Abstract
Aims
There are limited data on the effect of low‐dose, intermittent inotropic therapy in an outpatient setting on the quality of life (QOL) in patients with advanced refractory heart failure (HF) symptoms. We aimed to analyse the effect of this treatment modality on QOL and subsequent survival.
Methods and results
The study population comprised 287 consecutive patients with advanced refractory HF symptoms who were treated with low‐dose, intravenous intermittent inotropic therapy in the HF Day Care Service at Sheba Medical Centre between September 2000 and September 2012. All patients completed a baseline Minnesota Living with Heart Failure Questionnaire (MLWHFQ), and 137 (48%) completed a 1 year follow‐up questionnaire. MLWHFQ scores' means ranged from 0 (better QOL) to 5 (worse QOL). Mean age was 68 ± 12, 86% were men, 77% had ischaemic cardiomyopathy, and the mean left ventricle ejection fraction (LVEF) was 26% ± 13. The mean baseline MLWHFQ score was 3.1 (±1), while the mean at 1 year of treatment was of 2.7 (±1.1), indicating an overall improvement in QOL associated with intermittent low‐dose inotrope therapy (p < 0.01). Multivariate analysis showed that younger age, non‐ischaemic cardiomyopathy, and worse renal function were independently associated with improvement in QOL at 1 year. Improvement in QOL was not associated with a significant survival benefit during subsequent follow‐up.
Conclusions
In patients with advanced refractory HF symptoms, treatment with low‐dose, intermittent intravenous inotropes in an outpatient setting is associated with significant improvement in QOL. However, improvement in QOL in this population does not appear to affect subsequent long‐term survival.
Keywords: Heart failure, Intermittent inotropes, Quality of life, Survival
Introduction
The prevalence of heart failure (HF) is gradually increasing, and it is a major reason for recurrent hospitalisations and mortality.1 Various estimates indicate that 1–2% of the world population in developed countries suffers from this syndrome, and HF rates after the age of 85 years may increase to over 10%.2
A subgroup of patients suffering from chronic HF may develop severe symptoms despite maximal optimal medical and interventional therapy.3 In this high‐risk population, treatment with inotropic drugs is associated with improvement of their haemodynamic profile. However, their positive impact on outcomes in patients with HF, whether given as continuous or as intermittent infusion, has not been proved yet.4, 5, 6, 7, 8, 9, 10, 11, 12, 13
Studies that examined the impact of inotropic treatment on outcomes of HF patients are scarce and had important limitations, including utilization of relatively high doses of inotropic agents, which may increase arrhythmic risk and overall mortality, low use of automated implantable cardioverter defibrillator (ICDs), and the evaluation of all‐cause mortality as the sole end point. Importantly, currently there is limited data on the effect of intermittent inotropic therapy on the quality of life (QOL) in patients with advanced refractory HF symptoms, which is a major therapeutic target in this population.
Accordingly, the present study was carried out in a population of patients with advanced refractory HF symptoms who received low‐dose intermittent inotropic therapy in a tertiary outpatient HF centre, and was designed to evaluate: (i) the effect of treatment with intermittent low‐dose intravenous dopamine or dobutamine on QOL in advanced HF patients; (ii) factors associated with improvement in QOL following treatment with intermittent inotropic therapy; and (iii) the association between baseline and follow‐up QOL and survival in this population.
Methods
Study population
The present study population comprised all 287 patients admitted to the HF Day Care Service of the Sheba Medical Centre between September 2000 and September 2012. The treatment protocol has been described before.14 Briefly, all patients had advanced HF symptoms (New York Heart Association Functional Class III–IV) despite optimal guideline‐based medical and device therapy. Exclusion criteria to participation in the programme included unstable angina, a history of heart surgery, or acute myocardial infarction of ≤3 months, uncontrolled systemic hypertension > 180/110 mmHg, malignant ventricular arrhythmias on 24 h Holter monitoring, history of current drug or alcohol abuse, inability to participate in the programme, non‐compliance, ongoing infection, or immediate life‐threatening extra cardiac disease or malignancy. All patients completed a baseline Minnesota Living with Heart Failure Questionnaire (MLWHFQ), and 137 (48%) patients who completed one‐year of treatment at the day‐care centre completed also a 1 year follow‐up questionnaire.
Data from the medical records of patients participating in the programme were prospectively collected, documented, and updated into a computerized database. The database included the patient's medical history, coronary artery disease risk factors, left ventricle ejection fraction (LVEF), concomitant medical and device therapy, laboratory results, and all invasive and non‐invasive procedures. The study was approved by the local Ethics Committee.
