Abstract
Quality of life (QoL) is an important outcome measure in clinical studies, but interpretation is hindered by incompleteness of data. We addressed this issue in a population-based cohort study of 146 patients with newly diagnosed rectal cancer. QoL was assessed by means of European Organization for the Research and Treatment of Cancer questionnaires at discharge from hospital after primary treatment and then every 3 months for 2 years. In parallel, objective clinical data were documented. Analyses were conducted in three steps: participants versus non-participants with QoL-assessment; poor compliers who filled in only one or two questionnaires (n=20) versus good compliers who filled in all or nearly all questionnaires (n=18); and the proportion of missing forms and critical (very poor) QoL scores in risk patients versus non-risk patients over the course of 2 years.
Non-participants and poor compliers were older, were more likely to receive palliative (rather than curative) treatment, and had worse scores for physical status. Tumour progression and therapeutic interventions were more frequent in poor compliers than in good-compliers. Patients with risk factors (age 475 years, poor physical status, palliative treatment) were more likely to have missing questionnaires and critical QoL scores in respect of physical functioning and global quality of life over the course of 2 years.
Missing values for QoL have clinical as well as methodological implications, because QoL scores can enhance a clinician's insight. Unwillingness to fill in a questionnaire is an indicator of serious illness. Studies that report sample statistics without specifying compliance rates and the characteristics of non-compliers will give a misleadingly positive picture.
INTRODUCTION
With improvements in the treatment of colorectal cancer, attention is moving from short-term endpoints to longer-term quality of life.1–3 Various standardized questionnaires have been developed and rigorously tested for reliability, validity and sensitivity. For patients with rectal cancer, the EORTC QLQ-C30 and FACT instruments are particularly suitable since they incorporate a cancer-specific core questionnaire and a supplementary colorectal symptom specific module.4,7 Technical drawbacks have been surmounted and QoL research has made the step to clinical application.8–11
Numerous papers have addressed QoL in rectal cancer,12–16 but the results are not consistent. For example, some groups report QoL to be better after sphincter-preserving surgery (no stoma) than after abdominoperineal extirpation with stoma, but others find no advantage.12,16–18 Part of the explanation for such discrepancies may lie in missing data, and statisticians have proposed various ways to adjust for the deficits.19–22 However, such approaches will not greatly aid understanding of QoL results until we know how the non-availability of data relates to the clinical and psychological state of the patient. There is already reason to think that healthier patients are more likely to answer the questionnaires.23–25 Either severely ill patients may feel too unwell to participate or the researchers may judge them too unwell. Whatever the reason, use of the available data will tend to overestimate quality of life and bias comparisons between treatment effects.26,27 We therefore explored this issue in a cohort of patients with rectal cancer. The hypothesis to be tested was that compliance with QoL testing is associated with physical status. We also examined the relation between compliance and the recording of critical values (very poor scores).
METHODS
Study design and endpoints
To evaluate quality of care in routine circumstances, the study was population-based, conducted in a defined geographic area28,29—a rural county with 252 000 inhabitants and three hospitals offering surgical treatment for rectal carcinoma. Inclusion criteria were newly diagnosed rectal carcinoma and primary treatment in the study area during 2 calendar years. Of 151 consecutive patients entered, 146 fulfilled all inclusion criteria.30 This cohort was followed up for 2 years.
