The presence of a functional histamine H4 receptor in neutrophils with anti-inflammatory properties.
Keywords: inflammation, signaling, innate immunity
Abstract
The histamine H4 receptor regulates the inflammatory response. However, it is not known whether this receptor has a functional role in human neutrophils. We found that fMLP (1 μM), but not histamine (0.1–1 μM), induced Mac-1-dependent adhesion, polarization, and degranulation (release of lactoferrin). A pretreatment of neutrophils with histamine (0.001–1 μM) or JNJ 28610244 (0.1–10 μM), a specific H4 receptor agonist, led to inhibition of degranulation. Total inhibition of degranulation was obtained with 0.1 μM histamine and 10 μM JNJ 28610244. Furthermore, such inhibition by histamine of degranulation was reversed by JNJ 7777120 and JNJ 28307474, two selective H4 receptor antagonists. However, neither histamine nor the H4 receptor agonist JNJ 28610244 prevented fMLP-induced, Mac-1-dependent adhesion, indicating that the H4 receptor may block signals emanating from Mac-1-controlling degranulation. Likewise, engagement of the H4 receptor by the selective agonist JNJ 28610244 blocked Mac-1-dependent activation of p38 MAPK, the kinase that controls neutrophil degranulation. We also show expression of the H4 receptor at the mRNA level in ultrapure human neutrophils and myeloid leukemia PLB-985 cells. We concluded that engagement of this receptor by selective H4 receptor agonists may represent a good, therapeutic approach to accelerate resolution of inflammation.
Introduction
PMNs are the most abundant leukocytes in blood and play a crucial role in host defense against bacteria and fungi. The recruitment of PMNs to sites of inflammation is a complex process orchestrated by chemokines and adhesion receptors [1]. β2 Integrins constitute a major group of adhesion molecules expressed in PMNs. These heterodimeric transmembrane receptors are composed of a common β chain, CD18, and one of four unique α chains, CD11 (a–d) [2]. CD11b/CD18 (Mac-1) is the prominent β2 integrin expressed in PMNs.
The recruitment of PMNs to the site of inflammation is tightly regulated by chemoattractants, chemokines, and proinflammatory cytokines. These soluble mediators, which are produced during episodes of infection, induce expression of β2 integrins on the membrane surface of PMNs [3] and switch the β2 integrins from a low- to a high-affinity, ligand-binding conformation (inside-out signaling) [2]. This change of conformation in CD11b/CD18 (Mac-1), as well as in CD11a/CD18 (LFA-1), is required for firm attachment to the endothelium, migration along the endothelial layer, and the subsequent extravasation.
Besides their role in adhesion and migration, β2 integrins also regulate inflammatory functions of PMNs, including production of ROS and degranulation. Degranulation is the process by which intracellular granules, which contain proteases and antimicrobial peptides, as well as the NADPH oxidase complex, fuse with the phagosome and plasma membrane. The release of the granule content into the phagosome and the extracellular milieu, coupled with the production of ROS, is responsible for the destruction of the microorganisms [4].
The signal transduction properties of β2 integrins are crucial for regulation of PMN functions. In human PMNs, adhesion-induced ligation of the β2 integrins activates various nonreceptor tyrosine kinases, including Src family members (Fgr, Hck, and Lyn) [5]. Genetic studies of knockout mice have shown that the Src tyrosine kinases Fgr and Hck are necessary for β2 integrin-dependent modulation of a number of functions in PMNs, including degranulation [5]. The crucial step in the regulation of PMN degranulation is phosphorylation of p38 MAPK by Src family tyrosine kinases [6].
Histamine is an important regulator of biological functions and is best known as an inflammatory mediator in allergic reactions [7]. Histamine mediates its biological effects through four pharmacologically distinct histamine receptors: H1 receptor, H2 receptor, H3 receptor, and H4 receptor, each with a different Ki [7]. Ki values for the H1 and H2 receptors range from 2 to 10 μM, whereas those for the H3 and H4 receptors range from 5 to 10 nM [7, 8]. The H4 receptor is of particular interest, as it may play a role in immune and inflammatory disorders [9]. Among the histamine receptor family, the H3 receptor is the closest member to the H4 receptor and shares only a 35% amino acid homology with the H4 receptor [7]. The development of specific agonists and antagonists of the H4 receptor has allowed the identification of immune responses regulated by the H4 receptor in eosinophils [10], mast cells [11], and invariant NKT cells [12].
Little information is available on the nature of the histamine receptors expressed in peripheral blood PMNs. Histamine H1 binding sites are present in human PMNs [13]. In contrast, other investigators reported the presence of H2- but not H1 binding sites in PMNs [14]. There is no report for the presence of H3 or H4 binding sites in human PMNs.
Histamine is a potent inhibitor of PMN inflammatory functions, as shown by its ability to block fMLP-induced superoxide production, release of β-glucuronidase [15, 16], and biosynthesis of leukotrienes [17]. Furthermore, these inhibitory effects of histamine are mediated via the H2 receptor, as they are reversed by antagonists of the H2 receptor [15, 16]. Recent data have revealed expression of the H4 receptor in human PMNs. Indeed, the mRNA encoding for the H4 receptor was found in human PMNs isolated from peripheral blood [18] and in HL-60 cells differentiated into granulocytes [19]. However, it is not known yet what functional role this receptor has in PMNs. Based on these findings, we sought to investigate whether the H4 receptor regulates inflammatory functions in human PMNs. By using pharmacological agonists and antagonists of the H4 receptor, we provide evidence that the H4 receptor is a negative regulator of adhesion-dependent PMN degranulation.
MATERIALS AND METHODS
Materials
Ficoll-Hypaque was purchased from GE Healthcare Biosciences AB (Uppsala, Sweden). Human lactoferrin, rabbit anti-lactoferrin antibodies, Dextran 500, and o-phenylenediamine were purchased from Sigma-Aldrich (Dorset, UK). The rabbit polyclonal anti-p-p38 MAPK antibody (Thr180/Tyr182; Cat. #9211S) and the rabbit polyclonal p38 MAPK antibody (Cat. #9212) were purchased from Cell Signaling Technology/New England Biolabs (Hitchin, UK). The primers for PCR reactions were synthesized by Eurofins Genomics (Ebersberg, Germany). All other chemicals were of analytical grade and came from Sigma-Aldrich. The H4 receptor antagonists JNJ 7777120 and JNJ 28307474, as well as the H4 receptor agonist JNJ 28610244, were synthesized as described previously [20–22].
Isolation of human PMNs and differentiation of PLB-985 cells
Venous blood was collected from healthy donors by venous puncture after obtaining informed consent. This study was approved by the Office for Research Ethics Committees Northern Ireland (Ref. 07/NIR03/86). PMNs were isolated from the blood using Dextran sedimentation and centrifugation through Ficoll-Hypaque [23]. The cells (97% purity) were resuspended in RPMI medium, supplemented with 20 mM Hepes (pH 7.4). Malignant myeloid leukemia PLB-985 cells were differentiated along the granulocytic pathway by adding 1.25% DMSO for 5 days to the culture medium (RPMI, supplemented with 10% FCS). Differentiation into PMN-like cells is accompanied by expression of the granulocytic marker p47phox [24].
