar between the co-twins. However, similarly as at the cluster level, 2 interesting patterns were observed, a converging pattern for metabolite levels significantly different between the co-twins at fasting but converging at 120 min end point, and a diverging pattern for those metabolites with no baseline differences but with significantly different metabolite concentrations at 120 min postprandial response (Supplemental Table S1). Twenty of 394 metabolites showed a converging pattern (17 polar metabolites and 3 lipids), and 22 showed a diverging pattern [14 polar metabolites, 2 lipids, and 2 BAs: glycine cholic acid (GCA) and glycine lithocholic acid (GLCA)]. The converging metabolites included organic acids such as 3-hydroxybenzoic acid, amino acids including isoleucine, and fatty acids including oleic acid. Most of the converging metabolites showed significantly higher fasting levels in DH as compared with DL co-twins, except for the fatty acids. The diverging metabolites included sugar derivatives such as arabinitol and organic acids such as acetic acid. While all diverging lipids and BA showed significantly lower levels for DH as compared with DL co-twins 120 min time point, 9 of 14 small polar metabolites showed higher levels in DH co-twins.
Significant differences between DH and DL co-twins were observed for glycine but not for taurine-conjugated BA forms or nonconjugated BA forms (Fig. 1B). As expected, the levels of many conjugated BAs significantly increased already 30 min after the BMT. Among the nonconjugated BA forms, only chenodeoxycholic acid (CDCA) showed between-group differences, with significantly higher levels at 30 min in DH co-twins as compared with other groups.
Intratwin pair similarity and diversity of bacterial profiles
There were no significant differences in the bacterial DGGE-profile similarities between the concordant and discordant twin pairs because of high individual differences. However, when comparing the similarity of the fecal predominant bacterial groups between the co-twins, there was a trend that discordant twins had less similar intra-twin pair Bfra-DGGE profiles than concordant co-twins (Fig. 2A). Similarly, no statistically significant differences were detected when comparing DH and DL co-twin fecal bacterial diversities as detected with group-specific DGGE methods. However, there was a trend that the heavier co-twin had lower diversity of Bif and Clept group bacteria (Fig. 2B).
Figure 2.
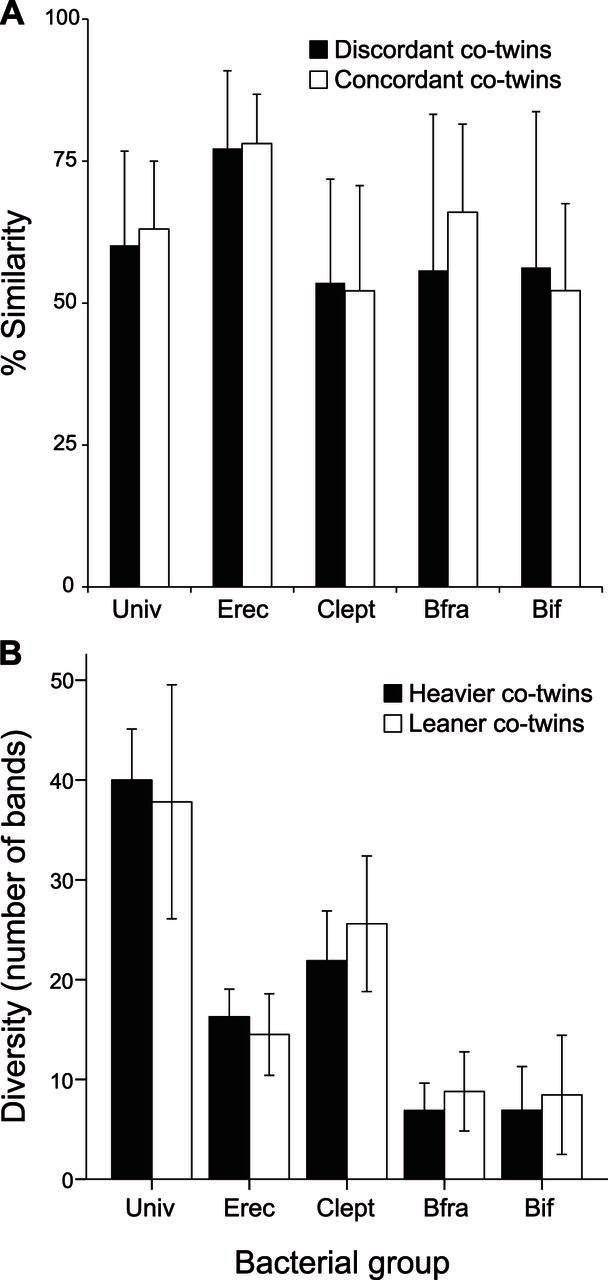
A) Difference in fecal bacterial diversity between heavier and leaner co-twins (in the discordant group). B) Intratwin pair similarity of the predominant bacterial groups in human stools in discordant and concordant twin pairs.
