Abstract
Purpose
To report the 12-months visual and anatomical outcomes of chronic diabetic macular oedema (DMO) treated with ILUVIEN in a real-world clinical practice in a single tertiary referral centre.
Method
Retrospective data collection and analysis of consecutive 28 eyes of 23 diabetic patients received ILUVIEN implant for refractory DMO. Standard assessment included visual acuity (VA), central retinal thickness (CRT), slit-lamp biomicroscopy, and Goldmann tonometry for intraocular pressure (IOP) at 1, 6, and 12 months.
Results
Baseline mean VA was 47 (SD 18) letters improved to 55 (SD 17) letters (P=0.004) at 12 months. VA was improved in 16 eyes (57%), stabilised in 9 eyes (32%), and decreased in 3 eyes (11%). Seven eyes (25%) gained ≥15 letters, and 10 eyes (36%) gained >10 letters from baseline. The percentage of eyes achieved driving vision (≥70 Early Treatment Diabetic Retinopathy Study letters) was doubled from baseline 18 to 36% at 6 months and 32% at 12 months. Mean CRT decreased by 198 μm from baseline 494 μm (SD 191) to 296 μm (SD 121) at 12 months (P<0.001). Two eyes received additional anti-vascular endothelial growth factor injections after 10 months. Complications: Raised IOP in three eyes (11%) controlled with IOP-lowering drops, vitreous haemorrhage in one eye and one endophthalmitis (1 year vision improved to 6/24).
Conclusion
Our real-world results show that the visual and the anatomical improvements achieved by a single ILUVIEN implant injection were maintained up to 12 months with minimal adjunctive therapy. IOP monitoring remains essential in ILUVIEN patients, although our study shows a relatively low risk of IOP elevation post ILUVIEN injection, even in existing controlled ocular hypertension. Our results demonstrate that ILUVIEN is an effective long-term option in treating chronic refractory DMO.
Introduction
Diabetic macular oedema (DMO), as the common complication in diabetic retinopathy, is the leading cause of blindness in the working population among patients aged 20 to 70 years in developed countries.1, 2 The pathophysiology of DMO is a complex process with numerous biochemical and histopathological abnormalities whereby hyperglycaemia initiates molecular pathways leading to dilated capillaries, retinal microaneurysms, and loss of pericytes.3 This results in impairment of the blood–retinal barrier and increased vascular permeability, causing fluid to accumulate in retinal tissue.4, 5, 6 At early disease stages, vascular endothelial growth factor (VEGF) is the major driver of retinal vascular permeability change. However, a large number of physiological and molecular factors, including angiogenesis, inflammation, and oxidative stress, are involved in the pathogenesis of DMO.5, 6, 7, 8 The underlying pathogenesis is usually multifactorial, especially in chronic and refractory DMO.
Current treatment options of DMO include focal/grid macular laser photocoagulation and the use of intravitreal drugs depending on the localisation of DMO. Macular laser photocoagulation was the standard of care for over 30 years, but visual acuity (VA) gain was only modest.9, 10, 11 In recent years, intravitreal drugs targeted towards VEGF has become first-line therapy in foveal-involving DMO. However, the blockade of one single pathway may not represent an optimum treatment strategy as anti-VEGF do not suppress all the inflammatory cytokines and pathways involved in DMO and this may explain the need of frequent retreatment or insufficient response.
There has been interest in intravitreal corticosteroids, which are not only able to attenuate some of the effects driven by overexpression of VEGF but also reduce inflammation, which is recommended for DMO with insufficient response to anti-VEGF agents.12 The two licensed and approved corticosteroid implants in the United Kingdom for treating pseudophakic DMO are Ozurdex (a biodegradable 700 μg of dexamethasone) and ILUVIEN (a non-biodegradable 0.2 μg per day fluocinolone acetonide). Whilst the treatment effect of Ozurdex lasts for up to 6 months, the effect of ILUVIEN is up to three years.13, 14, 15, 16 While the efficacy and safety of ILUVIEN are well studied and established in clinical trials, there is however no long-term real-world published literature available on chronic DMO treated with ILUVIEN.15, 16 We report for the first time a larger series and a longer term of 12-month results on the efficacy and safety of chronic DMO treated with ILUVIEN in a real-world clinical practice in a tertiary referral eye centre in the United Kingdom.
