Abstract
With the prevalence of hepatitis C virus expected to decline, the proportion of hepatocellular carcinoma (HCC) related to non-alcoholic steatohepatitis (NASH) is anticipated to increase exponentially due to the growing epidemic of obesity and diabetes. The annual incidence rate of developing HCC in patients with NASH-related cirrhosis is not clearly understood with rates ranging from 2.6%-12.8%. While multiple new mechanisms have been implicated in the development of HCC in NASH; further prospective long-term studies are needed to validate these findings. Recent evidence has shown a significant proportion of patients with non-alcoholic fatty liver disease and NASH progress to HCC in the absence of cirrhosis. Liver resection and transplantation represent curative therapeutic options in select NASH-related HCC patients but have placed a significant burden to our healthcare resources and utilization. Currently NASH-related HCC is the fastest growing indication for liver transplant in HCC candidates. Increased efforts to implement effective screening and preventative strategies, particularly in non-cirrhotic NASH patients, are needed to reduce the future impact imposed by NASH-related HCC.
Keywords: Non-alcoholic steatohepatitis, Cirrhosis, Non-alcoholic fatty liver disease, Obesity, Hepatocellular carcinoma
Core tip: Non-alcoholic steatohepatitis (NASH) is anticipated to account for a greater proportion of hepatocellular carcinoma (HCC) incidence due to the growing epidemic of obesity and diabetes. Currently NASH-related HCC is the fastest growing indication for liver transplant in HCC candidates. Increased efforts to implement effective screening and preventative strategies particularly in non-cirrhotic NASH patients possibly based on genetic susceptibility are needed to reduce the future impact imposed by NASH-related HCC.
INTRODUCTION
Hepatocellular carcinoma (HCC) accounts for 90% of primary liver cancers Worldwide, HCC being the sixth most common cancer, and is the second leading cause of cancer-related death[1]. HCC largely occurs in the background of chronic liver disease and cirrhosis of the liver[2]. The leading liver disease etiologies for cirrhosis in patients with HCC include but are not limited to chronic hepatitis B, chronic hepatitis C virus (HCV), and alcoholic liver disease. With advent of curative treatments for HCV, the risk of progression to cirrhosis and development of HCC secondary to HCV is anticipated to decline. However, in recent years, non-alcoholic fatty liver disease (NAFLD) has quickly risen as one of leading etiologies for liver disease. NAFLD is a spectrum of chronic liver disease ranging from simple hepatic steatosis to liver cell injury and inflammation known as non-alcoholic steatohepatitis (NASH). The rising incidence of NAFLD/NASH has subsequently led to a dramatic rise in NASH-related HCC incidence[3]. Numerous studies have demonstrated that NASH can lead to advanced fibrosis and cirrhosis, thereby increasing the risk of developing HCC[4-6]. Among patients with NAFLD or NASH, liver disease is the third leading cause of death[4], while HCC represents the main cause of death in this group[7]. The cumulative annual incidence rate for developing HCC in patients with NASH-related cirrhosis is approximately 2.4%-12.8%[8]. In the absence of NASH or cirrhosis, NAFLD can present with HCC. These patients usually present with less aggressive tumors and are less likely to diagnosed by surveillance compared to HCC that develops in the setting of viral hepatitis[9-11]. A similar rising trend has been reported in NASH progressing to HCC in the absence of cirrhosis[12-14]. In NASH, several risk factors for HCC development have been identified including metabolic syndrome and insulin resistance causing changes in serum cytokines, persistent inflammation, and altered gut microflora and bile composition[15].
EPIDEMIOLOGY
Currently, NAFLD affects more than 80 million Americans, making it the most common etiology for liver disease in the United States. With the incidence of obesity, diabetes and metabolic syndrome continuing to increase in the United States and Europe, NAFLD/NASH may become the most common cause of HCC in developed countries in the near future[16]. In 2012, primary liver cancer was recognized overall as the second most common cause of cancer-related death in the world. In the United States, HCC is the most rapidly rising cause of cancer and cancer-related deaths with an incidence that has tripled over the last decade. This high likelihood for mortality reflects a poor prognosis without therapeutic intervention[17]. HCC is the most prevalent histological subtype accounting for 70%-85% of primary liver malignancies[18]. Compared to HCC in alcoholic liver disease and viral hepatitis, there is a lack of strong epidemiological data regarding the incidence and prevalence of HCC in NAFLD[19]. While the prevalence of NAFLD is thought to be highest among Hispanics and Caucasians, the ethnic distribution among NAFLD/NASH-related HCC patients has yet to be defined[20]. NASH-related HCC patients are predominantly male; however, gender has not been proven to be a statistical risk factor NASH progression to HCC[21]. Studies analyzing demographic and clinical characteristics of NASH-related HCC patients are outlined in Table 1. Reports indicate that NASH can be verified by histological evaluation in up to 47% of all NAFLD cases among obese individuals[22,23]. Amongst a growing population of diabetes which has surpassed 26 million in the United States, the prevalence of biopsy-proven NAFLD and NASH has been reported to be as high as 74% and 11%, respectively[24,25].
Table 1.
Reported studies of hepatocellular carcinoma in patients with cirrhotic and non-cirrhotic non-alcoholic fatty liver disease/non-alcoholic steatohepatitis, and their clinical characteristics
| Ref. | All (n) | NASH/NAFLD (n) | Study type | Clinical characteristics |
Cirrhotic NASH with HCC |
Non-cirrhotic NASH with HCC |
||
| Histological diagnosis | Clinical diagnosis | Histological diagnosis | Clinical diagnosis | |||||
| Cotrim et al[97] | 110 | 110 | Cohort | Age, 67 ± 11 yr; male, 72 (65.5%); non-Hispanic white, N/A | 32 (29.1%) | 58 (52.7%) | 20 (18.2%) | 0 |
| Van Meer et al[98] | 933 | 911 | Cohort | Age, 64 yr; male, 60 (66%); non-Hispanic white, N/A | N/A | N/A | 91 (100%) | N/A |
| Shrager et al[99] | 9 | 9 | Case series | Age, 58 yr; male, 8 (88.9%); non-Hispanic white, N/A | 5 (55.5%) | N/A | 4 (44.4%) | N/A |
| Kikuchi et al[93] | 42 | 38 | Case series | Age, 66.5 yr; male 26 (62%); non-Hispanic white, N/A | 34 | N/A | 4 | N/A |
| Chagas et al[100] | 394 | 7 | Prospectiv | Age, 63 ± 13 yr; Male 4 (57%); non-Hispanic white, N/A | 6 | N/A | 1 | N/A |
| Ertle et al[101] | 150 | 36 | Cohort | Age, 68.6 ± 8.4 yr; male 32 (88.9%); non-Hispanic white, N/A | 5 | 142 | 10 | 72 |
| Tokushige et al[102] | 2299 | 292 | Cohort | Age, 72 ± 8.4 yr; male, 181 (62%) | 1813 | N/A | 1113 | N/A |
| Hashizume et al[103] | 1310 | 10 | Case series | Age 71.5 yr; male 6 (66.7%) | 5 | N/A | 4 | N/A |
| Kawada et al[13] | 807 | 8 | Cohort | Age 73 yr; male 3/6 (50%); non-Hispanic white, N/A | 2 | N/A | 6 | N/A |
| Malik et al[104] | 143 | 143 | Case control | Age 59 ± 7.6 yr; male 44 (44.9%); 16 non-Hispanic White, 1 Asian | 17 | N/A | 0 | N/A |
| Takuma et al[105] | 11 | 11 | Case series/Literature review | Age 73.8 ± 4.9 yr; male 5 (45%) | 4 | N/A | 7 | N/A |
| Perumpail et al[106] | 44 | 6 | Cohort | Age 72 ± 8 yr; male 5 (83.3%) | NA | NA | 6 | N/A |
| Ascha et al[107] | 510 | 195 | Cohort | Age 56.5 yr; male 86 (44.1%) | NA | NA | N/A | 254 |
| Mohamad et al[108] | 83 | 83 | Cohort retrospective | Age 64.8 ± 10.4 yr; male 54 (65.1%); non-Hispanic White, 77 (92.8%) | 47 | N/A | 36 | N/A |
Histological data available in 86 patients only;
AASLD Radiological criteria used for diagnosis;
Results based on both liver biopsy and abdominal imaging. Differentiating data not available in the study;
Histologic confirmation obtained in 59% of the patients diagnosed with HCC. HCC: Hepatocellular carcinoma; EMT: Epithelial to mesenchymal transition; NAFLD: Non-alcoholic fatty liver disease; NASH: Non-alcoholic steatohepatitis; N/A: Not available.
