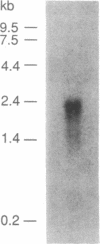
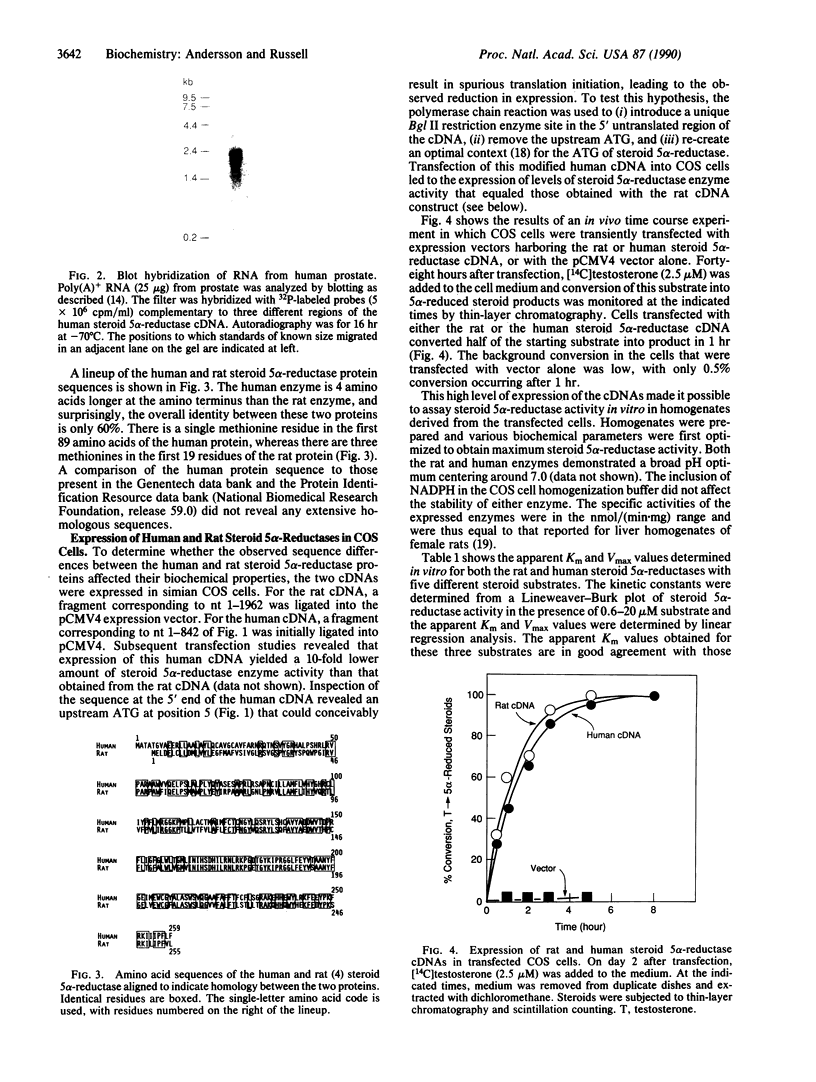
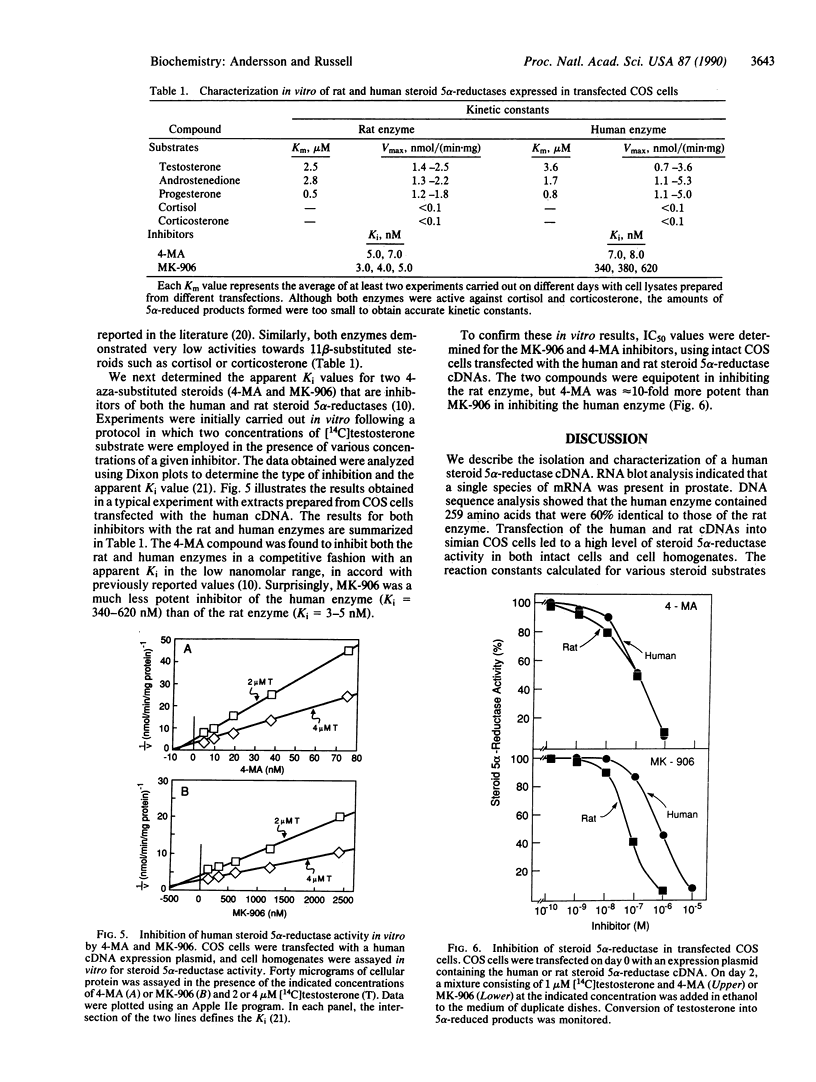
Abstract
The microsomal enzyme steroid 5 alpha-reductase is responsible for the conversion of testosterone into the more potent androgen dihydrotestosterone. In man, this steroid acts on a variety of androgen-responsive target tissues to mediate such diverse endocrine processes as male sexual differentiation in the fetus and prostatic growth in men. Here we describe the isolation, structure, and expression of a cDNA encoding the human steroid 5 alpha-reductase. A rat cDNA was used as a hybridization probe to screen a human prostate cDNA library. A 2.1-kilobase cDNA was identified and DNA sequence analysis indicated that the human steroid 5 alpha-reductase was a hydrophobic protein of 259 amino acids with a predicted molecular weight of 29,462. A comparison of the human and rat protein sequences revealed a 60% identity. Transfection of expression vectors containing the human and rat cDNAs into simian COS cells resulted in the synthesis of high levels of steroid 5 alpha-reductase enzyme activity. Both enzymes expressed in COS cells showed similar substrate specificities for naturally occurring steroid hormones. However, synthetic 4-azasteroids demonstrated marked differences in their abilities to inhibit the human and rat steroid 5 alpha-reductases.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson S., Bishop R. W., Russell D. W. Expression cloning and regulation of steroid 5 alpha-reductase, an enzyme essential for male sexual differentiation. J Biol Chem. 1989 Sep 25;264(27):16249–16255. [PMC free article] [PubMed] [Google Scholar]
- Andersson S., Davis D. L., Dahlbäck H., Jörnvall H., Russell D. W. Cloning, structure, and expression of the mitochondrial cytochrome P-450 sterol 26-hydroxylase, a bile acid biosynthetic enzyme. J Biol Chem. 1989 May 15;264(14):8222–8229. [PubMed] [Google Scholar]