Endpoints of the study were self-reported quality of life and quality indicators for primary (operative and adjuvant) treatment for rectal cancer derived from clinical practice guidelines.31–33 Secondary endpoints were compliance rates for QoL assessment and clinically relevant events in the course of the disease during the follow-up period. These were defined as events that contributed to mortality or had therapeutic consequences (change/discontinuation of adjuvant therapy, readmission to hospital, or surgical intervention).34
The study was explicitly observational—i.e. patients were free to take part in the routine follow-up programme or not, and to choose the hospital or the medical practitioner offering this service. To optimize comprehensive data acquisition, an organizational system based on a quality circle and a managing study team was established. The quality circle consisted of surgeons of each hospital, representatives of all occupational groups caring for rectal cancer patients and representatives of the patient self-help groups. The circle functioned as a forum to facilitate clinical adoption of the QoL concept, to discuss local options of rectal cancer care, and to monitor performance of the study. The managing study team consisted of a surgical trainee, a psychologist and a data manager. The team was responsible for providing information on and advice to participating physicians and patients, logistic support (questionnaires and clinical documentation charts), data management and implementation of the study concept. The implementation strategy included three methods—continuous medical education via the quality circle, outreach visits to the hospitals and practices and approaches to local opinion leaders.35
Patients with rectal cancer were identified from hospital electronic data systems and by visits of the study team. To identify migration effects and patients who did not receive in-hospital treatment, doctors' practices in the study area and hospitals in the neighbouring counties were surveyed. Patients who fulfilled the inclusion criteria were informed about the study and received the information leaflet36 from their hospital surgeon before discharge after primary treatment. After obtaining consent, the study team secured primary documentation and contacted the institutions chosen by the patients to do the follow-up, so as to obtain follow-up information. The study team evaluated all clinical data for completeness and consistency, recontacting those who submitted the data when necessary.
Data assessment and analysis
QoL data and clinical data were collected at discharge from the hospital after primary treatment and at follow-up visits every 3 months over the study period. QoL was assessed with the self-administered EORTC QLQ-C30 and CR38 questionnaires.4,5,17 Primary documentation of clinical data included sociodemographic details, standardized clinical and histopathological classification of the tumour,37 physical status of the patient and concomitant diseases, diagnostic procedures, nature of treatment and complications. Follow-up documentation included diagnostic findings and therapeutic interventions.
To test for an association between compliance with QoL assessment and physical status and treatment in the course of the disease, we applied the methods of correlational studies.38 First, we compared characteristics of the patients who participated in QoL assessment (i.e. those who returned at least one complete questionnaire, n=98) with those who did not (n=48). Second, we analysed two extreme groups—patients who filled in only one or two QoL-questionnaires (poor compliance group, n=20) and patients who filled in all or eight of the nine questionnaires (good compliance group, n=18). These analyses led to the identification of demographic and clinical risk factors for not filling in questionnaires (such as age or tumour stage).
In a third step we analysed whether risk versus no-risk patients differed in the rate of returned questionnaires over the 2-year period and whether the two patient groups differed in the proportion of critical QoL scores over time. On the basis of earlier work9 we defined a score as critical when the value was under 50 on a scale of 0 (very bad) to 100 (very good). For this analysis we chose six QoL scores representative of somatic, psychological and social well-being—physical functioning, role functioning, emotional functioning, future perspective, social functioning and global quality of life. The QoL scores were computed according to the EORTC manual.5
Summary statistics are presented as means and standard deviations, percentages and graphs over time. The following statistical tests were used: independent t-test, χ2 test, Pearson correlations. The two-sided significance level (a) for observed differences was set at 0.05. All analyses were conducted with SPSS version 10.39
RESULTS
Clinical documentation charts were completed for all patients (n=146) at discharge from the hospital. Follow-up documentation of clinical data (objective health status) could be obtained from 95% of the cohort (139 patients).
Of the 146 patients fulfilling the inclusion criteria, 98 participated in the QoL assessment and filled in at least one questionnaire during the study period. The remaining 48 did not participate in QoL assessment, for the following reasons: advanced disease (supportive care only, n=5); death within 30 days postoperatively (n=6), refusal to fill in a questionnaire (n=17); physical or mental inability to fill in a questionnaire (n=13). In 7 cases reasons for non-compliance were unclear.
The overall questionnaire response rate was 59% at discharge from the hospital and 36% at the end of follow-up for the cohort (n=146). The mortality rates were 4% (postoperative) and 27% (2 years). Thus, theoretically (taking into account survival) response rates could have reached 94% and 73%, respectively.