Materials and methods
Retrospective analysis of consecutive 28 eyes of 23 patients treated with ILUVIEN (0.2 μg per day FAc) implant during the period from 2014 April to 2015 April. Data collection was from patients' medical records and included baseline characteristics, general and ocular history, and previous DMO treatment. Patients were examined before ILUVIEN treatment (baseline) and then regularly at 1 (for the detection of early intraocular pressure (IOP) change), 3, 6, 9, and 12 months post ILUVIEN implant. Standard ophthalmic assessment included VA (measured in Snellen), slit-lamp biomicroscopy, central retinal thickness (CRT) measurement using Topcon ocular coherence tomography SD-OCT (3D OCT-2000; Topcon Corporation, Tokyo, Japan) and Goldmann tonometry (Haag-Streit, Koeniz, Switzerland) for IOP.
Using a pre-agreed Microsoft Excel form data collection was from patients' medical records of visits at baseline, 1, 6, and 12 months post ILUVIEN implant and ±2 weeks was accepted as visit window. The mean number of follow-up visits in the first year was SD 4±1. The primary end point was the change in VA at 12 months. The other secondary outcomes assessed were the change in CRT, the change in IOP, adverse events, and the need for rescue treatments. Statistical analyses were performed using Wilcoxon's signed-rank test and the paired-sample t-test, with a level of P<0.05 being accepted as statistically significant using SPSS software (version 22.0; SPSS Inc., Chicago, IL, USA) programme. Snellen VA were converted into Early Treatment Diabetic Retinopathy Study (ETDRS) letter scores as described by Gregori et al,17 to facilitate statistical calculation.
The intravitreal procedure of ILUVIEN implant was performed in an aseptic theatre setting and postoperative chloramphenicol eye drops four times a day was advised for 1 week. Four eyes received ILUVIEN implant as a concurrent procedure with phacoemulsification and intraocular lens implant (eyes no. 5, 6, 8 and 28). As this analysis was part of our hospital clinical audit requirement, no ethical approval was needed. All patients received informed consent for investigations and procedures.
Results
Demographic and baseline characteristics are listed in Table 1. Twenty-three patients were identified: 9 (39%) males and 14 (61%) females, with a mean age of 67 (SD 11) years; 19 (83%) and 4 (17%) had type II and type I diabetes mellitus, respectively.
Table 1. Demographic and baseline characteristics.
| Patient number | Gender | Age (years) | Ethnicity | Diabetes type | Eye number | Laterality left/right | DR grade | DMO duration (years) |
Previous treatment for DMO |
IOP-lowering eye drops | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Macular laser /vitrectomised | IVB | IVR | IVTA | Ozurdex | ||||||||||
| 1 | M | 55 | Bangladeshi | I | 1 | L | R2M1P1 | 7 | Focal 1x | 0 | 6x | 0 | 0 | N |
| 2 | F | 82 | Caribbean | II | 2 | R | R3M1P1 | 7 | Focal 3x, grid 2x | 2x | 0 | 1x | 0 | N |