Dependency network analysis
Next, we assessed the direct associations of metabolic, microbial, and selected clinical variables in acquired obesity. To distinguish direct and indirect interactions of these variables, we utilized undirected Gaussian graphical model where the variables are connected if and only if their partial correlation is significantly nonzero (using FDR multiple testing for the selection of edgesl; ref. 29). The data was twin normalized, i.e., the within-pair differences between the co-twins were used as variables. Unlike the pairwise measures of associations, e.g., Pearson correlation coefficient, partial correlation provides a stronger criterion for dependency by adjusting for confounding effects, and thus removing spurious associations to a large extent. This is particularly favorable for an integration of multiple layers of information as in our study, because it inherently filters out false positives by discovering only those direct interactions with high confidence.
Three networks were constructed for weight-discordant twin pairs, including the metabolomic variables measured at baseline (Fig. 3A), at 120 min end point (Fig. 3B) as well as their differences between the 120 min and baseline (Fig. 3C). BMI was negatively associated with diversity of predominant bacteria (as detected with Univ-DGGE) in discordant twin pairs (Fig. 3), in agreement with earlier studies (14). At baseline, bifidobacterial diversity (Bif-DGGE) was negatively correlated with clusters M2 (carbohydrates and amino acids) and L11 (long-chain TGs). The diversities of Lachnospiraceae (Erec-DGGE) and predominant bacteria (Univ-DGGE) were positively correlated to cluster M5 (amino acids and TCA cycle metabolites). The Bfra diversity (Bfra-DGGE) was positively correlated to BA lithocholic acid (LCA) and to the cluster L8 (phosphatidylethanolamines). Diversity of Ruminococcaceae (Clept-DGGE) was positively associated with cluster L7 [long-chain polyunsaturated fatty acid (PUFA)-containing TGs; Fig. 3A]. At baseline, liver fat was positively correlated to clusters L9 and L14 [saturated fatty acid (SFA)- and monounsaturated fatty acid (MUFA)-containing TGs], in line with previous findings (31), and to cholic acid (CA). At 120 min time point, liver fat was positively correlated with postprandial levels of glycine ursodeoxycholic acid (GUDCA) and negatively correlated with M1 and L13 clusters (mainly sugars and microbiota derived-organic acids, and medium-chain TGs containing SFA and MUFA), while the CA associated with insulin and inversely with MI (Fig. 3B).
Figure 3.
Dependency network analyses for discordant co-twins for weight. A) Dependency network analysis of baseline concentration levels of metabolite and lipid clusters with BAs; gut microbial diversity; BMI; percentage body fat; intra-abdominal, subcutaneous, and liver fat; glucose and insulin concentrations; and MI. Data were twin normalized by using the within-pair difference between the heavier and the leaner co-twins for clusters, BMI, MI, and microbiota diversity and by using the log2 of the ratio between the heavier and the leaner co-twins for the rest of variables. B) Dependency network analysis using twin-normalized variables and including the end-point concentration levels of metabolite and lipid clusters with BAs; gut microbial diversity; BMI; percentage body fat; intra-abdominal, subcutaneous, and liver fat; glucose and insulin concentrations; and MI. C) Dependency network analysis using twin-normalized variables and including the level differences in metabolic variables (clusters, BAs) between the 120 min end point together with the gut microbial diversity; BMI; percentage body fat; intra-abdominal, subcutaneous, and liver fat; glucose and insulin concentrations; and MI. Node shapes represent different types of variables; node color corresponds to significance and direction of regulation comparing heavier versus leaner co-twins. NS, not significant.