Table 1 shows the demographic and clinical details of the cohort and the subgroups of participants and non-participants with QoL assessment. Non-participants were older, were more likely to be receiving palliative (as opposed to curative) treatment, showed greater variance in surgical treatment strategies and were more likely to have American Society of Anesthesiologists (ASA) scores III and IV signifying poor physical status.40
Table 1.
Patient characteristics
| Study population n=146 | No participation n=48 | Participation in QoL study n=98 | P (no participation versus participation) | |
|---|---|---|---|---|
| Age | ||||
| Mean | 65.6 | 70.3 | 63.2 | 0.001 |
| Range | 33-92 | 40-92 | 33-88 | |
| Gender | ||||
| Female | 58 | 21 | 38 | NS |
| Male | 88 | 27 | 60 | |
| UICC cancer stage | ||||
| I (pT1-2 N0 M0) | 48 | 15 | 33 | NS |
| II (pT3-4 N0 M0) | 34 | 14 | 20 | |
| III (all pT N+ M0) | 37 | 6 | 31 | |
| IV (all pT/N M+) | 21 | 7 | 14 | |
| No UICC classification | 6 | 6 | - | |
| ASA grade | ||||
| I | 9 | - | 9 | |
| II | 51 | 10 | 41 | 0.055* |
| III | 41 | 13 | 28 | |
| IV | 12 | 4 | 8 | |
| No classification | 33 | 21 | 12 | |
| Surgical therapy | ||||
| None | 5 | 5 | - | |
| Low anterior resection | 89 | 20 | 69 | <0.001 |
| Rectal extirpation | 33 | 10 | 23 | |
| Other | 19 | 13 | 6 | |
| Postoperative adjuvant therapy | ||||
| Yes | 46 | 11 | 35 | NS |
| No | 94 | 31 | 63 | |
| Not reported | 6 | 6 | - | |
| Intention of primary treatment | ||||
| Curative | 118 | 31 | 87 | <0.001 |
| Palliative | 28 | 17 | 11 |
QoL=Quality of life; UICC=International Union Against Cancer; ASA=American Society of Anesthesiologists; NS=not significant
Pearson's χ2 test for low-risk patients (ASA I+II) versus high-risk patients (ASA III+IV)
Good compliers versus poor compliers
Table 2 compares the characteristics of good (n=18) and poor (n=20) compliers with QoL questionnaires as defined above. Poor compliers were older, more severely ill according to tumour stage and ASA classification and more likely to receive palliative treatment. The groups differed significantly regarding therapeutic interventions and progression of tumour disease. In the 2-year observation period, 9 patients died in the poor-compliance group and none in the good compliance group; consequently, survival differed significantly from the time of first operation. Causes of death were progression of tumour disease in 8 cases and cardiopulmonary disease in 1 case. Clearly, one reason for the lower responsiveness of poor compliers could have been that they died sooner; therefore, we looked at individual data. Among the 18 good compliers, all of whom survived the 2 years, the total possible number of questionnaires completed was 9618=162. In fact they returned 142 (mean 4 per patient). In the 20 poor compliers mean survival was 8.6 months, so 141 questionnaires (7 per patient) were in theory returnable until death or the end of follow-up. Actually they returned only 34 (mean 1.5 per patient). The possible number of questionnaires in the poor compliance group is almost identical to the number of questionnaires actually returned by the high compliance group thus, length of survival is not a sufficient explanation for the difference between these two groups in number of questionnaires completed.
Table 2.