Quality of life assessment
The QOL of patients at baseline and after 1 year of treatment was assessed using the MLWHFQ. The questionnaire's content reflects most significant aspects of patient's physical and emotional life affected by HF, includes 21 questions, and items are scored from 0 to 5 in a Likert scale. We calculated each patient's mean score at the beginning and after 1 year of treatment when appropriate, and the relative difference of the two means to assess relative improvement or worsening in the QOL. The test's reliability, reproducibility, and internal consistency have been previously described.15, 16, 17
For the analysis of QOL improvement or worsening after 1 year of treatment, we calculated the difference between the MLWHFQ mean after 1 year of treatment and at the beginning of treatment divided by the MLWHFQ mean at 1 year, expressing the ‘percent’ change in QOL. Those with a negative (below ‘0’) value were defined as having improved their QOL (‘improvers’) while those with a positive value (i.e. over ‘0’) were defined as having worsened or ‘non‐improvers’.
Heart failure day care protocol
Treatment schedule was based on one to two visits/week and included an intravenous infusion of furosemide and low‐dose positive inotrope or vasodilator agents. Dobutamine started at 3 µg/kg/min and increased to 5 µg/kg/min. Dopamine 1–3 µg/kg/min was given for dobutamine intolerance and to patients with advanced renal failure and inadequate urinary response. Nitroprusside, up to 60 µg/min, was used as a vasodilator agent. During each session, heart rhythm, blood pressure, capillary oxygen saturation, and urine output were continuously monitored. Renal function tests and electrolytes were determined at each visit. Patients continued to attend the day care sessions as long as they had (i) symptomatic benefit from therapy, and (ii) remained in NYHA class III–IV and required intravenous therapy for their clinical stability. Patients discontinued attendance if they underwent heart transplantation (a detailed complete description of the treatment protocol at the HF day‐care centre is presented in the Appendix S1).
Statistical analysis
Baseline characteristics of the study population are expressed as mean ± SD for continuous variables and as frequencies and percentages for categorical variables. For the purpose of graphically showing our results, in Figure 1 A, we showed the mean MLWHFQ score with the standard error of the mean. Differences between baseline characteristics of HF patients with either better or worse QOL at the beginning of the treatment (defined as MLWHFQ, categorized at the median value) or in subjects who improved or worsened their QOL after 1 year of treatment (as defined above, and dichotomized by the improvement or worsening of their QOL) were generated by using the independent Samples t‐test or χ2 test for continuous or categorical variables, respectively. The statistical difference between baseline and follow‐up QOL was assessed using paired t‐test analysis.
Figure 1.
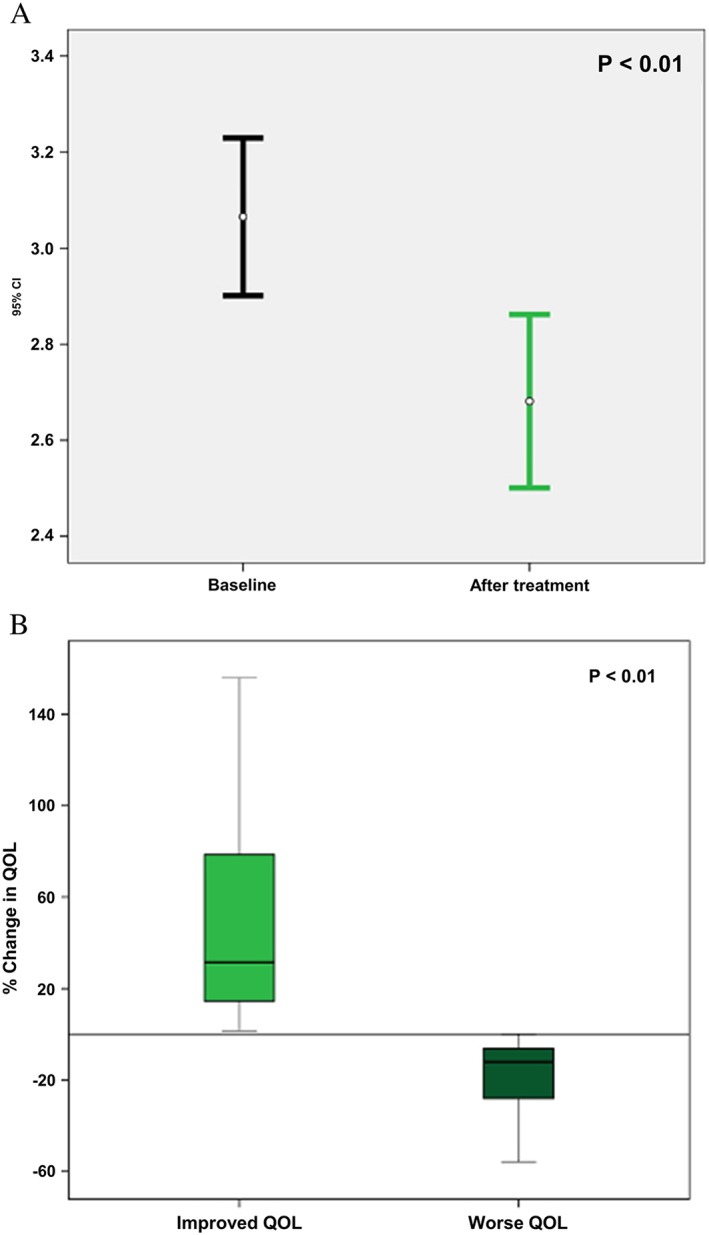
Improvement in quality of life after 1 year of therapy: (A) overall improvement; (B) average change among ‘improvers’ vs. ‘non‐improvers’.