| 3 | L | R2M1P1 | 7 | Focal 1x, grid 2x | 0 | 0 | 2x | 0 | N | |||||
| 3 | F | 81 | Indian | II | 4 | R | R1M1P1 | 6 | Focal 3x, grid 1x | 5x | 6x | 0 | 0 | N |
| 4 | F | 68 | Indian | II | 5 | R | R2M1P1 | 7 | Focal 4x, grid 2x | 3x | 6x | 0 | 1x | N |
| 6 | L | R2M1P1 | 7 | Focal 4x, grid 3x | 3x | 0 | 0 | 1x | N | |||||
| 5 | M | 71 | Bangladeshi | II | 7 | L | R1M1P0 | 2 | No | 0 | 3x | 0 | 0 | N |
| 6 | M | 70 | Caucasian | II | 8 | R | R3M1P1 | 6 | Focal 1x, grid 2x | 6x | 6x | 1x | 0 | N |
| 7 | M | 57 | Caucasian | II | 9 | R | R3M1P1 | 6 | Focal 1x | 3x | 0 | 6x | 0 | Cosopt |
| 10 | L | R3M1P1 | 6 | No | 3x | 0 | 6x | 0 | Cosopt | |||||
| 8 | F | 74 | Pakistani | II | 11 | R | R1M1P1 | 6 | Focal 4x | 0 | 5x | 0 | 0 | N |
| 9 | F | 79 | Caribbean | II | 12 | R | R3M1P1 | 14 | Focal 4x, grid 3x | 6x | 2x | 0 | 0 | N |
| 10 | M | 63 | Caribbean | II | 13 | R | R1M1P1 | 11 | Focal 5x | 3x | 5x | 0 | 0 | Xalatan |
| 11 | M | 48 | Caucasian | I | 14 | L | R3M1P1 | 5 | Focal 1x | 11x | 0 | 7x | 0 | N |
| 15 | R | R3M1P1 | 6 | Grid 1x, vitrectomised | 12x | 0 | 4x | 1x | N | |||||
| 12 | F | 66 | Chinese | II | 16 | R | R3M1P1 | 4 | Grid 1x | 8x | 2x | 1x | 0 | N |
| 13 | F | 59 | Indian | II | 17 | L | R1M1P1 | 5 | Focal 2x, vitrectomised | 4x | 3x | 2x | 0 | N |
| 14 | F | 70 | Pakistani | II | 18 | R | R2M1P1 | 5 | Focal 3x | 0 | 0 | 0 | 0 | N |
| 15 | F | 66 | Caucasian | II | 19 | R | R1M1P1 | 4 | Focal 1x, grid 2x | 3x | 0 | 2x | 0 | N |
| 16 | M | 59 | Caucasian | II | 20 | R | R1M1P0 | 3 | No | 7x | 6x | 0 | 0 | N |
| 17 | F | 53 | Caucasian | I | 21 | L | R3M1P1 | 5 | Grid 1x | 7x | 3x | 2x | 0 | Tiopex |
| 18 | M | 55 | Indian | I | 22 | L | R3M1P1 | 4 | Focal 1x, grid 1x | 2x | 3x | 1x | 0 | Azopt |
| 19 | F | 59 | Other Black background | II | 23 | R | R3M1P1 | 5 | Focal 3x, grid 2x | 3x | 9x | 8x | 0 | N |
| 20 | F | 82 | Caribbean | II | 24 | R | R1M1P1 | 10 | Focal 1x, grid 7x | 6x | 0 | 0 | 0 | Cosopt |
| 21 | F | 82 | Caribbean | II | 25 | R | R1M1P1 | 5 | Focal 1x | 5x | 7x | 2x | 0 | N |
| 26 | L | R1M1P1 | 3 | Focal 1x | 3x | 7x | 2x | 0 | N | |||||
| 22 | F | 68 | Bangladeshi | II | 27 | R | R3M1P1 | 6 | Focal 1x | 3x | 3x | 0 | 0 | N |
| 23 | M | 68 | Caucasian | II | 28 | R | R1M1P1 | 5 | Focal 1x | 10x | 9x | 5x | 0 | Latanoprost |
Abbreviations: DMO, diabetic macular oedema; DR, diabetic retinopathy; F, female; IOP, intraocular pressure; IVB, intravitreal bevacizumab; IVR, intravitreal ranibizumab; IVTA, intravitreal triamcinolone acetonide; L, left; M, male; N, none; R, right; RMP, retinopathy, maculopathy, photocoagulation.
Mean duration of DMO was 6 (SD 2) years before the ILUVIEN implant. Table 1 summarises various treatment modalities and frequencies each patient received before ILUVIEN treatment. Seven eyes (25%) had controlled ocular hypertension (OHT), two eyes (7%) had previous vitrectomy procedure, two eyes (21%) had existing epiretinal membrane and two eyes (7%) also had viteromacular traction.