Next, we studied how the differences of twin-normalized metabolic variables between the 120 min end point and baseline associated with other clinical and microbial diversity variables (Fig. 3C). Notably, diversity of each bacterial group was associated with a distinct group of metabolic variables. The postprandial changes of secondary BAs ursodeoxycholic acid (UDCA) and CDCA were negatively correlated with the bifidobacterial diversity (Bif-DGGE). In addition to their negative association with BMI, differences in diversity of predominant bacteria (Univ-DGGE) between discordant co-twins were positively associated with the cluster L9 (TGs with low carbon number and double bond content). The MI was negatively associated with the postprandial changes of the glycine-conjugated BA GUDCA, which was directly associated with liver fat. The postprandial changes of L13 cluster were both negatively associated with liver fat and with intra-abdominal fat. Postprandial changes of cluster L14 (TGs with odd-chain fatty acids, i.e., the nonendogenously synthesized fatty acids) were negatively associated with diversity of Lachnospiraceae (Erec-DGGE), thus suggesting that this group may be involved in the metabolism of dietary lipids. Diversity of Bacteroides spp. (Bfra-DGGE) was associated positively with M1 cluster changes (sugars and microbiota-derived organic acids) and negatively with postprandial changes of L15 cluster (unsaturated medium-chain TGs). The diversity of Ruminococcaceae (Clept-DGGE) was directly associated with changes in the levels of the clusters L2 and L8 (sphingomyelins and other phospholipids).
DISCUSSION
We applied a twin study design using MZ twin pairs discordant and concordant for weight sampled from the population in a standardized Big Mac meal challenge test to elucidate the postprandial response in obesity, independent of genetic factors. The comprehensive plasma metabolic profiling revealed that within-pair similarity is a dominant factor in the metabolic postprandial response, independent of acquired obesity. In addition, our study showed that diversities of specific bacterial groups, as detected with different bacterial group-specific DGGE methods, were associated with postprandial changes of specific metabolites.
When comparing the time-dependent metabolite response profiles between the co-twins discordant for weight, the plasma metabolic profiling revealed that the majority of metabolites did not show any significant differences in their fasting or postprandial levels when comparing twin individuals from twin pairs discordant for weight, and a fraction of the metabolites displayed a converging or diverging time profiles when comparing the weight-discordant co-twins. Among the converging metabolites were two BCAAs, valine and isoleucine, and fatty acids. While the fasting levels of BCAAs were higher and those of fatty acids were lower in DH co-twins, respectively, their levels were similar at 120 min after the Big Mac meal when comparing DH and DL co-twins. Elevated fasting concentrations of BCAAs are associated with acquired obesity, insulin resistance, and risk of type 2 diabetes (32–34). Our data suggest that postprandial response of BCAAs as well as fatty acids is under tight homeostatic control, independent of acquired obesity. However, the postprandial response to the meal challenge cannot be considered to be exclusively governed by genetic factors. As shown in a recent genome-wide association study on Finnish individuals, where the MZ twin pairs of our study were included, the heritability of many metabolites analyzed was modest (35), and the fraction of variance accounted for by known single-nucleotide polymorphism genotypes is mostly very small. Therefore, in addition to inheritance, also environmental factors, such as childhood diet and medication (especially antibiotics), possible prenatal exposures, and effects inducing epigenetic changes, might be responsible for the observed response. Furthermore, effects may be different in younger or older subjects, as our twins were young and healthy adults.
Microbial fermentation of polysaccharides may affect host adiposity by several mechanisms (12). In this study, Bfra diversity as detected with Bfra-DGGE was positively associated with postprandial changes in sugar- and microbiota-derived organic acids. This finding is in agreement with studies in mice, which showed that the metagenome of ob/ob and Western diet-fed wild-type mice is enriched in genes that encode the catabolism of complex polysaccharides when compared with their lean wild-type littermates (36). Obese mice exhibit increased capacity for gut luminal short-chain fatty acid production, suggesting that the gut microbiota may affect lipid metabolism and obesity by increasing substrates for energy metabolism in the liver and peripheral tissues (37). Accordingly, obese subjects exhibit enrichment in hydrogen gas-producing bacteria and hydrogen-consuming methanogenic archaea, which may increase energy availability to the obese host (38, 39). Moreover, we showed in our previous study with these same twins that their habitual intake of soluble fiber had a significant positive association with the Bfra numbers as detected with Bfra-specific quantitative PCR (qPCR; ref. 40). In addition, individuals with high energy intake had significantly lower numbers of Bfra as detected with qPCR (40).
