Abstract
Background
The identification of four Consensus Molecular Subtypes (CMS1–4) of colorectal cancer forms a new paradigm for the design and evaluation of subtype-directed therapeutic strategies. The most aggressive subtype - CMS4 - has the highest chance of disease recurrence. Novel adjuvant therapies for patients with CMS4 tumours are therefore urgently needed. CMS4 tumours are characterized by expression of mesenchymal and stem-like genes. Previous pre-clinical work has shown that targeting Platelet-Derived Growth Factor Receptors (PDGFRs) and the related KIT receptor with imatinib is potentially effective against mesenchymal-type colon cancer. In the present study we aim to provide proof for the concept that imatinib can reduce the aggressive phenotype of primary CMS4 colon cancer.
Methods
Tumour biopsies from patients with newly diagnosed stage I-III colon cancer will be analysed with a novel RT-qPCR test to pre-select patients with CMS4 tumours. Selected patients (n = 27) will receive treatment with imatinib (400 mg per day) starting two weeks prior to planned tumour resection. To assess treatment-induced changes in the aggressive CMS4 phenotype, RNA sequencing will be performed on pre- and post-treatment tissue samples.
Discussion
The development of effective adjuvant therapy for primary colon cancer is hindered by multiple factors. First, new drugs that may have value in the prevention of (early) distant recurrence are almost always first tested in patients with heavily pre-treated metastatic disease. Second, measuring on-target drug effects and biological consequences in tumour tissue is not commonly a part of the study design. Third, due to the lack of patient selection tools, clinical trials in the adjuvant setting require large patient populations. Finally, the evaluation of recurrence-prevention requires a long-term follow-up. In the ImPACCT trial these issues are addressed by including newly diagnosed pre-selected patients with CMS4 tumours prior to primary tumour resection, rather than non-selected patients with late-stage disease. By making use of the pre-operative window period, the biological effect of imatinib treatment on CMS4 tumours can be rapidly assessed. Delivering proof-of-concept for drug action in early stage disease should form the basis for the design of future trials with subtype-targeted therapies in colon cancer patients.
Trial registration
ClinicalTrials.gov: NCT02685046. Registration date: February 9, 2016.
Keywords: Colon carcinoma, Targeted therapy, Imatinib, Proof-of-concept, Pre-operative window, RNA sequencing
Background
Mortality from colon cancer is almost invariably due to the development of distant metastases. In current practice, pathological (TNM stage) and clinical characteristics (age, co-morbidity) mainly determine the choice of adjuvant chemotherapy. However, these features have limited value in predicting which patients are at risk of developing metastases. In clinical trials, the five-year recurrence rate in stage III colon cancer patients is approximately 50% without adjuvant chemotherapy. With adjuvant treatment this is reduced to ~35%, implying that such treatment is only effective in a subgroup of patients [1]. Consequently, the majority of patients are currently being under- or over-treated. It is therefore important to be able to identify patients who are at high risk of recurrence and to develop more effective therapies to prevent relapse. Relapse-prevention trials in the adjuvant setting are challenging however, due to the long follow-up periods and the large numbers of patients that are required for sufficient statistical power. Prior evidence of drug activity and the availability of a companion diagnostic tool for patient selection could greatly facilitate the design and increase the quality of such studies.
Novel adjuvant therapies should be based on an understanding of the pathways that drive metastasis formation. Recent studies on molecular classification of colon cancer have provided insight into these pathways. Several research groups have independently developed classification systems for primary colon cancer based on gene expression profiles [2–8]. Cross-cohort analysis of the results has led to the identification of four consensus molecular subtypes (CMS1–4). Of these, CMS4 (~25% of colon cancers) is associated with a significantly worse disease-free and overall survival [9]. Novel treatment strategies for this subtype are thus particularly needed.
The pro-metastatic pathways that are upregulated in CMS4 provide opportunities for subtype-targeted therapy. CMS4 tumours are characterised by high expression of stem cell and mesenchymal genes, and a high stromal content [9]. We have previously shown that Platelet-Derived Growth Factor Receptors (PDGFRs) and KIT are highly expressed in mesenchymal-type colon tumours and that their expression strongly correlates with disease-free survival. Moreover, in vitro and in vivo inhibition of PDGFR and KIT tyrosine kinase signalling reduced invasiveness, metastatic potential and stem-like characteristics of mesenchymal-type colon cancer [10–12]. Based on these findings we hypothesise that patients with poor-prognosis mesenchymal-type colon cancers could benefit from treatment with imatinib, a tyrosine kinase inhibitor with high selectivity for PDGFR and KIT.
