L-plastin participates in NKG2D clustering and NKG2D-mediated inhibition of NK cell chemotaxis.
Keywords: membrane rafts, chemotaxis
Abstract
Membrane rafts are microdomains of the plasma membrane that have multiple biological functions. The involvement of these structures in the biology of T cells, namely in signal transduction by the TCR, has been widely studied. However, the role of membrane rafts in immunoreceptor signaling in NK cells is less well known. We studied the distribution of the activating NKG2D receptor in lipid rafts by isolating DRMs in a sucrose density gradient or by raft fractionation by β-OG-selective solubility in the NKL cell line. We found that the NKG2D-DAP10 complex and pVav are recruited into rafts upon receptor stimulation. Qualitative proteomic analysis of these fractions showed that the actin cytoskeleton is involved in this process. In particular, we found that the actin-bundling protein L-plastin plays an important role in the clustering of NKG2D into lipid rafts. Moreover, coengagement of the inhibitory receptor NKG2A partially disrupted NKG2D recruitment into rafts. Furthermore, we demonstrated that L-plastin participates in NKG2D-mediated inhibition of NK cell chemotaxis.
Introduction
Membrane rafts (also called “lipid rafts”) are microdomains of the plasma membrane that are enriched in cholesterol, glycolipids, phospholipids, and other lipids containing preferentially saturated fatty acids. These domains also contain lipid-modified signaling proteins, phospholipid-binding proteins, and transmembrane proteins, most of them palmitoylated [1]. Membrane rafts have been proposed as being important platforms for the enrichment of molecules involved in signal transduction and other aspects of leukocyte biology. The importance of these structures was demonstrated in a number of studies in T lymphocytes [2]. As a result of their specific lipid composition, membrane rafts are relatively resistant to certain detergents (e.g., Triton X-100; some of the Brij series) and upon solubilization, produce DRM complexes that are believed to correspond more or less exactly to the raft microdomains in intact membranes. However, generally, DRMs should not be equated with membrane rafts because of various, possible detergent-induced artifacts. With this reservation in mind, DRMs are widely used as an approximate biochemical equivalent of membrane rafts.
The actin cytoskeleton is functionally, closely related to lipid rafts, as it controls membrane raft dynamics, formation, and maintenance of these membrane microdomains [3]. The majority of studies in this area has focused on the role of membrane rafts in T cell activation. However, less is known about these compartments in NK cells. The final outcome of NK cell–target interaction is determined by the integration of activating and inhibitory signals delivered by a wide repertoire of receptors. NK cell cytotoxicity has been shown to require CD2-associated protein, a protein that localizes into lipid rafts [4]. The phosphorylation of ERK and the production of IFN-γ in NK cells are enhanced by the recruitment of IL-12R and FcγRIIIa to membrane rafts [5]. Moreover, the adhesion between the NK cell and the target cell, based on the LFA-1–ICAM-1 interaction, results in Vav1 phosphorylation, inducing cytoskeletal rearrangements that lead to clustering of 2B4 in lipid rafts. Coengagement of 2B4 with LFA-1 induces activation of NK cell cytotoxicity, and activation of a MHC-I inhibitory receptor abrogates 2B4 recruitment, thereby preventing NK cell activation [6]. Ligation of inhibitory receptors on NK cells results in the immobilization of the receptors at the contact sites, accompanied by lipid raft exclusion from these areas. For instance, ligation of the CD94/NKG2A inhibitory receptor leads to polarization and stabilization of the receptor at the contact site but does not associate with lipid rafts, thus impeding the development of activating signals [7]. Consistent with this, it has been described that inhibitory signaling via NKG2A disrupts raft recruitment and actin reorganization at the IS, thus impairing NK cell activation [8]. Furthermore, the killer cell Ig-like inhibitory receptor is able to block actin-dependent recruitment of the activating receptor 2B4 to lipid rafts, preventing the initiation of activating, signaling cascades [9].
The role of membrane rafts in NKG2D-mediated signaling has not been fully explored. It has been demonstrated that NKG2D is recruited to DRM domains upon receptor activation and that inhibitory signals triggered by the CD94/NKG2A receptor can prevent NKG2D association with membrane rafts and the formation of the IS [10]. Thus, interaction of membrane rafts with cytoskeleton is important for NKG2D-mediated NK cell activation.
In this study, we analyze the raft proteome following NKG2D activation in NK cells. We demonstrate that the actin-bundling protein L-plastin participates in NKG2D receptor clustering upon activation and that its disruption affects NKG2D-mediated regulation of NK cell chemotaxis.
MATERIALS AND METHODS
Cell culture and receptor activation
The human NKL cell line was kindly donated by Dr. Jerome Ritz (Dana-Farber Cancer Institute, Boston, MA, USA) and cultured as described elsewhere [11]. The C1R (human B cell lymphoma) cell line was grown in RPMI-1640 medium plus 10% heat-inactivated FBS, 100 U/ml penicillin, 100 μg/mL streptomycin, and 25 ng/mL amphoterycin (PAA, Pasching, Austria). The C1R class I-negative cell line stably transfected with the NKG2D ligand MICA*004 (C1R-MICA*004) [12] was maintained in complete medium, supplemented with hygromycin B at 800 μg/ml (Invitrogen, Carlsbad, CA, USA). Human NK cells were isolated from PBMC by negative selection using a MACS NK-cell isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany). The purity was tested by flow cytometry, and cells were used when purity was >95%. Human NK cells were cultured for 48 h with 50 U/mL IL-2 (Roche, Mannheim, Germany).
For detergent extraction, NKL or human NK cells were stimulated with 10 μg/mL anti-NKG2D antibody (R&D Systems, Minneapolis, MN, USA), 10 μg/mL anti-NKG2A antibody (R&D Systems), or control IgG (Jackson ImmunoResearch, Suffolk, UK) in medium on ice for 30 min, and then 2 μg/mL goat anti-mouse antibody was added, and cells were transferred to 37°C for 5 min. For chemotaxis assays, human NK cells were stimulated with 10 μg/mL plate-bound mAb against NKG2D, or IgG as a control.
Density gradient ultracentrifugation
Cells (5×107) were solubilized in 50 mM Tris, pH 7.5, 150 mM NaCl and 1% Brij 98 containing phosphatases and protease inhibitors (10 mM NaF, 2 mM EDTA, 1 mM Pefabloc, 1 mM Na3VO4, and 5 mM iodoacetamide) for 30 min on ice. Lysates were mixed 1:1 with 80% sucrose and placed at the bottom of a polyallomer centrifuge tube (Beckman Coulter, Palo Alto, CA, USA) and then overlaid carefully with 35% sucrose and lysis buffer. Samples were subjected to ultracentrifugation (50,000 g/18 h/2°C). Eight fractions (0.5 mL each) were collected from the top of the gradient and analyzed by Western blot or subjected to proteomic analysis.
Gel-filtration chromatography
Gel-filtration experiments were performed using Sepharose 4B (Sigma-Aldrich, St. Louis, MO, USA), as described previously [13].