This rise in the incidence of NASH-related HCC has impacted trends in liver transplantation as well. A retrospective cohort study amongst adult liver transplant recipients from 2002-2012 indicated that there was 4-fold increase in patients undergoing liver transplant for NASH-related HCC compared to 2-fold increase in number of patients undergoing transplantation for HCV-related HCC[26]. During this 10-year span, NASH also became the second leading cause of HCC-related liver transplantation in America, steadily increasing from 8.3% in 2002 to 10.3% in 2007 and to 13.5% in 2012[16], and most likely will surpass 15% by 2017.
PROGRESSION OF NASH/NAFLD TO HCC
NAFLD is the hepatic manifestation of metabolic syndrome, with insulin resistance driving the alteration in physiology. As mentioned earlier, it ranges from isolated hepatic steatosis, to NASH with or without cirrhosis, and progression to HCC. The diagnosis of NASH is based on histological evidence of hepatic steatosis or magnetic resonance spectroscopic evidence > 5% fat accumulation of liver weight without the presence of secondary causes such as alcohol abuse, endocrine disorders, chronic HCV infection or familial hypobetalipoproteinemia[27]. Recent evidence has demonstrated an association between NASH and HCC to be exclusive to patients who had progressed to cirrhosis, suggesting causality[8].
Compared to benign course of simple steatosis, patients with NASH are more likely to develop progressive advanced liver disease. Matteoni et al[28] demonstrated increased rates of cirrhosis in patients with NASH compared to those with fatty liver without NASH (25% vs 3%, respectively), and increased risk of liver disease-related death (11% vs 2%, respectively). In a much larger study across the entire spectrum of NAFLD which included 420 patients, Rafiq et al[29] demonstrated a higher mortality in those with NASH/NAFLD when compared to the general population; liver-related deaths occurred in 13% vs < 1% in general population, and 3% of those with NAFLD developed cirrhosis. Another study further confirmed increased rate of liver-related deaths among patients with NASH when compared with those without NASH (17.5% vs 3%, respectively). In patients with compensated cirrhosis, NASH-related cirrhosis patients had better survival outcomes compared to HCV-related cirrhosis patients. However, in decompensated cirrhosis both cohorts had comparable poor outcomes[30,31]. Currently, both the American Association for the Study of Liver Diseases, and the European Association for the Study of Liver Disease recommend screening for HCC in patients with NASH related cirrhosis every 6-12 mo[32].
HCC IN NON-CIRRHOTIC NAFLD/NASH
Emerging evidence suggests that a significant proportion of patients with NAFLD-associated HCC, do not have histologic evidence of cirrhosis. In a study conducted by Kawada et al[13], of 1168 patients who underwent hepatic resection for HCC, 6 of 8 patients with NASH-related HCC did not demonstrate cirrhosis. This study suggested that the presence of cirrhosis in NASH-related HCC was lower compared to HCV-related HCC. These data suggest that compared to patients HCV, HCC may develop at an earlier stage those with NASH. Paradis et al[33] analyzed 128 HCC patients who were recruited over 12 years, and reported significant number of patients with NASH developed HCC in the absence of fibrosis when compared to HCC in the setting of other underlying chronic liver disease (65% with F0-F2 in NASH group vs 26% in chronic liver disease)[33,34]. To explain this phenomenon in non-cirrhotic NAFLD patients, one proposed hypothesis is the malignant transformation of hepatic adenoma. Few published reports have suggested that in the presence of metabolic syndrome, hepatocellular adenoma may incur a malignant transformation[19,35].
HCC IN CIRRHOTIC NAFLD/NASH
During the last two decades, various studies have tried to determine the relationship between NAFLD/NASH, cryptogenic cirrhosis and HCC. A recent meta-analyses by White et al[8] showed that approximately 60% HCC cases attributed to NAFLD/NASH had cirrhosis either before or at the time of diagnosis. This meta analyses also included review of cohort and longitudinal studies which showed that NASH-associated cirrhosis consistently carried an increased HCC risk ranging between 2.4% and 12.8%[8]. Additionally, this study reported the risk of developing HCC is lower in patients with cirrhosis due to NAFLD/NASH when compared to those with chronic HCV (NAFLD/NASH, 26.9% vs HCV, 19.7%).
The true prevalence of NASH and NASH-related HCC is likely underestimated. In up to 6.9%-29% of HCC, the underlying etiology of liver disease is unknown and is considered secondary to cryptogenic cirrhosis[19]. Features suggestive of NASH are more frequently observed in HCC arising in patients with cryptogenic cirrhosis than in age- and sex-matched HCC patients of well-defined viral or alcoholic etiology[36]. Although the prevalence of NAFLD/NASH-related HCC is not well defined, the increasing incidence of obesity and diabetes, suggests the impact of NAFLD/NASH-related HCC will continue to grow.
MORTALITY IN NAFLD/NASH
Long term outcomes in NAFLD and NASH has been evaluated in several studies and distinctive differences between NASH and non-NASH subtypes of NAFLD have been shown[28,29,37-42]. Type 2 diabetes mellitus has been shown to increase the risk of both liver- related mortality and overall mortality in NAFLD patients[43,44]. In light of these findings NAFLD patients with type II diabetes should be prioritized in future treatment protocols[44]. A population-based study published in 1996 followed 153852 subjects and found that diabetic patients had a standardized incidence ratio of 4.1 for HCC[45]. However, another retrospective analysis from United States Veteran Registry noted increased the risk of primary liver cancer in patients with diabetes only in the presence of other risk factors such as hepatitis C or B or alcoholic cirrhosis[46]. These observations were not supported by further analysis that found an incremented HCC risk in diabetic patients independently from alcoholic liver disease and viral hepatitis[47,48]. In a recent meta-analyses, Younossi et al[49] reported that in NAFLD patients, annual incidence of HCC was 0.44 per 1000 person-years (95%CI: 0.29-0.66), whereas for those with NASH, the annual HCC incident rate was 5.29 per 1000 person-years (95%CI: 0.75-37.56). Among NAFLD cohort, the pooled liver-specific and overall mortality incidence rates were 0.77 per 1000 person-years (95%CI: 0.33-1.77 events) and 15.44 per 1000 person-years (95%CI: 11.72-20.34 events), respectively. Among the NASH cohort, the pooled liver-specific and overall mortality incidence rates were 11.77 per 1000 person-years (95%CI: 7.10-19.53 events) and 25.56 per 1000 person-years (95%CI: 6.29-103.8 events), respectively.
Although cardio-vascular (CV) events remain the major cause of death in patients with NAFLD and NASH, the CV mortality rate amongst the NASH and non-NASH subtypes of NAFLD is similar[42,50-52]. Since patients with NASH have significantly higher liver-related mortality than those with non-NASH NAFLD, treatment strategies should be designed to ameliorate the risks for cardiovascular mortality[28,29,38,40-42,49,50]. Further, patients with NASH and type 2 diabetes mellitus, will need increased attention and linkage of care to reduce liver disease-related compliactions and to reduce their risk of HCC[53-55].