The gut microbiota has also a profound effect on BA metabolism by promoting deconjugation, dehydrogenation, and dehydroxylation of primary BA in the distal small intestine and colon (41). BAs are predominantly conjugated to glycine in humans and to taurine in mice. Bile acids are critical regulators of hepatic lipid and glucose metabolism and influence signaling pathways via 2 receptors with pleiotropic functions, the nuclear farnesoid X receptor (FXR), and the G-protein-coupled BA receptor TGR5 (41). A recent gnotobiotic study identified muricholic acid (MCA) as a potent FXR antagonist (14), suggesting that the gut microbiota modulates BA synthesis by changing the BA pool composition and by alleviating FXR inhibition in the small intestine. Both humans and mice synthesize CA, but humans synthesize CDCA instead of MCA (42). In our study, GCA and GLCA (secondary BAs derived from CDCA) showed significant differences at the end of the BMT (120 min) when comparing DH and DL co-twins. Both BAs followed a diverging pattern, with significantly higher levels in DL co-twins, suggesting that the response of these specific BAs to the nutritional challenge is primarily driven by the acquired obesity, independently of genetic factors, in contrast to the other BAs analyzed in this study. Our BA profiling data are also consistent with the observed increase of the levels of GCA, GCDCA, and taurine CDCA (TCDCA) following glucose ingestion that was reported by a metabolic profiling study of the response to an oral glocuse tolerance test in healthy subjects (5).
Our study identified the association between the postprandial changes in the glycine form of UDCA and liver fat content as well as MI, an indicator of insulin sensitivity. GUDCA has not been previously linked to insulin sensitivity. Only the taurine-conjugated TUDCA has been reported to improve liver and muscle but not adipose tissue insulin sensitivity in obese subjects (43). The conjugated forms of the secondary BA UDCA and other BA species are present in a low amount in human plasma, and a complete plasma BA profile was lacking in previous challenge studies. The potential role of postprandial GUDCA in the regulation of insulin resistance remains to be elucidated in future studies.
One limitation of the present study is the small sample size. Despite a population-based screening of 10 full-birth cohorts of young adult twins, only 16 MZ twin pairs with major discordance for weight not attributable to or associated with comorbidities or medication use were identified from a cohort containing >1200 MZ twin pairs. This is consistent with the evidence that obesity is significantly influenced by genetic factors. Another potential limitation is the duration of the postprandial challenge. The lack of between-group differences observed in the lipid species may be due to the short duration of the challenge. The microbial analyses of this study were performed using 5 different DGGE methods rather than presently commonly applied next-generation sequencing (NGS) techniques. However, the detection limit for Lachnospiraceae (Erec)- Ruminococcaceae, (Clept)-, and Bfra-specific DGGEs is 0.1% of the total bacterial population (25, 26), whereas resolution for Bif-DGGE may even be as low as 0.01% of the total bacterial population (27). Moreover, sequencing to a depth of ≥10,000 reads/sample is commonly applied in NGS studies. At this sequencing depth, taxa present at an abundance ≥1:1000 (0.1%) can be detected with a precision of 0.78 ± 0.051 (44). It should therefore be noted that the resolutions of our bacterial group-specific DGGE methods are also 0.1% of the total bacterial population.
In summary, we identified different fasting and postprandial response metabolic profiles in MZ twins discordant for weight and found that within-pair similarity is a dominant factor in the metabolic postprandial response to the nutritional challenge, independent of acquired obesity. In particular, specific BAs were found to associate with liver fat and insulin resistance in fasting or postprandial states. Our findings underline important roles of genetic and early life factors in the regulation of postprandial metabolite levels. Different microbial groups appear to modulate postprandial responses of different metabolites, thus suggesting that despite the observed tight control of postprandial metabolite levels their metabolism may be altered via modulation of gut microbiota.