To test this hypothesis in a proof-of-concept study, we designed the ImPACCT trial (Imatinib as Pre-operative Anti-Colon Cancer Targeted therapy). In ImPACCT, patients with CMS4 colon cancer are identified with a recently developed 4-gene RT-qPCR test that measures PDGFRA, PDGFRB, PDGFC and KIT expression levels in diagnostic tumour biopsies [13]. Pre-selected patients with CMS4 tumours are then treated with imatinib during the pre-operative window period (the time between initial cancer diagnosis and surgery). This allows comparison of pre-treatment diagnostic tumour biopsies with biopsies obtained from the resection specimen after treatment. The primary objective is to assess whether imatinib treatment reduces the aggressive phenotype of CMS4 tumours in colon cancer patients. ImPACCT may not only form the basis for future adjuvant studies with imatinib, but could also serve as a blueprint for other proof-of-concept studies with subtype-targeted therapies.
Methods
Study design
The ImPACCT trial is an open-label, multi-centre proof-of-concept study. The primary endpoint of this trial is the effect of imatinib treatment on tumour biology, which is a pharmacodynamic endpoint, and as such this trial could be deemed a phase II/translational trial. A study flow chart is depicted in Fig. 1.
Fig. 1.
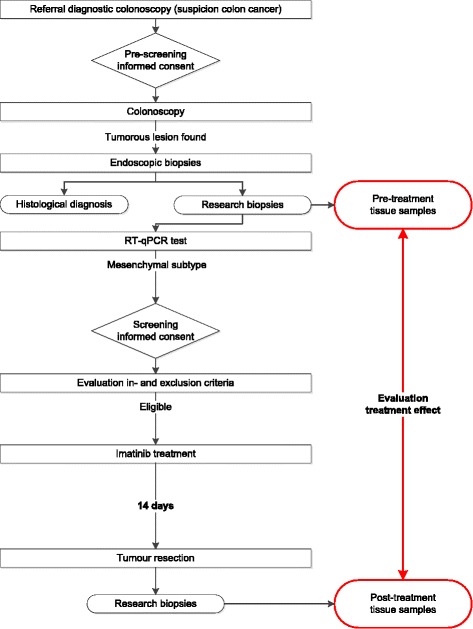
Study flow chart
Objectives
The primary objective of this trial is to investigate the effects of treatment on pro-metastatic pathways in aggressive primary CMS4 tumours. RNA sequencing will be performed on pre- and post-treatment tissue samples to document imatinib-induced genome-wide gene expression changes.
Secondary objectives include: to assess the extent of inhibition of PDGFR- and KIT after imatinib treatment; to relate intra-tumoural imatinib pharmacokinetics (PK) to systemic imatinib concentrations; to relate the level of inhibition of PDGFR/KIT signalling and the extent of changes in gene expression to the systemic and intra-tumoural PK of imatinib and its active metabolite CGP74588; to assess changes in tumour markers during treatment by measuring the concentrations of plasma-CEA and circulating tumour DNA (ctDNA) and to study the effects of imatinib on organoid-forming potential. Finally, the effect of short-term exposure to imatinib immediately followed by bowel surgery on the complication rate will be monitored.
Study population
All patients who are scheduled for a diagnostic colonoscopy on account of clinical suspicion of colon carcinoma will be approached for permission to obtain extra biopsies for this study in case a tumour is found in the colon. These biopsies are pre-screened with the new RT-qPCR test that predicts the chance of a tumour being CMS4, based on the combined expression of imatinib targets PDGFRA, PDGFRB, PDGFC and KIT [13]. If the predicted chance of CMS4 in the biopsies is 50% or higher, patients will be approached for imatinib therapy. The study population that undergoes treatment with imatinib will thus consist of treatment-naïve newly diagnosed colon cancer patients with a tumour with a high probability of having the CMS4 phenotype. In- and exclusion criteria for enrolment in the second part of the trial (imatinib therapy) are listed in Table 1.