RISK FACTORS AND PROPOSED MECHANISMS FOR NASH-RELATED HCC
Development of HCC in the setting of chronic liver disease is a complex but gradual process that requires transition through a dysplasia-carcinoma sequence. Several putative oncogenic mechanisms has been incriminated that lead to genomic instability, including telomere erosion, chromosome segregation defects and alterations in the DNA-damage-response pathways[56,57]. Obesity and diabetes are involved in the mechanisms involved in the development of HCC in NAFLD. The development of HCC in NAFLD is likely multifactorial; involving low grade chronic systemic inflammatory response, increased lipid storage and lipotoxicity, gut disbiosis with elevated levels of lipopolysaccharide (LPS) and hyperinsulinemia with insulin resistance and increased IGF levels[19]. In addition patients with HCC from NAFLD in general has a distinctive phenotype with presentation in older age, being less aggressive and less likely to be diagnosed by surveillance compared with HCC caused by viral hepatitis[9-11]. Other factors such as genetic polymorphism and, increased iron absorption may also lead to development of HCC in NASH[14]. Proposed mechanisms for NASH-related HCC are depicted in Figure 1.
Figure 1.
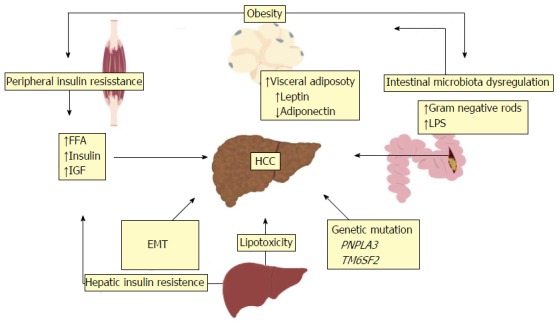
Risk factors and proposed mechanisms for non-alcoholic fatty liver disease and non-alcoholic steatohepatitis-related hepatocellular carcinoma. The development of NAFLD and NASH-related HCC is multifactorial. Proposed pathogenic mechanisms include obesity, peripheral and hepatic insulin resistance from type 2 diabetes, increased hepatic lipid storage and lipotoxicity, EMT, genetic mutations and intestinal mibrobiota dysregulation. HCC: Hepatocellular carcinoma; EMT: Epithelial to mesenchymal transition; NAFLD: Non-alcoholic fatty liver disease; NASH: Non-alcoholic steatohepatitis; FFA: Free fatty acid; IGF: Insulin-like growth factor; LPS: Lipopolysaccharide; PNPLA3: Patatin-like phospholipase domain-containing 3; TM6SF2: Transmembrane 6 superfamily member 2.
Cytokines carry out the intercellular communication signals, cellular interactions along with growth and differentiation. Disease states cause imbalances in cytokine levels promoting aberrant signaling and modulating inflammatory responses seen in epithelial to mesenchymal transition pathologic process[15]. Imbalances in the levels of cytokines such as tumor necrosis factor (TNF)-alpha, leptin, adiponectin and interleukin-6 (IL-6) play a pivotal role in NASH[58-60].
Obesity
Obesity is a significant risk for the development of HCC particularly in patients with NASH, who have a higher predisposition for obesity. Obese (body mass index > 30 kg/m2) patients have a reported 1.93-fold higher risk of developing primary liver cancer. Obesity and excessive visceral adipose tissue has been associated with a chronic inflammatory state due to increased levels of leptin. Leptin, a profibrotic and proangiogenic cytokine, activates the Janus kinase (JAK) pathway, thereby initiating an intracellular signaling cascade of pro-inflammatory cytokines[61,62]. Obesity has also been associated reduced level of adiponectin, an anti-inflammatory cytokine. Additionally, obesity has been associated with other risk factors including insulin resistance, increased hepatic lipid storage and alteration of intestinal microflora.
Insulin resistance
Diabetes has shown to be an independent risk factor for the development of HCC in NASH[61,63]. Excessive fat accumulation and obesity lead to hepatic and peripheral insulin resistance causing compensatory hyperinsulinemia. Evidence supports that insulin and insulin-like growth factor (IGF) may promote the development of primary liver cancer by activating various oncogenic pathways[61]. Both IGF-1 and insulin receptor substrate stimulates growth by activating the mitogen-activate protein kinase (MAPK) pathway and increases the transcription of c-fos and c-jun, known proto-oncogenes. Activation of MAPK pathway subsequently activates the Wnt/β-catenin signaling cascade leading to fibrosis and hepatocarcinogenesis[61,62].
Lipotoxicity
Increased lipid accumulation in the liver arises from lipolysis within peripheral adipose tissue, dietary sources and de novo hepatic lipogenesis[19,64]. This increased lipid accumulation causes hepatic lipotoxicity resulting in the excessive production of saturated and monounsaturated free fatty acids (FFAs)[65]. These FFAs undergo β-oxidation leading to formation of reactive oxygen species. Reactive oxygen species induce endothelial reticulum stress, mitochondrial damage and gene transcription promoting inflammatory cell signaling pathways.
Intestinal microflora dysregulation
Other novel pathogenic pathway between the gut and liver has been demonstrated, which is driven by dietary changes leading to gut dysbiosis that has the potential to generate hepatic inflammation can ultimately influence HCC. In NASH patients, small intestinal bacterial overgrowth[66,67] and increased TNF-α levels, elevated expression of Toll-like receptor (TLR) 4 and increased levels of serum IL-8[67] has been demonstrated.
LPS, a major component of outer membrane of gram-negative bacteria, is an endotoxin that causes inflammation upon entering the systemic circulation. The involvement of LPS in the development of HCC is suspected by the observation that LPS removal by gut sterilization results in diminished tumor growth in patients with chronic liver injury[68,69]. In two recent studies, the investigators observed in NASH patients, increased levels of TNF-alpha, interleukin-8 and elevated expression of TLR 4 and small intestinal bacterial overgrowth[66,67]. NASH patients also have less gut gram-negative Bacteroidetes and an increase in alcohol producing bacteria when compared to patients with simple steatosis, which raises a question as to whether these strains are involved in the pathogenesis of NASH[70,71].
Several recent studies have identified potential link between gut dysbiosis and NAFLD in both in animal models and human[66-72]. There is incremental evidence for gut microbiome in the pathogenesis of NASH based on these findings, suggesting potential therapeutic role of correcting of gut dysbiosis to a more healthy phenotype in limiting progression of NASH. Evidence linking gut microbiota, NASH, and HCC development is reported from Dapito et al[69]. They treated mice with diethylnitrosamine (DEN) followed by carbon tetrachloride (CCL4) to promote fibrosis-driven HCC[69]. They found that TLR4-deficient mice had limited HCC growth; DEN/CCL4-treated wild-type mice that received antibiotics also had reduced tumor growth, suggesting that the microbiota played a role in HCC progression possibly via LPS-TLR4 axis.
Gut microbiota can catalyse generation of secondary bile acids such a sDCA, which is known to induce DNA damage[72]. Yoshimoto et al[68] found that DCA can promote the activation of a senescence-associated secretory phenotype in HSCs, reflected by the secretion of IL-1β. Further they observed limited obesity-induced HCC development in the absence of IL-1β, and alleviation of HCC development with antibiotic treatment. In addition, lowering of DCA or feeding of DCA, limited or enhanced HCC growth respectively. Although the role for bile acids in NASH HCC progression need further exploration, these studies certainly lay the foundation for future exploratory studies in both animal models and human.