Supplementary Material
Acknowledgments
This work was supported by the EU FP7 Projects TORNADO (project 222720) and βBat (project 277713), Academy of Finland Centre of Excellence in Molecular Systems Immunology and Physiology Research (SyMMyS, 2012–2017, decision 250114), Novo Nordisk Foundation, Finnish Foundation for Cardiovascular Research, Diabetes Research Foundation, Helsinki University Central Hospital Research Funds, and an Academy of Finland grant (decision 266286). I.B.-P. is grateful to the Carlos III Health Institute of the Spanish Ministry of Health for a Sara Borrell postdoctoral contract.
Marko Sysi-Aho and Peddinti Gopalacharyulu are acknowledged for assistance in data analysis. Anna-Liisa Ruskeepää, Leena Öhrnberg, Ulla Lahtinen, and Sirkku Jäntti are acknowledged for excellent contribution to metabolite analysis and Marja-Liisa Jalovaara for excellent assistance in the microbiological analyses.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- BA
- bile acid
- BCAA
- branched chain amino acid
- Bfra
- Bacteroides spp.
- Bif
- Bifidobacterium spp.
- BMT
- Big Mac test
- C
- concordant control
- CA
- cholic acid
- CDCA
- chenodeoxycholic acid
- CL
- concordant leaner
- Clept
- Clostridium leptum
- DCA
- deoxycholic acid
- DEXA
- dual-energy X-ray absorptiometry
- DGGE
- denaturing gradient gel electrophoresis
- DH
- discordant heavier
- DL
- discordant leaner
- Erec
- Eubacterium rectale
- FDR
- false discovery rate
- FXR
- farnesoid X receptor
- GC×GC-TOFMS
- 2-dimensional gas chromatography coupled to time-of-flight mass spectrometry
- GLCA
- glycine lithocholic acid
- GUCDA
- glycine ursodeoxycholic acid
- LCA
- lithocholic acid
- MI
- Matsuda index
- MUFA
- monounsaturated fatty acid
- MZ
- monozygotic
- PUFA
- polyunsaturated fatty acid
- SFA
- saturated fatty acid
- TCDCA
- taurine chenodeoxycholic acid
- TG
- triglyceride
- UDCA
- ursodeoxycholic acid
- Univ-DGGE
- universal PCR-denaturing gradient gel electrophoresis
- UPLC-QqQMS
- ultraperformance liquid chromatography coupled to triple-quadrupole mass spectrometry
- UPLC-QTOFMS
- ultraperformance liquid chromatography coupled to quadrupole-time of flight mass spectrometry
- UPGMA
- unweighted-pair group method with arithmetic mean
REFERENCES
- 1. Catenacci V. A., Hill J. O., Wyatt H. R. (2009) The obesity epidemic. Clin. Chest Med. 30, 415–444, vii [DOI] [PubMed] [Google Scholar]
- 2. Cruz-Teno C., Perez-Martinez P., Delgado-Lista J., Yubero-Serrano E. M., Garcia-Rios A., Marin C., Gomez P., Jimenez-Gomez Y., Camargo A., Rodriguez-Cantalejo F., Malagon M. M., Perez-Jimenez F., Roche H. M., Lopez-Miranda J. (2012) Dietary fat modifies the postprandial inflammatory state in subjects with metabolic syndrome: the LIPGENE study. Mol. Nutr. Food Res. 56, 854–865 [DOI] [PubMed] [Google Scholar]
- 3. Krug S., Kastenmuller G., Stuckler F., Rist M. J., Skurk T., Sailer M., Raffler J., Romisch-Margl W., Adamski J., Prehn C., Frank T., Engel K. H., Hofmann T., Luy B., Zimmermann R., Moritz F., Schmitt-Kopplin P., Krumsiek J., Kremer W., Huber F., Oeh U., Theis F. J., Szymczak W., Hauner H., Suhre K., Daniel H. (2012) The dynamic range of the human metabolome revealed by challenges. FASEB J. 26, 2607–2619 [DOI] [PubMed] [Google Scholar]