Raft fractionation by β-OG-selective solubility
After stimulation, NKL or human NK cells were lysed in 25 mM Tris, pH 7.4, 150 mM NaCl, and 0.2% Triton X-100 containing protease and phosphatase inhibitors, and then the pellet fraction was solubilized with 1% Triton X-100 plus 60 mM β-OG (a raft-disrupting detergent preserving protein–protein interaction) at 37°C to analyze surface rafts associated with the cytoskeleton. Solubilized raft components were separated from nuclei and protein cytoskeletal network by centrifugation. The raft fractions were then subjected to Western blot or proteomic analysis.
Protein digestion and MS
Membrane raft fractions from sucrose density gradient ultracentrifugation experiments were precipitated in methanol-chloroform [14], subjected to SDS-PAGE using a 12.5% gel, and stained with Coomassie blue (Bio-Rad, Hercules, CA, USA). Protein bands were excised in 10 pieces of the gel. Proteins were reduced with 10 mM DTT, alkylated with 50 mM iodoacetamide, and trypsin-digested. The peptides were analyzed by LC-MS/MS in a QTRAP MS (Applied Biosystems, Foster City, CA, USA), coupled to a nano-HPLC (Nano LC UltiMate; LC Packings, Dionex, Sunnyvale, CA, USA). The same procedure was followed with the raft fractions obtained by solubilization in β-OG. All MS data sets were searched using the MASCOT search engine (v.2.4.01; Matrix Science, London, UK) under the Homo sapiens taxonomy. Trypsin was selected as the enzyme (one missed cleavage allowed). The peptide mass tolerance was set at ±0.5 amu, and the fragment mass tolerance was set at ±0.3 amu. The MASCOT score obtained for each protein was >32, indicating identity or extensive homology at a significance level of P < 0.05. Protein functions and characteristics were obtained from UniProt Knowledgebase (www.expasy.org).
Western blot
The samples subjected to SDS-PAGE were transferred to the Immobilon-P membrane (Millipore, Billerica, MA, USA). Blots were developed with the ECL detection system (Amersham, GE Healthcare, Munich, Germany). The following antibodies were used: anti-DAP10, anti-pVav, anti-L-plastin, and anti-GAPDH (Santa Cruz Biotechnology, Santa Cruz, CA, USA); anti-cofilin (Abcam, Cambridge, UK); anti-ERM (Cell Signaling Technology, Danvers, MA, USA); anti-Annexin-2 (BD Biosciences, Erembodegem, Belgium); and anti-NKG2D (clone 3.1.1.1; Millipore). Secondary antibodies (HRP-conjugated) were from Dako (Glostrup, Denmark).
RNA interference
Human NK cells and NKL cells were transfected using specific ON-TARGETplus SMARTpool siRNA for L-plastin (L-011716-00-0005) or ON-TARGETplus Non-targeting Pool (Dharmacon, Thermo Fisher Scientific, Lafayette, CO, USA), according to the manufacturer's instructions. Cell viability was determined by flow cytometry using 7-AAD (Immunostep, Salamanca, Spain), and dead cells were removed by centrifugation before each experiment. The effects of siRNA on levels of L-plastin expression were analyzed by Western blot, and immunoblot with GAPDH was included as a loading control.
Degranulation assays and IFN-γ production
Human NK cells transfected with control siRNA or L-plastin siRNA were incubated with C1R or C1R-MICA target cells at different E:T ratios. Monoclonal CD107a antibody conjugated to allophycocyanin was added to the wells. After 1 h, brefeldin A and monensin (BioLegend, San Diego, CA, USA) were added for an additional 5 h. After the culture, cells were stained with PE-conjugated anti-CD16 and anti-CD56 (BioLegend). Cells were fixed, permeabilized, and stained with intracellular IFN-γ-FITC (BioLegend). Isotype controls were used as negative controls. CD107a expression on NK cells and IFN-γ production were analyzed by BD FACSCalibur with CellQuest Pro software (BD Biosciences, San Jose, CA, USA).
Migration assays
All assays were performed in Transwell chambers (6.5 mm diameter, 3 μm pore; Costar, Corning, NY, USA). NK cells (3×105–5×105) were incubated with plate-bound antibodies and then removed from the plates and allowed to migrate through transwell membranes coated with 10 μg/mL fibronectin (Sigma-Aldrich) in medium containing 50 ng/mL CXCL12 (PeproTech, London, UK). Migrated NK cells were counted after 2.5 h in a Casy Counter (Roche Innovatis AG, Reutlingen, Germany).
F-Actin content
F-actin levels were determined by flow cytometry using TRITC-phalloidin (Sigma-Aldrich), as described previously [15].
Statistical analysis
Differences in the observed means between two groups were analyzed with Student's t-test. Comparisons among more than two groups were analyzed using a general linear model ANOVA, with treatments (IgG; mAb against NKG2D and NKG2A, alone or in combination) and/or pretreatments (transfections with siRNA) as fixed factors and experiments as a random factor. Interactions between the factors were considered. When significant differences were found, treatments were compared using the post hoc Duncan test. Values are expressed as mean + sem. Statistical significance was concluded for values of P < 0.05.
RESULTS
NKG2D-DAP10 complex is recruited to DRMs upon ligation
Signaling downstream of the NKG2D receptor involves the phosphorylation of the tyrosine-isoleucine-asparagine-methionine motif of the adaptor protein DAP10 [16]. This process is analogous to the phosphorylation of the ITAM-containing receptors by Src kinases present in membrane rafts. Thus, it was of interest to gain insight into the function of these structures in NKG2D-mediated signaling in NK cells.
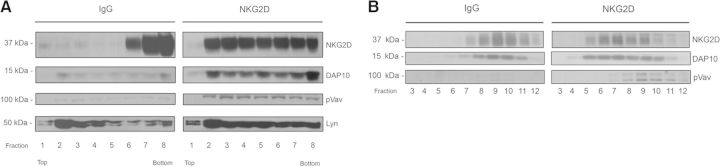
To study the distribution of NKG2D and its adaptor protein in lipid rafts, we separated DRM fractions upon stimulation in a sucrose density gradient. As large numbers of cells are required for this approach, we used the NKL cell line. We found that antibody-mediated cross-linking apparently induces the recruitment of the NKG2D receptor into rafts, as it is recovered in the low-density DRM fractions, whereas under control conditions, most of NKG2D is located in the lowest fractions of the gradient (Fig. 1A). The adaptor protein DAP10 also associates with DRMs after NKG2D activation. Moreover, the pVav, which regulates actin polymerization and clustering of NKG2D [10], was found in the low-density fractions upon NKG2D activation. In contrast, only a small fraction of pVav was detected under control conditions. Furthermore, similar results were obtained from gel filtration experiments on Sepharose 4B, as the receptor, DAP10, and pVav were all recovered in large detergent-resistant complexes upon lysis in Brij 98 (Fig. 1B).
Figure 1. Recruitment of the NKG2D–DAP10 complex and pVav to DRMs upon NKG2D activation.
(A) NKL cells were stimulated by anti-NKG2D cross-linking or an isotype control (IgG) and lysed. The DRM fractions were then isolated by sucrose gradient ultracentrifugation. Fractions were analyzed by Western blot using the indicated antibodies; Lyn served as a control for the membrane fraction. (B) Stimulated NKL cells were lysed and fractionated by gel filtration on Sepharose 4B. The distributions of NKG2D, DAP10, and pVav in the samples were determined by Western blot.
