Abstract
Aims
A considerable proportion of hospitalized patients for acute decompensated heart failure will be readmitted or die in short‐term follow‐up. In the present study, we aimed to assess the role of admission sodium (Na) and uric acid (UA) levels in the prediction of 30 day post‐discharge heart failure readmission or all‐cause mortality in advanced heart failure patients admitted with acute decompensation.
Methods and results
One hundred and forty consecutive advanced heart failure patients who were admitted for a recent cardiac decompensation were enrolled in this prospective study. Serum Na and UA levels remained statistically unchanged during index admission (P = 0. 54 and 0.19, respectively). Within 30 days post‐discharge, composite end point of heart failure rehospitalization or all‐cause death occurred in 62 (44.3%) patients (event group). Length of stay was statistically similar between patients in the event and non‐event groups (P = 0.38). No correlations were also found between length of stay and left ventricular ejection fraction, serum Na, UA, erythrocyte sedimentation rate (ESR), high‐sensitivity C‐reactive protein (hs‐CRP), creatinine, and N‐terminal pro b‐type natriuretic peptide (NT‐proBNP) levels (all P > 0.05). Lower left ventricular ejection fraction and Na and higher UA on admission were significantly associated with 30 day event both in univariate and multivariate analyses.
Conclusions
Given the predictive role of baseline Na and UA for early post‐discharge outcome and the absence of significant changes in their levels during initial hospitalization, admission Na and UA can be considered as prognosticators of acute decompensated heart failure, which their prognostic significance cannot be affected by routine acute heart failure therapy.
Keywords: Acute heart failure, Outcomes, Sodium, Uric acid
Introduction
Acute decompensated heart failure (ADHF) is one of the leading causes of hospitalization in the USA. Annually, more than one million patients are hospitalized with a primary diagnosis of heart failure (HF) in the USA.1 The majority of hospitalized patients with ADHF will be readmitted with recurrent symptoms or will die within a few months. As defined by the Carvedilol prospective randomized cumulative survival (COPERNICUS) study, frequent (three or more) hospitalizations for acute decompensation within a year is one of the major characteristics of advanced HF.2 As a result, a growing body of interest is seen in recent literature investigating the application of various physical examination findings, laboratory measurements, and ultrasonographic and echocardiographic indices in the prediction of both short‐term and long‐term rehospitalization and death in ADHF patients.
The pathophysiology of hyponatremia in HF has been described as a result of renal dysfunction, neurohumoral activation, and associated increases in antidiuretic hormone (ADH), angiotensin II, and norepinephrine levels, which in complex interactions impair water excretion (i.e. dilutional hyponatremia) in an attempt to return perfusion pressure to normal.3 This condition is also exacerbated by diuretic agents, which enhance sodium excretion (i.e. depletional hyponatremia). In a subanalysis from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure registry, on admission, hyponatremia has been reported in ∼20% patients with ADHF.4 Moreover, hospital‐acquired hyponatremia has been reported to occur in approximately 15–25% ADHF patients as a result of decongestive treatment.5, 6 Whatever the aetiology, hyponatremia has been suggested as a predictor of short‐term outcomes in hospitalized patients with worsening HF.7, 8, 9
In addition, the prognostic role of serum uric acid (UA) has been established in patients with chronic HF.10, 11, 12, 13 In a recent investigation in patients with acute HF, admission hyperuricemia was shown to be associated with higher risk of death or HF rehospitalization at 6 months.14
However, adequate evidence regarding the role of admission serum sodium and UA levels in the prediction of short‐term prognosis of advanced HF are still limited. As a result, in the present study, we aimed to assess the role of admission sodium and UA levels, as routine laboratory measurements, in the prediction of 30 day post‐discharge HF readmission or all‐cause mortality in advanced HF patients admitted with acute decompensation. We also assessed the changes in sodium and UA during initial hospitalization to investigate whether adequate HF treatment could cause significant changes in these biochemical measurements. On the other hand, we also investigated the association of length of stay (LOS) with short‐term prognosis of ADHF patients.
Methods
Study population
One hundred and forty consecutive advanced HF patients who were admitted at Rajaei Cardiovascular Medical and Research Center between April 2015 and December 2015 for a recent cardiac decompensation were enrolled in this prospective study. Advanced HF was defined according to the criteria applied by COPERNICUS study2 as one or more of the following: the presence of pulmonary rales, ascites, or peripheral edema; frequent (≥3) hospitalizations for ADHF within the previous year; the need for an intravenous positive inotrope or an intravenous vasodilator within 2 weeks before enrollment; or a left ventricular ejection fraction (LVEF) of equal or less than 15%.