Table 1.
In- and exclusion criteria for treatment with imatinib
| Inclusion criteria | Exclusion criteria |
|---|---|
| - Male or female aged ≥18 years; - Histologically proven adenocarcinoma of the colon; - Completed cancer staging with CT-abdomen and CT-thorax/X-thorax according to hospital’s standard of care; - Confirmed eligibility for surgery with curative intent as deemed by the hospital’s multidisciplinary board review; - Test positive for CMS4 subtype; - ≥4 properly stored pre-treatment biopsies for gene expression analysis/ELISA; - WHO performance status 0 or 1; - Adequate haematology status and organ function (defined as: normal creatinine clearance (≥60 ml/min (MDRD)), ALAT within 2.5× upper limit of normal (ULN), PT-INR < 1.5, leukocytes >1,5*10^9/L, Hb > 6.0 mmol/L, platelets >100*10^9/L); - Willingness and ability to comply with scheduled visits, treatment plans and laboratory tests; - Provision of written informed consent. |
- The presence of synchronous distant metastases; - Current hospital standard of care dictates that subject should undergo any neoadjuvant therapy; - Concurrent participation in another clinical trial using any medicinal product, or participation in such a trial in the period of three months prior to the current trial; - Women who are pregnant, plan to become pregnant or are lactating during the study or for up to 30 days after the last dose of imatinib; - Known HIV or Hepatitis B/C infection; - Known symptomatic congestive heart failure; - Co-morbidity requiring concomitant treatment with drugs that act as strong inducers of CYP3A4 or with drugs with a narrow therapeutic range influenced by imatinib. |
Study procedures
Patients pre-selected with the RT-qPCR test will be screened by a medical oncologist for inclusion in the second part of this study. Included patients will receive treatment with imatinib starting two weeks prior to planned tumour resection. Imatinib will be administered orally at a daily dosage of 400 mg for two weeks, with the last dose given within 12 h before surgery. Patients are requested to register drug intake and any adverse events in a patient diary. Before the start of imatinib therapy and at the end of the treatment period, blood samples will be obtained to measure ctDNA and plasma-CEA. Plasma imatinib trough levels will be determined on the day of surgery. Immediately following tumour resection, biopsies will be taken from the surgical specimen (post-treatment biopsies). Gene and protein expression of the pre-treatment biopsies (from colonoscopy) and post-treatments biopsies will be compared to assess the effects of imatinib therapy on PDGFR- and KIT-signalling and on the mesenchymal gene expression profile. After surgery, patients will be monitored according to standard of care. Any postoperative adverse events up until 14 days after discontinuation of study medication (end of study) will be documented.
Sample size calculation
This study is designed as a proof-of-concept study with multiple outcomes of interest. We expect the effect size of imatinib treatment on the various parameters to be very high, since we specifically select patients who express high levels of the drug targets. However, we are aware of possible factors that may reduce the observed effect size. This includes intra-tumour heterogeneity in target expression – causing potential misclassification – and variation in drug distribution throughout the tumour and between patients. Therefore, we anticipate a medium to high Cohen effect size for the primary endpoint (i.e. Cohen’s effect size of 0.65). To demonstrate such an effect, we need to include 27 (evaluable) patients, based on a two-sided paired-samples t-test with a significance level α of 0.05 and power 1-β of 90%. We specifically chose this high power in order not to dismiss effects that are potentially relevant for further development of imatinib therapy in colorectal cancer patients. Given that approximately 25% of colon cancers are CMS4, at least 4*27 = 108 eligible patients with newly diagnosed colon cancer will need to be pre-screened with the RT-qPCR test. Since <10% of the patients undergoing colonoscopy are diagnosed with colon cancer, at least 1.100 patients will have to be approached for pre-screening informed consent.