Engagement of β2 integrins
Easy Grip Petri dishes were incubated overnight at 4°C or for 2 h at room temperature with 20 μg/ml fibrinogen in PBS. Thereafter, the Petri dishes were blocked for 30 min by adding 5% FCS, prepared in PBS and washed twice with PBS and once with RPMI medium. PMNs were subsequently allowed to adhere to the fibrinogen-coated dishes at 37°C in the presence of fMLP (0.1 μM) to engage the β2 integrin Mac-1 [23].
Degranulation assays
PMNs (1×106) were incubated at 37°C on 24-well plates coated with fibrinogen in the absence or presence of fMLP (0.1 μM) or other histamine receptor agonists/antagonists. The supernatants, free of PMNs, were transferred to Eppendorf tubes and kept on ice. The concentration of lactoferrin in the supernatants was determined using an ELISA, as described [5]. Color was developed by the o-phenylenediamine/H2O2 system. Absorbance of the wells was read at 490 nm with a Versa max microplate reader (Molecular Devices, Sunnyvale, CA, USA). The concentration of lactoferrin in each well was calculated based on the human lactoferrin calibration curve. For each experimental condition, the mean concentration of lactoferrin was calculated from three wells.
Adhesion assays
PMNs (0.5×106) were allowed to adhere onto fibrinogen-coated dishes at 37°C. After 30 min, nonattached cells were removed by aspiration, and plates were washed three times with PBS. Adherent cells were fixed for 30 min with 4% paraformaldehyde. Thereafter, cells were washed once with PBS and stained from 15 min with crystal violet (0.1% crystal violet in 10% methanol). The plates were washed three times with PBS. The stain was eluted with 0.1% SDS in PBS. OD of the elution was measured at 570 nm using a spectrophotometer [23]. For each experimental condition, the mean OD value was calculated from three wells.
Western blot analysis
PMNs (5×106) were lysed on ice by adding a 5× concentrated lysis buffer [500 mM Tris-HCl, pH 7.5, 5% NP-40, 25 mM EDTA, 25 mM EGTA, 250 mM NaCl, 25 mM NaF, 5 mM Na3VO4, and protease inhibitors (100 μg/ml aprotinin, 5 μg/ml leupeptin, 10 mM benzamidine, 10 mM Pefabloc)]. Cell lysates were spun down (10,000 g, 10 min), and supernatants were collected into Eppendorf tubes. Concentrated Laemmli sample buffer (5×), containing 1 mM DTT was added, and lysates were boiled for 5 min. Proteins were subjected to electrophoresis on 8% or 10% SDS-PAGE and transferred to PVDF transfer membranes, which were blocked in PBS, supplemented with 0.2% Tween 20 and 3% skimmed milk, and then incubated overnight in a cold room with a 1:1000 dilution of the rabbit anti-p-p38 MAPK antibody or the rabbit anti-p38 MAPK antibody (after stripping off the p-p38 MAPK antibody). Thereafter, membranes were washed and subsequently incubated for 1 h with peroxidase-conjugated goat anti-rabbit IgGs (1:10,000). Antibody binding was visualized by ECL.
RNA extraction, RT, and PCR
PMNs or PLB-985 cells (10×106) were pelleted by centrifugation (190 g, 10 min). RNA was extracted with TRIzol Reagent, according to the protocol provided by the manufacturer (Life Technologies, Paisley, UK). RNA (1 μg) was used for RT. cDNA synthesis was carried out using the iScript cDNA synthesis kit (Bio-Rad, Hemel Hempstead, UK). PCR was performed using the following primers: β-actin, 5′-ATGGATGATGATATCGCCGCG-3′ (forward), 5′-CTAGAAGCATTTGCGGTGGACGATGGAGGGGCC-3′ (reverse); H4 receptor, 5′-CATCCCTCACACGCTGTTCG-3′ (forward), 5′-GCAGGAAGGAACTTCTGTCGATGC-3′ (reverse). The PCR cycles were described in detail in ref. [25]. The PCR products were separated on 3% agarose gel containing ethidium bromide. The separated bands were visualized by UV transillumination, and images were captured with a MultiDoc-It digital imaging system (Transilluminator Model M-26).
Flow cytometry and cell sorting
Autofluorescence-based flow sorting has been carried out as described [26].
Statistical analysis
Experimental data are presented as mean ± sem from the number (n) of independent experiments. Unpaired Student's t-test or one-way ANOVA was used to assess statistical difference between samples.
RESULTS AND DISCUSSION
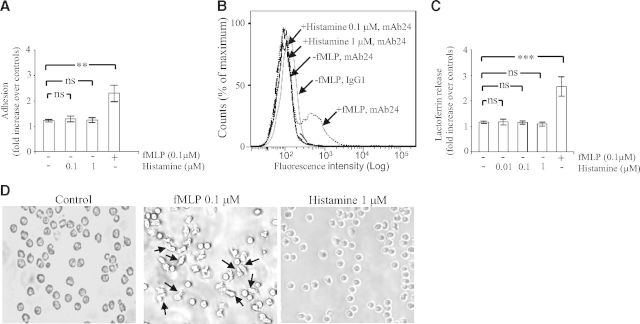
The H4 receptor is expressed in many immune cells, including eosinophils [10], mast cells [11], invariant NKT cells [12], and Langerhans cells [27]. In these cells, the H4 receptor regulates immune functions. Thus, the H4 receptor potentiates secretion of IL-6 in mast cells [11] and synthesis of IL-4 and IFN-γ in invariant NKT cells [12] and controls emigration of human Langerhans cells from the epidermis [27]. In eosinophils, the H4 receptor is a chemotactic receptor, in that it controls expression of Mac-1 on the membrane surface and thereby, controls eosinophil adhesion and migration in response to histamine [10]. Accordingly, we investigated whether histamine was a positive regulator of the β2 integrins Mac-1 and LFA-1 in PMNs. To this end, we compared the ability of bacterial fMLP and histamine to induce Mac-1-dependent adhesion. fMLP augmented adhesion of PMNs on plates coated with fibrinogen (2.2-fold increase over nonstimulated control cells), whereas histamine (0.1–1 μM) did not (Fig. 1A). As fibrinogen is a ligand for Mac-1 but not for LFA-1 [2], augmented PMN adhesion in response to fMLP is explained by the ability of the chemoattractant to induce conformational activation of Mac-1 [2]. This implies that histamine is unable to switch Mac-1 into its high-affinity, ligand-binding conformation. We next investigated whether histamine regulated LFA-1. To this end, we measured by flow cytometry the expression of the mAb24 epitope of the CD11a subunit. This epitope is a marker for the high-affinity, ligand-binding conformation of LFA-1 [28]. Incubation of suspended PMNs with fMLP (0.1 μM, 30 min) resulted in a shift of the histogram peak corresponding to the mAb24 epitope, thus reflecting conformational change of LFA-1 (Fig. 1B). In contrast, no such change of conformation of LFA-1 was observed when PMNs were incubated with histamine (0.1–1 μM, 30 min; Fig. 1B).