Patients with new onset (de novo) HF, pulmonary hypertension and/or right‐sided HF, end‐stage renal disease, or undergoing renal replacement therapy were excluded. Patients with in‐hospital death or major cardiovascular event (including acute coronary syndromes, endotracheal intubation, invasive or surgical revascularization, cardiac transplantation, and mechanical circulatory support) at the index admission were also excluded.
The present study was conducted in accordance with the ethical guidelines of the Declaration of Helsinki. Institutional Review Boards at Rajaei Cardiovascular Medical and Research Center approved the study protocol, and written informed consent was obtained from all participants.
Laboratory measurements
Serum samples were obtained from an antecubital vein within 30 min from admission and at discharge. All tests were performed at the same day or stored at 2–8 °C for analysis in the next day. Serum creatinine was measured using the photometric Jaffe method with commercially available kits (Pars Azmoon Co., Tehran, Iran). Serum hs‐CRP levels were measured via the immunoturbidimetry method using commercially available hs‐CRP reagent kit (Audit Diagnostics, Cork, Ireland). Serum UA was measured by enzymatic colorimetery method using an autoanalyser (Selectra‐2, Paderno d'Adda, Italy) and commercially available kits (Pars Azmoon Co., Tehran, Iran). Plasma NT‐proBNP levels were measured using the ELISA method (Biomedia Corp, Bratislava, Slovakia).
Outcome measures
The outcome of interest was a post‐discharge 30 day event. Serum Na and UA levels were compared between event and non‐event groups. A 30 day event was defined as a composite of emergency department (ED)/hospital HF readmission or all‐cause mortality within 1–30 days post‐discharge from the index hospitalization. At day 30, study co‐ordinators performed follow‐up telephone interviews to document events. If any event of interest has reported to occurred, it was then verified by assessing the medical records by a registered nurse. Serum Na and UA levels were compared between event and non‐event groups.
The secondary outcomes were changes in laboratory measurements during initial hospitalization (from admission to discharge). Acute kidney injury (AKI) during index admission was defined as a rise in serum creatinine of >0.3 mg/dL or >25% of baseline creatinine level.
The other secondary outcome was LOS during initial hospitalization, which was compared between event and non‐event groups.
Statistical analysis
All data were initially assessed for normality using the Kolmogorov–Smirnov test. Continuous variables with and without normal distribution are presented as means ± standard deviation and median (interquartile range), respectively, and were compared using the Student's t‐test, the Mann–Whitney U‐test, and the Kruskal–Wallis test, as appropriate. Categorical data are presented as numbers and percentages and were compared by the χ 2 test. All reported probability values were two‐tailed, and a P‐value < 0.05 was considered statistically significant. Relationships between LOS and LVEF and laboratory measurements were assessed using Pearson tests. The univariable associations between predictor variables and 30 day event were modelled using logistic regression. Variables with statistical significance of association (i.e. LVEF, Na, and UA) were included in multivariable logistic regression model.
Results
Patient demographics
A total of 140 patients with New York Heart Association (NYHA) classes III (n = 83, 59.3%) and IV (n = 57, 40.7%) consisted of 97 (69%) men with a mean age of 49 ± 19 years were included. Median LVEF was 15% (interquartile range (IQR), 10–25%). Baseline characteristics of participants are summarized in Table 1.
Table 1.