Extreme group analyses: poor compliers versus good compliers with quality of life assessment
| Poor compliers n=20 | Good compliers n=18 | P | |
|---|---|---|---|
| Age | |||
| Mean | 64.3 | 60.9 | 0.03 |
| Range | 36-88 | 48-73 | |
| Gender | |||
| Female | 9 | 5 | NS |
| Male | 11 | 13 | |
| Cancer stage | |||
| I (pT1-2 NO MO) | 3 | 8 | |
| II (pT3-4 NO MO) | 6 | 3 | 0.04 |
| III (all pT N+ MO) | 4 | 6 | |
| IV (all pT/N M+) | 7 | 1 | |
| ASA grade | |||
| I | 2 | - | |
| II | 5 | 12 | 0.05* |
| III | 5 | 5 | |
| IV | 3 | - | |
| No ASA classification | 5 | 1 | |
| Surgical therapy | |||
| None | - | - | NS |
| Low anterior resection | 13 | 10 | |
| Rectal extirpation | 7 | 8 | |
| Other | - | - | |
| Postoperative adjuvant therapy | |||
| Yes | 8 | 5 | |
| No | 12 | 13 | |
| Intention of primary treatment | |||
| Curative | 14 | 17 | 0.05 |
| Palliative | 6 | 1 | |
| Tumour progression | 13 | 5 | 0.022 |
| After curative primary treatment | |||
| local recurrence | 4 | 2 | |
| Distant metastases | 3 | 3 | |
| After palliative primary treatment | |||
| General progression | 6 | - | |
| Therapeutic interventions | 7 | 1 | 0.026 |
| Resection of metastases/local recurrence | 2 | 1 | |
| Palliative chemotherapy | 5 | 00 | |
| Death | 9 | 0 | 0.001 |
| Mean survival (months) | 18.6 | 24.0 | 0.001 |
ASA=American Society of Anesthesiologists; NS=not significant
Pearson's χ2 test for low-risk patients (ASA I+II) versus high-risk patients (ASA III+IV)
Clinical risk factors for non-compliance
Tables 1 and 2 suggest three main risk factors for non-compliance—age 475, ASA score III or IV, and palliative treatment intention. The cut-offs were chosen on the basis of earlier work.40–43 In the whole cohort 81 patients (55%) presented with one or more of these risk criteria and thus were assigned to the risk group. The remaining 65 fell into the non-risk group.
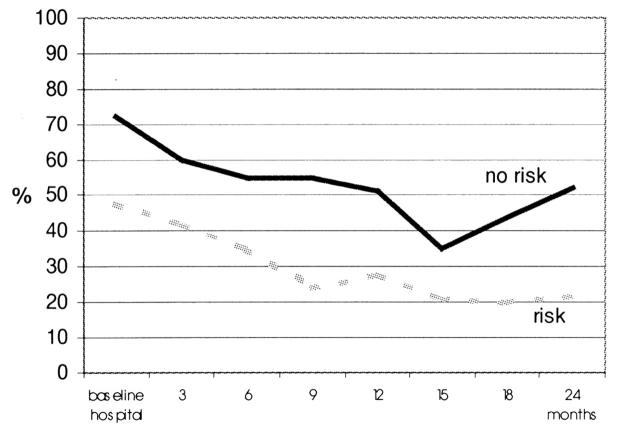
Figure 1 shows the percentages of patients filling in questionnaires across the 2-year observation period. Patients in the non-risk group were consistently more likely to fill in questionnaires than risk patients. Non-risk patients filled in a total of 276 questionnaires, risk patients a total of 194 questionnaires. The difference in completion rates (53% versus 30%) was significant—χ2 (df=1)=64.71, P<0.001. The proportions of risk and non-risk patients with score values 550 in selected domains of QoL are presented in Figure 2. Risk patients were more likely to have critical physical functioning scores than non-risk patients—27% versus 12%, χ2 (df=1)=7.73, P<0.01. Risk patients were more likely to have critical global QoL scores than non-risk patients—26% versus 18%, χ2 (df=1)=3.49, P<0.06. We also examined critical score values regarding emotional functioning, role functioning, social functioning and future perspective, but significant differences between risk and non-risk patients did not emerge.
Figure 1.
Clinical risk factors and compliance with quality of life assessment: proportions of patients with complete questionnaires. Risk factors are defined as age 475 years or ASA III/IV or palliative treatment
Figure 2.