Figure 1. Histamine does not induce a Mac-1-dependent adhesion and degranulation.
(A) Human PMNs (0.5×106) were incubated on plates coated with fibrinogen in the absence (−) or presence (+) of fMLP (0.1 μM) or histamine (0.1–1 μM). After 30 min, nonadherent cells were removed by aspiration, and adherent cells were fixed with paraformaldehyde and stained with crystal violet. Subsequently, the OD of the eluted dye was read by spectrophotometry at 570 nm. Adhesion is expressed as fold increase over nonstimulated control cells incubated for 30 min on BSA-coated plates (taken as 1 U). The data represent means ± sem of four separate experiments. Measurements for each experimental condition were performed in triplicate. (B) Human PMNs in suspension, stimulated or not with fMLP (0.1 μM) or histamine (0.1–1 μM), were incubated for 30 min at 37°C in the presence of the mAb24 (1 μg/ml) or an isotype-matched control IgG1 antibody (1 μg/ml). Thereafter, cells were collected, washed, and resuspended in medium containing FITC-labeled anti-mouse IgGs. Representative flow cytometry histograms (out of at least three), depicting mean fluorescence intensity of mAb24 epitope expression on human PMNs, are shown. (C) Human PMNs (1×106) were incubated on plates coated with fibrinogen in the absence (−) or presence (+) of fMLP (0.1 μM) or histamine (0.01–1 μM). After 30 min, the concentration of lactoferrin in the extracellular milieu was measured by ELISA as described in Materials and Methods. The data represent means ± sem of six separate experiments. Lactoferrin release is expressed as fold increase over nonstimulated control cells incubated for 30 min on BSA-coated plates (taken as 1 U). Measurements for each experimental condition were performed in triplicate. (D) PMNs were incubated on plates coated with fibrinogen in the absence (control; left) or presence of fMLP (0.1 μM; middle) or histamine (1 μM; right). After 30 min, adhered cells were visualized using a Leitz Diaplan microscope and a 5-megapixel Leica DFC420 digital camera (Leica Microsystems, Buffalo Grove, IL, USA). Arrows show polarized and elongated PMNs. **P < 0.01, and ***P < 0.001; ns, not significant by one-way ANOVA with post hoc Bonferroni's test compared with controls.
The release of lactoferrin (a marker of specific granules) was used as a readout for degranulation. Mac-1 was engaged on PMNs by incubating the leukocytes on plates coated with fibrinogen in the presence of fMLP (0.1 μM) [23]. Engagement of Mac-1 for 30 min led to an ∼2.5-fold increase in the level of degranulation when compared with cells in which Mac-1 was not engaged (cells incubated on fibrinogen-coated plates for 30 min in the absence of fMLP; Fig. 1C). In contrast, incubation of PMNs on plates coated with fibrinogen, in the presence of histamine (0.01–1 μM), did not lead to augmented lactoferrin concentration in the extracellular milieu (Fig. 1C). We confirmed that degranulation was dependent on engagement of Mac-1 by showing that fMLP (0.1 μM) did not induce degranulation in PMNs incubated on plates coated with casein, which is not a ligand for β2 integrins (lactoferrin release in fMLP-stimulated cells=0.97±0.14-fold increase over controls). In addition, stimulation of PMNs in suspension with fMLP (0.1 μM) did not result in the release of lactoferrin, unless cytochalasin B was added (lactoferrin release in fMLP-stimulated cells=1.31±0.17; in fMLP- and cytochalasin B-treated cells=4.25±1; in cytochalasin B-treated cells=0.83±0.15).
Engagement of β2 integrins on PMNs generates signals activating spreading and polarization [29]. Spreading of PMNs is a pivotal event in their ability to respond to physiological agonists by releasing oxidants and granule proteases [29]. Figure 1D shows images of PMNs incubated for 30 min on plates coated with fibrinogen. Unstimulated cells have settled on the bottom of the wells, appear rounded, and can be washed off easily from the plates (Fig. 1D, left). In response to fMLP, PMNs underwent a marked polarization. The vast majority of the cells shows extended pseudopods (Fig. 1D, middle). These elongated PMNs are firmly attached to the bottom of the plate. In contrast, cells incubated with histamine did not exhibit polarization, had a rounded morphology, and could be detached easily from the wells, as observed in control cells (Fig. 1D, right). We concluded that histamine did not switch Mac-1 or LFA-1 into their active ligand-binding conformations, and this explains why PMNs do not adhere, polarize, and exhibit degranulation in response to histamine.
Histamine inhibits degranulation in response to fMLP [15] or serum-activated zymosan particles [16] in nonadherent human PMNs treated with cytochalasin B. In the present study, we investigated the effect of histamine on Mac-1-dependent degranulation. A 5-min preincubation of PMNs with histamine resulted in a dose-dependent inhibition of fMLP-induced, Mac-1-dependent degranulation (Fig. 2A). Total inhibition was achieved with concentrations of histamine ranging between 0.1 μM and 1 μM. Other investigators also described inhibition of PMN functions by histamine [15, 16]. For example, in PMNs treated with cytochalasin B, histamine totally blocked fMLP-induced superoxide production and secretion of β-glucuronidase with concentrations of 10 μM and 100 μM, respectively [15]. Furthermore, these inhibitory effects of histamine were prevented by cimetidine [15] or metiamide [16], two antagonists of the H2 receptor, and reproduced by dimaprit, a specific agonist of the H2 receptor [15]. In comparison, we found that total inhibition of Mac-1-dependent degranulation was obtained with much lower concentrations of histamine (ranging between 0.1 μM and 1 μM). This finding suggested the possible involvement of the H4 receptor, the high-affinity, histamine-binding receptor.
Figure 2. Effect of histamine and JNJ 28610244 on lactoferrin release.