Baseline characteristics of the study population
| Variable | All (n = 140) | Event (n = 62) | Non‐event (n = 78) | P‐value |
|---|---|---|---|---|
| Male gender, n | 97 (69) | 43 (69) | 54 (69) | 0.98 |
| Age (years) | 49 ± 19 | 47 ± 20 | 52 ± 19 | 0.10 |
| Left ventricular ejection fraction, % | 15 (10–25) | 12 (10–20) | 20 (15–25) | 0.002 |
| New York Heart Association class, n | 0.19 | |||
| III | 83 (59) | 33 (53) | 50 (64) | |
| IV | 57 (41) | 29 (47) | 28 (36) | |
| Aetiology of heart failure, n | 0.21 | |||
| Ischaemic | 56 (40) | 26 (42) | 30 (38.5) | |
| Idiopathic | 84 (60) | 36 (58) | 48 (61.5) | |
| Risk factors, n | ||||
| Diabetes mellitus | 28 (20) | 12 (19) | 16 (20.5) | 0.43 |
| Hypertension | 22 (16) | 9 (14.5) | 13 (16.5) | 0.68 |
| Dyslipidemia | 44 (31.5) | 17 (27.5) | 27 (34.5) | 0.07 |
| Cigarette smoking | 32 (23) | 13 (21) | 19 (24) | 0.49 |
| Treatment, n | ||||
| Diuretic | 136 (97) | 61 (98) | 75 (96) | 0.52 |
| Angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker | 118 (84) | 54 (87) | 64 (82) | |
| Beta blocker | 119 (85) | 52 (84) | 67 (86) | |
| Spironolactone | 83 (59) | 35 (56) | 48 (61.5) | |
| Inotropes | 5 (3.5) | 4 (6) | 1 (1) | |
| (Dopamine and milrinone) | ||||
| Digoxin | 29 (21) | 21 (34) | 8 (10) | |
| Calcium channel blocker | 0 (0) | 0 (0) | 0 (0) | |
| Cardiac resynchronization therapy (CRT) | 12 (8.5) | 4 (6) | 8 (10) | |
| Implantable cardiac device (ICD) | 23 (16.5) | 9 (14.5) | 14 (18) | |
| Ventricular assisted device (VAD) | 0 (0) | 0 (0) | 0 (0) | |
| Length of stay (days) | 12 (8–16) | 12 (7–15) | 12 (8–19) | 0.38 |
| Laboratory measurements | ||||
| White blood cell (cells/mcl) | 8100.00 (6100–10 500) | 8100 (7000–10 400) | 8100 (5700–10 800) | 0.39 |
| Haemoglobin, g/dL | 13.40 (12.10–14.10) | 13.40 (12.25–14.10) | 13.40 (11.90–14.00) | 0.40 |
| ESR, mm/h | 9.5 (6–16.75) | 9 (6–23) | 10 (6–15) | 0.54 |
| Hs‐CRP, mg/dL | 6 (5–12.25) | 6 (5–13) | 6 (5–10.75) | 0.75 |
| Na, mEq/L | 135 (131–137) | 133 (130–135.25) | 136 (131–139) | <0.001 |
| Uric acid, mg/dL | 8.0 (7.0–8.8) | 8.0 (7.8–9.8) | 7.75 (7–8.62) | <0.001 |
| Creatinine, mg/dL | 1.2 (0.9–1.6) | 1.3 (0.9–1.6) | 1.1 (0.9–1.5) | 0.13 |
| NT‐proBNP, ng/dL | 7632 (2263–15 839) | 8750 (2555–18 600) | 7620 (2247.25–11 442.75) | 0.13 |
| Prolactin, ng/mL | 17.67 (14.40–22.80) | 19.67 (15.71–21.07) | 15.93 (11.84–24) | 0.058 |
| Thyroid‐stimulating hormone (TSH), mU/L | 2.95 (1.32–4.50) | 3.25 (1.82–4.40) | 2.8 (1.25–4.57) | 0.66 |
| Vitamin D, ng/mL | 15.90 (11.22–36.85) | 13.30 (9.80–43.20) | 16.40 (13–36.40) | 0.68 |
The 30 day heart failure rehospitalization or all‐cause mortality prediction
Within 30 days post‐discharge, composite end point of HF rehospitalization or all‐cause death occurred in 62 (44.3%) patients (event group). Demographic and biochemical variables are compared between event and non‐event groups (Table 1).
Lower LVEF, hyponatremia, and hyperuricemia on admission were significantly associated with the 30 day event both in univariate and multivariate analyses (Table 2).
Table 2.
Logistic regression modelsa
| Univariable model | Multivariable model | |||||
|---|---|---|---|---|---|---|
| OR | 95% Confidence interval | P‐value | OR | 95% Confidence interval | P‐value | |
| Age | 0.986 | 0.969–1.004 | 0.127 | |||
| Left ventricular ejection fraction | 0.944 | 0.903–0.986 | 0.010 | 0.951 | 0.908–0.997 | 0.035 |
| New York Heart Association function class | 1.569 | 0.795–3.099 | 0.194 | |||
| White blood cell | 1.000 | 1.000–1.000 | 0.267 | |||
| Haemoglobin | 1.187 | 0.889–1.586 | 0.245 | |||
| Na | 0.892 | 0.829–0.961 | 0.002 | 0.905 | 0.839–0.977 | 0.010 |
| ESR | 1.018 | 0.974–1.064 | 0.434 | |||
| Hs‐CRP | 0.997 | 0.960–1.035 | 0.866 | |||
| NT‐proBNP | 1.000 | 1.000–1.000 | 0.188 | |||
| Prolactin | 0.998 | 0.974–1.022 | 0.867 | |||
| Uric acid | 1.320 | 1.074–1.622 | 0.008 | 1.276 | 1.031–1.580 | 0.025 |
| Creatinine | 1.474 | 0.714–3.046 | 0.294 | |||
| Acute kidney injuryb | 1.725 | 0.879–3.386 | 0.113 | |||
| Length of stay | 0.969 | 0.907–1.035 | 0.350 | |||
Dependent variable: 30 day event (composite of rehospitalization or death).