VA results
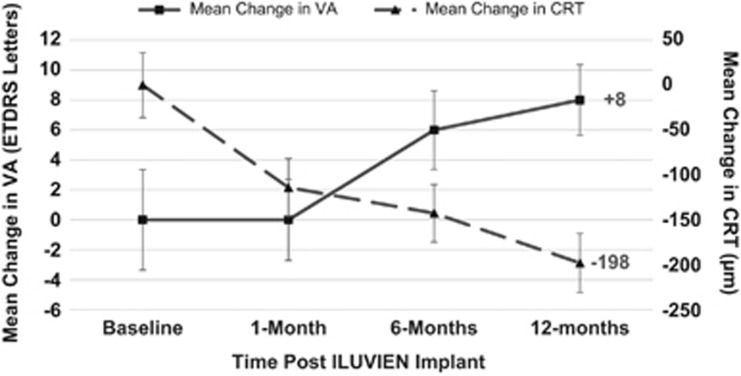
Baseline mean VA was 47 (SD 18) letters, ranged from −4 to 76 letters (Snellen equivalent 1/60 to 6/9), improved to 55 (SD 17) letters at 12 months (Figure 1). Compared with baseline VA, mean VA change was statistically significant at months 6 and 12 but not at first month (Table 2). However, eyes with poor baseline VA because of chronic DMO (ETDRS letter score of ≤35, Snellen equivalent 6/60 or worse) achieved the greatest mean VA improvement at 12 months (+16 letters), and the ‘relatively good' baseline VA group achieved the least VA gain (Table 3).
Figure 1.
Mean changes in VA and CRT compared with baseline.
Table 2. Changes in baseline vision and central retinal thickness.
| Number of eyesa | Mean VA in letters | Mean CRT (μm) | Eyes with VA ≥70 letters (%) |
|---|---|---|---|
| Baseline | |||
| 28 | 47 (SD 18) | 494 (SD 191) | 5/28 (18%) |
| 24 | 46 (SD 19) | 495 (SD 191) | |
| One month post ILUVIEN | |||
| 28 | 47 (SD 21) P=0.913 | 323 (SD 143) P=0.003 | 6/28 (21%) |
| 24 | 46 (SD 21) P=0.797 | 379 (SD 157) P=0.018 | |
| 6 months post ILUVIEN | |||
| 28 | 53 (SD 17) P=0.021 | 351 (SD 149) P<0.001 | 10/28 (36%) |
| 24 | 53 (SD 17) P=0.009 | 358 (SD 161) P=0.002 | |
| 12 months post ILUVIEN | |||
| 28 | 55 (SD 17) P=0.004 | 296 (SD 121) P<0.001 | 9/28 (32%) |
| 24 | 54 (SD 17) P=0.004 | 301 (SD 131) P<0.001 | |
Abbreviations: CRT, central retinal thickness; SD, standard deviation; VA, visual acuity in Early Treatment Diabetic Retinopathy Study (ETDRS) letter score.
Subanalysis of 24 eyes—excluded eyes underwent concurrent procedures of ILUVIEN with phacoemulsification.
Table 3. Mean change in vision at 12 months for different baseline visual acuity.
| Baseline VA | Number of eyes | Mean change in VA compared with baseline |
|---|---|---|
| ≥70 letters (Snellen 6/12 or better) | 5 | +2 letters |
| 36–69 letters (Snellen >6/12 to <6/60) | 11 | +12 letters |
| ≤35 letters (Snellen 6/60 or worse) | 12 | +16 letters |
Abbreviation: VA, visual acuity in Early Treatment Diabetic Retinopathy Study (ETDRS) letter score.