Proteomic analysis of DRMs upon NKG2D activation
Qualitative and quantitative proteomic approaches have improved our understanding of the proteins involved in membrane raft clustering and signaling. Indeed, several investigations have addressed the protein composition of lipid rafts in various cell lines and primary cells [17]. However, the characterization of the raft proteome in NK cells has not been fully studied. We therefore carried out a proteomic study to identify which proteins are recruited to rafts upon NKG2D activation.
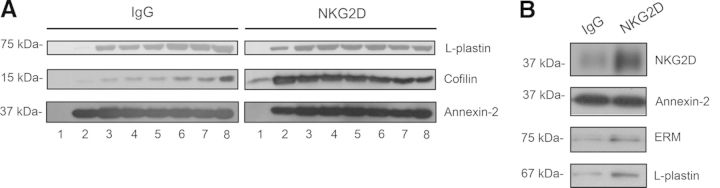
Proteins of the low-density fractions of sucrose gradient experiments were precipitated and separated by SDS-PAGE. Ten bands were then excised for subsequent in-gel digestion and analysis by LC-MS/MS. A selection of the most relevant proteins identified in all three independent experiments under control conditions and after NKG2D cross-linking is shown in Table 1. We found actin-binding proteins, including cofilin, profilin, and plastin-2. The presence of some of the proteins was confirmed by Western blot (Fig. 2A); in the case of annexin-2, a protein that participates in cytoskeleton remodeling [18], it was identified under control conditions in two out of three experiments and therefore, was not listed in Table 1. However, we could find in Western blot analysis that it is equally recruited into DRMs. Rho GDI proteins, which regulate the GDP/GTP exchange reaction of the Rho GTPases [19], have also been identified in the fractions isolated upon NKG2D cross-linking. Overall, proteins related to the actin cytoskeleton comprise the largest category of proteins identified in the DRM fractions, which suggests that the cytoskeleton may be important for organizing membrane domains upon NKG2D activation. Therefore, to investigate the rafts associated with the cytoskeleton further, raft fractionation experiments involving β-OG-selective solubility were performed. OG is a nonionic detergent that solubilizes DRMs efficiently but does not disrupt protein associations with the cytoskeleton [20]. Upon NKG2D activation, NKL cells were lysed, and the raft fractions were solubilized with Triton X-100 plus β-OG. Proteins of the isolated fractions were separated by SDS-PAGE and then digested as indicated above. Table 2 depicts a selection of the proteins identified in all three independent experiments. We identified some of the proteins found previously in the DRM fractions, i.e., annexin-2, cofilin, profilin, and plastin-2. In addition, other proteins that regulate cytoskeleton dynamics were found. Moesin is a member of the ERM protein family. ERM proteins act as linkers between the actin cytoskeleton and the plasma membrane. The protein IQGAP1 is a key regulator of the cytoskeleton involved in the formation of actin-filament structures, such as lamellipodia and membrane ruffling [21]. α-Actinin 4 and coronin 1A are actin-binding proteins that are also crucial in a variety of intracellular structures. The Rho GTPase RhoA was also identified. Some of the proteins identified were detected by Western blot, as in DRM proteomic analysis (Fig. 2B).
Table 1. Identified Proteins in DRMs Isolated by Sucrose Gradient Ultracentrifugation.
| Code | Protein name | Molecular weight (kDa) | Score | Queries matched | % Protein |
|---|---|---|---|---|---|
| IgG | |||||
| P60709 | Actin, cytoplasmic 1 | 41.737 | 325 | 11 | 34 |
| P23528 | Cofilin-1 | 18.502 | 59 | 2 | 18 |
| P04406 | GAPDH | 36.053 | 199 | 7 | 22 |
| P13796 | Plastin-2 | 70.289 | 124 | 4 | 12 |
| Q71U36 | Tubulin α-1A chain | 50.136 | 198 | 6 | 11 |
| P07347 | Tubulin β chain | 49.671 | 143 | 9 | 24 |
| NKG2D | |||||
| P60709 | Actin, cytoplasmic 1 | 41.737 | 472 | 13 | 37 |
| P07355 | Annexin A2 | 38.604 | 62 | 3 | 10 |
| P27797 | Calreticulin | 48.182 | 105 | 4 | 14 |
| P23528 | Cofilin-1 | 18.502 | 373 | 8 | 42 |
| P04406 | GAPDH | 36.053 | 214 | 8 | 28 |
| P13796 | Plastin-2 | 70.289 | 620 | 21 | 37 |
| P07737 | Profilin-1 | 15.054 | 74 | 2 | 20 |
| P52565 | Rho GDP-dissociation inhibitor 1 | 23.207 | 42 | 2 | 11 |
| P52566 | Rho GDP-dissociation inhibitor 2 | 22.988 | 50 | 2 | 19 |
| Q71U36 | Tubulin α-1A chain | 50.136 | 265 | 11 | 37 |
| P07347 | Tubulin β chain | 49.671 | 332 | 13 | 35 |
Selection of the most relevant proteins identified in three independent experiments, indicating the molecular weight, score, number of queries matched, and percentage of the identified protein.
Figure 2. Western blot analysis of DRM fractions.
(A) Analysis of sucrose density gradient fractions of L-plastin, cofilin, and annexin-2, proteins identified in the proteomic analysis. (B) Analysis in DRM fractions isolated by β-OG-selective solubility showing the distribution of NKG2D and the proteins identified by qualitative proteomics (annexin-2, ERM, and L-plastin).
Table 2. Proteins Identified in DRM Fractions Isolated by β-OG-Selective Solubility.
| Code | Protein name | Molecular weight (kDa) | Score | Queries matched | % Protein |
|---|---|---|---|---|---|
| IgG | |||||
| P60709 | Annexin A2 | 38.604 | 285 | 12 | 41 |
| P23528 | Coronin-1A | 51.026 | 117 | 5 | 16 |
| P04406 | Galectin-1 | 14.716 | 68 | 1 | 11 |
| P13796 | Plastin-2 | 70.289 | 178 | 8 | 16 |
| P07737 | Profilin-1 | 15.054 | 93 | 3 | 31 |
| P07347 | Tubulin β chain | 49.671 | 88 | 8 | 20 |
| P08670 | Vimentin | 53.652 | 216 | 40 | 56 |
| NKG2D | |||||
| O43707 | α-Actinin-4 | 104.854 | 233 | 13 | 21 |
| P07355 | Annexin A2 | 38.604 | 399 | 16 | 49 |
| P23528 | Cofilin-1 | 18.502 | 129 | 5 | 38 |
| P31146 | Coronin-1A | 51.026 | 160 | 9 | 20 |
| P04406 | Galectin-1 | 14.716 | 73 | 2 | 18 |
| P26038 | Moesin | 67.820 | 108 | 6 | 13 |
| P13796 | Plastin-2 | 70.289 | 466 | 19 | 38 |
| P07737 | Profilin-1 | 15.054 | 206 | 6 | 42 |
| P46940 | Ras GTPase-activating-like protein IQGAP1 | 189.252 | 187 | 7 | 10 |
| P61586 | Transforming protein RhoA | 21.768 | 61 | 2 | 11 |
| O75083 | WD repeat-containing protein 1 | 66.194 | 66 | 6 | 18 |
Selection of the most relevant proteins identified in three independent experiments, indicating the molecular weight, score, number of queries matched, and percentage of the identified protein.