During index hospitalization.
Comparisons of admission of LVEF, Na, and UA values between event and non‐event groups are depicted in Figure 1.
Figure 1.
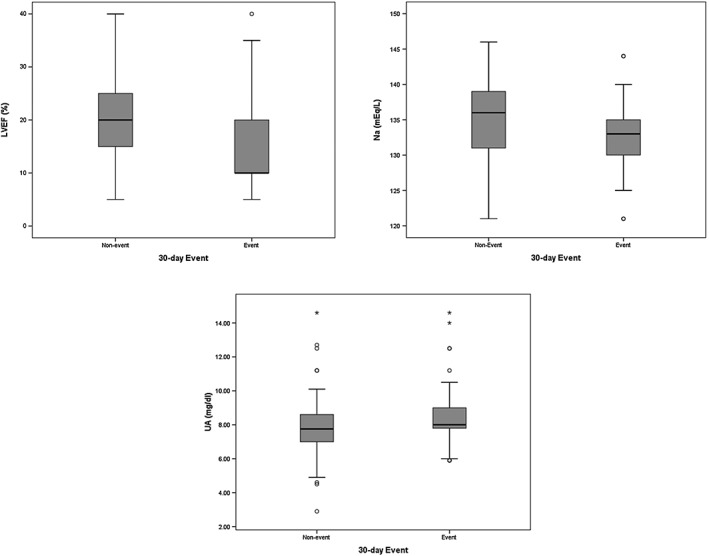
The left ventricular ejection fraction (LVEF, %), sodium (Na, mEq/L), and uric acid (UA, mg/dL) in patients with and without the 30‐day event (heart failure re‐hospitalization or all‐cause death).
Changes during initial hospitalization
On admission, biochemical measurements were compared with discharge values in Table 3. The only laboratory measurement that significantly changed during hospitalization was serum creatinine, which increased from 1.20 mg/dL (0.90–1.60) to 1.40 mg/dL (1.30–1.64) (P < 0.001). Because the changes in serum creatinine during hospitalization were statistically significant, the incidence of AKI was determined and compared between event and non‐event groups. AKI was seen in 31 (39.74%) patients in the non‐event group and 33 (53.22%) patients in the event group (P = 0.12).
Table 3.
Changes in laboratory measurements during index hospitalization
| On admission | At discharge | P‐value | |
|---|---|---|---|
| White blood cell | 8100.00 (6100.00–10 500.00) | 8350.00 (6725.00–11 050.00) | 0.96 |
| Haemoglobin | 13.4 (12.10–14.10) | 11.80 (11.00–13.80) | 0.10 |
| Sodium | 135 (131–137) | 134 (132–137.7) | 0.54 |
| Uric acid | 8.00 (7.00–8.80) | 8.00 (7.50–9.00) | 0.19 |
| ESR | 11.00 (6.00–20.00) | 12.00 (9–18.75) | 0.39 |
| Hs‐CRP | 9.50 (6.00–16.75) | 9.00 (6.00–13.75) | 0.14 |
| Prolactin | 17.67 (14.40–22.80) | 18.50 (14.00–25.02) | 0.15 |
| NT‐ProBNP | 7632.00 (2263.00–15 839.00) | 8123.50 (2555.00–12 060.25) | 0.76 |
| Creatinine | 1.20 (0.90–1.60) | 1.40 (1.30–1.64) | <0.001 |
Length of stay
Length of stay was statistically similar between patients in the event [12 (7–15)] and non‐event groups [12 (8–19)] (P = 0.38). No correlations were found between LOS and LVEF (r = 0.03, P = 0.73), Na (r = −0.16, P = 0.14), UA (r = −0.01, P = 0.92), ESR (r = 0.09, P = 0.41), hs‐CRP (r= −0.03, P = 0.78), creatinine (r = 0.04, P = 0.66), and NT‐proBNP (r = 0.13, P = 0.25).