Genetic polymorphisms
Genetic polymorphism is also one of the factors that may account for development of HCC in NAFLD. Genetic predisposition plays an important role in susceptibility to the metabolic syndrome and NASH. Recent genome-wide association studies have identified a single nucleotide polymorphism in the patatin-like phospholipase domain-containing 3 (PNPLA3) gene. Specifically, a C-to-G genotype in the rs738409 gene, encoding the I148M protein variant, determines differences in hepatic fat accumulation[73]. Although the physiological and biological functions of PNPLA3 within the liver, which effect fat accumulation and NASH, remain unclear, the association of rs738409 polymorphisms with HCC is evident[74].
It has also been suggested a polymorphism in the transmembrane 6 superfamily member 2 gene (TM6SF2) may increase the risk of NASH progression to HCC[75]. TM6SF2 mutation encodes for a loss of function substitution of lysine to glutamic acid. This TM6SF2 variant was associated with liver injury in NAFLD and NASH patients. While there is an increased prevalence of the TM6SF2 variant in NAFLD and NASH patients, conflicting preliminary data exists regarding its role in the progression to HCC.
Other risk factors
Increased intrahepatic iron accumulation has been associated with NASH progression to HCC. Although clinical data is limited, Sorrentino et al[76] demonstrated higher hepatic iron storage levels among NASH-related HCC patients compared to NASH patients. The underlying mechanism of increased iron absorption in NASH patients may be related to oxidative DNA damage but further studies are required to understand the role of iron accumulation in NAFLD and HCC[19,76].
Other significant risk factors for NASH progression to HCC include advanced age and concomitant chronic alcohol consumption[77]. Alcohol consumption among NASH patients has an associated 3.6-fold increased risk for development of HCC. Also emerging evidence has suggested a possible correlation between obstructive sleep apnea and NAFLD and NASH but its association to development of HCC has not been investigated[78].
SURVEILLANCE
With the increasing prevalence of NAFLD/NASH and associated HCC, chemopreventive and perhaps reconsideration of current surveillance guidelines are needed[16]. The current AASLD guidelines recommend screening for HCC every 6 mo in patients with cirrhosis. However, the current guidelines lack recommendations for surveillance of NASH patients without cirrhosis who are at risk for developing HCC. This is further supported by a study performed by Mittal et al[79] in which the data collected on about 1500 HCC patients where HCC related to NASH received less surveillance and treatment compared with HCC arising in underlying etiologies related to HCV and alcohol.
The lack of longitudinal data in the non-cirrhotic NASH population makes it difficult to develop good evidence - based screening guideline. There is a need for studies addressing the screening guidelines for surveillance of HCC in NASH particularly for non-cirrhotic individuals. We suspect that earlier screening may be needed in patients with NASH who have multiple risk factors for HCC[19].
CURRENT THERAPEUTIC OPTIONS
The biological heterogeneity of HCC makes it difficult to clarify the key mechanisms of cancer development and thus to develop and implement effective therapies[80]. A few chemopreventive agents have shown promise in the prevention and treatment of steatohepatitis and fibrosis; however these are small individual studies and thus there is a lack of a general consensus due to paucity of data. There is currently no effective chemoprevention to decrease the incidence of HCC. Exceptions include nucleoside analogues used to reduce viral replication in those with hepatitis B, and DAAs for HCV which have very high cure rates[81].
Medical therapy
Regular exercise and controlled caloric intake is the mainstay of therapy for NAFLD, however the extent to which these are effective to prevent the development of HCC is unclear. Physical activity has been reported to have a preventive effect on development of HCC. A large prospective cohort study, which included over 400000 participants suggested that increased physical activity might have a role in HCC prevention that is independent of weight reduction[82]. Preliminary data suggests that statins, metformin and S-Adenosylmethonine are potential chemopreventive agents[16].
Patients with NASH have been found to be deficient in vitamin E and D; vitamin D deficiency is thought to play a role in hepatic carcinogenesis[83,84]. Other dietary antioxidants such as vitamin C, selenium, coenzyme Q12 and certain phytochemicals have also been touted have chemopreventive potential[85]. NASH patients have been shown to have low levels of serum lycopene[83]. There is a strong inverse relationship between serum lycopene levels and the risk of GI cancers[86].
Metformin has an antitumor effect in HCC via suppression of mTOR pathway[87]. Although it may not have a role in the treatment of NASH, metformin may have a role in decreasing the incidence of HCC in NASH[16]. A review of two recent meta-analyses included 22650 cases of HCC in approximately 334000 patients with type 2 diabetes revealed that metformin reduced incidence of HCC by 50% whereas sulfonylurea and insulin increased incidence of HCC by 62% and 161% respectively[88]. The use of metformin has also been shown to increase survival of HCC patients who have cirrhosis[89].
Statins have shown a protective effect in individuals who are at risk for development of steatohepatiits and F2-F4 fibrosis[90]. The protective effect of statins in diabetics is thought to be due to anti-inflammatory properties of statins mediated through the inhibition of JAK[91]. A recent Swedish case control study which evaluated almost 4000 HCC patients treated with statin that were matched with 19970 controls showed that the odds ratio for HCC amongst statin users was 0.88, suggesting a modest but beneficial effect of statins in reducing the risk of HCC[92].
The heterogeneity of HCC makes it difficult to clarify the mechanism of cancer development and to develop effective therapeutics. However, an integrative functional genomics approach will contribute to the discovery of potential molecular features critical for HCC development. These studies will provide us with better treatment strategies that may be effective to treat all HCC patients including those with NASH.
Surgical therapy
Curative treatment options including liver resection and liver transplantation in select early-stage HCC candidates. The Barcelona Clinic Liver Cancer staging system and therapeutic algorithm has been applied to HCC candidates including those with NASH-related HCC[93]. Non-cirrhotic NASH-related HCC patients who underwent curative surgical resection have shown to have superior survival than those with HCV and alcohol-related HCC[11].
Since the implementation of the Model for End-Stage Liver Disease (MELD) system for liver allocation in 2002 the number of HCC liver transplantations has dramatically increased. In 2012, they accounted for 23.2% of all liver transplantations in the United States[26]. HCC liver candidates are eligible to receive a MELD exception which upgrades their priority and thus, increases their likelihood of receiving liver transplant and survival. Subsequently, a higher number of HCC candidates have sought listing for liver transplant. A recent study using United Network for Organ Sharing data from 2004-2013[94] demonstrated that NASH-related HCC candidates have lower rates for receiving MELD exception and have longer time to transplant compared to HCV-related HCC. Despite this, NASH-related HCC was the fastest growing indication for liver transplantation from 2002-2012[26]. NASH-related HCC liver transplant recipients have better outcomes compared HCV-related HCC with a 5-year post-transplant survival approaching 68%[95]. NASH-related HCC liver transplant recipients with morbid obesity and CV risk factors tend to have poorer outcomes[96]. Further research is needed to evaluate NASH-related HCC post liver transplant survival risk factors and exploring why this growing cohort is less likely to receive a MELD exception.
CONCLUSION
With the prevalence of HCV expected to decline, NASH is anticipated to account for a greater proportion of HCC incidence in the near future due to the growing epidemic of obesity and diabetes. The annual incidence rate of developing HCC in patients with NASH-related cirrhosis is not clearly understood with rates ranging from 2.6%-12.8%. Recent evidence has shown a significant proportion of patients with NAFLD and NASH progress to HCC in the absence of cirrhosis. While liver resection and transplantation represent curative therapeutic options in select NASH-related HCC candidates, they also have placed a significant burden to our healthcare resources and utilization. Currently NASH-related HCC is the fastest growing indication for liver transplant in HCC candidates. Increased efforts to implement effective screening and preventative strategies, particularly in non-cirrhotic NASH cohort, are needed to reduce the future impact imposed by NASH and NASH-related HCC.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): E
Conflict-of-interest statement: All authors have no conflicts of interest to disclose related to the research or data presented in this manuscript. There was no funding for this study. This manuscript is not being considered for publication elsewhere.