- 4. Pellis L., van Erk M. J., van Ommen B., Bakker G. C., Hendriks H. F., Cnubben N. H., Kleemann R., van Someren E. P., Bobeldijk I., Rubingh C. M., Wopereis S. (2012) Plasma metabolomics and proteomics profiling after a postprandial challenge reveal subtle diet effects on human metabolic status. Metabolomics 8, 347–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shaham O., Wei R., Wang T. J., Ricciardi C., Lewis G. D., Vasan R. S., Carr S. A., Thadhani R., Gerszten R. E., Mootha V. K. (2008) Metabolic profiling of the human response to a glucose challenge reveals distinct axes of insulin sensitivity. Mol. Syst. Biol. 4, e214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wopereis S., Rubingh C. M., van Erk M. J., Verheij E. R., van Vliet T., Cnubben N. H., Smilde A. K., van der Greef J., van Ommen B., Hendriks H. F. (2009) Metabolic profiling of the response to an oral glucose tolerance test detects subtle metabolic changes. PLoS One 4, e4525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zivkovic A. M., Wiest M. M., Nguyen U., Nording M. L., Watkins S. M., German J. B. (2009) Assessing individual metabolic responsiveness to a lipid challenge using a targeted metabolomic approach. Metabolomics 5, 209–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Van Dijk S. J., Mensink M., Esser D., Feskens E. J., Muller M., Afman L. A. (2012) Responses to high-fat challenges varying in fat type in subjects with different metabolic risk phenotypes: a randomized trial. PLoS One 7, e41388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pietiläinen K. H., Sysi-Aho M., Rissanen A., Seppänen-Laakso T., Yki-Järvinen H., Kaprio J., Oresic M. (2007) Acquired obesity is associated with changes in the serum lipidomic profile independent of genetic effects–a monozygotic twin study. PLoS One 2, e218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Naukkarinen J., Rissanen A., Kaprio J., Pietilainen K. H. (2012) Causes and consequences of obesity: the contribution of recent twin studies. Int. J. Obes. (Lond.) 36, 1017–1024 [DOI] [PubMed] [Google Scholar]
- 11. Bäckhed F., Manchester J. K., Semenkovich C. F., Gordon J. I. (2007) Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc. Natl. Acad. Sci. U. S. A. 104, 979–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tremaroli V., Bäckhed F. (2012) Functional interactions between the gut microbiota and host metabolism. Nature 489, 242–249 [DOI] [PubMed] [Google Scholar]
- 13. Turnbaugh P. J., Hamady M., Yatsunenko T., Cantarel B. L., Duncan A., Ley R. E., Sogin M. L., Jones W. J., Roe B. A., Affourtit J. P., Egholm M., Henrissat B., Heath A. C., Knight R., Gordon J. I. (2009) A core gut microbiome in obese and lean twins. Nature 457, 480–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sayin S. I., Wahlstrom A., Felin J., Jantti S., Marschall H. U., Bamberg K., Angelin B., Hyotylainen T., Oresic M., Bäckhed F. (2013) Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell. Metab. 17, 225–235 [DOI] [PubMed] [Google Scholar]
- 15. Yokota A., Fukiya S., Islam K. B., Ooka T., Ogura Y., Hayashi T., Hagio M., Ishizuka S. (2012) Is bile acid a determinant of the gut microbiota on a high-fat diet? Gut Microbes 3, 455–459 [DOI] [PubMed] [Google Scholar]
- 16. Kaprio J., Pulkkinen L., Rose R. J. (2002) Genetic and environmental factors in health-related behaviors: studies on Finnish twins and twin families. Twin Res. 5, 366–371 [DOI] [PubMed] [Google Scholar]
- 17. Graner M., Seppala-Lindroos A., Rissanen A., Hakkarainen A., Lundbom N., Kaprio J., Nieminen M. S., Pietilainen K. H. (2012) Epicardial fat, cardiac dimensions, and low-grade inflammation in young adult monozygotic twins discordant for obesity. Am. J. Cardiol. 109, 1295–1302 [DOI] [PubMed] [Google Scholar]
- 18. Pietilainen K. H., Rissanen A., Kaprio J., Makimattila S., Hakkinen A. M., Westerbacka J., Sutinen J., Vehkavaara S., Yki-Jarvinen H. (2005) Acquired obesity is associated with increased liver fat, intra-abdominal fat, and insulin resistance in young adult monozygotic twins. Am. J. Physiol. Endocrinol. Metab. 288, E768–774 [DOI] [PubMed] [Google Scholar]