The impact of various variables on improvement in QOL was measured using a logistic regression model. Pre‐specified candidate covariates in the multivariate models are listed in Table 2. Kaplan–Meier survival analysis was carried out in order to evaluate the association between baseline QOL (dichotomized at the median value) and the cumulative probability for all‐cause mortality. ‘Landmark’ analysis was similarly employed to evaluate the association between QOL (dichotomized by improvement vs. non‐improvement of QOL after 1 year of treatment—see above) and subsequent mortality. Multivariate Cox proportional hazards regression modelling was utilized to identify factors independently associated with mortality risk during follow‐up. Pre‐specified candidate covariates in the Cox model are also listed in Table 3. Renal function was expressed as the estimated glomerular filtration rate as measured by creatinine clearance using the Modification of Diet in Renal Disease formula. A p value of <0.05 was considered significant. IBM SPSS Statistics software 22 and SAS version 9.4 were used.
Table 2.
Multivariate analysis: Independent predictors of improvement in QOL after 1 year of low‐dose intermittent intravenous inotropic therapy
| Predictor | Odds ratio | 95% confidence interval | P value |
|---|---|---|---|
| Age (per 1 year decrement) | 1.06 | 1.02–1.10 | 0.02 |
| Non‐ischaemic cardiomyopathy | 2.77 | 1.01–7.51 | 0.04 |
| Creatinine clearance per mL/min/1.73 m2 decrement | 1.03 | 1.01–1.06 | <0.01 |
The model was further adjusted for BMI > 30 kg/m2, treatment with beta blockers, or presence of CRT‐D.
Abbreviations: CRT‐D, Cardiac Resynchronization Therapy‐Defibrillator; QOL, Quality of Life.
Table 3.
Independent predictors of mortality after 1 year of treatment
| Predictor | HR | 95% confidence interval | P value |
|---|---|---|---|
| Creatinine clearance per mL/min/1.73 m2 decrement | 1.02 | 1.01–1.03 | 0.048 |
| Ischaemic cardiomyopathy | 1.93 | 1.09–3.44 | 0.03 |
| Improvement in QOL* | 1.14 | 0.74–1.83 | 0.37 |
The model was further adjusted for age and left ventricular ejection fraction as a continuous variable.
Abbreviation: HR, Hazards ratio; QOL, Quality of Life.
Results
Among the 287 study participants, mean age was 68 years (±12) years and 14% were females. Patients participated in the programme for a mean of 16 months (±21). Mean overall follow‐up time was 3.2 years. Patients attended the outpatient centre for an average of 84 sessions per patient/year. The clinical characteristics of study patients by baseline QOL prior to entering the programme (defined by MLWHFQ score's mean dichotomized at the median value) are presented in Table 1. One hundred and eight patients (38%) were also treated with vasodilators. Compared with those with a high baseline QOL, patients with a worse baseline QOL were significantly younger, had a lower frequency of systemic hypertension, and lower baseline serum sodium levels. There were no statistically significant differences between the two groups in respect of the following variables: sex, mean baseline LVEF, HF function class, BMI, presence of diabetes mellitus, ischaemic aetiology, baseline medications, device therapy, mean time in treatment, and the use of vasodilators during the study period.
Table 1.