Peer-review started: July 31, 2016
First decision: September 8, 2016
Article in press: March 14, 2017
P- Reviewer: Chetty R, Chiu KW, Chuang WL, Hernanda PY, Kamiyama T, Zhang X S- Editor: Kong JX L- Editor: A E- Editor: Li D
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 3.Michelotti GA, Machado MV, Diehl AM. NAFLD, NASH and liver cancer. Nat Rev Gastroenterol Hepatol. 2013;10:656–665. doi: 10.1038/nrgastro.2013.183. [DOI] [PubMed] [Google Scholar]
- 4.Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, Angulo P. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–121. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 5.Jansen PL. Non-alcoholic steatohepatitis. Eur J Gastroenterol Hepatol. 2004;16:1079–1085. doi: 10.1097/00042737-200411000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Nagaoki Y, Hyogo H, Aikata H, Tanaka M, Naeshiro N, Nakahara T, Honda Y, Miyaki D, Kawaoka T, Takaki S, et al. Recent trend of clinical features in patients with hepatocellular carcinoma. Hepatol Res. 2012;42:368–375. doi: 10.1111/j.1872-034X.2011.00929.x. [DOI] [PubMed] [Google Scholar]
- 7.Yatsuji S, Hashimoto E, Tobari M, Taniai M, Tokushige K, Shiratori K. Clinical features and outcomes of cirrhosis due to non-alcoholic steatohepatitis compared with cirrhosis caused by chronic hepatitis C. J Gastroenterol Hepatol. 2009;24:248–254. doi: 10.1111/j.1440-1746.2008.05640.x. [DOI] [PubMed] [Google Scholar]
- 8.White DL, Kanwal F, El-Serag HB. Association between nonalcoholic fatty liver disease and risk for hepatocellular cancer, based on systematic review. Clin Gastroenterol Hepatol. 2012;10:1342–1359.e2. doi: 10.1016/j.cgh.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marrero JA, Fontana RJ, Su GL, Conjeevaram HS, Emick DM, Lok AS. NAFLD may be a common underlying liver disease in patients with hepatocellular carcinoma in the United States. Hepatology. 2002;36:1349–1354. doi: 10.1053/jhep.2002.36939. [DOI] [PubMed] [Google Scholar]
- 10.Guzman G, Brunt EM, Petrovic LM, Chejfec G, Layden TJ, Cotler SJ. Does nonalcoholic fatty liver disease predispose patients to hepatocellular carcinoma in the absence of cirrhosis? Arch Pathol Lab Med. 2008;132:1761–1766. doi: 10.5858/132.11.1761. [DOI] [PubMed] [Google Scholar]
- 11.Reddy SK, Steel JL, Chen HW, DeMateo DJ, Cardinal J, Behari J, Humar A, Marsh JW, Geller DA, Tsung A. Outcomes of curative treatment for hepatocellular cancer in nonalcoholic steatohepatitis versus hepatitis C and alcoholic liver disease. Hepatology. 2012;55:1809–1819. doi: 10.1002/hep.25536. [DOI] [PubMed] [Google Scholar]
- 12.Starley BQ, Calcagno CJ, Harrison SA. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology. 2010;51:1820–1832. doi: 10.1002/hep.23594. [DOI] [PubMed] [Google Scholar]
- 13.Kawada N, Imanaka K, Kawaguchi T, Tamai C, Ishihara R, Matsunaga T, Gotoh K, Yamada T, Tomita Y. Hepatocellular carcinoma arising from non-cirrhotic nonalcoholic steatohepatitis. J Gastroenterol. 2009;44:1190–1194. doi: 10.1007/s00535-009-0112-0. [DOI] [PubMed] [Google Scholar]
- 14.Karagozian R, Derdák Z, Baffy G. Obesity-associated mechanisms of hepatocarcinogenesis. Metabolism. 2014;63:607–617. doi: 10.1016/j.metabol.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 15.Jiang CM, Pu CW, Hou YH, Chen Z, Alanazy M, Hebbard L. Non alcoholic steatohepatitis a precursor for hepatocellular carcinoma development. World J Gastroenterol. 2014;20:16464–16473. doi: 10.3748/wjg.v20.i44.16464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noureddin M, Rinella ME. Nonalcoholic Fatty liver disease, diabetes, obesity, and hepatocellular carcinoma. Clin Liver Dis. 2015;19:361–379. doi: 10.1016/j.cld.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Satapathy SK, Sanyal AJ. Epidemiology and Natural History of Nonalcoholic Fatty Liver Disease. Semin Liver Dis. 2015;35:221–235. doi: 10.1055/s-0035-1562943. [DOI] [PubMed] [Google Scholar]
- 18.El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118–1127. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 19.Margini C, Dufour JF. The story of HCC in NAFLD: from epidemiology, across pathogenesis, to prevention and treatment. Liver Int. 2016;36:317–324. doi: 10.1111/liv.13031. [DOI] [PubMed] [Google Scholar]
- 20.Fleischman MW, Budoff M, Zeb I, Li D, Foster T. NAFLD prevalence differs among hispanic subgroups: the Multi-Ethnic Study of Atherosclerosis. World J Gastroenterol. 2014;20:4987–4993. doi: 10.3748/wjg.v20.i17.4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dongiovanni P, Romeo S, Valenti L. Hepatocellular carcinoma in nonalcoholic fatty liver: role of environmental and genetic factors. World J Gastroenterol. 2014;20:12945–12955. doi: 10.3748/wjg.v20.i36.12945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gholam PM, Flancbaum L, Machan JT, Charney DA, Kotler DP. Nonalcoholic fatty liver disease in severely obese subjects. Am J Gastroenterol. 2007;102:399–408. doi: 10.1111/j.1572-0241.2006.01041.x. [DOI] [PubMed] [Google Scholar]
- 23.Merriman RB, Ferrell LD, Patti MG, Weston SR, Pabst MS, Aouizerat BE, Bass NM. Correlation of paired liver biopsies in morbidly obese patients with suspected nonalcoholic fatty liver disease. Hepatology. 2006;44:874–880. doi: 10.1002/hep.21346. [DOI] [PubMed] [Google Scholar]
- 24.Byrne CD, Olufadi R, Bruce KD, Cagampang FR, Ahmed MH. Metabolic disturbances in non-alcoholic fatty liver disease. Clin Sci (Lond) 2009;116:539–564. doi: 10.1042/CS20080253. [DOI] [PubMed] [Google Scholar]
- 25.Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, Landt CL, Harrison SA. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124–131. doi: 10.1053/j.gastro.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 26.Wong RJ, Cheung R, Ahmed A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology. 2014;59:2188–2195. doi: 10.1002/hep.26986. [DOI] [PubMed] [Google Scholar]
- 27.Ong JP, Younossi ZM. Epidemiology and natural history of NAFLD and NASH. Clin Liver Dis. 2007;11:1–16, vii. doi: 10.1016/j.cld.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 28.Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–1419. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 29.Rafiq N, Bai C, Fang Y, Srishord M, McCullough A, Gramlich T, Younossi ZM. Long-term follow-up of patients with nonalcoholic fatty liver. Clin Gastroenterol Hepatol. 2009;7:234–238. doi: 10.1016/j.cgh.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 30.Hui JM, Kench JG, Chitturi S, Sud A, Farrell GC, Byth K, Hall P, Khan M, George J. Long-term outcomes of cirrhosis in nonalcoholic steatohepatitis compared with hepatitis C. Hepatology. 2003;38:420–427. doi: 10.1053/jhep.2003.50320. [DOI] [PubMed] [Google Scholar]