In both proteomic approaches, we could observe that although some proteins are found under control conditions and upon NKG2D cross-linking, the scores, the number of queries matched, and the percentage of the proteins detected were generally higher in DRM fractions isolated upon NKG2D activation. This correlated with an enrichment of some proteins in comparison with control conditions when their levels were analyzed by Western blot (i.e., L-plastin, cofilin, ERM).
L-plastin is required for NKG2D-DAP10 recruitment to lipid rafts
Our proteomic analysis revealed that many proteins involved in the actin cytoskeleton rearrangement are actively recruited into lipid rafts upon NKG2D activation. Since the cytoskeleton helps establish membrane rafts, favoring raft-associated signaling events [3], we therefore explored cytoskeletal proteins that might be involved in NKG2D recruitment to rafts. We focused on the protein plastin-2, which was identified in DRM isolation experiments and in rafts isolated by β-OG-selective solubility. Plastin-2 (also known as L-plastin or LPL) belongs to a subclass of actin-binding proteins called actin-bundling proteins. L-plastin is an isoform exclusively expressed in leukocytes [22], that is required for several immune functions, such as integrin activation in neutrophils, T cell activation, and B and T cell chemotaxis [23].
L-plastin expression was silenced in the NKL cell line using siRNA (Fig. 3A), and then raft fractionation experiments by β-OG-selective solubility were performed. Depletion of L-plastin did not affect the expression of NKG2D or DAP10 (data not shown). Raft fractions were analyzed by Western blot to detect NKG2D, DAP10, pVav, L-plastin, and annexin-2. We observed that the partial depletion of L-plastin significantly affected the levels of these proteins. The amounts of NKG2D, DAP10, and pVav recruited to DRM fractions, following receptor activation, decreased in the cells transfected with L-plastin siRNA relative to controls (P=0.012, P=0.005, and P=0.008, respectively; Fig. 3B and C). Recruitment of L-plastin to DRMs was also affected significantly (P=0.023) and was even more severe upon NKG2D cross-linking, whereas no differences were found in the levels of annexin-2. These results suggest a role for L-plastin in the clustering of the NKG2D receptor into rafts. We investigated further whether inhibitory signals could interfere with raft association of NKG2D in human NK cells transfected with L-plastin siRNA (Fig. 4A). NK cells were stimulated with anti-NKG2D, anti-NKG2A, or both together, and DRM fractions isolated by β-OG-selective solubility were analyzed by Western blot. The levels of NKG2D, DAP10, pVav, L-plastin, and annexin-2 were quantified (Fig. 4B and C). We observed no association of NKG2D with rafts upon NKG2A activation, whereas simultaneous engagement of NKG2D and NKG2A partially abrogated NKG2D recruitment to rafts. Reduction of L-plastin levels significantly affected receptor association with DRMs depending on the experimental condition (P<0.001). The post hoc analysis showed that knockdown of L-plastin impaired NKG2D recruitment upon NKG2D cross-linking and upon costimulation of NKG2A, whereas it did not alter receptor levels upon NKG2A activation and under control conditions. We observed similar results for DAP10 (P<0.001) and pVav (P<0.001). In the case of L-plastin, its levels decreased under all experimental conditions (P<0.001); a post hoc Duncan's test revealed that this reduction was more severe upon NKG2D activation and after coengagement of NKG2A.
Figure 3. L-plastin in NKG2D recruitment to rafts.
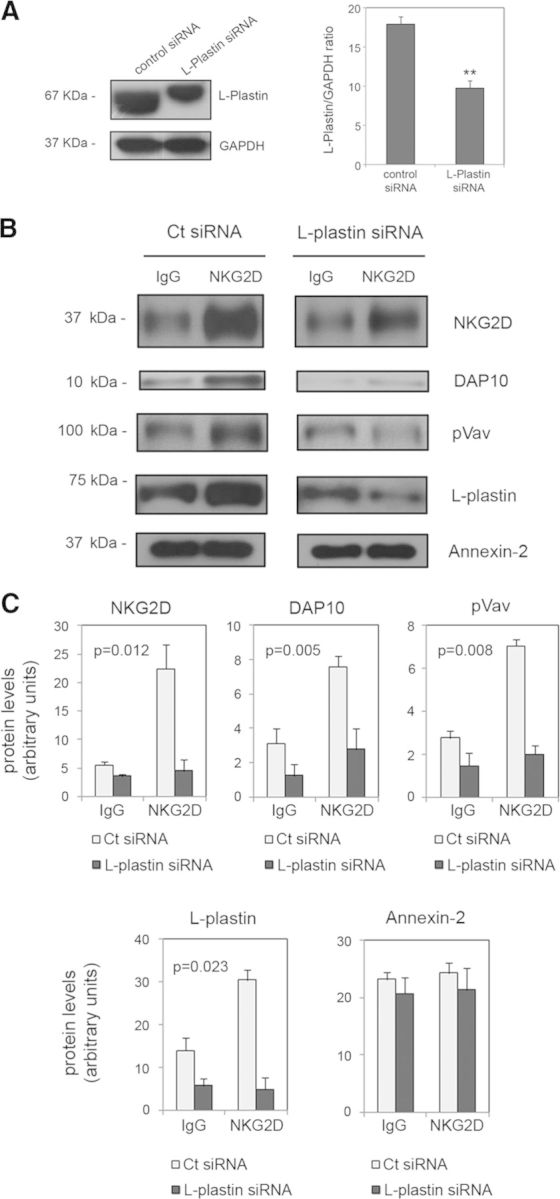
(A) NKL cells were transfected with control or L-plastin siRNA, and levels of L-plastin expression were analyzed by Western blot. Anti-GAPDH blots were included as a protein-loading control. The graph shows the mean and sem of three independent experiments (**P<0.005; Student's t-test). (B) Twenty-four hours after transfection, DRM fractionation by β-OG-selective solubilization upon NKG2D activation was performed. DRM fractions were subjected to SDS-PAGE, and NKG2D, DAP10, pVav, L-plastin, and annexin-2 were detected by Western blot. (C) Protein levels were quantified by densitometry in control (Ct) siRNA- and L-plastin siRNA-transfected cells. The graphs show the mean and sem of three independent experiments. Data were examined by ANOVA. The statistical significance for the effect of L-plastin depletion upon receptor activation on protein levels is shown in each graph.
Figure 4. Coengagement of NKG2A partially impairs NKG2D recruitment into rafts.