Baseline characteristics of study population (N = 287) at the beginning of treatment comparing patients according to their QOL (according to the MLWHFQ's median)
| Worse QOL (median > 3.14) | Better QOL (median ≤ 3.14) | P value | |
|---|---|---|---|
| N = 148 | N = 139 | ||
| Age, mean | 66 (±12) | 70 (±12) | 0.03 |
| Male gender | 126 (85%) | 122 (88%) | 0.51 |
| LVEF % mean | 25 (±12) | 27 (±14) | 0.10 |
| NYHA Functional Class IV | 85 (57%) | 67 (48%) | 0.11 |
| BMI (kg/m2) | 28 (±5) | 28 (±5) | 0.6 |
| Systemic hypertension | 71 (48%) | 90 (65%) | 0.04 |
| Diabetes mellitus | 72 (49%) | 71 (51%) | 0.68 |
| Ischaemic aetiology | 113 (76%) | 108 (78%) | 0.78 |
| Beta‐blockers | 111 (75%) | 101 (73%) | 0.65 |
| Furosemide | 144 (97%) | 137 (99%) | 0.45 |
| ACE‐I | 69 (47%) | 63 (45%) | 0.82 |
| ARB | 41 (28%) | 42 (30%) | 0.64 |
| Digoxin | 80 (54%) | 71 (51%) | 0.6 |
| CRT‐D | 30 (20%) | 36 (26%) | 0.26 |
| Haemoglobin mean (g/dl) | 13 (±2) | 12 (±2) | 0.07 |
| eGFR mean | 46 (±19) | 42 (±23) | 0.16 |
| Sodium (mEq/L) | 137 (±4) | 138 (±4) | 0.03 |
| Mean time in treatment (months) | 17.8 (±22.2) | 13.4 (20.4) | 0.08 |
| Vasodilators | 57 (39%) | 51 (37%) | 0.8 |
Abbreviations: ACEI, angiotensin‐converting‐enzyme inhibitor; ARB, angiotensin‐receptor blocker; BMI, Body Mass Index; CRT‐D, Cardiac Resynchronization Therapy‐Defibrillator; eGFR, Estimated Glomerular Filtration Rate using the MDRD formula; LVEF, Left Ventricle Ejection Fraction; MLWHFQ, Minnesota Living with Heart Failure Questionnaire; NYHA, New York Heart Association; QOL, Quality of Life. [Correction added after online publication on 22 September 2016: bold font removed from Table 1]
Quality of life at 1 year following participation in the heart failure day‐care programme
All 137 study participants who survived for at least 1 year and continued treatment at the HF programme completed the MLWHFQ at the end of that period (48% of the initial cohort). Vasodilators were administered in 61 patients (45%). The MLWHFQ mean score at baseline was 3.1 (±1), while the mean at 1 year of treatment was of 2.7 (±1.1), indicating a mean improvement in QOL of 8.4 MLWHFQ points at 1 year among study patients (p < 0.01, using a paired t‐test, Figure 1 A). Notably, the change in QOL after 1 year of treatment between improvers ranged from 2 to 219%, while in those who worsened, their QOL change ranged from 1% to 93% (p < 0.01, Figure 1 B).
The characteristics of the patients who completed the 1 year QOL follow‐up questionnaire classified according to their change in MLWHFQ score at 1 year (defined as ‘improvers’ vs. ‘non‐improvers’), are presented in Appendix S1. Patients who improved their QOL after 1 year of treatment with intermittent low‐dose inotropic therapy at the HF programme (N = 82) were younger and had a worse baseline functional class compared with ‘non‐improvers’ (N = 55), whereas other clinical characteristics including mean treatment time and the use of intravenous vasodilators during the treatment period were similar between the two groups (Appendix S1)
Multivariate logistic regression analysis showed that independent predictors for improvement in QOL after 1 year of treatment in the HF day‐care centre (Table 2) included a younger age (6% greater likelihood for improvement per 1 year decrement in age; p = 0.02), the presence of non‐ischaemic vs. ischaemic cardiomyopathy (2.8‐fold greater likelihood for improvement; p = 0.04), and reduced renal function (3% greater likelihood for improvement per 1‐unit decrement in the estimated glomerular filtration rate; p < 0.01; Table 2). Other candidate predictors, including obesity, treatment with beta‐blockers, or presence of CRT‐D, were not shown to be significantly associated with improvement in QOL at 1 year among study patients.
Association between baseline and follow‐up quality of life and subsequent mortality
We used Kaplan–Meier analysis to compare survival between patients with a lower vs. higher QOL at baseline, prior to entering the HF day‐care programme (Figure 2). This analysis showed that at 1 year of follow‐up, mortality rates were somewhat higher among patients with a lower baseline QOL (35%) as compared with those with a higher score at baseline (25%). However, at 5 years of follow‐up, the respective rates were virtually identical (78% and 76%, respectively; Log‐Rank p = 0.37 for the overall difference during follow‐up [Figure 2]). Similarly, change in QOL of life after 1 year of treatment at the HF day‐care centre was not associated with significant mortality difference between the groups during subsequent follow‐up. Thus, ‘improvers’ vs. ‘non‐improvers’ after 1 year of treatment experienced similar survival rates during subsequent follow‐up (Figure 3). Consistent with those findings, Cox proportional hazards regression modelling showed that change in QOL at 1 year was not an independent predictor for all‐cause mortality during follow‐up (HR = 1.14; p = 0.37; Table 3). This analysis showed that independent predictors for mortality among study patients included worse renal function and an ischaemic vs. non‐ischaemic aetiology of HF (93% risk‐increase [Table 3]).
Figure 2.