- 31.Sanyal AJ, Banas C, Sargeant C, Luketic VA, Sterling RK, Stravitz RT, Shiffman ML, Heuman D, Coterrell A, Fisher RA, et al. Similarities and differences in outcomes of cirrhosis due to nonalcoholic steatohepatitis and hepatitis C. Hepatology. 2006;43:682–689. doi: 10.1002/hep.21103. [DOI] [PubMed] [Google Scholar]
- 32.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paradis V, Zalinski S, Chelbi E, Guedj N, Degos F, Vilgrain V, Bedossa P, Belghiti J. Hepatocellular carcinomas in patients with metabolic syndrome often develop without significant liver fibrosis: a pathological analysis. Hepatology. 2009;49:851–859. doi: 10.1002/hep.22734. [DOI] [PubMed] [Google Scholar]
- 34.Charrez B, Qiao L, Hebbard L. Hepatocellular carcinoma and non-alcoholic steatohepatitis: The state of play. World J Gastroenterol. 2016;22:2494–2502. doi: 10.3748/wjg.v22.i8.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Farges O, Ferreira N, Dokmak S, Belghiti J, Bedossa P, Paradis V. Changing trends in malignant transformation of hepatocellular adenoma. Gut. 2011;60:85–89. doi: 10.1136/gut.2010.222109. [DOI] [PubMed] [Google Scholar]
- 36.Bugianesi E, Leone N, Vanni E, Marchesini G, Brunello F, Carucci P, Musso A, De Paolis P, Capussotti L, Salizzoni M, et al. Expanding the natural history of nonalcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology. 2002;123:134–140. doi: 10.1053/gast.2002.34168. [DOI] [PubMed] [Google Scholar]
- 37.Teli MR, James OF, Burt AD, Bennett MK, Day CP. The natural history of nonalcoholic fatty liver: a follow-up study. Hepatology. 1995;22:1714–1719. [PubMed] [Google Scholar]
- 38.Younossi ZM, Stepanova M, Rafiq N, Makhlouf H, Younoszai Z, Agrawal R, Goodman Z. Pathologic criteria for nonalcoholic steatohepatitis: interprotocol agreement and ability to predict liver-related mortality. Hepatology. 2011;53:1874–1882. doi: 10.1002/hep.24268. [DOI] [PubMed] [Google Scholar]
- 39.Adams LA, Sanderson S, Lindor KD, Angulo P. The histological course of nonalcoholic fatty liver disease: a longitudinal study of 103 patients with sequential liver biopsies. J Hepatol. 2005;42:132–138. doi: 10.1016/j.jhep.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 40.Ekstedt M, Franzén LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, Kechagias S. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865–873. doi: 10.1002/hep.21327. [DOI] [PubMed] [Google Scholar]
- 41.Söderberg C, Stål P, Askling J, Glaumann H, Lindberg G, Marmur J, Hultcrantz R. Decreased survival of subjects with elevated liver function tests during a 28-year follow-up. Hepatology. 2010;51:595–602. doi: 10.1002/hep.23314. [DOI] [PubMed] [Google Scholar]
- 42.Stepanova M, Rafiq N, Makhlouf H, Agrawal R, Kaur I, Younoszai Z, McCullough A, Goodman Z, Younossi ZM. Predictors of all-cause mortality and liver-related mortality in patients with non-alcoholic fatty liver disease (NAFLD) Dig Dis Sci. 2013;58:3017–3023. doi: 10.1007/s10620-013-2743-5. [DOI] [PubMed] [Google Scholar]
- 43.Younossi ZM, Otgonsuren M, Venkatesan C, Mishra A. In patients with non-alcoholic fatty liver disease, metabolically abnormal individuals are at a higher risk for mortality while metabolically normal individuals are not. Metabolism. 2013;62:352–360. doi: 10.1016/j.metabol.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 44.Younossi ZM, Gramlich T, Matteoni CA, Boparai N, McCullough AJ. Nonalcoholic fatty liver disease in patients with type 2 diabetes. Clin Gastroenterol Hepatol. 2004;2:262–265. doi: 10.1016/s1542-3565(04)00014-x. [DOI] [PubMed] [Google Scholar]
- 45.Adami HO, Chow WH, Nyrén O, Berne C, Linet MS, Ekbom A, Wolk A, McLaughlin JK, Fraumeni JF. Excess risk of primary liver cancer in patients with diabetes mellitus. J Natl Cancer Inst. 1996;88:1472–1477. doi: 10.1093/jnci/88.20.1472. [DOI] [PubMed] [Google Scholar]
- 46.El-Serag HB, Richardson PA, Everhart JE. The role of diabetes in hepatocellular carcinoma: a case-control study among United States Veterans. Am J Gastroenterol. 2001;96:2462–2467. doi: 10.1111/j.1572-0241.2001.04054.x. [DOI] [PubMed] [Google Scholar]
- 47.Turati F, Talamini R, Pelucchi C, Polesel J, Franceschi S, Crispo A, Izzo F, La Vecchia C, Boffetta P, Montella M. Metabolic syndrome and hepatocellular carcinoma risk. Br J Cancer. 2013;108:222–228. doi: 10.1038/bjc.2012.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bugianesi E. Non-alcoholic steatohepatitis and cancer. Clin Liver Dis. 2007;11:191–207, x-xi. doi: 10.1016/j.cld.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 49.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 50.Musso G, Gambino R, Cassader M, Pagano G. Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med. 2011;43:617–649. doi: 10.3109/07853890.2010.518623. [DOI] [PubMed] [Google Scholar]
- 51.Targher G, Arcaro G. Non-alcoholic fatty liver disease and increased risk of cardiovascular disease. Atherosclerosis. 2007;191:235–240. doi: 10.1016/j.atherosclerosis.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 52.Targher G, Marra F, Marchesini G. Increased risk of cardiovascular disease in non-alcoholic fatty liver disease: causal effect or epiphenomenon? Diabetologia. 2008;51:1947–1953. doi: 10.1007/s00125-008-1135-4. [DOI] [PubMed] [Google Scholar]
- 53.Lam B, Younossi ZM. Treatment options for nonalcoholic fatty liver disease. Therap Adv Gastroenterol. 2010;3:121–137. doi: 10.1177/1756283X09359964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Younossi ZM, Venkatesan C. A 2012 clinical update for internists in adult nonalcoholic fatty liver disease. Panminerva Med. 2012;54:29–37. [PubMed] [Google Scholar]
- 55.Venkatesan C, Younossi ZM. Potential mechanisms underlying the associations between liver enzymes and risk for type 2 diabetes. Hepatology. 2012;55:968–970. doi: 10.1002/hep.24769. [DOI] [PubMed] [Google Scholar]
- 56.Thorgeirsson SS, Grisham JW. Molecular pathogenesis of human hepatocellular carcinoma. Nat Genet. 2002;31:339–346. doi: 10.1038/ng0802-339. [DOI] [PubMed] [Google Scholar]
- 57.Farazi PA, DePinho RA. Hepatocellular carcinoma pathogenesis: from genes to environment. Nat Rev Cancer. 2006;6:674–687. doi: 10.1038/nrc1934. [DOI] [PubMed] [Google Scholar]
- 58.Schett G, Elewaut D, McInnes IB, Dayer JM, Neurath MF. How cytokine networks fuel inflammation: Toward a cytokine-based disease taxonomy. Nat Med. 2013;19:822–824. doi: 10.1038/nm.3260. [DOI] [PubMed] [Google Scholar]
- 59.Maddur MS, Miossec P, Kaveri SV, Bayry J. Th17 cells: biology, pathogenesis of autoimmune and inflammatory diseases, and therapeutic strategies. Am J Pathol. 2012;181:8–18. doi: 10.1016/j.ajpath.2012.03.044. [DOI] [PubMed] [Google Scholar]
- 60.Grivennikov SI, Karin M. Inflammatory cytokines in cancer: tumour necrosis factor and interleukin 6 take the stage. Ann Rheum Dis. 2011;70 Suppl 1:i104–i108. doi: 10.1136/ard.2010.140145. [DOI] [PubMed] [Google Scholar]