- 19. Wallace T. M., Levy J. C., Matthews D. R. (2004) Use and abuse of HOMA modeling. Diabetes Care 27, 1487–1495 [DOI] [PubMed] [Google Scholar]
- 20. Friedewald W. T., Levy R. I., Fredrickson D. S. (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 18, 499–502 [PubMed] [Google Scholar]
- 21. Castillo S., Mattila I., Miettinen J., Oresic M., Hyotylainen T. (2011) Data analysis tool for comprehensive two-dimensional gas chromatography/time-of-flight mass spectrometry. Anal. Chem. 83, 3058–3067 [DOI] [PubMed] [Google Scholar]
- 22. Nygren H., Seppanen-Laakso T., Castillo S., Hyotylainen T., Oresic M. (2011) Liquid chromatography-mass spectrometry (LC-MS)-based lipidomics for studies of body fluids and tissues. Methods Mol. Biol. 708, 247–257 [DOI] [PubMed] [Google Scholar]
- 23. Mättö J., Maunuksela L., Kajander K., Palva A., Korpela R., Kassinen A., Saarela M. (2005) Composition and temporal stability of gastrointestinal microbiota in irritable bowel syndrome–a longitudinal study in IBS and control subjects. FEMS Immunol. Med. Microbiol. 43, 213–222 [DOI] [PubMed] [Google Scholar]
- 24. Nubel U., Engelen B., Felske A., Snaidr J., Wieshuber A., Amann R. I., Ludwig W., Backhaus H. (1996) Sequence heterogeneities of genes encoding 16S rRNAs in Paenibacillus polymyxa detected by temperature gradient gel electrophoresis. J. Bacteriol. 178, 5636–5643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Maukonen J., Matto J., Satokari R., Soderlund H., Mattila-Sandholm T., Saarela M. (2006) PCR DGGE and RT-PCR DGGE show diversity and short-term temporal stability in the Clostridium coccoides-Eubacterium rectale group in the human intestinal microbiota. FEMS Microbiol. Ecol. 58, 517–528 [DOI] [PubMed] [Google Scholar]
- 26. Maukonen J., Simoes C., Saarela M. (2012) The currently used commercial DNA-extraction methods give different results of clostridial and actinobacterial populations derived from human fecal samples. FEMS Microbiol. Ecol. 79, 697–708 [DOI] [PubMed] [Google Scholar]
- 27. Satokari R. M., Vaughan E. E., Akkermans A. D., Saarela M., de Vos W. M. (2001) Bifidobacterial diversity in human feces detected by genus-specific PCR and denaturing gradient gel electrophoresis. Appl. Environ. Microbiol. 67, 504–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fraley C., Raftery A. E. (2007) Model-based methods of classification: Using the mclust software in chemometrics. J. Stat. Soft. 18, 1–13 [Google Scholar]
- 29. Schafer J., Strimmer K. (2005) An empirical Bayes approach to inferring large-scale gene association networks. Bioinformatics 21, 754–764 [DOI] [PubMed] [Google Scholar]
- 30. Brohee S., Faust K., Lima-Mendez G., Vanderstocken G., van Helden J. (2008) Network Analysis Tools: from biological networks to clusters and pathways. Nat. Protoc. 3, 1616–1629 [DOI] [PubMed] [Google Scholar]
- 31. Oresic M., Hyotylainen T., Kotronen A., Gopalacharyulu P., Nygren H., Arola J., Castillo S., Mattila I., Hakkarainen A., Borra R. J., Honka M. J., Verrijken A., Francque S., Iozzo P., Leivonen M., Jaser N., Juuti A., Sorensen T. I., Nuutila P., Van Gaal L., Yki-Jarvinen H. (2013) Prediction of non-alcoholic fatty-liver disease and liver fat content by serum molecular lipids. Diabetologia 56, 2266–2274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Newgard C. B., An J., Bain J. R., Muehlbauer M. J., Stevens R. D., Lien L. F., Haqq A. M., Shah S. H., Arlotto M., Slentz C. A., Rochon J., Gallup D., Ilkayeva O., Wenner B. R., Yancy W. S., Jr., Eisenson H., Musante G., Surwit R. S., Millington D. S., Butler M. D., Svetkey L. P. (2009) A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 