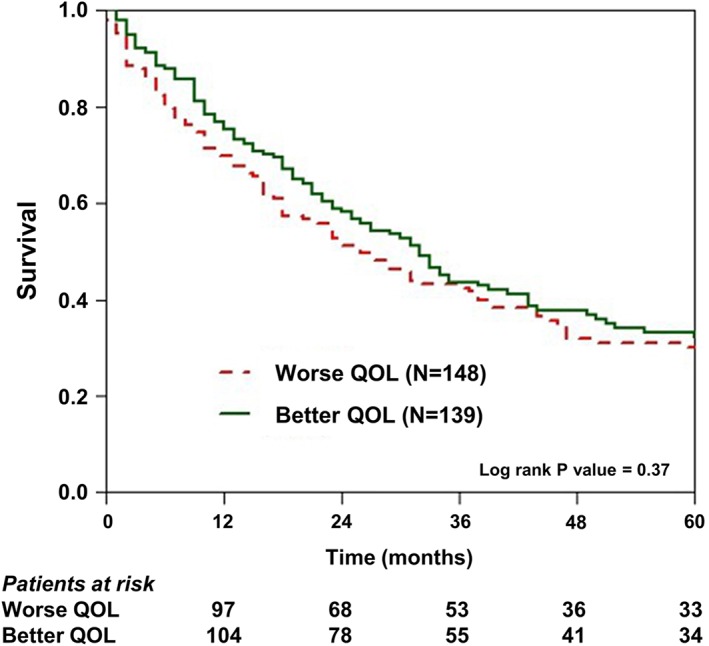
Kaplan–Meier survival estimates of patients with quality of life below and over the median (represented by the Minnesota Living with Heart Failure Questionnaire's mean.
Figure 3.
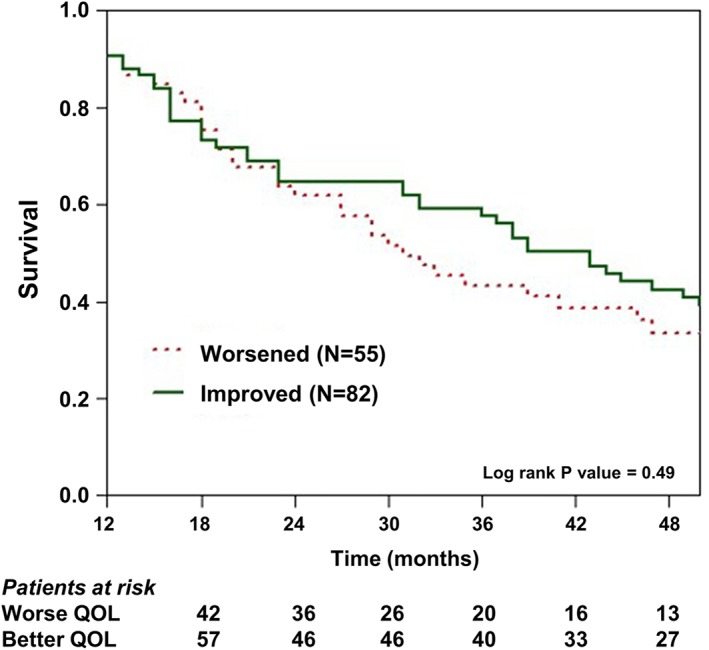
Kaplan–Meier survival estimates of patients after 1 year of treatment, comparing patients by change in quality of life after the programme.
Discussion
To our knowledge, the present study is the first to assess the impact of treatment with low‐dose intermittent inotropic therapy on the QOL of patients with advanced refractory HF, and its relationship to subsequent long‐term survival in a real world setting of patients with advanced refractory HF symptoms. Our results suggest that: (i) treatment with low‐dose inotropic therapy in a tertiary HF clinical is associated with a significant improvement in QOL; (ii) baseline or follow‐up QOL does not appear to be related to survival in this high‐risk population; (iii) independent predictors of improvement in QOL among patients with advanced HF treated with low‐dose intermittent inotropic therapy in a tertiary HF day‐care centre include a younger age, non‐ischaemic cardiomyopathy, and worse renal function; and (iv) factors independently associated with improved survival in this population include better renal function and a non‐ischaemic aetiology of HF.
Our cohort represents the 12 year experience of a unique tertiary outpatient day‐care HF setting in Israel that utilises low‐dose, intermittent, intravenous inotrope therapy as a central part of the treatment protocol, in addition to close clinical, laboratory, and electrocardiographic monitoring, and appropriate patient selection and supervision by experienced cardiologists specialised in the care of HF. Accordingly, the present findings provide unique and contemporary data on the effect of this modality of medical management on the QOL of patients with advanced refractory HF symptoms.