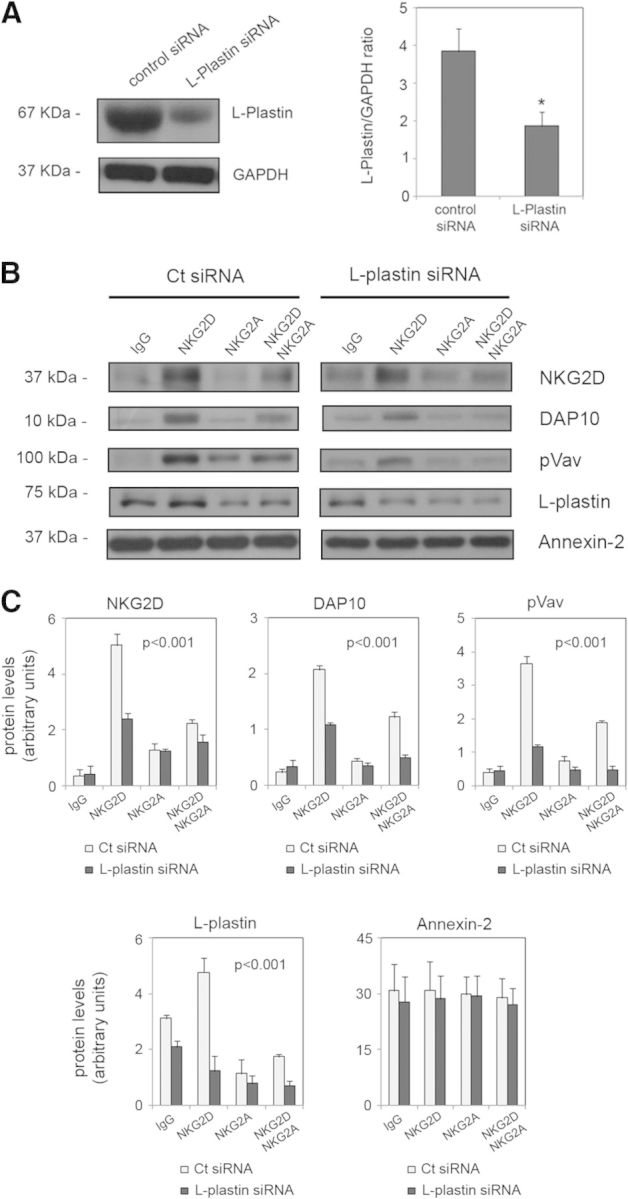
(A) Human NK cells were transfected with control or L-plastin siRNA, and levels of L-plastin expression were analyzed by Western blot. Anti-GAPDH blots were included as a protein-loading control. The graph shows the mean and sem of three independent experiments (*P<0.05; Student's t-test) (B) Twenty-four hours after transfection, DRM fractionation by β-OG-selective solubilization, upon NKG2D and NKG2A activation, alone or in combination, was performed. DRM fractions were subjected to SDS-PAGE, and NKG2D, DAP10, pVav, L-plastin, and annexin-2 were detected by Western blot. (C) Protein levels were quantified by densitometry in control siRNA- and L-plastin siRNA-transfected cells. The graphs show the mean and sem of three independent experiments. The effect of L-plastin depletion upon activation was assessed by ANOVA and the Duncan post hoc test. The statistical significance is shown in each graph.
L-plastin is involved in NKG2D-mediated NK cell chemotaxis
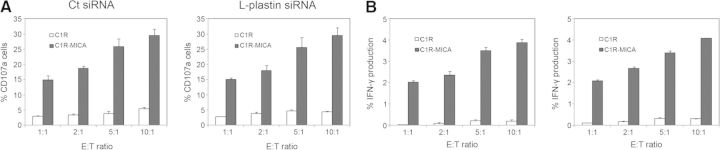
L-plastin contributes to important functions in cells of innate and adaptive immunity. In T cells, L-plastin has been described as an important component in cell activation and motility [24–26]. However, its implication in NK cell function has been poorly studied. Because knockdown of L-plastin expression negatively affected NKG2D clustering upon activation, we next investigated whether L-plastin could alter NKG2D function. We quantified CD107a expression to determine NK cell functional activity [27, 28] and the intracellular IFN-γ production by flow cytometry. Human NK cells transfected with control siRNA or L-plastin siRNA were incubated with the C1R class I-negative cell line stably transfected with the NKG2D ligand MICA*004 (C1R-MICA*004) for 6 h at different E:T ratios. As control cells, the C1R cell line transfected with the vector alone was used. We found no differences in degranulation assays in L-plastin-transfected cells compared with NK cells transfected with control siRNA, as similar percentages of NK cells expressing CD107a were found when coincubated with C1R-MICA*004 cells (Fig. 5A). IFN-γ production was not affected significantly by knockdown of L-plastin either, indicating that the partial depletion of this protein is not sufficient to affect NKG2D-mediated function (Fig. 5B).
Figure 5. Knockdown of L-plastin in CD107a degranulation assays and IFN-γ production.
Human NK cells cultured for 48 h in the presence of IL-2 were transfected with control siRNA or L-plastin siRNA. Cells were incubated for 6 h with C1R or C1R-MICA target cells at different E:T ratios, and the expression of CD107a (A) and IFN-γ production (B) was determined by flow cytometry. Results are represented as mean values of percentages and sem of two independent experiments.
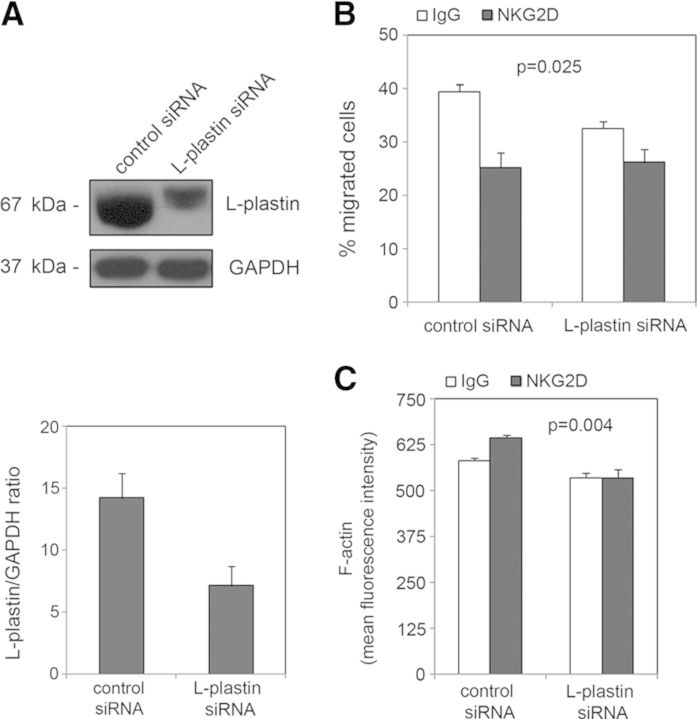
It has been reported recently that L-plastin regulates CXCL12-induced T cell migration [26], and we previously found that NKG2D activation impairs NK cell chemotaxis toward a CXCL12 gradient [29]. Nevertheless, specific roles of membrane rafts in NK cell migration have not been studied so far. The knowledge that knockdown of L-plastin expression negatively affects NKG2D clustering upon activation prompted us to investigate whether L-plastin could regulate NKG2D-mediated NK cell migration. To this end, L-plastin expression was knocked down in human primary NK cells isolated from peripheral blood using siRNA (Fig. 6A). Cells were activated with plate-bound IgG, and anti-NKG2D antibodies and chemotaxis assays toward CXCL12 were then performed (Fig. 6B). We observed that reduction of L-plastin expression affected the migratory ability depending on the experimental condition (P=0.025). The partial depletion of L-plastin impaired the migratory ability of control-treated cells. NKG2D activation inhibited NK cell chemotaxis in cells transfected with control siRNA, consistent with our previous study. Conversely, activation of NKG2D in cells transfected with L-plastin siRNA did not affect the chemotaxtic response, as the percentage of inhibition decreased from 35.9% to 19.4%. Given that binding of L-plastin to actin has been proposed as stabilizing and protecting F-actin from depolymerization [30], we explored whether L-plastin knockdown affected F-actin content. Levels of F-actin in human NK cells, transfected with control or L-plastin siRNA, were determined by flow cytometry (Fig. 6C). Reduction of L-plastin levels affected the F-actin content (P=0.004). Thus, depletion of L-plastin resulted in a significant decrease in the mean fluorescence intensity of F-actin, and this reduction was greater upon NKG2D activation.