- Brooks J. R., Baptista E. M., Berman C., Ham E. A., Hichens M., Johnston D. B., Primka R. L., Rasmusson G. H., Reynolds G. F., Schmitt S. M. Response of rat ventral prostate to a new and novel 5 alpha-reductase inhibitor. Endocrinology. 1981 Sep;109(3):830–836. doi: 10.1210/endo-109-3-830. [DOI] [PubMed] [Google Scholar]
- Cunha G. R., Donjacour A. A., Cooke P. S., Mee S., Bigsby R. M., Higgins S. J., Sugimura Y. The endocrinology and developmental biology of the prostate. Endocr Rev. 1987 Aug;8(3):338–362. doi: 10.1210/edrv-8-3-338. [DOI] [PubMed] [Google Scholar]
- Fisher L. K., Kogut M. D., Moore R. J., Goebelsmann U., Weitzman J. J., Isaacs H., Jr, Griffin J. E., Wilson J. D. Clinical, endocrinological, and enzymatic characterization of two patients with 5 alpha-reductase deficiency: evidence that a single enzyme is responsible for the 5 alpha-reduction of cortisol and testosterone. J Clin Endocrinol Metab. 1978 Sep;47(3):653–664. doi: 10.1210/jcem-47-3-653. [DOI] [PubMed] [Google Scholar]
- Frederiksen D. W., Wilson J. D. Partial characterization of the nuclear reduced nicotinamide adenine dinucleotide phosphate: delta 4-3-ketosteroid 5 alpha-oxidoreductase of rat prostate. J Biol Chem. 1971 Apr 25;246(8):2584–2593. [PubMed] [Google Scholar]
- Gonzalez F. J. The molecular biology of cytochrome P450s. Pharmacol Rev. 1988 Dec;40(4):243–288. [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Liang T., Cascieri M. A., Cheung A. H., Reynolds G. F., Rasmusson G. H. Species differences in prostatic steroid 5 alpha-reductases of rat, dog, and human. Endocrinology. 1985 Aug;117(2):571–579. doi: 10.1210/endo-117-2-571. [DOI] [PubMed] [Google Scholar]
- Metcalf B. W., Levy M. A., Holt D. A. Inhibitors of steroid 5 alpha-reductase in benign prostatic hyperplasia, male pattern baldness and acne. Trends Pharmacol Sci. 1989 Dec;10(12):491–495. doi: 10.1016/0165-6147(89)90048-5. [DOI] [PubMed] [Google Scholar]