- 61.Chettouh H, Lequoy M, Fartoux L, Vigouroux C, Desbois-Mouthon C. Hyperinsulinaemia and insulin signalling in the pathogenesis and the clinical course of hepatocellular carcinoma. Liver Int. 2015;35:2203–2217. doi: 10.1111/liv.12903. [DOI] [PubMed] [Google Scholar]
- 62.Siddique A, Kowdley KV. Insulin resistance and other metabolic risk factors in the pathogenesis of hepatocellular carcinoma. Clin Liver Dis. 2011;15:281–296, vii-x. doi: 10.1016/j.cld.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ueyama M, Nishida N, Korenaga M, Korenaga K, Kumagai E, Yanai H, Adachi H, Katsuyama H, Moriyama S, Hamasaki H, et al. The impact of PNPLA3 and JAZF1 on hepatocellular carcinoma in non-viral hepatitis patients with type 2 diabetes mellitus. J Gastroenterol. 2016;51:370–379. doi: 10.1007/s00535-015-1116-6. [DOI] [PubMed] [Google Scholar]
- 64.Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD) Metabolism. 2016;65:1038–1048. doi: 10.1016/j.metabol.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 65.Hirsova P, Ibrabim SH, Gores GJ, Malhi H. Lipotoxic lethal and sublethal stress signaling in hepatocytes: relevance to NASH pathogenesis. J Lipid Res. 2016;57:1758–1770. doi: 10.1194/jlr.R066357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wigg AJ, Roberts-Thomson IC, Dymock RB, McCarthy PJ, Grose RH, Cummins AG. The role of small intestinal bacterial overgrowth, intestinal permeability, endotoxaemia, and tumour necrosis factor alpha in the pathogenesis of non-alcoholic steatohepatitis. Gut. 2001;48:206–211. doi: 10.1136/gut.48.2.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shanab AA, Scully P, Crosbie O, Buckley M, O'Mahony L, Shanahan F, Gazareen S, Murphy E, Quigley EM. Small intestinal bacterial overgrowth in nonalcoholic steatohepatitis: association with toll-like receptor 4 expression and plasma levels of interleukin 8. Dig Dis Sci. 2011;56:1524–1534. doi: 10.1007/s10620-010-1447-3. [DOI] [PubMed] [Google Scholar]
- 68.Yoshimoto S, Loo TM, Atarashi K, Kanda H, Sato S, Oyadomari S, Iwakura Y, Oshima K, Morita H, Hattori M, et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature. 2013;499:97–101. doi: 10.1038/nature12347. [DOI] [PubMed] [Google Scholar]
- 69.Dapito DH, Mencin A, Gwak GY, Pradere JP, Jang MK, Mederacke I, Caviglia JM, Khiabanian H, Adeyemi A, Bataller R, et al. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell. 2012;21:504–516. doi: 10.1016/j.ccr.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mouzaki M, Comelli EM, Arendt BM, Bonengel J, Fung SK, Fischer SE, McGilvray ID, Allard JP. Intestinal microbiota in patients with nonalcoholic fatty liver disease. Hepatology. 2013;58:120–127. doi: 10.1002/hep.26319. [DOI] [PubMed] [Google Scholar]
- 71.Zhu L, Baker SS, Gill C, Liu W, Alkhouri R, Baker RD, Gill SR. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatology. 2013;57:601–609. doi: 10.1002/hep.26093. [DOI] [PubMed] [Google Scholar]
- 72.Ridlon JM, Alves JM, Hylemon PB, Bajaj JS. Cirrhosis, bile acids and gut microbiota: unraveling a complex relationship. Gut Microbes. 2013;4:382–387. doi: 10.4161/gmic.25723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sookoian S, Pirola CJ. Meta-analysis of the influence of I148M variant of patatin-like phospholipase domain containing 3 gene (PNPLA3) on the susceptibility and histological severity of nonalcoholic fatty liver disease. Hepatology. 2011;53:1883–1894. doi: 10.1002/hep.24283. [DOI] [PubMed] [Google Scholar]
- 74.Yopp AC, Choti MA. Non-Alcoholic Steatohepatitis-Related Hepatocellular Carcinoma: A Growing Epidemic? Dig Dis. 2015;33:642–647. doi: 10.1159/000438473. [DOI] [PubMed] [Google Scholar]
- 75.Chen LZ, Xia HH, Xin YN, Lin ZH, Xuan SY. TM6SF2 E167K Variant, a Novel Genetic Susceptibility Variant, Contributing to Nonalcoholic Fatty Liver Disease. J Clin Transl Hepatol. 2015;3:265–270. doi: 10.14218/JCTH.2015.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sorrentino P, D'Angelo S, Ferbo U, Micheli P, Bracigliano A, Vecchione R. Liver iron excess in patients with hepatocellular carcinoma developed on non-alcoholic steato-hepatitis. J Hepatol. 2009;50:351–357. doi: 10.1016/j.jhep.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 77.Khan FZ, Perumpail RB, Wong RJ, Ahmed A. Advances in hepatocellular carcinoma: Nonalcoholic steatohepatitis-related hepatocellular carcinoma. World J Hepatol. 2015;7:2155–2161. doi: 10.4254/wjh.v7.i18.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mirrakhimov AE, Polotsky VY. Obstructive sleep apnea and non-alcoholic Fatty liver disease: is the liver another target? Front Neurol. 2012;3:149. doi: 10.3389/fneur.2012.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mittal S, Sada YH, El-Serag HB, Kanwal F, Duan Z, Temple S, May SB, Kramer JR, Richardson PA, Davila JA. Temporal trends of nonalcoholic fatty liver disease-related hepatocellular carcinoma in the veteran affairs population. Clin Gastroenterol Hepatol. 2015;13:594–601.e1. doi: 10.1016/j.cgh.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Takai A, Dang HT, Wang XW. Identification of drivers from cancer genome diversity in hepatocellular carcinoma. Int J Mol Sci. 2014;15:11142–11160. doi: 10.3390/ijms150611142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hosaka T, Suzuki F, Kobayashi M, Seko Y, Kawamura Y, Sezaki H, Akuta N, Suzuki Y, Saitoh S, Arase Y, et al. Long-term entecavir treatment reduces hepatocellular carcinoma incidence in patients with hepatitis B virus infection. Hepatology. 2013;58:98–107. doi: 10.1002/hep.26180. [DOI] [PubMed] [Google Scholar]
- 82.Yun YH, Lim MK, Won YJ, Park SM, Chang YJ, Oh SW, Shin SA. Dietary preference, physical activity, and cancer risk in men: national health insurance corporation study. BMC Cancer. 2008;8:366. doi: 10.1186/1471-2407-8-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Erhardt A, Stahl W, Sies H, Lirussi F, Donner A, Häussinger D. Plasma levels of vitamin E and carotenoids are decreased in patients with Nonalcoholic Steatohepatitis (NASH) Eur J Med Res. 2011;16:76–78. doi: 10.1186/2047-783X-16-2-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fedirko V, Duarte-Salles T, Bamia C, Trichopoulou A, Aleksandrova K, Trichopoulos D, Trepo E, Tjønneland A, Olsen A, Overvad K, et al. Prediagnostic circulating vitamin D levels and risk of hepatocellular carcinoma in European populations: a nested case-control study. Hepatology. 2014;60:1222–1230. doi: 10.1002/hep.27079. [DOI] [PubMed] [Google Scholar]
- 85.Montella M, Crispo A, Giudice A. HCC, diet and metabolic factors: Diet and HCC. Hepat Mon. 2011;11:159–162. [PMC free article] [PubMed] [Google Scholar]