Figure 6. Role of L-plastin in NKG2D-mediated cell migration.
(A) Human NK cells were transfected with siRNA targeting L-plastin or a siRNA control. L-plastin expression levels were analyzed by Western blot, and anti-GAPDH blots were included as protein-loading controls. (B) Twenty-four hours after transfection, cells were incubated with anti-NKG2D or IgG, and their ability to migrate toward a CXCL12 gradient was assayed. Data shown are the mean and sem of two replicates pooled from three independent experiments. The effect of L-plastin depletion upon activation was assessed by ANOVA (P=0.025). (C) F-actin content was measured in human NK cells transfected with siRNA for L-plastin or a siRNA control. After activations, cells were fixed, permeabilized, and stained with phalloidin-TRITC, and the mean fluorescence intensity was determined. Results indicate the mean and sem of three independent experiments. Data were examined by ANOVA. Statistical significance for the effect of L-plastin depletion upon receptor activation on F-actin content was P=0.004.
DISCUSSION
In this study, we describe the involvement of the actin-bundling protein L-plastin in the clustering of the activating receptor NKG2D into lipid rafts and in the NKG2D-mediated inhibition of NK cell chemotaxis.
In NK cells, the recruitment of activating receptors into membrane rafts is required to ensure their correct organization at the site of target cell contact and thereby, NK cell effector functions. Prevention of this recruitment by inhibitory receptors is known to impair NK cell activation [6–9, 31, 32]. The association of the actin cytoskeleton with membrane rafts is essential for their structure and function and maintains the clustering of the raft-associated proteins [3].
We explored the role of lipid rafts in NKG2D-mediated signaling in more detail. This receptor is recruited to DRMs upon activation [10]. We show that the NKG2D-DAP10 complex localized in DRMs together with the phosphorylated form of a protein that is activated downstream of NKG2D and is essential for raft clustering [6, 33]. To understand the overall dynamic changes of proteins within lipid rafts upon NKG2D activation in NK cells, we analyzed the lipid raft proteome using LC-MS/MS. We found several proteins to be involved in actin cytoskeleton dynamics, as shown previously by other proteomic analyses in a variety of cell types [34–38]. Thus, our results indicate the existence of interactions between the actin cytoskeleton and membrane rafts.
To analyze these interactions further, we performed raft fractionation by β-OG-selective solubilization to dissolve DRMs without disrupting the protein associations with the cytoskeleton. We found additional cytoskeletal proteins that support a role of the actin cytoskeleton in NKG2D recruitment to rafts. Moreover, some of the proteins identified are closely related to Rho GTPases, as Rho GDI proteins or IQGAP1, which interacts with Rac1 and Cdc42 and controls actin polymerization through the activation of N-WASp [39]. In addition, we reported previously that the activity of Rac1 and Cdc42 increased upon NKG2D stimulation and that WASp and N-WASp are involved in NKG2D-mediated inhibition of NK cell chemotaxis [29].
A close interaction between rafts and the actin cytoskeleton is necessary for T cell activation [40–42]. We explored whether the cytoskeletal proteins identified in our analysis could be actively involved in NKG2D recruitment into rafts. We specifically investigated L-plastin, as it was identified by both proteomic approaches. L-plastin depletion impaired NKG2D-DAP10 and pVav recruitment to lipid rafts, as we observed a significant decrease in their levels in the DRM fractions isolated by β-OG. These results suggest a role for this protein in activation-induced recruitment of the NKG2D receptor to rafts. In addition, we showed that activation of NKG2D along with NKG2A partially disrupted the association of the NKG2D-DAP10 complex and pVav with DRMs. This effect even increased when L-plastin was silenced using siRNA. Together, these results are consistent with other reports describing that inhibitory signals prevent recruitment of activating receptors to DRMs [9, 10].
Several studies have addressed the role of L-plastin in a wide variety of cells of the immune system. In T cells, L-plastin promotes leukocyte integrin-mediated adhesion and contributes to the stabilization of T cell/APC contact [43–45]. Furthermore, L-plastin is required for cell proliferation and cytokine production [24]. However, the requirement for L-plastin in NK cell functions remains unclear to date. We evaluated whether L-plastin silencing might impair NKG2D function by measuring CD107a expression and IFN-γ production after incubation of human NK cells with NKG2D ligand-expressing target cells (C1R-MICA). Partial siRNA depletion of L-plastin did not affect NK cell degranulation or cytokine production. However, this partial deficiency of the protein was sufficient to diminish NK cell chemotaxis significantly toward CXCL12 under basal conditions. Other works have reported previously a defective chemotaxis in L-plastin knockdown Jurkat cells and in L-plastin null murine (LPL−/−) T cells [25, 38]. L-plastin regulates CXCL12-induced chemotaxis of human T lymphocytes [26]. NKG2D activation impairs NK cell migration toward a CXCL12 gradient [29]. In this study, we show that this inhibition is abrogated in cells transfected with L-plastin siRNA. Therefore, our results complement previous works describing a role for L-plastin in T cell motility, as this protein also contributes to chemoattractant-mediated migration in NK cells. In addition, this study extends the role of L-plastin, as we demonstrate that it is involved in NKG2D-mediated inhibition of NK cell chemotaxis. The activation of L-plastin by phosphorylation positively regulates its F-actin-binding activity [46], as well as F-actin localization, and is required for polarization and migration of chemokine-stimulated T lymphocytes [26]. We observed that knockdown of L-plastin was correlated with lower F-actin levels in control-treated NK cells. NKG2D cross-linking promotes an increase in F-actin content, but the effect is abrogated after silencing L-plastin expression. These results are consistent with the protective role of L-plastin preventing F-actin depolymerization or reducing actin filament turnover [30, 47].
In summary, our results provide new insights into the role of lipid rafts in NKG2D-mediated signaling, demonstrating a pivotal role of actin cytoskeletal proteins, as observed in the qualitative proteomic analyses. Our study also highlights the role of L-plastin in NK cell chemotaxis, which is essential for NKG2D recruitment into rafts and for the regulation of NKG2D-mediated NK cell migration. Given the emerging knowledge about L-plastin functions in cells of the immune system, it would be of interest to study a role for this protein under pathological conditions in NK cell-mediated responses.
ACKNOWLEDGMENTS
This work was supported by grants from the Spanish Fondo de Investigaciones Sanitarias (FIS)-Fondos Fonds Européen de Développement Régional (FEDER) European Union (PI12/02587) and the Red de Investigación Renal (REDinREN; RD12/0021/0021) and by the Czech Science Foundation (P302/12/G101). E.S-P. was supported by a “Severo Ochoa” fellowship from FICYT (Asturias, Spain). E.C-M. was supported by the Clarin Program (FICYT).