- Mooradian A. D., Morley J. E., Korenman S. G. Biological actions of androgens. Endocr Rev. 1987 Feb;8(1):1–28. doi: 10.1210/edrv-8-1-1. [DOI] [PubMed] [Google Scholar]
- Moore R. J., Griffin J. E., Wilson J. D. Diminished 5alpha-reductase activity in extracts of fibroblasts cultured from patients with familial incomplete male pseudohermaphroditism, type 2. J Biol Chem. 1975 Sep 25;250(18):7168–7172. [PubMed] [Google Scholar]
- Moore R. J., Wilson J. D. Localization of the reduced nicotinamide adenine dinucleotide phosphate: 4 -3-ketosteroid 5 -oxidoreductase in the nuclear membrane of the rat ventral prostate. J Biol Chem. 1972 Feb 10;247(3):958–967. [PubMed] [Google Scholar]
- Moore R. J., Wilson J. D. Steroid 5alpha-reductase in cultured human fibroblasts. Biochemical and genetic evidence for two distinct enzyme activities. J Biol Chem. 1976 Oct 10;251(19):5895–5900. [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Tilley W. D., Marcelli M., Wilson J. D., McPhaul M. J. Characterization and expression of a cDNA encoding the human androgen receptor. Proc Natl Acad Sci U S A. 1989 Jan;86(1):327–331. doi: 10.1073/pnas.86.1.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen A., Giagulli V. A., De Schepper P., Buntinx A., Stoner E. Hormonal effects of an orally active 4-azasteroid inhibitor of 5 alpha-reductase in humans. Prostate. 1989;14(1):45–53. doi: 10.1002/pros.2990140106. [DOI] [PubMed] [Google Scholar]
- Wilson J. D. Sexual differentiation. Annu Rev Physiol. 1978;40:279–306. doi: 10.1146/annurev.ph.40.030178.001431. [DOI] [PubMed] [Google Scholar]
- Wilson J. D. The pathogenesis of benign prostatic hyperplasia. Am J Med. 1980 May;68(5):745–756. doi: 10.1016/0002-9343(80)90267-3. [DOI] [PubMed] [Google Scholar]
- YATES F. E., HERBST A. L., URQUHART J. Sex difference in rate of ring A reduction of delta 4-3-keto-steroids in vitro by rat liver. Endocrinology. 1958 Dec;63(6):887–902. doi: 10.1210/endo-63-6-887. [DOI] [PubMed] [Google Scholar]