Current knowledge on the safety and efficacy of intermittent inotropic therapy in advanced heart failure patients
The use of intravenous inotropes in patients with advanced, symptomatic HF is limited by current guidelines to bridging therapy in patients with cardiogenic shock, patients awaiting Mechanical Circulatory Support or cardiac transplantation, or as a continuous infusion for palliative care of patients not eligible to those treatment options.1 Historically, long‐term use of intermittent intravenous inotropes in the absence of specific indications other than palliative care was and still is defined as ‘potentially harmful’,1 based on data that pointed towards lack of efficacy and increased mortality (mainly because of arrhythmia) in patients receiving inotropes.18
However, prior studies that evaluated the safety and efficacy of this management strategy have important limitations, including the use of inotropic agents that are currently not in use (i.e. enoximone, xamoterol, vesnarinone, and ibopamine), and evaluation of this modality of therapy in a non‐contemporary setting, prior to the routine use of automatic cardioverter–defibrillators in advanced HF patients.
Previous studies which suggested a compromise in survival rates with intermittent dobutamine therapy19 did not use a rigorous protocol to minimize the risks of dobutamine administration: electrolyte levels were not routinely monitored, dobutamine concentrations were not titrated for intra‐study changes in body weight, and most importantly, they utilized relatively high doses of dobutamine,20 all factors being specifically addressed in our protocol (see Appendix S1), increasing the safety of the treatment given.
The lack of up‐to‐date, well‐designed investigations is related, at least in part, to difficulties performing placebo‐controlled trials in advanced, symptomatic HF patients.
On the other hand, there are studies that concluded that the use of intermittent intravenous inotropes is safe and has a positive impact on improving symptoms, reducing the number of emergency room visits, hospitalizations, and haemodynamic parameters.11, 14, 15, 21, 22, 23, 24 Accordingly, in a recent review, Guglin et al. 25 concluded that the evidence is insufficient to link inotropes and increased mortality in low output HF.
Altenberger et al. 26 for the LevoRep trial showed in a prospective, randomized, double‐blind, placebo‐controlled multicentre that infusion of pulsed intravenous levosimendan (a calcium sensitizer) in 120 advanced HF patients did not improved the 6 min walk test and QOL when compared with placebo. Nonetheless, levosimendan was safely administered. These findings stress the need for a larger, well‐powered trial with intermittent, outpatient administration of levosimendan.
The impact of inotropes on quality of life
Although previous research showed clinical improvement with the infusion of inotropes,27, 28, 29, 30 there is a paucity of investigations evaluating the impact of low‐dose, intermittent, intravenous inotropes in an outpatient setting for this population on QOL. These studies, however, were carried out more than two decades ago and therefore did not utilize contemporary medical and device management in heart failure patients. In addition, these studies did not evaluate the relationship between baseline and follow‐up QOL and subsequent long‐term survival. Their benefit was shown mainly using high doses of inotropes, and in general, they included small cohorts of patients.
López‐Candales et al. 31 randomized a small cohort (N = 29) of symptomatic, low‐output HF patients to receive intravenous milrinone, dobutamine, or matching placebo. Among other parameters, the study sought to examine QOL improvement or worsening after treatment. A statistically significant improvement in QOL scores was seen, although the study failed to show a significant difference in the improvement in QOL between the intervention and the placebo groups. This study, however, was limited by sample size and therefore a direct effect of inotrope improvement on QOL could not be inferred. In contrast, the present study, although not randomized, showed that in a large population of advanced HF patients treatment with low‐dose intermittent inotropic therapy in a real world setting is associated with a significant improvement in QOL at 1 year of follow up.
Predictors of improvement in quality of life and mortality
We have identified three independent predictors of improvement in QOL after 1 year of treatment with low‐dose inotropic intermittent inotropic therapy: younger age, the presence of worse renal function, and the presence of non‐ischaemic cardiomyopathy.
Younger patients may be more likely to improve in their QOL possibly because older patients might have less ‘vital reserve’ than younger patients, and accordingly, may be less likely to respond to inotropes.
Worse renal function was related to improvement in QOL. This finding may be explained by the fact that by improving the cardiac output and renal perfusion, inotropes can help mitigate fluid overload better. On the other hand, as expected, worse renal function was shown to be an independent predictor of mortality after 1 year of treatment.
Our results also suggest that ischaemic cardiomyopathy was not associated with improvement in QOL, and it was an independent predictor of mortality in multivariate analysis (Table 3). It is known that patients with ischaemic aetiology of HF have a worse prognosis,32, 33 and our findings showed that their QOL did not improve following treatment with low‐dose inotropic therapy as their non‐ischaemic counterparts. The less impressive improvement in QOL in patients with ischaemic cardiomyopathy may be because of the fact that these patients suffer from significant scarring of the left ventricle, a fact that may condition the ability of the failing cardiac muscle to respond to inotropes compared with the non‐ischaemic, failing heart.