Discussion
In the present study, we assessed the role of baseline LVEF, NYHA function class, and biochemical measurements in the prediction of early post‐discharge event in patients admitted with ADHF. Our results showed that despite symptomatic improvement in all patients admitted with ADHF during a median LOS of 12 days,8, 9, 10, 11, 12, 13, 14, 15, 16 30 day rehospitalization or death occurred in nearly half of patients (44.3%). The main finding of our study was that admission LVEF, serum Na, and UA levels were independent predictors of HF readmission or all‐cause death during a 30 day period after discharge.
The vast majority of patients who are admitted with primary diagnosis of ADHF symptomatically improve during hospitalization; however, rehospitalization and death are seen in a substantial proportion of these patients during the first few weeks of discharge. Gheorghiade and his colleagues showed that deterioration in signs and symptoms, neurohormonal profile, and renal function during the first few weeks after discharge (‘vulnerable’ phase) is responsible for rehospitalization or death during the early post‐discharge phase.15 They demonstrated that this deterioration occurs despite standard HF therapy, including beta‐blockers, angiotensin‐converting enzyme inhibitors, or angiotensin receptor blocker, and aldosterone‐blocking agents.15 Accordingly, a growing body of research is trying to characterize this population of patients who are at increased risk of early post‐discharge mortality and hospitalization. Presumed prognosticators comprise a wide range of history and physical examination findings, laboratory measurements, electrocardiographic and echocardiographic indices, and ultrasound assessments and include anaemia, diabetes mellitus, new sustained arrhythmias, non‐use of neurohormonal antagonists, presence of coronary heart disease (CHD), jugular venous distension, admission systolic blood pressure, serum albumin levels, lymphocyte counts, troponin release, blood urea nitrogen (BUN) and BUN/creatinine ratio, natriuretic peptides, 6‐min walk distance (6MWD), LVEF, pulmonary capillary wedge pressure, diameter of inferior vena cava, and diuretic response and hemoconcentration during hospitalization among many others.16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28 However, there is no general consensus regarding the majority of these indices, and application of most of them is subject to several disadvantages in routine clinical practice.
In the present investigation, we showed that admission serum Na and UA are independent predictors of early post‐discharge outcome. We showed that a single measurement of Na and UA upon admission can predict the 30 day event both in univariate and multivariate analyses. Similarly, Davison and his colleagues in an analysis on 1990 patients enrolled in the PROTECT (Placebo‐Controlled Randomized Study of the Selective A1 Adenosine Receptor Antagonist Rolofylline for Patients Hospitalized With Acute Decompensated Heart Failure and Volume Overload to Assess Treatment Effect on Congestion and Renal Function) study showed that lower sodium was associated with both 30 day HF rehospitalization and 90 day death.29
In addition, our results demonstrated that although HF treatment during initial hospitalization resulted in symptomatic improvement in ADHF patients, the serum Na and UA levels did not change significantly during admission. However, the evidence to support vigorous treatment of mild‐to‐moderate hyponatremia with sodium, fluid restriction, and vasopressin receptor antagonist (either a V 2 receptor selective or non‐selective vasopressin antagonist) in ADHF patients and their beneficial effects on short‐term or long‐term outcome is inconclusive.30, 31 Thus, currently, the main indications for specific therapy to correct hyponatremia are a serum sodium concentration below 120 mEq/L (severe hyponatremia) and/or the presence of symptoms that might be due to hyponatremia.
Furthermore, our results demonstrated that event and non‐event groups had comparable LOS during initial hospitalization. Recently, results from the PROTECT study also revealed that longer LOS is a strong predictor of 90 day post‐discharge mortality but not of 30 day HF readmission.29 Their results also demonstrated that longer LOS was association with more severe HF (assessed by orthopnea and jugular venous pressure), history of ischaemic heart disease, history of diabetes mellitus, and higher BUN and UA.29 However, our results found no association between LOS and none of LVEF, Na, UA, creatinine, NT‐proBNP, and inflammatory markers.
In addition, to investigate the effects of AHF therapy on biochemical measurements, we assessed their changes during initial hospitalization. We showed that AHF therapy did not changed serum levels of Na, UA (as predictors of early outcome in our study), and NT‐proBNP level or inflammatory markers. The only laboratory measurement that significantly changed during initial hospitalization was the serum creatinine level from 1.20 mg/dL (0.90–1.60) to 1.40 mg/dL (1.30–1.64) giving rise to the occurrence of AKI in 64 (46%) patients. However, the incidence of AKI was comparable in both event and non‐event groups.