Statistical analyses
Imatinib-induced changes in the expression of specific gene signatures associated with CMS4 will be assessed by RNA-sequencing analysis. The signatures will be obtained from published literature and our own data. In addition, imatinib-induced gene expression changes will be analysed in a non-biased way by gene ontology and gene enrichment analyses. Using the appropriate statistical tests depending on distribution of the data (paired samples t-test in case of normal distribution or Wilcoxon signed-rank test if the distribution remains non-normal even after transformation) we will test whether there is a significant change in gene signature expression. Phosphorylation of PDGFRα/β and KIT will be quantitatively assessed as a fraction of the total amount of PDGFRα/β and KIT present in a sample. Post-treatment imatinib-induced inhibition of PDGFR phosphorylation will be compared relative to the pre-treatment sample (i.e. the patient’s internal control) using the appropriate statistical tests. Correlations between plasma trough levels and intratumoural concentration of imatinib, and between the extent of PDGFR and KIT inhibition and systemic/intratumoural imatinib concentrations will be evaluated using Pearson correlation coefficient. The Wilcoxon matched pairs signed rank test will be used to compare serial ctDNA and CEA levels. Organoid-forming potential of the tumours after imatinib therapy will be compared with the general success rate of organoid-establishment from colon cancers, using a Fisher exact test. In case of missing data, samples will be excluded pair-wise.
Discussion
Targeted anti-cancer therapies that seem promising in pre-clinical and early-phase clinical trials often fail to show benefit in phase III randomized controlled trials. Up to 60 % of phase III trials have negative outcomes [14, 15]. This high failure rate underscores the need for optimization of trial design, including better patient selection. Since the successful addition of oxaliplatin to the adjuvant chemotherapy regimen in 2004 [16], no further advances have been made in the outcome of patients with stage II/III colon cancer. The lack of recent positive adjuvant chemotherapy trials is partly due to the fact that hardly any new drugs are actually tested in the adjuvant setting. Novel treatments are generally first tested in patients with metastatic disease who have no regular treatment options left, and who have often received multiple lines of systemic treatment. Therapies aimed at preventing the development or outgrowth of metastases are probably most effective at early disease stages, but their clinical development may be terminated due to lack of efficacy in late-stage disease [17]. Moreover, new (combination) treatments that are effective in metastatic disease do not necessarily have value in the adjuvant setting, as exemplified by trials PETACC-03 (5-FU/LV plus irinotecan) [18], NSABP C-08 (FOLFOX6 plus bevacizumab) [19] and N0147 (FOLFIRI plus cetuximab) [20]. To address this problem, design and approval of clinical trials in which promising drugs are tested in treatment-naïve patients with early-stage disease, rather than in late-stage patients who progressed under standard treatment, is needed.
Prospective stratification and/or inclusion based on predictive molecular biomarkers will presumably improve trial results by enrichment for responsive patients [21]. However, microsatellite instability is currently the only molecular marker that is being used in the clinical decision process for adjuvant chemotherapy in colon cancer [22]. The four recently identified CMSs in colon cancer show marked differences in the activity of various biological pathways, which could provide the basis for subtype-specific targeted therapy [9]. CMS4 (the mesenchymal/stem-like/stroma-rich subtype) has the poorest prognosis and, importantly, this subtype seems associated with a lack of benefit from oxaliplatin treatment [23]. These findings call for clinical trials with novel therapies specifically targeting CMS4 tumours. In ImPACCT we aim to deliver proof-of-concept that PDGFR/KIT inhibition with imatinib reduces the aggressive phenotype of newly diagnosed primary CMS4 colon tumours. Delivering proof-of-concept for drug activity in early stages of drug development is pivotal to prevent unfounded trial phase transition [14, 15].
ImPACCT is conducted with treatment-naïve patients during the pre-operative window period, which allows us to obtain high quality tissue material before and after imatinib therapy without additional interventions: pre-treatment samples will be obtained during diagnostic colonoscopy and post-treatment biopsies are collected from the resection specimen; both procedures are part of standard of care. By comparison of pre- and post-treatment tissue samples, the effects of imatinib on CMS4 tumours can be evaluated at a cellular level. The treatment period of 14 days is within the normal time frame from diagnosis to surgery (the pre-operative window). We expect that two weeks of treatment will be sufficient to induce changes in gene expression: given that steady state plasma concentrations will be attained within four days (the plasma half-life of imatinib is 18 h [24]), patients will be exposed to the full dose for ten days. The chosen daily dose of 400 mg is the standard dose for chronic myeloid leukaemia, gastrointestinal stromal tumours (GIST) and myelodysplastic syndrome.