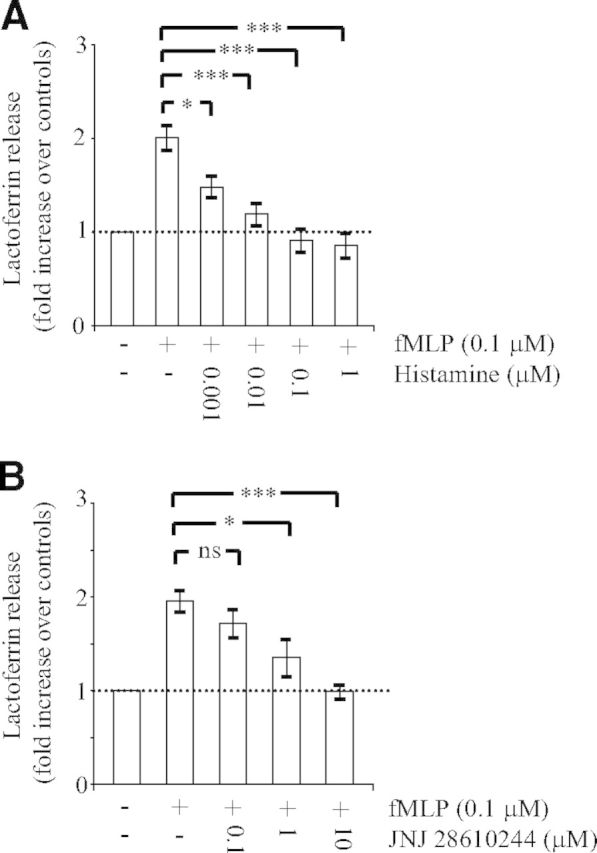
Human PMNs (1×106) were pretreated for 5 min without (−) or with (+) the indicated concentrations of histamine (A) or the H4 receptor agonist JNJ 28610244 (B). Thereafter, PMNs were incubated on plates coated with fibrinogen in the presence of fMLP (0.1 μM) to engage Mac-1. After 30 min, the concentration of lactoferrin in the extracellular milieu was measured by ELISA, as described in Materials and Methods. The data are expressed as fold increase over controls (cells incubated for 30 min on plates coated with fibrinogen in the absence of fMLP) and are the mean ± sem of four to eight (A) or six to eight (B) separate experiments. Measurements for each experimental condition were performed in triplicate. *P < 0.05, and ***P < 0.001; ns, not significant by one-way ANOVA with post hoc Bonferroni's test compared with fMLP-stimulated adherent cells.
It is important to report that the studies describing the requirement of 100 μM histamine to inhibit degranulation totally [15, 16] were carried out with human PMNs treated with cytochalasins, which is an artificial means of inducing activation of PMN inflammatory functions. Indeed, activation of PMN degranulation and production of ROS depend on adhesion by Mac-1 and spreading in response to soluble agonists [30]. Cytochalasins have been shown to modify the signaling capacity of G protein-coupled receptors. For example, in PMNs, cytochalasin B augments the coupling between fMLPR and Gi proteins [31] but also potentiates fMLP-induced desensitization of receptors for IL-8 and C5a [32]. In other cell types, cytochalasin B blocks surface expression of the chemokine receptors CXCR1 and CXCR2 by preventing internalization and recycling of these receptors [33]. Accordingly, we cannot exclude the possibility that in the experiments in which PMNs were treated with cytochalasins [15, 16], the expression and/or the signaling capacity of histamine receptors are altered.
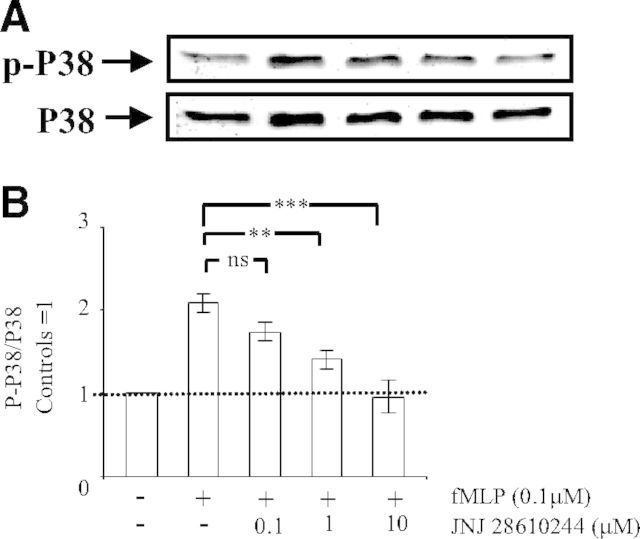
We next used the specific H4 receptor oxime agonist JNJ 28610244 to investigate a possible role of the H4 receptor in this inhibitory process. JNJ 28610244 has a tenfold lower affinity than histamine for the H4 receptor (Ki value of 53 nM) and exhibits at least 1000-fold selectivity over the H1 receptor, H2 receptor, or H3 receptor [22]. We found that similarly to histamine, JNJ 28610244 inhibited Mac-1-dependent degranulation in a dose-dependent manner. Total inhibition was observed with 10 μM JNJ 28610244 (Fig. 2B). The dose shift of JNJ 28610244 relative to histamine can be accounted for by it having tenfold less affinity for the human H4 receptor and the fact that in some donors, it may act only as a partial agonist, most likely reflecting differences in H4 receptor expression levels [18]. Accordingly, H4 receptor mRNA is detected in some, but not all, peripheral blood PMN preparations [10, 18]. For histamine and JNJ 28610244, the concentrations required to block degranulation totally are much greater than the reported affinities at the receptor. This most likely reflects low levels of H4 receptor expression in human PMNs. Our finding is consistent with other cell types, such as mast cells, that express low levels of the receptor [11].
To confirm further the involvement of the H4 receptor in the inhibition by histamine of Mac-1-dependent degranulation, we performed a series of experiments with two structurally different antagonists of the H4 receptor: JNJ 7777120 and JNJ 28307474 [20, 21]. JNJ 7777120 is an N-methylpiperazine analog, whereas JNJ 28307474 is a benzoimidazole. JNJ 7777120 exhibits at least 1000-fold selectivity for the H4 receptor (Ki of 4.5 nM) over the H1 receptor, H2 receptor, or H3 receptor [34]. JNJ 28307474 has a Ki of 4.9 nM for the human H4 receptor compared with Ki values of 159 nM, 2501 nM, and >1000 nM for the human H3 receptor, H1 receptor, and H2 receptor, respectively [21]. We found that JNJ 7777120 (Fig. 3A) blocked the inhibitory effect of 1 μM histamine on Mac-1-dependent degranulation with an IC50 value of ∼0.05 μM. A similar inhibition was observed with JNJ 28307474 (Fig. 3B), with an IC50 value of ∼0.13 μM.
Figure 3. Effect of histamine receptor antagonists on histamine-induced inhibition of lactoferrin release.