9, 311–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pietilainen K. H., Naukkarinen J., Rissanen A., Saharinen J., Ellonen P., Keranen H., Suomalainen A., Gotz A., Suortti T., Yki-Jarvinen H., Oresic M., Kaprio J., Peltonen L. (2008) Global transcript profiles of fat in monozygotic twins discordant for BMI: pathways behind acquired obesity. PLoS Med. 5, e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang T. J., Larson M. G., Vasan R. S., Cheng S., Rhee E. P., McCabe E., Lewis G. D., Fox C. S., Jacques P. F., Fernandez C., O'Donnell C. J., Carr S. A., Mootha V. K., Florez J. C., Souza A., Melander O., Clish C. B., Gerszten R. E. (2011) Metabolite profiles and the risk of developing diabetes. Nat. Med. 17, 448–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kettunen J., Tukiainen T., Sarin A. P., Ortega-Alonso A., Tikkanen E., Lyytikainen L. P., Kangas A. J., Soininen P., Wurtz P., Silander K., Dick D. M., Rose R. J., Savolainen M. J., Viikari J., Kahonen M., Lehtimaki T., Pietilainen K. H., Inouye M., McCarthy M. I., Jula A., Eriksson J., Raitakari O. T., Salomaa V., Kaprio J., Jarvelin M. R., Peltonen L., Perola M., Freimer N. B., Ala-Korpela M., Palotie A., Ripatti S. (2012) Genome-wide association study identifies multiple loci influencing human serum metabolite levels. Nat. Genet. 44, 269–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Turnbaugh P. J., Ley R. E., Mahowald M. A., Magrini V., Mardis E. R., Gordon J. I. (2006) An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444, 1027–1031 [DOI] [PubMed] [Google Scholar]
- 37. Bäckhed F., Crawford P. A. (2010) Coordinated regulation of the metabolome and lipidome at the host-microbial interface. Biochim. Biophys. Acta 1801, 240–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Samuel B. S., Hansen E. E., Manchester J. K., Coutinho P. M., Henrissat B., Fulton R., Latreille P., Kim K., Wilson R. K., Gordon J. I. (2007) Genomic and metabolic adaptations of Methanobrevibacter smithii to the human gut. Proc. Natl. Acad. Sci. U. S. A. 104, 10643–10648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhang H., DiBaise J. K., Zuccolo A., Kudrna D., Braidotti M., Yu Y., Parameswaran P., Crowell M. D., Wing R., Rittmann B. E., Krajmalnik-Brown R. (2009) Human gut microbiota in obesity and after gastric bypass. Proc. Natl. Acad. Sci. U. S. A. 106, 2365–2370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Simoes C. D., Maukonen J., Kaprio J., Rissanen A., Pietilainen K. H., Saarela M. (2013) Habitual dietary intake is associated with stool microbiota composition in monozygotic twins. J. Nutr. 143, 417–423 [DOI] [PubMed] [Google Scholar]
- 41. de Aguiar Vallim T. Q., Tarling E. J., Edwards P. A. (2013) Pleiotropic roles of bile acids in metabolism. Cell Metab. 17, 657–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Russell D. W. (2003) The enzymes, regulation, and genetics of bile acid synthesis. Annu. Rev. Biochem. 72, 137–174 [DOI] [PubMed] [Google Scholar]
- 43. Kars M., Yang L., Gregor M. F., Mohammed B. S., Pietka T. A., Finck B. N., Patterson B. W., Horton J. D., Mittendorfer B., Hotamisligil G. S., Klein S. (2010) Tauroursodeoxycholic acid may improve liver and muscle but not adipose tissue insulin sensitivity in obese men and women. Diabetes 59, 1899–1905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Faith J. J., Guruge J. L., Charbonneau M., Subramanian S., Seedorf H., Goodman A. L., Clemente J. C., Knight R., Heath A. C., Leibel R. L., Rosenbaum M., Gordon J. I. (2013) The long-term stability of the human gut microbiota. Science 341, 1237439. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.