Study limitations
The present study had a small size. It was conducted in a referral centre including relatively advanced HF patients [median LVEF of 15 (10–25)] with a substantial proportion of patients being in the NYHA function class of IV. The resultant selection bias may overestimate the LOS as well as the incidence of the 30 day event in AHDF patients compared with previous reports and may limit the generalizability of our findings in all AHDF patients.
Conclusions
Given the predictive role of baseline Na and UA for early post‐discharge outcome and the absence of significant changes in their levels during initial hospitalization, admission Na and UA can be considered as prognosticators of ADHF, which their prognostic significance cannot be affected by routine AHF therapy. In addition, serum Na and UA are low‐cost, easily available, and routine biochemical measurements in any ED and/or hospital settings in both developing and developed countries. These characteristics make Na and UA as unique prognosticators that can be used efficiently in routine clinical practice.
Conflict of interest
None declared.
Funding
None.
Amin, A. , Chitsazan, M. , Shiukhi Ahmad Abad, F. , Taghavi, S. , and Naderi, N. (2017) On admission serum sodium and uric acid levels predict 30 day rehospitalization or death in patients with acute decompensated heart failure. ESC Heart Failure, 4: 162–168. doi: 10.1002/ehf2.12135.
References
- 1. Blecker S, Paul M, Taksler G, Ogedegbe G, Katz S. Heart failure—associated hospitalizations in the United States. J Am Coll Cardiol 2013; 61: 1259–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Packer M, Coats AJ, Fowler MB, Katus HA, Krum H, Mohacsi P, Rouleau JL, Tendera M, Castaigne A, Roecker EB, Schultz MK, DeMets DL; Carvedilol Prospective Randomized Cumulative Survival Study Group . Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med 2001; 344: 1651–1658. [DOI] [PubMed] [Google Scholar]
- 3. Verbrugge FH, Steels P, Grieten L, Nijst P, Tang WH, Mullens W. Hyponatremia in acute decompensated heart failure: depletion versus dilution. J Am Coll Cardiol 2015; 65: 480–492. [DOI] [PubMed] [Google Scholar]
- 4. Gheorghiade M, Abraham WT, Albert NM, Gattis Stough W, Greenberg BH, O'Connor CM, She L, Yancy CW, Young J, Fonarow GC; OPTIMIZE‐HF Investigators and Coordinators . OPTIMIZE‐HF investigators and coordinators relationship between admission serum sodium concentration and clinical outcomes in patients hospitalized for heart failure: an analysis from the OPTIMIZE‐HF registry. Eur Heart J 2007; 28: 980–988. [DOI] [PubMed] [Google Scholar]
- 5. Konishi M, Haraguchi G, Ohigashi H, Sasaoka T, Yoshikawa S, Inagaki H, Ashikaga T, Isobe M. Progression of hyponatremia is associated with increased cardiac mortality in patients hospitalized for acute decompensated heart failure. J Card Fail 2012; 18: 620–625. [DOI] [PubMed] [Google Scholar]
- 6. Shchekochikhin DY, Schrier RW, Lindenfeld J, Price LL, Jaber BL, Madias NE. Outcome differences in community—versus hospital‐acquired hyponatremia in patients with a diagnosis of heart failure. Circ Heart Fail 2013; 6: 379–386. [DOI] [PubMed] [Google Scholar]
- 7. Klein L, O'Connor CM, Leimberger JD, Gattis‐Stough W, Piña IL, Felker GM, Adams KF Jr, Califf RM, Gheorghiade M, OPTIME‐CHF Investigators . Lower serum sodium is associated with increased short‐term mortality in hospitalized patients with worsening heart failure: results from the Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure (OPTIME‐CHF) study. Circulation 2005; 111: 2454–2460. [DOI] [PubMed] [Google Scholar]
- 8. Hamaguchi S, Kinugawa S, Tsuchihashi‐Makaya M, Matsushima S, Sakakibara M, Ishimori N, Goto D, Tsutsui H. Hyponatremia is an independent predictor of adverse clinical outcomes in hospitalized patients due to worsening heart failure. J Cardiol 2014; 63: 182–188. [DOI] [PubMed] [Google Scholar]