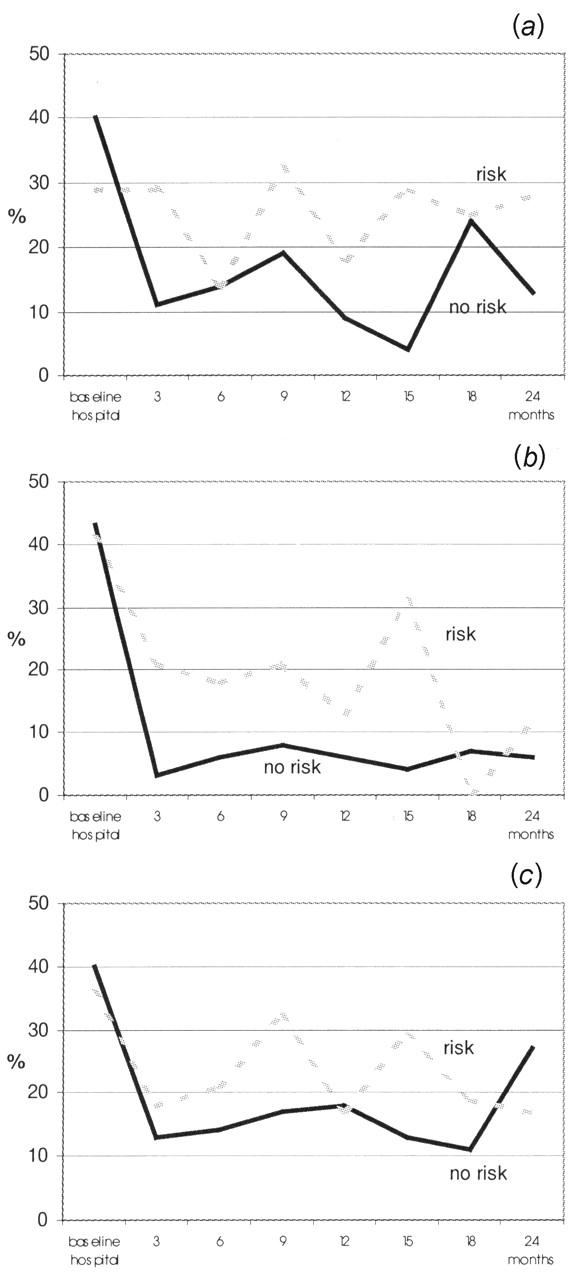
Clinical risk factors and quality of life. (a) Patients with global quality of life score 550; (b) patients with physical functioning score 550; (c) patients with emotional functioning score 550. Risk factors are defined as age 475 years or ASA III/IV or palliative treatment
DISCUSSION
As hypothesized, non-compliance with QoL assessment was strongly associated with patient characteristics, therapeutic interventions and tumour progression during follow-up. The most severely ill patients were the least likely to fill in QoL questionnaires. The clinical risk factors for poor compliance were age, high ASA grade and treatment with palliative intent. After 2 years' follow-up, patients with these risk factors were underrepresented in the sample. Patients with at least one risk factor scored significantly worse on physical functioning and global QoL.
The failure to obtain completed questionnaires occurred despite rigorous adherence by the study team to algorithms for data collection and management.36 Recruitment of eligible rectal cancer patients was 100% as indicated by a calculated incidence rate of 22.7/100 000/year and characteristics corresponding to data from the German Cancer Register and population-based studies.44–46
From the methodological point of view, our results indicate that the unobserved data were not missing at random. The risk factors for non-compliance resemble those noted in work from other countries and in patients with different cancers.24,25 The important contribution of the present study is that the risk factors that characterize non-compliant patients are associated with poor scores for QoL. Consequently, application of sample statistics (means, medians) to such data sets may lead to wrong conclusions. This difficulty applies particularly to cross-sectional studies including 'convenience samples' in which the population of origin is not specified, and to cohort studies with high drop-out rates. Any statistical imputation method for missing values has to take into account the strong associations between clinical risk factors, non-compliance with QoL and poor QoL. The handling of missing data should be pre-planned and described in the study protocol.