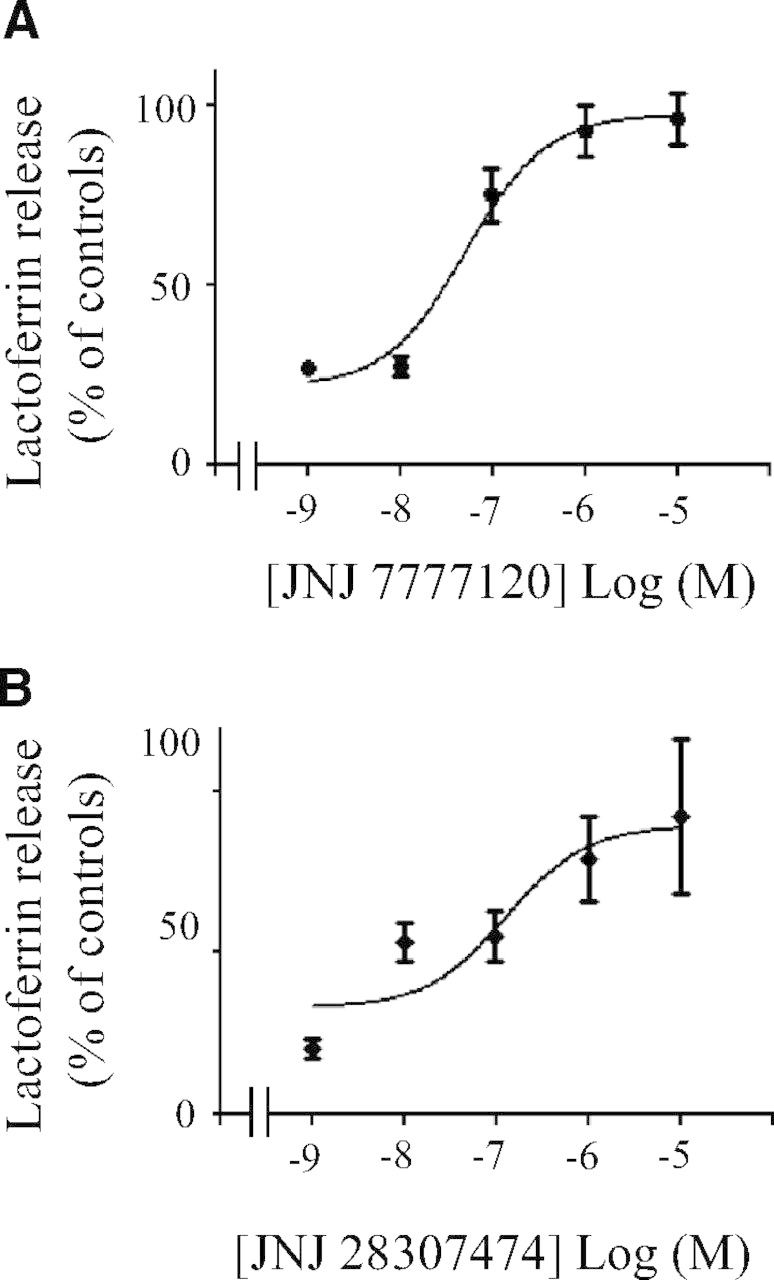
Human PMNs (1×106) were preincubated for 5 min, without or with the indicated concentrations of the H4 receptor antagonists JNJ 7777120 (A), or JNJ 28307474 (B). Thereafter, histamine (1 μM) was added for 5 min. PMNs were then incubated on plates coated with fibrinogen in the presence of fMLP (0.1 μM). After 30 min, the concentration of lactoferrin in the extracellular milieu was measured by ELISA as described in Materials and Methods. The data are expressed as percent of controls (adherent cells stimulated with fMLP) and represent means ± sem of three to six separate experiments, each performed in triplicates.
JNJ 7777120 has been used extensively to characterize biological effects of the H4 receptor. JNJ 7777120 blocked the change of shape and chemotaxis of eosinophils in response to histamine with IC50 values of 0.3 μM and 0.086 μM, respectively [10]. In comparison, we observed reversion by JNJ 7777120 of inhibition by histamine of Mac-1-dependent degranulation with an IC50 value of ∼0.05 μM. In murine mast cells, 10 μM JNJ 7777120 entirely blunted histamine-induced IL-6 synthesis [11]. We observed total reversion of degranulation with 1 μM JNJ 7777120 in human PMNs. Furthermore, we showed that JNJ 28307474 also prevented inhibition by histamine of degranulation with a maximal effect at 10 μM.
It was proposed that histamine inhibits PMN functions by decreasing but not abolishing fMLP-induced expression of Mac-1 on the membrane surface, which would lead to decreased adherence [35]. We provided evidence that neither histamine (1 μM; Fig. 4A) nor the H4 receptor agonist JNJ 28610244 (10 μM; Fig. 4B) inhibited Mac-1-dependent adhesion in response to fMLP. This result indicates that engagement of the H4 receptor does not prevent conformational activation of Mac-1 induced by fMLP but may blunt signaling events emanating from Mac-1 regulating PMN degranulation.
Figure 4. Engagement of the H4 receptor does not prevent Mac-1-dependent adhesion.
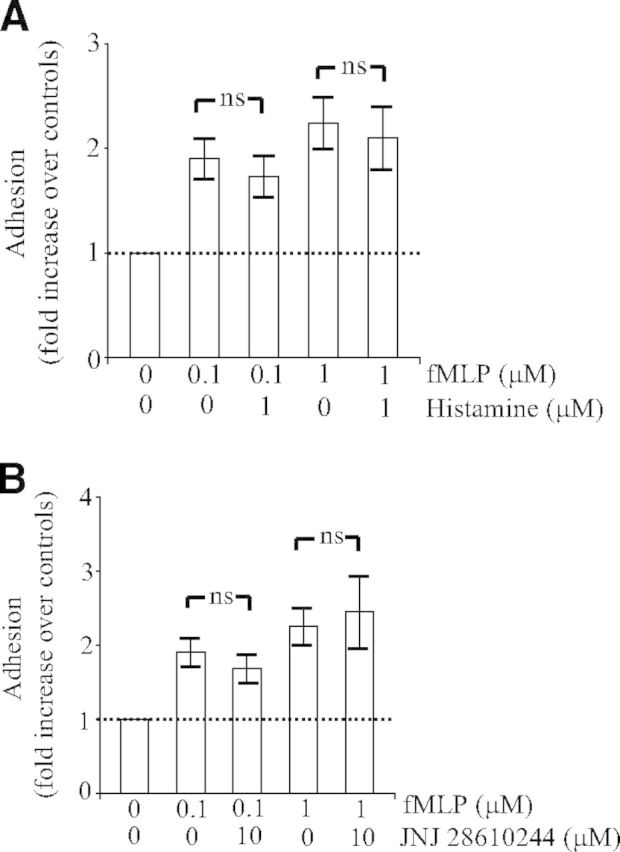
Human PMNs (0.5×106) were pretreated for 5 min without or with histamine (1 μM; A) or the H4 receptor agonist JNJ 28610244 (10 μM; B). Thereafter, PMNs were incubated on plates coated with fibrinogen in the absence or presence of fMLP (0.1–1 μM). After 30 min, nonadherent cells were removed by aspiration, and adhesion was quantified as described in Materials and Methods. Adhesion is expressed as fold increase over nonstimulated control cells (OD of control cells is taken as 1 U). The data represent means ± sem of five separate experiments. ns, Not significant by unpaired Student's t-test.