- 9. Sato N, Gheorghiade M, Kajimoto K, Munakata R, Minami Y, Mizuno M, Aokage T, Asai K, Sakata Y, Yumino D, Mizuno K, Takano T, ATTEND Investigators . Hyponatremia and in‐hospital mortality in patients admitted for heart failure (from the ATTEND registry). Am J Cardiol 2013; 111: 1019–1025. [DOI] [PubMed] [Google Scholar]
- 10. Tamariz L, Harzand A, Palacio A, Verma S, Jones J, Hare J. Uric acid as a predictor of all‐cause mortality in heart failure: a meta‐analysis. Congest Heart Fail 2011; 17: 25–30. [DOI] [PubMed] [Google Scholar]
- 11. Kim H, Shin HW, Son J, Yoon HJ, Park HS, Cho YK, Han CD, Nam CW, Hur SH, Kim YN, Kim KB. Uric acid as prognostic marker in advanced nonischemic dilated cardiomyopathy: comparison with N‐terminal pro B‐type natriuretic peptide level. Congest Heart Fail 2010; 16: 153–158. [DOI] [PubMed] [Google Scholar]
- 12. Levy WC, Mozaffarian D, Linker DT, Sutradhar SC, Anker SD, Cropp AB, Anand I, Maggioni A, Burton P, Sullivan MD, Pitt B, Poole‐Wilson PA, Mann DL, Packer M. The Seattle Heart Failure Model: prediction of survival in heart failure. Circulation 2006; 113: 1424–1433. [DOI] [PubMed] [Google Scholar]
- 13. Manzano L, Babalis D, Roughton M, Shibata M, Anker SD, Ghio S, van Veldhuisen DJ, Cohen‐Solal A, Coats AJ, Poole‐Wilson PP, Flather MD, SENIORS Investigators . Predictors of clinical outcomes in elderly patients with heart failure. Eur J Heart Fail 2011; 13: 528–536. [DOI] [PubMed] [Google Scholar]
- 14. Palazzuoli A, Ruocco G, Pellegrini M, Beltrami M, Giordano N, Nuti R, McCullough PA. Prognostic significance of hyperuricemia in patients with acute heart failure. Am J Cardiol 2016; 117: 1616–1621. [DOI] [PubMed] [Google Scholar]
- 15. Gheorghiade M, Filippatos G, Pang PS, Blair JEA, Burnett JC Jr., Grinfeld L, Maggioni AP, Swedberg K, Udelson JE, Zannad F, Traver TCB, Krasa H, Zimmer C, Konstam M. Changes in clinical, neurohormonal, electrolyte, renal, and hepatic profiles during and after hospitalization for acute decompensated heart failure: analysis from the EVEREST trial (late breaking clinical trial). Paper presented at: European Society of Cardiology; September 2, 2008; Munich, Germany.
- 16. Fujino M, Takahama H, Hamasaki T, Sekiguchi K, Kusano K, Anzai T, Noguchi T, Goto Y, Kitakaze M, Yokoyama H, Ogawa H, Yasuda S. Risk stratification based on nutritional screening on admission: three‐year clinical outcomes in hospitalized patients with acute heart failure syndrome. J Cardiol 2016; 68: 392–398. [DOI] [PubMed] [Google Scholar]
- 17. Gheorghiade M, Klein L, Abraham W, Albert NM, Greenberg BH, O'Connor CM, She L, Stough S, Yancy CW, Young JB, Fonarow GC. The relation between admission systolic blood pressure and outcomes in hospitalized patients with heart failure with reduced or preserved systolic function: an OPTIMIZE‐HF analysis. Circulation 2005; 112(suppl II): II‐599. Abstract. [Google Scholar]
- 18. Horwich TB, Patel J, MacLellan WR, Fonarow GC. Cardiac troponin I is associated with impaired hemodynamics, progressive left ventricular dysfunction, and increased mortality rates in advanced heart failure. Circulation 2003; 108: 833–838. [DOI] [PubMed] [Google Scholar]
- 19. Aronson D, Mittleman MA, Burger AJ. Elevated blood urea nitrogen level as a predictor of mortality in patients admitted for decompensated heart failure. Am J Med 2004; 116: 466–473. [DOI] [PubMed] [Google Scholar]
- 20. Felker GM, Leimberger JD, Califf RM, Cuffe MS, Massie BM, Adams KF Jr, Gheorghiade M, O'Connor CM. Risk stratification after hospitalization for decompensated heart failure. J Card Fail 2004; 10: 460–466. [DOI] [PubMed] [Google Scholar]