Experimental therapy during the pre-operative window period could potentially lead to an increase in postoperative complications. However, based on two phase II trials that evaluated the use of neoadjuvant imatinib therapy for otherwise irresectable or metastatic GIST, we believe it is safe to administer the last dose of imatinib within 12 h before surgery. In these trials imatinib was administered for several months and stopped one day prior to surgery. Both studies concluded that this approach was feasible, and the reported postoperative complications were acceptable and not out of the ordinary considering the extensive abdominal surgery [25, 26].
Instead of assessing a clinical endpoint (e.g. disease-free survival), this proof-of-concept trial investigates a surrogate endpoint (gene expression changes) to demonstrate a ‘biological’ treatment effect. This endpoint requires a relatively limited sample size and can be determined after only two weeks of therapy, which limits the burden placed on participants. If treatment effects are indeed demonstrated, knowledge of the size of the effect on tumour biology can be used to drive the design of a larger randomised phase II trial.
The design of the ImPACCT trial presented here allows rapid evaluation of the mechanism of action of a targeted therapy in a subtype-stratified patient population by analysis of paired pre- and post-treatment biopsies, without interfering with or delaying standard-of-care, by making use of the pre-operative window period. This design can serve as a blueprint for subtype-directed proof-of-concept trials in colon cancer, with the ultimate goal of designing effective adjuvant therapy that eradicates occult metastases.
Acknowledgements
We wish to acknowledge Joyce van Kuik and Shivanand Richardson for implementing and performing the RT-qPCR test at the Laboratory of Molecular Pathology, UMC Utrecht.
Funding
This study is supported by grants from the Dutch Cancer Society (UU2014–6617) and ZonMw (95104001). The funders had no involvement in the study design, in writing of this manuscript or in the decision to submit the paper for publication.
Availability of data and materials
Not applicable.
Authors’ contributions
IU and OK wrote the manuscript; IU, HJB, SGE, RHJM, WMUG, MK, MPL, OK, and IHMBR designed the study; SGE provided statistical counselling in clinical trial design; IU, HJB, MAB, MPS, YCWH, PMV, AWB, WWJL and RAW participate in conducting the study; All authors have read, critically reviewed and approved the final manuscript for publication.
Competing interest
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The study has been approved by the medical ethical committee of the University Medical Centre Utrecht, the Netherlands (15/527) and the Central Committee on Research Involving Human Subjects (NL50620.041.15). The trial will be performed in accordance with the protocol and the Medical Research Involving Human Subjects Act (WMO). The medicinal part of the trial will be conducted in agreement with the International Conference on Harmonization Good Clinical Practice (ICH GCP) guidelines and the World Medical Association (WMA) declaration of Helsinki (version 2013). Written informed consent will be obtained from all study participants.
Sponsor
This is an investigator-driven trial, sponsored by the University Medical Center Utrecht The sponsor had no involvement in the study design, in writing of this manuscript or in the decision to submit the paper for publication.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- ALAT
Alanine transaminase
- CEA
Carcino-embryonic antigen
- CMS
Consensus molecular subtype
- ctDNA
Circulating tumour DNA
- ELISA
Enzyme Linked Immuno-Sorbent Assay
- GIST
Gastrointestinal stromal tumours
- HIV
Human immunodeficiency virus
- ImPACCT
Imatinib as Pre-operative Anti-Colon Cancer Targeted therapy
- MDRD
Modification of Diet in Renal Disease
- PDGF(R)
Platelet-Derived Growth Factor (Receptor)
- PK
Pharmacokinetics
- PT-INR
Protrombine time - international normalized ratio
- RT-qPCR