Release of primary and secondary PMN granules in response to fMLP is mediated by p38 MAPK [6]. We therefore investigated whether the H4 receptor controlled p38 MAPK. To this end, we measured p-p38 MAPK on residues Thr180 and Tyr182 by Western blot analysis using a specific p-p38 MAPK antibody. Such p-p38 MAPK reflects its activation by Src tyrosine kinases [6]. We found that basal p-p38 MAPK increased by approximately twofold upon engagement of Mac-1. Interestingly, a preincubation of PMNs with the H4 receptor agonist JNJ 28610244 resulted in a dose-dependent inhibition of Mac-1-dependent p-p38 MAPK. Total inhibition of Mac-1-dependent p-p38 MAPK was obtained with 1 μM JNJ 28610244 (Fig. 5A and B). In contrast, other investigators showed that engagement of the H4 receptor by JNJ 28610244 on bone marrow-derived mast cells induced phosphorylation of the MAPK ERK, an event that may be associated with production of IL-6 [11]. Thus, depending on the cell type, the H4 receptor either positively or negatively regulates different MAPKs and the associated downstream immune functions. In human and murine PMNs, adhesion-dependent degranulation requires the Src family tyrosine kinases [5]. Indeed, the Src tyrosine kinase inhibitor PP1 prevents degranulation induced by spreading over fibrinogen, and PMNs from Fgr−/− Hck−/− mice exhibit a similar inhibition of degranulation. Furthermore, activation of p38 MAPK is blocked by PP1 and the Fgr−/− Hck−/− Lyn−/− mutation [6]. This has led to the conclusion that fMLP-induced degranulation is mediated by p38 MAPK, activated via the Src family tyrosine kinase [6]. Based on these studies, we propose that engagement of the H4 receptor by the selective H4 receptor agonist JNJ 28610244 may result in the inhibition of Src tyrosine kinase family members, the upstream regulators of p38 MAPK. In that case, inhibition of Src tyrosine kinases by JNJ 28610244 would prevent Mac-1-dependent signaling but would not impair PMN adhesion on fibrinogen [36].
Figure 5. Engagement of the H4 receptor prevents Mac-1-dependent activation of p38 MAPK.
Human PMNs (5×106) were preincubated for 5 min without (−) or with (+) the indicated concentrations of the H4 receptor agonist JNJ 28610244. Thereafter, PMNs were incubated on plates coated with fibrinogen, and fMLP (0.1 μM) was added to engage Mac-1. After 30 min, the cells were lysed. Proteins (40 μg) in lysate extracts were separated on 10% SDS-PAGE and transferred onto a PVDF membrane, which was incubated with a mouse anti-p-p38 MAPK antibody, followed by secondary goat anti-rabbit peroxidase-conjugated antibodies. Detection of the signals was carried out by ECL. Membranes were stripped off anti-p-p38 MAPK antibodies, and Western blot analysis was carried out with a rabbit anti-p38 MAPK antibody, followed by secondary goat anti-rabbit peroxidase-conjugated antibodies and ECL detection. (A) Representative Western blot; the arrows indicate the position of p-p38 MAPK and p38 MAPK. (B) Results after densitometric analysis (in arbitrary units) of the p-p38 MAPK/p38 MAPK ratio for each individual blot. The densitometric analysis of the p-p38 MAPK/p38 MAPK ratio is expressed as fold increase over control cells and represents means ± sem of three separate experiments. **P < 0.01, and ***P < 0.001; ns, not significant by one-way ANOVA with post hoc Bonferroni's test compared with fMLP-stimulated adherent cells.
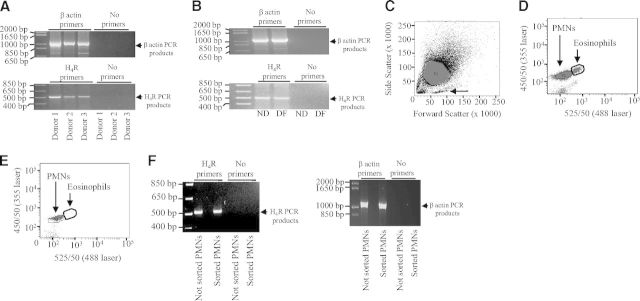
We carried out PCR experiments by using validated primers for H4 receptor cDNA [25] to assess the presence of the H4 receptor mRNA in PMNs. Similarly to what was reported by Ikawa and coworkers in synovial cells [25], we found expression of the H4 receptor mRNA in human PMNs isolated from three different healthy donors (Fig. 6A). The amplified PCR product band from H4 receptor cDNA is 530–550 bp (compared with standard molecular weights), which is the size of the PCR products obtained with human synovial cells using similar primers [25]. In parallel, the H4 receptor mRNA was also detected in differentiated PMN-like PLB-985 cells (Fig. 6B), which exhibit similar inflammatory functions to human PMNs [37]. To prove further that the H4 receptor mRNA is present in PMNs and not in contaminating cells (i.e., mononuclear cells and eosinophils), we used an autofluorescence-based sorting method [26] to obtain ultrapure (99.95%) PMNs. Forward- and side-scatter profiles separated granulocytes from contaminating mononuclear cells (Fig. 6C). Eosinophils are distinguished from PMNs when excited with 488 nm laser (525/50 nm) and 355 nm laser (450/50 nm; Fig. 6D). Eosinophils can be removed by autofluorescence-based sorting, resulting in a pure PMN population (Fig. 6E). This population was collected and used for PCR experiments. We found H4 receptor PCR products (530–550 bp) in both not-pure (not-sorted PMNs) and ultrapure (sorted PMNs) PMN populations (Fig. 6F).
Figure 6. Expression of the H4 receptor in human PMNs.
RNA was extracted from nondifferentiated PLB-985 cells (ND), PLB-985 cells differentiated along the granulocytic pathway (DF), and human PMNs derived from three different donors (Donors 1–3). Thereafter, 1 μg of each RNA preparation was reverse-transcribed. The obtained cDNAs were used for PCR reactions using validated primers for H4 receptor (H4R) or β-actin. The amplified PCR products were separated on a 3% agarose gel containing ethidium bromide. The bands were visualized by UV illumination. The positions of (A) H4 receptor and (B) β-actin PCR products are indicated by arrows (right) in each. (C) Human PMNs (P1) were analyzed by flow cytometry. Forward- and side-scatter profiles are shown. Contaminating mononuclear cells are indicated by an arrow. (D) Eosinophils and PMNs (P2) can be distinguished when excited with 488 nm laser (525/50 nm) and 355 nm laser (450/50 nm). (E) Analysis of the sorted PMNs (P2) shows removal of autofluorescent cells. (F) The positions of H4 receptor PCR products (left) and β-actin PCR products (right) in not-sorted and sorted PMNs are indicated by arrows (right) in each.
The biological significance of the H4 receptor in the negative regulation of PMN degranulation is currently unknown. One possible role that could be ascribed to the H4 receptor is in the resolution of inflammation. Indeed, PMNs produce histamine upon exposure to gram-negative bacteria, and this is driven by engagement of TLR4 [38]. Histamine produced by PMNs in response to bacteria could represent an important signal to halt PMN degranulation and production of ROS to avoid tissue damage at a time when pathogens have been cleared. Hence, pharmacological activation of the H4 receptor may be used as a strategy to reduce PMN-driven chronic and acute inflammation, whereas antagonists of the H4 receptor are therapeutic molecules for the treatment of itch and allergic asthma [7].