- 21. Lee DS, Austin PC, Rouleau JL, Liu PP, Naimark D, Tu JV. Predicting mortality among patients hospitalized for heart failure: derivation and validation of a clinical model. JAMA 2003; 290: 2581–2587. [DOI] [PubMed] [Google Scholar]
- 22. Klein L, O'Connor CM, Leimberger JD, Gattis‐Stough W, Pina IL, Felker GM, Adams KF Jr, Califf RM, Gheorghiade M, for the OPTIME‐CHF Investigators . Lower serum sodium is associated with increased short‐term mortality in hospitalized patients with worsening heart failure: results from the OPTIME‐CHF study. Circulation 2005; 111: 2454–2460. [DOI] [PubMed] [Google Scholar]
- 23. Maisel A, Hollander JE, Guss D, McCullough P, Nowak R, Green G, Saltzberg M, Ellison SR, Bhalla MA, Bhalla V, Clopton P, Jesse R, for the Rapid Emergency Department Heart Failure Outpatient Trial Investigators . Primary results of the Rapid Emergency Department Heart Failure Outpatient Trial (REDHOT): a multicenter study of B‐type natriuretic peptide levels, emergency department decision making, and outcomes in patients presenting with shortness of breath. J Am Coll Cardiol 2004; 44: 1328–1333. [DOI] [PubMed] [Google Scholar]
- 24. Felker GM, Gattis WA, Leimberger JD, Adams KF, Cuffe MS, Gheorghiade M, O'Connor CM. Usefulness of anemia as a predictor of death and rehospitalization in patients with decompensated heart failure. Am J Cardiol 2003; 92: 625–628. [DOI] [PubMed] [Google Scholar]
- 25. Benza RL, Tallaj JA, Felker GM, Zabel KM, Kao W, Bourge RC, Pearce D, Leimberger JD, Borzak S, O'Connor CM, Gheorghiade M, for the OPTIME‐CHF Investigators . Arrhythmias in acute decompensated heart failure: results from the OPTIME‐CHF study. J Card Fail 2004; 10: 279–284. [DOI] [PubMed] [Google Scholar]
- 26. Chernomordik F, Berkovitch A, Schwammenthal E, Goldenberg I, Rott D, Arbel Y, Elis A, Klempfner R. Short‐ and long‐term prognostic implications of jugular venous distension in patients hospitalized with acute heart failure. Am J Cardiol 2016; 118: 226–231. [DOI] [PubMed] [Google Scholar]
- 27. Ter Maaten JM, Valente MA, Damman K, Cleland JG, Givertz MM, Metra M, O'Connor CM, Teerlink JR, Ponikowski P, Bloomfield DM, Cotter G, Davison B, Subacius H, van Veldhuisen DJ, van der Meer P, Hillege HL, Gheorghiade M, Voors AA. Combining diuretic response and hemoconcentration to predict rehospitalization after admission for acute heart failure. Circ Heart Fail. 2016. jun; 9. pii: e002845. doi: 10.1161/CIRCHEARTFAILURE.115.002845. [DOI] [PubMed] [Google Scholar]
- 28. Cubo‐Romano P, Torres‐Macho J, Soni NJ, Reyes LF, Rodríguez‐Almodóvar A, Fernández‐Alonso JM, González‐Davia R, Casas‐Rojo JM, Restrepo MI, de Casasola GG. Admission inferior vena cava measurements are associated with mortality after hospitalization for acute decompensated heart failure. J Hosp Med 2016. Jun 6. doi: 10.1002/jhm.2620. [DOI] [PubMed] [Google Scholar]
- 29. Davison BA, Metra M, Senger S, Edwards C, Milo O, Bloomfield DM, Cleland JG, Dittrich HC, Givertz MM, O'Connor CM, Massie BM, Ponikowski P, Teerlink JR, Voors AA, Cotter G. Patient journey after admission for acute heart failure: length of stay, 30‐day readmission and 90‐day mortality. Eur J Heart Fail 2016; 18: 1041–1050. [DOI] [PubMed] [Google Scholar]
- 30. Travers B, O'Loughlin C, Murphy NF, Ryder M, Conlon C, Ledwidge M, McDonald K. Fluid restriction in the management of decompensated heart failure: no impact on time to clinical stability. J Card Fail 2007; 13: 128–132. [DOI] [PubMed] [Google Scholar]
- 31. Aliti GB, Rabelo ER, Clausell N, Rohde LE, Biolo A, Beck‐da‐Silva L. Aggressive fluid and sodium restriction in acute decompensated heart failure: a randomized clinical trial. JAMA Intern Med 2013; 173: 1058–1064. [DOI] [PubMed] [Google Scholar]