Reverse Transcriptase quantitative Polymerase Chain Reaction
- WHO
World Health Organisation
Footnotes
Principal Investigator: I. H. M. Borel Rinkes
Contributor Information
O. Kranenburg, Email: o.kranenburg@umcutrecht.nl
I. H. M. Borel Rinkes, Email: i.h.m.borelrinkes@umcutrecht.nl
References
- 1.Bockelman C, Engelmann BE, Kaprio T, Hansen TF, Glimelius B. Risk of recurrence in patients with colon cancer stage II and III: a systematic review and meta-analysis of recent literature. Acta Oncol. 2015;54(1):5–16. doi: 10.3109/0284186X.2014.975839. [DOI] [PubMed] [Google Scholar]
- 2.Marisa L, de Reynies A, Duval A, Selves J, Gaub MP, Vescovo L, Etienne-Grimaldi MC, Schiappa R, Guenot D, Ayadi M, et al. Gene expression classification of colon cancer into molecular subtypes: characterization, validation, and prognostic value. PLoS Med. 2013;10(5):e1001453. doi: 10.1371/journal.pmed.1001453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roepman P, Schlicker A, Tabernero J, Majewski I, Tian S, Moreno V, Snel MH, Chresta CM, Rosenberg R, Nitsche U, et al. Colorectal cancer intrinsic subtypes predict chemotherapy benefit, deficient mismatch repair and epithelial-to-mesenchymal transition. Int J Cancer. 2014;134(3):552–562. doi: 10.1002/ijc.28387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schlicker A, Beran G, Chresta CM, McWalter G, Pritchard A, Weston S, Runswick S, Davenport S, Heathcote K. Castro DA et al Subtypes of primary colorectal tumors correlate with response to targeted treatment in colorectal cell lines. BMC Med Genet. 2012;5:66. doi: 10.1186/1755-8794-5-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Budinska E, Popovici V, Tejpar S, D'Ario G, Lapique N, Sikora KO, Di Narzo AF, Yan P, Hodgson JG, Weinrich S, et al. Gene expression patterns unveil a new level of molecular heterogeneity in colorectal cancer. J Pathol. 2013;231(1):63–76. doi: 10.1002/path.4212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Sousa EMF, Wang X, Jansen M, Fessler E, Trinh A, de Rooij LP, de Jong JH, de Boer OJ, van Leersum R, Bijlsma MF, et al. Poor-prognosis colon cancer is defined by a molecularly distinct subtype and develops from serrated precursor lesions. Nat Med. 2013;19(5):614–618. doi: 10.1038/nm.3174. [DOI] [PubMed] [Google Scholar]
- 7.Sadanandam A, Lyssiotis CA, Homicsko K, Collisson EA, Gibb WJ, Wullschleger S, Ostos LCG, Lannon WA, Grotzinger C, Del Rio M, et al. A colorectal cancer classification system that associates cellular phenotype and responses to therapy. Nat Med. 2013;19(5):619–625. doi: 10.1038/nm.3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cancer Genome Atlas N Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487(7407):330–337. doi: 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guinney J, Dienstmann R, Wang X, de Reynies A, Schlicker A, Soneson C, Marisa L, Roepman P, Nyamundanda G, Angelino P, et al. The consensus molecular subtypes of colorectal cancer. Nat Med. 2015;21(11):1350–1356. doi: 10.1038/nm.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steller EJA, Raats DA, Koster J, Rutten B, Govaert KM, Emmink BL, Snoeren N, van Hooff SR, Holstege FCP, Maas C, et al. PDGFRB promotes liver metastasis formation of mesenchymal-like colorectal tumor cells. Neoplasia. 2013;15(2):204–217. doi: 10.1593/neo.121726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fatrai S, van Schelven SJ, Ubink I, Govaert KM, Raats D, Koster J, Verheem A, Borel Rinkes IH, Kranenburg O. Maintenance of Clonogenic KIT(+) human colon tumor cells requires secretion of stem cell factor by differentiated tumor cells. Gastroenterology. 2015;149(3):692–704. doi: 10.1053/j.gastro.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 12.Kitadai Y, Sasaki T, Kuwai T, Nakamura T, Bucana CD, Fidler IJ. Targeting the expression of platelet-derived growth factor receptor by reactive stroma inhibits growth and metastasis of human colon carcinoma. Am J Pathol. 2006;169(6):2054–2065. doi: 10.2353/ajpath.2006.060653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ubink I, Elias SG, Moelans CB, Laclé MM, van Grevenstein WMU, van Diest PJ, Borel Rinkes IHM, Kranenburg O A novel diagnostic tool for selecting patients with mesenchymal-type colon cancer reveals intra-tumor subtype heterogeneity. J Natl Cancer Inst. 2017;109(8). doi: 10.1093/jnci/djw303. [DOI] [PubMed]