For clinicians, QoL scores can be valuable in explaining discrepancies between clinical status and wellbeing,47,48 but it is not difficult to think of reasons why severely ill patients are sometimes unkeen to participate in such assessments—lack of concentration, lack of motivation, a move to alternative treatment.10,24 The present study was not designed to disentangle these, but our results could serve as a starting-point for more specific work on the nature of the link. One provocative hypothesis concerns the mooted existence of a 'having fun' stereotype of quality of life. This might cause patients and doctors to believe that QoL is important for the relatively healthy but no longer an issue for the seriously ill. At worst, QoL-related therapeutic interventions11,16,48 might then be withheld in the very patients who stand most to benefit.
Acknowledgments
This study was conducted with the financial support of the German Ministry of Health (grant No.: FB 2-43332-70/6). The sponsor had no involvement in study design, data collection, data analysis, data interpretation or writing of the report.
We thank Susanne Hainbach for management of the data files, computer support and graphical assistance. We are indebted to the members of the quality circle for their valuable comments on practical aspects of regional cancer care and support: U-S Albert, P Berressem, H Böhm, I Dittrich-Scheerer, C Eichler, M Ernst, N Fenner, C Hämmerle, K Fischer, C Heitmann, R Herpers, J Hermanns, A Hochgrebe, M Hoffmann, H Hofmann, M Kuenneke, A Krehbiel, G Leiber, B Marcovici, P Müller, C Nies, J Rosenberger, K-D Schulz, B Städter, B Stinner, F-J Strombach, S Thommes, A Vogel, G-E von Manteuffel, R Weber, F Weidenbach, P Wilke.
References
- 1.Calman KC. Quality of life in cancer patients—an hypothesis. J Med Ethics 1984;10: 124-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allison PJ, Locker D, Feine S. Quality of life: a dynamic construct. Soc Sci Med 1997;45: 221-30 [DOI] [PubMed] [Google Scholar]
- 3.Fayers PM, Machin D. Quality of life: Assessment, Analysis and Interpretation. Chichester: Wiley, 2000
- 4.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Ins 1993;85: 365-76 [DOI] [PubMed] [Google Scholar]
- 5.Fayers P, Aaronson N, Bjordal K, Groenvold M, Curran D, Bottomley A. EORTC QLQ-C30 Scoring Manual, 3rd edn. Brussels: EORTC Study Group on Quality of Life, 2001
- 6.Ware JE, Sherbourne CD. The MOS 36-item Short-Form Health Survey I: conceptual framework and item selection. Med Care 1992;30: 473-83 [PubMed] [Google Scholar]
- 7.Cella DF, Tulsky DS, Gray G, et al. The functional assessment of cancer therapy (FACT) scale: development and validation of the general measure. J Clin Oncol 1993;11: 570-9 [DOI] [PubMed] [Google Scholar]
- 8.Koller M, Kussmann J, Lorenz W, Rothmund M. Die Messung von Lebensqualität in der chirurgischen Tumornachsorge: Methoden, Probleme und Einsatzmöglichkeiten. Chirurg 1994;65: 333-9 [PubMed] [Google Scholar]
- 9.Koller M, Lorenz W. Quality of life: a deconstruction for clinicians. J R Soc Med 2002;95: 481-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sprangers MAG. Quality-life-assessment in oncology. Achievements and challenges. Acta Oncol 2002;41: 229-37 [DOI] [PubMed] [Google Scholar]
- 11.Albert US, Koller M, Lorenz W, et al. Quality of life profile: from measurement to clinical application. Breast 2002;11: 324-34 [DOI] [PubMed] [Google Scholar]