ACKNOWLEDGMENTS
This work was supported by the Faculty of Medicine, Dentistry, and Health Sciences, Queen's University of Belfast (to K.D. and M.E.) and by the FP7 European Cooperation in Science and Technology (COST) Action (BM0806; to T.P., M.K., M.E., and K.D.). T.P. was part of an exchange program between the Slovak Academy of Sciences in Bratislava (Slovakia) and Queen's University Belfast (UK). T.P. has been awarded a Young Investigator Award by the European Histamine Research Society. T.S. was supported by the European Community Action Scheme for the Mobility of University Students (ERASMUS) program and was part of an exchange program between Queen's University Belfast and the University of Fulda (Germany). This study was funded, in part, by the Royal College of Anaesthesia (UK; to M.K. and P.L.C.) and Grant Projects APVV-0052-10 and CZ.1.07/2.3.00/30.0030 (to T.P.).
We thank the blood donors from the Centre of Infection and Immunity, Queen's University Belfast. We thank Nancy Hogg (London Research Institute, UK) for the gift of the mAb24. Stepan Gambaryan (University of Würzburg, Germany) is acknowledged for his contribution to the study of p38 MAPK. Lauren Kerrigan carried out degranulation assays as part of her honors project. Päivi Järvinen (Ludwig-Maximilians-Universität München, Germany) is acknowledged for providing samples during the revision of the manuscript and Ralf Zenke (core facility of the Max Planck Institute of Biochemistry, Martinsried, Germany) for performing autofluorescence-based sorting.
Footnotes
- −/−
- knockout
- Fgr
- p58c-fgr
- Hck
- p59/61hck
- Ki
- dissociation constant
- Lyn
- p53/56lyn
- Mac-1
- macrophage 1 antigen
- OD
- optical density
- p
- phosphorylated
- PMN
- polymorphonuclear neutrophil
- PVDF
- polyvinylidene difluoride
- ROS
- reactive oxygen species
AUTHORSHIP
K.D., T.P., V.J., C.M., D.C., V.B., M.K., and T.S. performed experiments. R.L.T. and P.L.C. provided experimental tools. K.D. and M.E. designed the study and supervised the work. K.D. wrote the manuscript. R.L.T., P.L.C., and M.E. contributed to the writing of the manuscript. All authors read and approved the final manuscript.
DISCLOSURES
The authors declare no conflict of interest.
REFERENCES
- 1. Ley K., Laudanna C., Cybulsky M. I., Nourshargh S. (2007) Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat. Rev. Immunol. 7, 678–689. [DOI] [PubMed] [Google Scholar]
- 2. Diamond M. S., Springer T. A. (1993) A subpopulation of Mac-1 (Cd11b/Cd18) molecules mediates neutrophil adhesion to ICAM-1 and fibrinogen. J. Cell Biol. 120, 545–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sengeløv H., Kjeldsen L., Diamond M. S., Springer T. A., Børregaard N. (1993) Subcellular localization and dynamics of Mac-1 (α M β 2) in human neutrophils. J. Clin. Invest. 92, 1467–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sheppard F. R., Kelher M. R., Moore E.E., Mclaughlin N. J., Baerjee A., Silliman C. (2005) Structural organization of the neutrophil NADPH oxidase: phosphorylation and translocation during priming and activation. J. Leukoc. Biol. 78, 1025–1042. [DOI] [PubMed] [Google Scholar]
- 5. Mócsai A., Ligeti E., Lowell C. A., Berton G. (1999) Adhesion-dependent degranulation of neutrophils requires the Src family kinases Fgr and Hck. J. Immunol. 162, 1120–1126. [PubMed] [Google Scholar]
- 6. Mócsai A., Jakus Z., Vantus T., Berton G., Lowell C. A., Ligeti E. (2000) Kinase pathways in chemoattractant-induced degranulation of neutrophils: the role of p38 mitogen-activated protein kinase activated by Src family kinases. J. Immunol. 164, 4321–4331. [DOI] [PubMed] [Google Scholar]
- 7. Thurmond R. L., Gelfand E., Dunford P. (2008) The role of histamine H1 and H4 receptors in allergic inflammation: the search for new antihistamines. Nat. Rev. 7, 41–53. [DOI] [PubMed] [Google Scholar]
- 8. Jones B. L., Kearns G. L. (2011) Histamine: new thoughts about a familiar mediator. Clin. Pharmacol. Ther. 89,189–197. [DOI] [PubMed] [Google Scholar]
- 9. De Esch I. J., Thurmond R. L., Jongejan A., Leurs R. (2005) The histamine H4 receptor as a new therapeutic target for inflammation. Trends Pharmacol. Sci. 26, 462–469. [DOI] [PubMed] [Google Scholar]
- 10. Ling P., Ngo K., Nguyen S., Thurmond R. L., Edwards J. P., Karlsson L., Fung-Leung W-P. (2004) Histamine H4 receptor mediates eosinophil chemotaxis with cell shape change and adhesion molecule upregulation. Br. J. Pharmacol. 142, 161–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Desai P., Thurmond R. L. (2011) Histamine H4 receptor activation enhances LPS-induced IL-6 production in mast cells via ERK and PI3K activation. Eur. J. Immunol. 41, 1764–1773. [DOI] [PubMed] [Google Scholar]
- 12. Leite-De-Moraes M. C., Diem S., Michel M.-L., Ohtsu H., Thurmond R. L., Schmeider E., Dy M. (2009) Histamine receptor H4 activation positively regulates in vivo IL-4 and IFN-γ production by invariant NKT cells. J. Immunol. 182, 1233–1236. [DOI] [PubMed] [Google Scholar]
- 13. Wescott S., Kaliner M. (1983) Histamine H-1 binding site on human polymorphonuclear leukocytes. Inflammation 7, 291–300. [DOI] [PubMed] [Google Scholar]
- 14. Petty R., Francis J. W. (1986) Polymorphonuclear leukocyte histamine receptors; occurrence in cell surface clusters and their redistribution during locomotion. Proc. Natl. Acad. Sci. USA 83, 4332–4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Seligmann B. E., Fletcher M. P., Gallin J. I. (1983) Histamine modulation of human neutrophil oxidative metabolism, locomotion, degranulation, and membrane potential changes. J. Biol. Chem. 130, 1902–1909. [PubMed] [Google Scholar]
- 16. Busse W. W., Sosman J. (1976) Histamine inhibition of neutrophil lysosomal enzyme release: an H2 histamine receptor response. Science 194, 737–738. [DOI] [PubMed] [Google Scholar]