We thank Dr. José Luis Fernández-Martín (Servicio de Metabolismo Óseo y Mineral, Hospital Universitario Central de Asturias, Spain) for his technical assistance with MS. We also thank Dr. Pablo Martínez-Camblor (Oficina de Investigación Biosanitaria, Asturias, Spain) for his assistance with statistical analysis.
Footnotes
- β-OG
- β-octylglucoside
- amu
- atomic mass unit
- DAP10
- DNAX-activation protein 10
- DRM
- detergent-resistant membrane
- ERM
- ezrin/radixin/moesin
- FICYT
- Fundación para el fomento en Asturias de la investigación científica aplicada y la tecnología
- GDI
- GDP dissociation inhibitor
- IQGAP1
- IQ motif containing GTPase-activating protein 1
- IS
- immunological synapse
- LC
- liquid chromatography
- LPL
- L-plastin
- MICA
- MHC class I chain-related gene A
- MS
- mass spectrometry
- N-WASp
- neuronal Wiskott-Aldrich syndrome protein
- NKL
- NK leukemia
- pVav
- phosphorylated form of Vav
- siRNA
- small interfering RNA
- TRITC
- tetramethyl rhodamine isothiocyanate
AUTHORSHIP
E.S-P. performed the experiments, analyzed the data, and wrote the manuscript. T.B. and V.H. analyzed and interpreted the data and revised the manuscript critically for important intellectual content. E.C-M. and C.L-L. designed and supervised the research.
DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES
- 1. Lingwood D., Kaiser H. J, Levental I., Simons K. (2009) Lipid rafts as functional heterogeneity in cell membranes. Biochem. Soc. Trans. 37, 955–960. [DOI] [PubMed] [Google Scholar]
- 2. Horejsi V. (2005) Lipid rafts and their roles in T-cell activation. Microbes Infect. 7, 310–316. [DOI] [PubMed] [Google Scholar]
- 3. Chichili G. R., Rodgers W. (2009) Cytoskeleton-membrane interactions in membrane raft structure. Cell. Mol. Life Sci. 66, 2319–2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ma Y., Yang H., Qi J., Liu D., Xiong P., Xu Y., Feng W., Zheng G., Li P., Fang M., Tan Z., Zheng F., Gong F. (2010) CD2AP is indispensable to multistep cytotoxic process by NK cells. Mol. Immunol. 47, 1074–1082. [DOI] [PubMed] [Google Scholar]
- 5. Kondadasula S. V., Roda J. M., Parihar R., Yu J., Lehman A., Caligiuri M. A., Tridandapani S., Burry R. W., Carson W. E., III (2008) Colocalization of the IL-12 receptor and FcγRIIIa to natural killer cell lipid rafts leads to activation of ERK and enhanced production of interferon-γ. Blood 111, 4173–4183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Riteau B., Barber D. F., Long E. O. (2003) Vav1 phosphorylation is induced by β2 integrin engagement on natural killer cells upstream of actin cytoskeleton and lipid raft reorganization. J. Exp. Med. 198, 469–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sanni T. B., Masilamani M., Kabat J., Coligan J. E., Borrego F. (2004) Exclusion of lipid rafts and decreased mobility of CD94/NKG2A receptors at the inhibitory NK cell synapse. Mol. Biol. Cell 15, 3210–3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Masilamani M., Nguyen C., Kabat J., Borrego F., Coligan J. E. (2006) CD94/NKG2A inhibits NK cell activation by disrupting the actin network at the immunological synapse. J. Immunol. 177, 3590–3596. [DOI] [PubMed] [Google Scholar]
- 9. Watzl C., Long E. O. (2003) Natural killer cell inhibitory receptors block actin cytoskeleton-dependent recruitment of 2B4 (CD244) to lipid rafts. J. Exp. Med. 197, 77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Endt J., McCann F. E., Almeida C. R., Urlaub D., Leung R., Pende D., Davis D. M., Watzl C. (2007) Inhibitory receptor signals suppress ligation-induced recruitment of NKG2D to GM1-rich membrane domains at the human NK cell immune synapse. J. Immunol. 178, 5606–5611. [DOI] [PubMed] [Google Scholar]
- 11. Robertson M. J., Cochran K. J., Cameron C., Le J. M., Tantravahi R., Ritz J. (1996) Characterization of a cell line, NKL, derived from an aggressive human natural killer cell leukemia. Exp. Hematol. 24, 406–415. [PubMed] [Google Scholar]
- 12. Suárez-Alvarez B., López-Vázquez A., Gonzalez M. Z., Fdez-Morera J. L., Díaz-Molina B., Blanco-Gelaz M. A., Pascual D., Martínez-Borra J., Muro M., Alvarez-López M. R., López-Larrea C. (2007) The relationship of anti-MICA antibodies and MICA expression with heart allograft rejection. Am. J. Transplant. 7, 1842–1848. [DOI] [PubMed] [Google Scholar]
- 13. Draber P., Vonkova I., Stepanek O., Hrdinka M., Kucova M., Skopcova T., Otahal P., Angelisova P., Horejsi V., Yeung M., Weiss A., Brdicka T. (2011) SCIMP, a transmembrane adaptor protein involved in major histocompatibility complex class II signaling. Mol. Cell. Biol. 31, 4550–4562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wessel D., Flügge U. I. (1984) A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal. Biochem. 138, 141–143. [DOI] [PubMed] [Google Scholar]
- 15. Burns S. O., Killock D. J., Moulding D. A., Metelo J., Nunes J., Taylor R. R., Forge A., Thrasher A. J., Ivetic A. (2010) A congenital activating mutant of WASp causes altered plasma membrane topography and adhesion under flow in lymphocytes. Blood 115, 5355–5365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wu J., Song Y., Bakker A. B., Bauer S., Spies T., Lanier L. L., Phillips J. H. (1999) An activating immunoreceptor complex formed by NKG2D and DAP10. Science 285, 730–732. [DOI] [PubMed] [Google Scholar]
- 17. Foster L. J., Chan Q. W. (2007) Lipid raft proteomics: more than just detergent-resistant membranes. Subcell. Biochem. 43, 35–47. [DOI] [PubMed] [Google Scholar]
- 18. Gerke V., Creutz C. E., Moss S. E. (2005) Annexins: linking Ca2+ signalling to membrane dynamics. Nat. Rev. Mol. Cell. Biol. 6, 449–461. [DOI] [PubMed] [Google Scholar]
- 19. Ridley A. J. (2001) Rho GTPases and cell migration. J. Cell Sci. 114, 2713–2722. [DOI] [PubMed] [Google Scholar]
- 20. Miettinen H. M., Jalkanen M. (1994) The cytoplasmic domain of syndecan-1 is not required for association with Triton X-100-insoluble material. J. Cell Sci. 107, 1571–1581. [DOI] [PubMed] [Google Scholar]
- 21. Brandt D. T., Grosse R. (2007) Get to grips: steering local actin dynamics with IQGAPs. EMBO Rep. 1, 1019–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Delanote V., Vandekerckhove J., Gettemans J. (2005) Plastins: versatile modulators of actin organization in (patho)physiological cellular processes. Acta Pharmacol. Sin. 26, 769–779. [DOI] [PubMed] [Google Scholar]