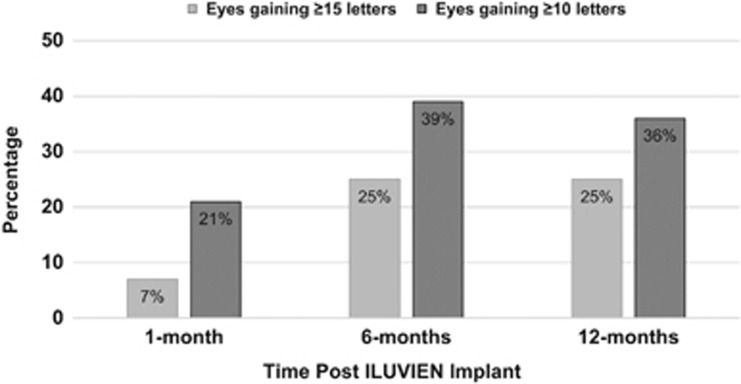
At 12 months VA improved in 16 eyes (57%), stabilised in 9 eyes (32%), and decreased in 3 eyes (11%), the percentage of eyes which gained ≥15 letters was 25% (7 eyes), and 10 eyes (36%) gained >10 letters from baseline (Figure 2). VA decreased only in 3 eyes (eyes no. 3, 5, and 16) at 12 months, with none of them losing more than 11 letters. VA of 35 letters or less was reported in 7 eyes (25%) at 12 months compared with 12 eyes (43%) at baseline.
Figure 2.
Visual outcome post ILUVIEN implant.
CRT results
Mean CRT reduction was statistically significant in all visits compared with the baseline CRT of 494 μm (SD 191); the greatest reduction was by 198 μm (P<0.001) at 12 months (Figure 1 and Table 2). At baseline, only 6 eyes (21%) had CRT <300 μm and 19 eyes (68%) had CRT >400 μm, which reported the greatest reduction in CRT at 12 months. At month 12, CRT reduction was evident in 24 eyes (86%) and increased in 4 eyes (14%).
Adverse events and rescue treatments results
Following ILUVIEN injection, only 3 eyes (11%) had raised IOP ≥10 mmHg at 6 months in eye no. 1, day 3 in eye no.16, and at 3 months in eye no. 21. Vitreous haemorrhage was reported in one eye (4% eye no. 3) after injection, which resolved over few weeks. A poorly controlled diabetic had culture-positive endophthalmitis (eye no.16) received intensive antibiotics treatment and recovered well to 6/24 vision at 1 year. Only two eyes (7%) received rescue treatment in the first year with Ranibizumab injection (at 10 months in eye no. 22 and at 12 months in eye no. 4).
Discussion
Our real-world 12-month results demonstrated compatible and potentially more favourable visual and anatomical outcomes than the pivotal FAME clinical trial at month 12: our study has a mean improvement of +8 letters in VA from baseline compared with +4.9 letters in the FAME trial (also based on chronic DMO subgroup).18 Twenty five per cent in our study gained ≥15 ETDRS letters vs 23.4% in the FAME trial.15 Our study has also shown a greater reduction or improvement of mean change in CRT of minus 198 μm vs minus 156.6 μm in the FAME trial.15 The favourable visual outcome in our study may possibly be explained by a better baseline VA in the FAME trial patients (hence less chance of ‘greater improvement'). In addition, most patients in our study had received previous anti-VEGF treatment unlike patients in the FAME trial.
In our cohort with relatively small numbers of study patients, we found no significant safety concern between eyes received ILUVIEN implant as concurrent procedure with phacoemulsification surgery (eyes no. 5, 6, 8, and 28), and eyes received ILUVIEN alone. Arguably, VA improvement in these four patients could be related to cataract surgery, but the reduction of CRT however is more suggestive of the efficacy of ILUVIEN, as more often DMO are likely to worsen or unchanged following cataract surgery: CRT at baseline vs CRT at 12 months were 825→350 (eye no. 5), 222→240 (eye no. 6), 426→218 (eye no. 8), and 475→246 (eye no. 28). Interestingly, the end statistical results remain unchanged if these four eyes were not included in the main analysis (Table 2). Our real-world study hence suggests the option of ILUVIEN implant may be delivered safely and at the earliest benefit, as a concurrent procedure with the cataract surgery.
As our practice serves a population with diverse ethnicity (Table 1), our study included 30.5% Caucasian, 26% Afro-Caribbean/Africans, and 43.5% Asian. There were also no significant outcome differences among the ethnic groups in this study; however, larger studies would be needed to address these interesting subanalyses.