- 14.Kola I, Landis J. Can the pharmaceutical industry reduce attrition rates? Nat Rev Drug Disc. 2004;3(8):711–715. doi: 10.1038/nrd1470. [DOI] [PubMed] [Google Scholar]
- 15.Seruga B, Ocana A, Amir E, Tannock IF. Failures in phase III: causes and consequences. Clin Cancer Res. 2015;21(20):4552–4560. doi: 10.1158/1078-0432.CCR-15-0124. [DOI] [PubMed] [Google Scholar]
- 16.Andre T, Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, Hickish T, Topham C, Zaninelli M, Clingan P, Bridgewater J, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350(23):2343–2351. doi: 10.1056/NEJMoa032709. [DOI] [PubMed] [Google Scholar]
- 17.Weber GF. Why does cancer therapy lack effective anti-metastasis drugs? Cancer Lett. 2013;328(2):207–211. doi: 10.1016/j.canlet.2012.09.025. [DOI] [PubMed] [Google Scholar]
- 18.Van Cutsem E, Labianca R, Bodoky G, Barone C, Aranda E, Nordlinger B, Topham C, Tabernero J, Andre T, Sobrero AF, et al. Randomized phase III trial comparing biweekly infusional fluorouracil/leucovorin alone or with irinotecan in the adjuvant treatment of stage III colon cancer: PETACC-3. J Clin Oncol. 2009;27(19):3117–3125. doi: 10.1200/JCO.2008.21.6663. [DOI] [PubMed] [Google Scholar]
- 19.Allegra CJ, Yothers G, O'Connell MJ, Sharif S, Petrelli NJ, Colangelo LH, Atkins JN, Seay TE, Fehrenbacher L, Goldberg RM, et al. Phase III trial assessing bevacizumab in stages II and III carcinoma of the colon: results of NSABP protocol C-08. J Clin Oncol. 2011;29(1):11–16. doi: 10.1200/JCO.2010.30.0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang J, Nair SG, Mahoney MR, Nelson GD, Shields AF, Chan E, Goldberg RM, Gill S, Kahlenberg MS, Quesenberry JT, et al. Comparison of FOLFIRI with or without cetuximab in patients with resected stage III colon cancer; NCCTG (Alliance) intergroup trial N0147. Clin Colorectal Cancer. 2014;13(2):100–109. doi: 10.1016/j.clcc.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hayashi K, Masuda S, Kimura H. Impact of biomarker usage on oncology drug development. J Clin Pharm Therap. 2013;38(1):62–67. doi: 10.1111/jcpt.12008. [DOI] [PubMed] [Google Scholar]
- 22.Sargent DJ, Marsoni S, Monges G, Thibodeau SN, Labianca R, Hamilton SR, French AJ, Kabat B, Foster NR, Torri V, et al. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J Clin Oncol. 2010;28(20):3219–3226. doi: 10.1200/JCO.2009.27.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song N, Pogue-Geile KL, Gavin PG, Yothers G, Kim SR, Johnson NL, Lipchik C, Allegra CJ, Petrelli NJ, O’Connell MJ et al Clinical Outcome From Oxaliplatin Treatment in Stage II/III Colon Cancer According to Intrinsic Subtypes: Secondary Analysis of NSABP C-07/NRG Oncology Randomized Clinical Trial. JAMA Oncol 2016;2(9):1162-1169 [DOI] [PMC free article] [PubMed]
- 24.Gotta V, Buclin T, Csajka C, Widmer N. Systematic review of population pharmacokinetic analyses of imatinib and relationships with treatment outcomes. Ther Drug Monit. 2013;35(2):150–167. doi: 10.1097/FTD.0b013e318284ef11. [DOI] [PubMed] [Google Scholar]
- 25.Doyon C, Sideris L, Leblanc G, Leclerc YE, Boudreau D, Dube P. Prolonged therapy with Imatinib Mesylate before surgery for advanced gastrointestinal Stromal tumor results of a phase II trial. Int J Surg Oncol. 2012;2012:761576. doi: 10.1155/2012/761576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eisenberg BL, Harris J, Blanke CD, Demetri GD, Heinrich MC, Watson JC, Hoffman JP, Okuno S, Kane JM, von Mehren M. Phase II trial of neoadjuvant/adjuvant imatinib mesylate (IM) for advanced primary and metastatic/recurrent operable gastrointestinal stromal tumor (GIST): early results of RTOG 0132/ACRIN 6665. J Surg Oncol. 2009;99(1):42–47. doi: 10.1002/jso.21160. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