- 23. Morley S. C. (2012) The actin-bundling protein L-plastin: a critical regulator of immune cell function. Int. J. Cell Biol. 2012, 935173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang C., Morley S. C., Donermeyer D., Peng I., Lee W. P., Devoss J., Danilenko D. M., Lin Z., Zhang J., Zhou J., Allen P. M., Brown E. J. (2010) Actin-bundling protein L-plastin regulates T cell activation. J. Immunol. 185, 7487–7497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Morley S. C., Wang C., Lo W. L., Lio C. W., Zinselmeyer B. H., Miller M. J., Brown E. J., Allen P. M. (2010) The actin-bundling protein L-plastin dissociates CCR7 proximal signaling from CCR7-induced motility. J. Immunol. 184, 3628–3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Freeley M., O'Dowd F., Paul T., Kashanin D., Davies A., Kelleher D., Long A. (2012) L-Plastin regulates polarization and migration in chemokine-stimulated human T lymphocytes. J. Immunol. 188, 6357–6370. [DOI] [PubMed] [Google Scholar]
- 27. Alter G., Malenfant J. M., Altfeld M. (2004) CD107a as a functional marker for the identification of natural killer cell activity. J. Immunol. Methods 294, 15–22. [DOI] [PubMed] [Google Scholar]
- 28. Aktas E., Kucuksezer U. C., Bilgic S., Erten G., Deniz G. (2009) Relationship between CD107a expression and cytotoxic activity. Cell. Immunol. 254, 149–154. [DOI] [PubMed] [Google Scholar]
- 29. Serrano-Pertierra E., Cernuda-Morollón E., López-Larrea C. (2012) Wiskott-Aldrich syndrome protein (WASp) and N-WASp are involved in the regulation of NK-cell migration upon NKG2D activation. Eur. J. Immunol. 42, 2142–2151. [DOI] [PubMed] [Google Scholar]
- 30. Lebart M. C., Hubert F., Boiteau C., Ventéo S., Roustan C., Benyamin Y. (2004) Biochemical characterization of the L-plastin-actin interaction shows a resemblance with that of α-actinin and allows a distinction to be made between the two actin-binding domains of the molecule. Biochemistry 43, 2428–2437. [DOI] [PubMed] [Google Scholar]
- 31. Lou Z., Jevremovic D., Billadeau D. D., Leibson P. J. (2000) A balance between positive and negative signals in cytotoxic lymphocytes regulates the polarization of lipid rafts during the development of cell-mediated killing. J. Exp. Med. 191, 347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fassett M. S., Davis D. M., Valter M. M., Cohen G. B., Strominger J. L. (2001) Signaling at the inhibitory natural killer cell immune synapse regulates lipid raft polarization but not class I MHC clustering. Proc. Natl. Acad. Sci. USA 98, 14547–14552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Villalba M., Bi K., Rodríguez F., Tanaka Y., Schoenberger S., Altman A. (2001) Vav1/Rac-dependent actin cytoskeleton reorganization is required for lipid raft clustering in T cells. J. Cell Biol. 155, 331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Von Haller P. D., Donohoe S., Goodlett D. R., Aebersold R., Watts J. D. (2001) Mass spectrometric characterization of proteins extracted from Jurkat T cell detergent-resistant membrane domains. Proteomics 1, 1010–1021. [DOI] [PubMed] [Google Scholar]
- 35. Nebl T., Pestonjamasp K. N., Leszyk J. D., Crowley J. L., Oh S. W., Luna E. J. (2002) Proteomic analysis of a detergent-resistant membrane skeleton from neutrophil plasma membranes. J. Biol. Chem. 277, 43339–43409. [DOI] [PubMed] [Google Scholar]
- 36. Bini L., Pacini S., Liberatori S., Valensin S., Pellegrini M., Raggiaschi R., Pallini V., Baldari C. T. (2003) Extensive temporally regulated reorganization of the lipid raft proteome following T-cell antigen receptor triggering. Biochem. J. 369, 301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yanagida M., Nakayama H., Yoshizaki F., Fujimura T., Takamori K., Ogawa H., Iwabuchi K. (2007) Proteomic analysis of plasma membrane lipid rafts of HL-60 cells. Proteomics 7, 2398–2409. [DOI] [PubMed] [Google Scholar]
- 38. Lin S. L., Chien C. W., Han C. L., Chen E. S., Kao S. H., Chen Y. J., Liao F. (2010) Temporal proteomics profiling of lipid rafts in CCR6-activated T cells reveals the integration of actin cytoskeleton dynamics. J. Proteome Res. 9, 283–297. [DOI] [PubMed] [Google Scholar]
- 39. Le Clainche C., Schlaepfer D., Ferrari A., Klingauf M., Grohmanova K., Veligodskiy A., Didry D., Le D., Egile C., Carlier M. F., Kroschewski R. (2007) IQGAP1 stimulates actin assembly through the N-Wasp-Arp2/3 pathway. J. Biol. Chem. 282, 426–435. [DOI] [PubMed] [Google Scholar]
- 40. Bunnell S. C., Kapoor V., Trible R. P., Zhang W., Samelson L. E. (2001) Dynamic actin polymerization drives T cell receptor-induced spreading: a role for the signal transduction adaptor LAT. Immunity 14, 315–329. [DOI] [PubMed] [Google Scholar]
- 41. Samstag Y., Eibert S. M., Klemke M., Wabnitz G. H. (2003) Actin cytoskeletal dynamics in T lymphocyte activation and migration. J. Leukoc. Biol. 73, 30–48. [DOI] [PubMed] [Google Scholar]
- 42. Gomez T. S., Billadeau D. D. (2008) T cell activation and the cytoskeleton: you can't have one without the other. Adv. Immunol. 97, 1–64. [DOI] [PubMed] [Google Scholar]
- 43. Jones S. L., Wang J., Turck C. W., Brown E. J. (1998) A role for the actin-bundling protein L-plastin in the regulation of leukocyte integrin function. Proc. Natl. Acad. Sci. USA 95, 9331–9336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wabnitz G. H., Lohneis P., Kirchgessner H., Jahraus B., Gottwald S., Konstandin M., Klemke M., Samstag Y. (2010) Sustained LFA-1 cluster formation in the immune synapse requires the combined activities of L-plastin and calmodulin. Eur. J. Immunol. 40, 2437–2449. [DOI] [PubMed] [Google Scholar]
- 45. De Clercq S., Zwaenepoel O., Martens E., Vandekerckhove J., Guillabert A., Gettemans J. (2013) Nanobody-induced perturbation of LFA-1/L-plastin phosphorylation impairs MTOC docking, immune synapse formation and T cell activation. Cell. Mol. Life Sci. 70, 909–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Janji B., Giganti A., de Corte V., Catillon M., Bruyneel E., Lentz D., Plastino J., Gettemans J., Friederich E. (2006) Phosphorylation on Ser5 increases the F-actin binding activity of L-plastin and promotes its targeting to sites of actin assembly in cells. J. Cell Sci. 119, 1947–1960. [DOI] [PubMed] [Google Scholar]
- 47. Al Tanoury Z., Schaffner-Reckinger E., Halavatyi A., Hoffmann C., Moes M., Hadzic E., Catillon M., Yatskou M., Friederich E. (2010) Quantitative kinetic study of the actin-bundling protein L-plastin and of its impact on actin turn-over. PLoS One 5, e9210. [DOI] [PMC free article] [PubMed] [Google Scholar]