Study limitations
According to the treatment protocol we utilized dopamine and dobutamine as the sole inotropes in the study. Although there is evidence that the calcium sensitizer levosimendan can be safely administered to advanced HF patients, the fact that it is not used in our centre in the outpatient setting because of administrative issues, but rather is given intermittently for 24 h during hospitalization for symptomatic HF patients, limits the findings of our paper. Another limitation is the lack of biomarkers in our study population.
The relative small number of participants limits our analysis. Although a larger cohort could describe better intra‐cohort differences in QOL and specific predictors of improvement in QOL with treatment, we showed a clear, statistically significant improvement. Another limitation is the absence of a control group. Further well‐designed studies randomising patients with placebo‐control groups should be pursued in order to analyse the relative benefit of inotropes in HF day‐care programmes. Such a trial would be challenging because of the difficulty of enrolling a cohort of patients large enough to attain statistical power. Bias is implicit in that the very reason for initiation of inotropic therapy may have been trying to increase QOL in symptomatic patients. At present, however, our findings provide important real world data on the QOL and survival associated with this mode of medical therapy.
While our study sought to describe the effects on QOL and all‐cause mortality of intermittent outpatient inotropes therapy, their haemodynamic effect was not directly assessed.
Conclusions and clinical implications
We found that in patients with advanced HF who remain symptomatic despite maximal medical and device therapy, treatment including intermittent, low‐dose intravenous inotropes in an outpatient setting is associated with significant improvement in QOL. However, improvement in QOL does not appear to affect subsequent long‐term survival. We believe that the use of these drugs in a multidisciplinary outpatient setting on properly selected patients represents a valid option for advanced patients and more outpatient units should be opened to address this special population. Further large‐scale, placebo‐controlled randomized controlled studies are needed in order to answer questions like the specific effect of inotropes on mortality.
Funding
This study received no funding.
Supporting information
Appendix S1. Baseline characteristics of study population receiving at least 1 year of treatment (N = 137) at the beginning of treatment comparing patients according to their change in QOL.
Supporting info item
Chernomordik, F. , Freimark, D. , Arad, M. , Shechter, M. , Matetzky, S. , Savir, Y. , Shlomo, N. , Peled, A. , Goldenberg, I. , and Peled, Y. (2017) Quality of life and long‐term mortality in patients with advanced chronic heart failure treated with intermittent low‐dose intravenous inotropes in an outpatient setting. ESC Heart Failure, 4: 122–129. doi: 10.1002/ehf2.12114.
The copyright line for this article was changed on 17 October 2016 after original online publication.
References
- 1. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012. Eur Heart J 2012; 33: 1787–1847. [DOI] [PubMed] [Google Scholar]
- 2. Mosterd A, Hoes AW. Clinical epidemiology of heart failure. Heart 2007; 93: 1137–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Metra M, Ponikowski P, Dickstein K, McMurray JJ, Gavazzi A, Bergh CH, Fraser AG, Jaarsma T, Pitsis A, Mohacsi P, Böhm M, Anker S, Dargie H, Brutsaert D, Komajda M. Heart Failure Association of the European Society of Cardiology. Advanced chronic heart failure: a position statement from the Study Group on Advanced Heart Failure of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2007; 9: 684–694. [DOI] [PubMed] [Google Scholar]
- 4. The Xamoterol in Severe Heart Failure Study Group. Xamoterol in severe heart failure. Lancet 1990; 336: 1–6. [PubMed] [Google Scholar]
- 5. Cohn JN, Goldstein SO, Greenberg BH, Lorell BH, Bourge RC, Jaski BE, Gottlieb SO, McGrew F 3rd, DeMets DL, White BG. Vesnarinone Trial Investigators. A dose‐dependent increase in mortality with vesnarinone among patients with severe heart failure. N Engl J Med 1998; 339: 1810–1816. [DOI] [PubMed] [Google Scholar]
- 6. Hampton JR, van Veldhuisen DJ, Kleber FX, Cowley AJ, Ardia A, Block P, Cortina A, Cserhalmi L, Follath F, Jensen G, Kayanakis J, Lie KI, Mancia G, Skene AM. Randomised study of effect of ibopamine on survival in patients with advanced severe heart failure: Second Prospective Randomised Study of Ibopamine on Mortality and Efficacy (PRIME II) Investigators. Lancet 1997; 349: 971–977. [DOI] [PubMed] [Google Scholar]