The percentage of eyes achieved driving vision (70 ETDRS letters or more) was doubled from baseline 18 to 36% at 6 months and 32% at 12 months. This is indeed a very significant and welcome result, especially for the younger diabetics who lead active working lives and are keen to maintain driving. Our study has also shown much reduced clinic visits needed in the first year with a mean of 4±SD1 visits; this is beneficial and appreciated by both patients and helps to relieve the overstretched hospital service from frequent monthly anti-VEGF treatment.19
Secondary OHT with ILUVIEN
Clinicians have indeed been reluctant to apply intraocular or periocular steroid as the first-line treatment for DMO based on the well-known adverse effect of OHT or secondary glaucoma up to 30–40%.15, 19, 20, 21, 22, 23, 24, 25 In our study, 11% (3 eyes) required initiation of IOP-lowering drops compared with 18.4% in the ILUVIEN Registry Safety Study (IRISS) and 22% in the FAME trial.26 The difference is possibly because of the small number of eyes in this study, and the other possibility is that patients already on IOP-lowering drops were excluded from the FAME trial; however, in our study, 25% (7 eyes) were known to have controlled OHT on topical treatment. None of our study eyes needed further invasive glaucoma procedures or surgery in the first year, although eye no. 21 had received selective laser trabeculoplasty at month 16 to maintain IOP control without IOP-lowering drops.
We are not aware of any published literature correlating risk and effect of ILUVIEN implant on patients with OHT. Our study shows the risk of IOP elevation secondary to ILUVIEN implant injection was not higher in patients with controlled OHT compared with patients without OHT. Nevertheless, a recent review of current literature proposed an algorithm to provide guidance for the monitoring and management of IOP following treatments with corticosteroids in DMO, suggesting imaging of optic nerve head fibres and visual field test for at-risk patients before corticosteroid intravitreal injections.27 Intraocular steroid is also associated with additional risk of cataract formation, but it was not shown in this study as ILUVIEN is approved to be used only in pseudophakic DMO eyes in the United Kingdom.
Rescue treatment
In this study, two eyes received additional anti-VEGF injections: eye no. 22 received Ranibizumab at month 10 when CRT did not improve and subsequent further VA reduction; eye no. 4 had initial good response with ILUVIEN with significant VA improvement, but CRT increased after month 6 and hence received intravitreal Ranibizumab injection at month 12. There is no guidance in any existing published literature (based on short-term results) on ‘rescue therapy', including any guidance from the manufacturer. In our series, the decision on ‘rescue therapy' was offered when recorded VA deterioration (5 letters or more, equivalent to one Snellen line) was due to increase in CRT (and CRT was ≥400 μm). Our paper is the first to suggest the window and safety of ‘rescue therapy' after ILUVIEN implant, which is close to 1-year window. Ranibizumab was the choice of ‘rescue therapy' as at the time of study, it was the only licenced anti-VEGF available.
In addition, this study had included two vitrectomised eyes (eyes no. 15 and 17), although the number is too small to draw any definitive conclusion, both vitrectomised eyes have shown a longer lasting favourable CRT reduction without any additional rescue therapy. One of the eyes (eye no.15) in this series had been reported earlier in published case-report.28
Larger real-life outcomes data may explain more the role of macular laser and anti-VEGF as rescue treatment and when to designate a patient as inadequate responder. The main strengths of our study are the availability of longer term result up to 12 months and a larger series representation of ‘real-world' experience in managing chronic struggling DMO and it shows the effect of ILUVIEN implant on patients with OHT. Our study limitations are its retrospective nature with no comparators and using non-refracted Snellen VA scores.
Conclusion
Our real-world single-centre results demonstrate that ILUVIEN offers a significant long-term benefit for patients with chronic DMO inadequately responsive to other available therapies. Favourable visual and anatomical improvements were achieved by a single ILUVIEN implant injection in the first year with minimal adjunctive therapy. Our study also shows relatively low risk of raised IOP following ILUVIEN injection in patients with controlled OHT. However, IOP monitoring remains essential in patients receiving ILUVIEN implant. Longer and larger real-world data may provide a comprehensive long-term management strategy.