- 86.Giovannucci E, Rimm EB, Liu Y, Stampfer MJ, Willett WC. A prospective study of tomato products, lycopene, and prostate cancer risk. J Natl Cancer Inst. 2002;94:391–398. doi: 10.1093/jnci/94.5.391. [DOI] [PubMed] [Google Scholar]
- 87.Bhat M, Sonenberg N, Gores GJ. The mTOR pathway in hepatic malignancies. Hepatology. 2013;58:810–818. doi: 10.1002/hep.26323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang P, Kang D, Cao W, Wang Y, Liu Z. Diabetes mellitus and risk of hepatocellular carcinoma: a systematic review and meta-analysis. Diabetes Metab Res Rev. 2012;28:109–122. doi: 10.1002/dmrr.1291. [DOI] [PubMed] [Google Scholar]
- 89.Zhang X, Harmsen WS, Mettler TA, Kim WR, Roberts RO, Therneau TM, Roberts LR, Chaiteerakij R. Continuation of metformin use after a diagnosis of cirrhosis significantly improves survival of patients with diabetes. Hepatology. 2014;60:2008–2016. doi: 10.1002/hep.27199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dongiovanni P, Petta S, Mannisto V, Mancina RM, Pipitone R, Karja V, Maggioni M, Kakela P, Wiklund O, Mozzi E, et al. Statin use and non-alcoholic steatohepatitis in at risk individuals. J Hepatol. 2015;63:705–712. doi: 10.1016/j.jhep.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 91.Zheng L, Yang W, Wu F, Wang C, Yu L, Tang L, Qiu B, Li Y, Guo L, Wu M, et al. Prognostic significance of AMPK activation and therapeutic effects of metformin in hepatocellular carcinoma. Clin Cancer Res. 2013;19:5372–5380. doi: 10.1158/1078-0432.CCR-13-0203. [DOI] [PubMed] [Google Scholar]
- 92.Ong JP, Pitts A, Younossi ZM. Increased overall mortality and liver-related mortality in non-alcoholic fatty liver disease. J Hepatol. 2008;49:608–612. doi: 10.1016/j.jhep.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 93.Kikuchi L, Oliveira CP, Alvares-da-Silva MR, Tani CM, Diniz MA, Stefano JT, Chagas AL, Alencar RS, Vezozzo DC, Santos GR, et al. Hepatocellular Carcinoma Management in Nonalcoholic Fatty Liver Disease Patients: Applicability of the BCLC Staging System. Am J Clin Oncol. 2016;39:428–432. doi: 10.1097/COC.0000000000000134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Young K, Aguilar M, Gish R, Younossi Z, Saab S, Bhuket T, Liu B, Ahmed A, Wong RJ. Lower rates of receiving model for end-stage liver disease exception and longer time to transplant among nonalcoholic steatohepatitis hepatocellular carcinoma. Liver Transpl. 2016;22:1356–1366. doi: 10.1002/lt.24507. [DOI] [PubMed] [Google Scholar]
- 95.Onaca N, Davis GL, Jennings LW, Goldstein RM, Klintmalm GB. Improved results of transplantation for hepatocellular carcinoma: a report from the International Registry of Hepatic Tumors in Liver Transplantation. Liver Transpl. 2009;15:574–580. doi: 10.1002/lt.21738. [DOI] [PubMed] [Google Scholar]
- 96.Conzen KD, Vachharajani N, Collins KM, Anderson CD, Lin Y, Wellen JR, Shenoy S, Lowell JA, Doyle MB, Chapman WC. Morbid obesity in liver transplant recipients adversely affects longterm graft and patient survival in a single-institution analysis. HPB (Oxford) 2015;17:251–257. doi: 10.1111/hpb.12340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cotrim HP, Oliveira CP, Coelho HS, Alvares-da-Silva MR, Nabuco L, Parise ER, Ivantes C, Martinelli AL, Galizzi-Filho J, Carrilho FJ. Nonalcoholic steatohepatitis and hepatocellular carcinoma: Brazilian survey. Clinics (Sao Paulo) 2016;71:281–284. doi: 10.6061/clinics/2016(05)07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.van Meer S, van Erpecum KJ, Sprengers D, Klümpen HJ, Jansen PL, Ijzermans JN, Siersema PD, de Man RA, Verheij J. Hepatocellular carcinoma in noncirrhotic livers is associated with steatosis rather than steatohepatitis: potential implications for pathogenesis. Eur J Gastroenterol Hepatol. 2016;28:955–962. doi: 10.1097/MEG.0000000000000641. [DOI] [PubMed] [Google Scholar]
- 99.Shrager B, Jibara GA, Tabrizian P, Roayaie S, Ward SC. Resection of nonalcoholic steatohepatitis-associated hepatocellular carcinoma: a Western experience. Int J Surg Oncol. 2012;2012:915128. doi: 10.1155/2012/915128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chagas AL, Kikuchi LO, Oliveira CP, Vezozzo DC, Mello ES, Oliveira AC, Cella LC, Herman P, Bachella T, Caldwell SH, et al. Does hepatocellular carcinoma in non-alcoholic steatohepatitis exist in cirrhotic and non-cirrhotic patients? Braz J Med Biol Res. 2009;42:958–962. doi: 10.1590/s0100-879x2009005000019. [DOI] [PubMed] [Google Scholar]
- 101.Ertle J, Dechêne A, Sowa JP, Penndorf V, Herzer K, Kaiser G, Schlaak JF, Gerken G, Syn WK, Canbay A. Non-alcoholic fatty liver disease progresses to hepatocellular carcinoma in the absence of apparent cirrhosis. Int J Cancer. 2011;128:2436–2443. doi: 10.1002/ijc.25797. [DOI] [PubMed] [Google Scholar]
- 102.Tokushige K, Hashimoto E, Horie Y, Taniai M, Higuchi S. Hepatocellular carcinoma in Japanese patients with nonalcoholic fatty liver disease, alcoholic liver disease, and chronic liver disease of unknown etiology: report of the nationwide survey. J Gastroenterol. 2011;46:1230–1237. doi: 10.1007/s00535-011-0431-9. [DOI] [PubMed] [Google Scholar]
- 103.Hashizume H, Sato K, Takagi H, Hirokawa T, Kojima A, Sohara N, Kakizaki S, Mochida Y, Shimura T, Sunose Y, et al. Primary liver cancers with nonalcoholic steatohepatitis. Eur J Gastroenterol Hepatol. 2007;19:827–834. doi: 10.1097/MEG.0b013e3282748ef2. [DOI] [PubMed] [Google Scholar]
- 104.Malik SM, Gupte PA, de Vera ME, Ahmad J. Liver transplantation in patients with nonalcoholic steatohepatitis-related hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2009;7:800–806. doi: 10.1016/j.cgh.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 105.Takuma Y, Nouso K. Nonalcoholic steatohepatitis-associated hepatocellular carcinoma: our case series and literature review. World J Gastroenterol. 2010;16:1436–1441. doi: 10.3748/wjg.v16.i12.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Perumpail RB, Wong RJ, Ahmed A, Harrison SA. Hepatocellular Carcinoma in the Setting of Non-cirrhotic Nonalcoholic Fatty Liver Disease and the Metabolic Syndrome: US Experience. Dig Dis Sci. 2015;60:3142–3148. doi: 10.1007/s10620-015-3821-7. [DOI] [PubMed] [Google Scholar]
- 107.Ascha MS, Hanouneh IA, Lopez R, Tamimi TA, Feldstein AF, Zein NN. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology. 2010;51:1972–1978. doi: 10.1002/hep.23527. [DOI] [PubMed] [Google Scholar]
- 108.Mohamad B, Shah V, Onyshchenko M, Elshamy M, Aucejo F, Lopez R, Hanouneh IA, Alhaddad R, Alkhouri N. Characterization of hepatocellular carcinoma (HCC) in non-alcoholic fatty liver disease (NAFLD) patients without cirrhosis. Hepatol Int. 2016;10:632–639. doi: 10.1007/s12072-015-9679-0. [DOI] [PubMed] [Google Scholar]