- 17. Flamand N., Plante H., Picard S., Laviolette M., Borgeat P. (2004) Histamine-induced inhibition of leukotriene biosynthesis in human neutrophils: involvement of the H2 receptor and cAMP. Br. J. Pharmacol. 141, 552–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Oda T., Morikawa N., Saito Y., Masuho Y., Matsumoto S-I. (2000) Molecular cloning and characterization of a novel type of histamine receptor preferentially expressed in leukocytes. J. Biol. Chem. 275, 36781–36786. [DOI] [PubMed] [Google Scholar]
- 19. Van Rijn R. M., Van Marle A., Chazot P. L., Langemeijer E., Qin Y., Shenton F. C., Lim H. D., Zuiderveld O. P., Sansuk K., Dy M., Smit M., Tensen C. P., Bakker R. A., Leurs R. (2008) Cloning and characterization of dominant negative splice variants of the human histamine H4 receptor. Biochem. J. 414, 121–131. [DOI] [PubMed] [Google Scholar]
- 20. Jablonowski J. A., Grice C. A., Chai W., Dvorak C. A., Venable J. D., Kwok A. K., Ly K. S., Wei J., Baker S. M., Desai P. J., Jiang W., Wilson S. J., Thurmond R. L., Karlsson L., Edwards J. P., Lovenberg T. W., Carruthers N. I. (2003) The first potent and selective non-imidazole human histamine H4 receptor antagonists. J. Med. Chem. 46, 3957–3960. [DOI] [PubMed] [Google Scholar]
- 21. Cowden J. M., Zhang M., Dunford P. J., Thurmond R. L. (2010) The histamine H4 receptor mediates inflammation and pruritus in Th2-dependent dermal inflammation. J. Invest. Dermatol. 130, 1023–1033. [DOI] [PubMed] [Google Scholar]
- 22. Yu F., Wolin R. L., Wei J., Desai P. J., Mcgovern P. M., Dunford P. J., Karlsson L., Thurmond R. L. (2010) Pharmacological characterization of oxime agonists of the histamine H4 receptor. J. Receptor Ligand Channel Res. 3, 37–49. [Google Scholar]
- 23. Deevi R. K., Koney-Dash M., Kissenpfennig A., Johnston J. A., Schuh K., Walter U., Dib K. (2010) Vasodilator-stimulated phospho-protein (VASP) regulates inside-out signaling of β2 integrins in neutrophils. J. Immunol. 184, 6575–6584. [DOI] [PubMed] [Google Scholar]
- 24. Koney-Dash M., Deevi R. K., Mcfarlane C., Dib K. (2011) Exchange protein directly activated by cAMP1 (EPAC1) is expressed in human neutrophils and mediates cAMP-dependent activation of the monomeric GTPase Rap1. J. Leukoc. Biol. 90, 741–749. [DOI] [PubMed] [Google Scholar]
- 25. Ikawa Y., Suzuki M., Shiono S., Ohki E., Moriya H., Negishi E., Ueno K. (2005) Histamine H4 receptor expression in human synovial cells obtained from patients suffering from rheumatoid arthritis. Biol. Pharm. Bull. 28, 2016–2018. [DOI] [PubMed] [Google Scholar]
- 26. Dorward D. A., Lucas C. D., Alessandri A. L., Marwick J. A., Rossi F., Dransfield I., Haslett C., Dhaliwal K., Rossi A. G. (2013) Autofluorescence-based sorting: rapid and nonperturbing isolation of ultrapure neutrophils to determine cytokine production. J. Leukoc. Biol. 94, 193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gschwandtner M., Rossbach K., Dijkstra D., Baumer W., Kietzmann M., Stark H., Werfel T., Gutzmer R. (2010) Murine and human Langerhans cells express a functional histamine H4 receptor: modulation of cell migration and function. Exp. Allergy Immunol. 65, 840–849. [DOI] [PubMed] [Google Scholar]
- 28. Dransfield I., Hogg N. (1989) Regulated expression of Mg2+ binding epitope on leukocyte integrin α subunit. EMBO J. 8, 3759–3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Berton G., Laudanna C., Sorio C., Rossi F. (1989) Generation of signals activating neutrophil functions by leukocyte integrins: LFA-1 and gp150/95, but not CR3, are able to stimulate the respiratory burst of human neutrophils. J. Cell Biol. 116, 1007–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nathan C. F. (1987) Neutrophil activation on biological surfaces. Massive secretion of hydrogen peroxide in response to products of macrophages and lymphocytes. J. Clin. Invest. 80, 1550–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Meyer Zu Heringdorf D., Liedel K., Kaldenberg-Stasch S., Michel M. C., Jakobs K. H., Wieland T. (1996) Translocation of microfilament-associated inhibitory guanine-nucleotide-binding proteins to the plasma membrane in myeloid differentiated human leukemia (HL-60) cells. Eur. J. Biochem. 235, 670–676. [DOI] [PubMed] [Google Scholar]
- 32. Tomhave E. D., Richardson R. M., Didsbury J. R., Menard L., Snyderman R., Ali H. (1994) Cross-desensitization of receptors for peptide chemoattractants. Characterization of a new form of leukocyte regulation. J. Immunol. 153, 3267–3275. [PubMed] [Google Scholar]
- 33. Zaslaver A., Feniger-Barish R., Ben-Baruch A. (2001) Actin filaments are involved in the regulation of trafficking of two closely related chemokine receptors, CXCR1 and CXCR2. J. Immunol. 166, 1272–1284. [DOI] [PubMed] [Google Scholar]
- 34. Thurmond R. L., Desai P. J., Dunford P. J., Fung-Leung W-P., Hofstra C. L., Jiang W., Nguyen S., Riley J. P., Sun S., Williams K. N., Edwards J. P., Karlsson L. (2004) A potent and selective histamine H4 receptor antagonist with anti-inflammatory properties. J. Pharmacol. Exp. Therapeutics 309, 404–413. [DOI] [PubMed] [Google Scholar]
- 35. Francis J. W., Todd R. F., Boxer L. A., Petty H. R. (1991) Histamine inhibits cell spreading and C3bi receptor clustering and diminishes hydrogen peroxide production by adherent human neutrophils. J. Cell. Physiol. 147, 128–137. [DOI] [PubMed] [Google Scholar]
- 36. Jenei V., Deevi R. K., Adams C. A., Axelsson L., Hirst D. G., Andersson T., Dib K. (2006) Nitric oxide produced in response to engagement of β2 integrins on human neutrophils activates the monomeric GTPases Rap1 and Rap2 and promotes adhesion. J. Biol. Chem. 281, 35008–35020. [DOI] [PubMed] [Google Scholar]
- 37. Pivot-Pajot C., Chouinard F. C., Amine El Azreq M., Harbour D., Bourgoin S. G. (2010) Characterization of degranulation and phagocytic capacity of a human neutrophilic cellular model, PLB-985 cells. Immunobiology 215, 38–52. [DOI] [PubMed] [Google Scholar]
- 38. Smuda C., Wechsler J. B., Bryce P. J. (2011) TLR-induced activation of neutrophils promotes histamine production via a PI3 kinase-dependent mechanism. Immunol. Lett. 141, 102–108. [DOI] [PMC free article] [PubMed] [Google Scholar]