Acknowledgments
This study did not receive financial support from any commercial companies.
Footnotes
Poster presentation at Annual RCOphth Congress 2016, Birmingham, UK.
FA, PLL and RC declare no conflict of interest. SE is an Advisory Board Member of and speaker for Alimera Science, Bayer, Novartis, and Alcon. AM is an Advisory Board Member of Alimera Sciences. BM is an Advisory Board Member of and speaker for Alimera Science, Allergan, Bayer, Novartis, and ORAYA therapeutics.
References
- Moss SE, Klein R, Klein BE. Ten-year incidence of visual loss in a diabetic population. Ophthalmology 1994; 101: 1061–1070. [DOI] [PubMed] [Google Scholar]
- Klein R, Lee KE, Gangnon RE, Klein BE. The 25-year incidence of visual impairment in type 1 diabetes mellitus the wisconsin epidemiologic study of diabetic retinopathy. Ophthalmology 2010; 117: 63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonetti DA, Lieth E, Barber AJ, Gardner TW. Molecular mechanisms of vascular permeability in diabetic retinopathy. Semin Ophthalmol 1999; 14: 240–248. [DOI] [PubMed] [Google Scholar]
- Klaassen I, Van Noorden CJ, Schlingemann RO. Molecular basis of the inner blood–retinal barrier and its breakdown in diabetic macular edema and other pathological conditions. Prog Retin Eye Res 2013; 34: 19–48. [DOI] [PubMed] [Google Scholar]
- Ehrlich R, Harris A, Ciulla TA, Kheradiya N, Winston DM, Wirostko B. Diabetic macular oedema: physical, physiological and molecular factors contribute to this pathological process. Acta Ophthalmol 2010; 88: 279–291. [DOI] [PubMed] [Google Scholar]
- Joussen AM, Poulaki V, Le ML, Koizumi K, Esser C, Janicki H et al. A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J 2004; 18: 1450–1452. [DOI] [PubMed] [Google Scholar]
- Funatsu H, Noma H, Mimura T, Eguchi S, Hori S. Association of vitreous inflammatory factors with diabetic macular edema. Ophthalmology 2009; 116: 73–79. [DOI] [PubMed] [Google Scholar]
- Tang J, Kern TS. Inflammation in diabetic retinopathy. Prog Retin Eye Res 2011; 30: 343–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diabetic Retinopathy Clinical Research NetworkDiabetic Retinopathy Clinical Research NetworkElman MJ, Diabetic Retinopathy Clinical Research NetworkAiello LP, Diabetic Retinopathy Clinical Research NetworkBeck RW, Diabetic Retinopathy Clinical Research NetworkBressler NM, Diabetic Retinopathy Clinical Research NetworkBressler SB et al. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology 2010; 117: 1064–1077 e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heng LZ, Comyn O, Peto T, Tadros C, Ng E, Sivaprasad S et al. Diabetic retinopathy: pathogenesis, clinical grading, management and future developments. Diabetic Medicine 2013; 30: 640–650. [DOI] [PubMed] [Google Scholar]
- Diabetic Retinopathy Clinical Research N. A randomized trial comparing intravitreal triamcinolone acetonide and focal/grid photocoagulation for diabetic macular edema. Ophthalmology 2008; 115: 1447–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart MW. Corticosteroid use for diabetic macular edema: old fad or new trend? Curr Diab Rep 2012; 12: 364–375. [DOI] [PubMed] [Google Scholar]
- Ozurdex Summary of Product Characteristics. Available at: https://www.medicines.org.uk/emc/medicine/23422 (last accessed 3 March 2016).
- ILUVIEN Summary of Product Characteristics. Available at: https://www.medicines.org.uk/emc/medicine/27636 (last accessed 9 November 2015).