- 12.Camilleri-Brennan J, Steele RJC. Quality of life after treatment for rectal cancer. Br J Sur 1998;85: 1036-43 [DOI] [PubMed] [Google Scholar]
- 13.Camilleri-Brennan J, Steele RJC. Prospective analysis of quality of life and survival following mesorectal excision for rectal cancer. Br J Surg 2001;88: 1617-22 [DOI] [PubMed] [Google Scholar]
- 14.Camilleri-Brennan J, Steele RJC. The impact of recurrent rectal cancer on quality of life. Eur J Surg Oncol 2001;27: 349-53 [DOI] [PubMed] [Google Scholar]
- 15.O'Leary DP, Fide CJ, Lucarotti ME. Quality of life after low anterior resection with total mesorectal excision and temporary loop ileostomy for rectal carcinoma. Br J Surg 2001;88: 1216-20 [DOI] [PubMed] [Google Scholar]
- 16.Koller M, Lorenz W. Quality of life research in patients with rectal cancer: traditional approaches versus a problem-solving oriented perspective. Langenbeck's Arch Surg 1998;383: 427-36 [DOI] [PubMed] [Google Scholar]
- 17.Sprangers MAG, Taal BG, Aaronson NK, te Velde A. Quality of life in colorectal cancer. Stoma vs. non-stoma patients. Dis Colon Rectum 1995;38: 361-9 [DOI] [PubMed] [Google Scholar]
- 18.Grumann MM, Noack EM, Hoffmann IA, Schlag PM. Comparison of quality of life in patients undergoing abdominoperineal extirpation or anterior resection for rectal cancer. Ann Surg 2001;233: 149-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Troxel AB, Fairclough DL, Curran D, Hahn E. Statistical analysis of quality of life with missing data in cancer clinical trials. Stat Med 1998;17: 653-66 [DOI] [PubMed] [Google Scholar]
- 20.Curran D, Bacchi M, Schmitz SF, Molenberghs G, Sylvester RJ. Identifying the types of missingness in quality of life data from clinical trials. Stat Med 1998;17: 739-56 [DOI] [PubMed] [Google Scholar]
- 21.Curran D, Molenberghs G, Fayers PM, Machin D. Incomplete quality of life data in randomized trials: missing forms. Stat Med 1998;17: 697-709 [DOI] [PubMed] [Google Scholar]
- 22.Fairclough DL. Summary measures and statistics for comparison of quality of life in a clinical trial of cancer therapy. Stat Med 1997;16: 1197-209 [DOI] [PubMed] [Google Scholar]
- 23.Ramsey SD, Andersen MR, Etzioni R, et al. Quality of life in survivors of colorectal carcinoma. Cancer 2000;88: 1294-303 [PubMed] [Google Scholar]
- 24.Italian Group for Evaluation of Outcomes in Oncology (IGEO). Patient compliance with quality of life questionnaires. Tumori 1999;85: 92-5 [PubMed] [Google Scholar]
- 25.Kaasa S, Jensen Hjermstad M, Jordhoy MS, Wisloff F, Loge JH. Compliance in quality of life data: a Norwegian experience. Stat Med 1998;17: 623-32 [DOI] [PubMed] [Google Scholar]
- 26.Raboud JM, Singer J, Thorne A, Schechter MT, Shafran SD. Estimating the effect of treatment on quality of life in the presence of missing data due to drop-out and death. Qual Life Res 1998;7: 487-94 [DOI] [PubMed] [Google Scholar]
- 27.Sprangers MA, Moinpour CM, Moynihan TJ, Patrick DL, Revicki DA, and the clinical significance consensus meeting group. Assessing meaningful change in quality of life over time: a user's guide for clinicians. Mayo Clin Proc 2002;77: 561-71 [DOI] [PubMed] [Google Scholar]
- 28.Feinstein AR. Clinical Epidemiology. The Architecture of Clinical Research. Philadelphia: Saunders, 1985
- 29.Wennberg JE, Gittelsohn AM. Small area variations in health care delivery. Science 1973;182: 1102-8 [DOI] [PubMed] [Google Scholar]