- Campochiaro PA, Brown DM, Pearson A, Chen S, Boyer D, Ruiz-Moreno J et al. Sustained delivery fluocinolone acetonide vitreous inserts provide benefit for at least 3 years in patients with diabetic macular edema. Ophthalmology 2012; 119: 2125–2132. [DOI] [PubMed] [Google Scholar]
- Campochiaro PA, Brown DM, Pearson A, Ciulla T, Boyer D, Holz FG et al. Long-term benefit of sustained-delivery fluocinolone acetonide vitreous inserts for diabetic macular edema. Ophthalmology 2011; 118: 626–635. [DOI] [PubMed] [Google Scholar]
- Gregori NZ, Feuer W, Rosenfeld PJ. Novel method for analyzing snellen visual acuity measurements. Retina 2010; 30: 1046–1050. [DOI] [PubMed] [Google Scholar]
- Cunha-Vaz J, Ashton P, Iezzi R, Campochiaro P, Dugel PU, Holz FG et al. FAME Study Group.. Sustained delivery fluocinolone acetonide vitreous implants: long-term benefit in patients with chronic diabetic macular edema. Ophthalmology 2014; 121(10): 1892–1903. [DOI] [PubMed] [Google Scholar]
- Prünte C, Fajnkuchen F, Mahmood S, Ricci F, Hatz K, Studnička J et al. RETAIN Study Group.. Ranibizumab 0.5 mg treat-and-extend regimen for diabetic macular oedema: the RETAIN study. Br J Ophthalmol 2016; 100(6): 787–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer DS, Yoon YH, Belfort R Jr, Bandello F, Maturi RK, Augustin AJ et al. Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology 2014; 121: 1904–1914. [DOI] [PubMed] [Google Scholar]
- Beck RW, Edwards AR, Aiello LP, Bressler NM, Ferris F, Glassman AR et al. Three-year follow-up of a randomized trial comparing focal/grid photocoagulation and intravitreal triamcinolone for diabetic macular edema. Arch Ophthalmol 2009; 127: 245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Googe J, Brucker AJ, Bressler NM, Qin H, Aiello LP, Antoszyk A et al. Randomized trial evaluating short-term effects of intravitreal ranibizumab or triamcinolone acetonide on macular edema after focal/grid laser for diabetic macular edema in eyes also receiving panretinal photocoagulation. Retina 2011; 31: 1009–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callanan DG, Gupta S, Boyer DS, Ciulla TA, Singer MA, Kuppermann BD et al. Dexamethasone intravitreal implant in combination with laser photocoagulation for the treatment of diffuse diabetic macular edema. Ophthalmology 2013; 120: 1843–1851. [DOI] [PubMed] [Google Scholar]
- Ramu J, Yang Y, Menon G, Bailey C, Narendran N, Bunce C et al. A randomized clinical trial comparing fixed vs pro-re-nata dosing of Ozurdex in refractory diabetic macular oedema (OZDRY study). Eye 2015; 29: 1603–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heng LZ, Sivaprasad S, Crosby-Nwaobi R, Saihan Z, Karampelas M, Bunce C et al. A prospective randomised controlled clinical trial comparing a combination of repeated intravitreal Ozurdex and macular laser therapy versus macular laser only in centre-involving diabetic macular oedema (OZLASE study). Br J Ophthalmol 2016; 100(6): 802–807. [DOI] [PubMed] [Google Scholar]
- Taylor S, Chakravarthy U, Bailey C, presented on behalf of the ILUVIEN Registry Safety Study (IRISS) Investigators Group. Changes in intraocular pressure after ILUVIEN (190 micrograms fluocinolone acetonide)—real-world experiences following usage in three European countries. Royal College of Ophthalmologist Annual Meeting; 4–26 May 2016. Birmingham, UK, 2016.
- Goñi FJ, Stalmans I, Denis P, Nordmann JP, Taylor S, Diestelhorst M et al. Elevated intraocular pressure after intravitreal steroid injection in diabetic macular edema: monitoring and management. Ophthalmol Ther 2016; 5(1): 47–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar Anil, AlFahad Q, Mitra A, Elsherbiny S, Lip PL. Intravitreal fluocinolone acetonide (Iluvien) for treatment of refractory diabetic macular oedema in vitrectomised eyes. Eye 2016; 30: 763–764. [DOI] [PMC free article] [PubMed] [Google Scholar]