- 30.Kopp I, Koller M, Stinner B, et al. Chirurgische Therapie des Rektumkarzinoms: Abbildung der realen Versorgungssituation im Rahmen einer kreisbezogenen Qualitätssicherungs-Studie. Chirurg 2001;72: 1467-77 [DOI] [PubMed] [Google Scholar]
- 31.Timmreck TC. Health Services Cyclopedic Dictionary—A Compendium of Health-care and Public Health Terminology. Sudbury, MA: Jones Bartlett, 1997
- 32.Hermanek P. Qualitätsmanagement bei Diagnose und Therapie kolorektaler Karzinome. Leber Magen Darm 1996;26: 20-4 [PubMed] [Google Scholar]
- 33.Eigler FW, Gabbert H, Herfarth C, et al. Leitlinien zur Therapie des Rektumkarzinoms. Forum DKG 1997;12: 292-7 [Google Scholar]
- 34.Troidl H, Wechsler AS, McKneally MF. How to choose a relevant endpoint. In: Troidl H, McKneally MF, Mulder DS, Wechsler AS, McPeek B, Spitzer WO, eds. Surgical Research. Basic Principles and Clinical Practice, 3rd edn. New York: Springer, 1998: 303-19
- 35.Gross PA, Greenfield S, Cretin S, et al. Optimal methods for guideline implementation: conclusions from Leeds Castle meeting. Med Care 2001;39: 85-92 [PubMed] [Google Scholar]
- 36.Fallowfield L. Compliance issues in quality of life assessment: Experiences of two cancer research campaign sponsored groups. Stat Med 1998;17: 541-6 [DOI] [PubMed] [Google Scholar]
- 37.Hermanek P, Sobin LH. TNM Classification of Malignant Tumours. Berlin: Springer, 1991
- 38.West SG, Wicklund RA. A Primer of Social Psychological Theories. Monterey: Brooks/Cole, 1980
- 39.Bühl A, Zöfel P. SPSS Version 10: Einführung in die moderne Datenanalyse unter Windows. München: Addison Wesley, 2000
- 40.Wolters U, Wolf T, Stützer H, Schröder T. ASA classification and perioperative variables as predictors of postoperative outcome. Br J Anaesth 1996;77: 217-22 [DOI] [PubMed] [Google Scholar]
- 41.Coebergh JWW, Janssen-Heijnen MLG, Post PN, Razenberg PPA. Serious co-morbidity among unselected cancer patients newly diagnosed in the southeastern part of the Netherlands in 1993–1996. J Clin Epidemiol 1999;52: 1131-6 [DOI] [PubMed] [Google Scholar]
- 42.Hermanek P, Wiebelt H, Staimmer D, Riedl S and the German Study Group Colorectal Carcinoma (SGCRC). Prognostic factors of rectum carcinoma—experience of the German multicentre study SGCRC. Tumori 1995;81(suppl.): 60-4 [PubMed] [Google Scholar]
- 43.Ohmann Ch, Lorenz W. Quantifizierung des perioperativen Risikos. Langenbecks Arch Chir Suppl Kongressbericht 1987;372: 211-16 [DOI] [PubMed] [Google Scholar]
- 44.Arbeitsgemeinschaft Bevölkerungsbezogener Krebsregister in Deutschland in Zusammenarbeit mit dem Robert Koch-Institut. Krebs in Deutschland—Häufigkeiten und Trends. Riegelsberg: Braun Druck, 2002
- 45.Goudet P, Roy P, Arveux I, Cougard P, Faivre J. Population-based study of the treatment and prognosis of carcinoma of the rectum. Br J Surg 1997;84: 1546-50 [PubMed] [Google Scholar]
- 46.Paszat LF, Brundage MD, Groome PA, Schulze K, Mackillop WJ. A population-based study of rectal cancer: Permanent colostomy as an outcome. Int J Radial Oncol Biol Phys 1999;45: 1185-91 [DOI] [PubMed] [Google Scholar]
- 47.Lorenz W, Troidl H, Solomkin JS, et al. Second step: testing—outcome measurements. World J Surg 1999;23: 768-80 [DOI] [PubMed] [Google Scholar]
- 48.Koller M, Lorenz W, Wagner K, et al. Expectations and quality of life of cancer patients undergoing radiotherapy. J R Soc Med 2000;93: 621-8 [DOI] [PMC free article] [PubMed] [Google Scholar]