- 7. Packer M, Carver JR, Rodeheffer RJ, Ivanhoe RJ, DiBianco R, Zeldis SM, Hendrix GH, Bommer WJ, Elkayam U, Kukin ML, Mallis GI, Sollano JA, Shannon J, Tandon PK, DeMets DL. for the PROMISE Study Research Group . Effect of oral milrinone on mortality in severe chronic heart failure. N Engl J Med 1991; 325: 1468–1475. [DOI] [PubMed] [Google Scholar]
- 8. Lubsen J, Just H, Hjalmarsson A, La Framboise D, Remme WJ, Heinrich‐Nols J, Dumont JM, Seed P. Effect of pimobendan on exercise capacity in patients with heart failure: main results from the Pimobendan in Congestive Heart Failure (PICO) trial. Heart 1996; 76: 223–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Metra M, Eichhorn E, Abraham WT, Linseman J, Böhm M, Corbalan R, DeMets D, De Marco T, Elkayam U, Gerber M, Komajda M, Liu P, Mareev V, Perrone SV, Poole‐Wilson P, Roecker E, Stewart J, Swedberg K, Tendera M, Wiens B, Bristow MR, ESSENTIAL Investigators . Effects of low‐dose oral enoximone administration on mortality, morbidity, and exercise capacity in patients with advanced heart failure: the randomized, double‐blind, placebo‐controlled, parallel group ESSENTIAL trials. Eur Heart J 2009; 30: 3015–3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Follath F, Yilmaz MB, Delgado JF, Parissis JT, Porcher R, Gayat E, Burrows N, McLean A, Vilas‐Boas F, Mebazaa A. Clinical presentation, management and outcomes in the acute heart failure global survey of standard treatment (ALARM‐HF). Intensive Care Med 2011; 37: 619–626. [DOI] [PubMed] [Google Scholar]
- 11. Rose EA, Gelijns AC, Moskowitz AJ, Heitjan DF, Stevenson LW, Dembitsky W, Long JW, Ascheim DD, Tierney AR, Levitan RG, Watson JT, Meier P, Ronan NS, Shapiro PA, Lazar RM, Miller LW, Gupta L, Frazier OH, Desvigne‐Nickens P, Oz MC, Poirier VL. Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) Study Group; long‐term use of a left ventricular assist device for end‐stage heart failure. New Engl J Med 2001; 345: 1435–1443. [DOI] [PubMed] [Google Scholar]
- 12. Krell MJ, Kline EM, Bates ER, Hodgson JM, Dilworth LR, Laufer N, Vogel RA, Pitt B. Intermittent ambulatory dobutamine therapy in patients with severe congestive heart failure. Am Heart J 1986; 112: 787–791. [DOI] [PubMed] [Google Scholar]
- 13. Goldberg LI. Cardiovascular and renal actions of dopamine. Potential clinical application. Pharmacol Rev 1972; 241: 1–29. [PubMed] [Google Scholar]
- 14. Freimark D, Arad M, Matetzky S, DeNeen I, Gershovitz L, Morag NK, Hochberg N, Makmal Y, Shechter M. An advanced chronic heart failure day care service: a 5 year single‐center experience. Isr Med Assoc J 2009; 11: 419–425. [PubMed] [Google Scholar]
- 15. Rector TS, Cohn JN. Assessment of Patient Outcome with the Minnesota Living with Heart Failure Questionnaire: reliability and validity during a randomized, double‐blind, placebo‐controlled trial of pimobendan. Am Heart J 1992; 124: 1017–1025. [DOI] [PubMed] [Google Scholar]
- 16. Bennett SJ, Oldridge NB, Eckert GJ, Embree JL, Browning S, Hou N, Deer M, Murray MD. Discriminant properties of commonly used quality of life measures in heart failure. Qual Life Res 2002; 11: 349–359. [DOI] [PubMed] [Google Scholar]
- 17. Gorkin L, Norvell NK, Rosen RC, Charles E, Schumaker SA, McIntyre KM, Capone RJ, Kostis J, Niaura R, Woods P, Hosking J, Garces C, Handberg E, Ahern DK, Follick MJ. For the SOLVD Investigators. Assessment of quality of life as observed from the baseline data of the Studies of Left Ventricular Dysfunction (SOLVD) trial quality‐of‐life substudy. Am J Cardiol 1993; 71: 1069–1073. [DOI] [PubMed] [Google Scholar]
- 18. Uretsky BF, Jessup M, Konstam MA, Dec GW, Leier CV, Benotti J, Murali S, Herrmann HC, Sandberg JA. Multicenter trial of oral enoximone in patients with moderate to moderately severe congestive heart failure. Lack of benefit compared with placebo. Enoximone Multicenter Trial Group. Circulation 1990; 82: 774–780. [DOI] [PubMed] [Google Scholar]
- 19. Dies F, Krell MJ, Whitlow P, Whitlow P, Liang CS, Goldenberg I, Applefeld MM, Gilbert EM. Intermittent dobutamine in ambulatory out‐patients with chronic cardiac failure. Circulation 1986; 74(suppl. II): 138. [Google Scholar]
- 20. Francis GS, Bartos JA, Adatya S. Inotropes. J Am Coll Cardiol 2014; 63: 2069–2078. [DOI] [PubMed] [Google Scholar]
- 21. Dubourg O, Delorme G, Hardy A, Beauchet A, Tarral A, Bourdarias JP. Placebo‐controlled trial of oral enoximone in end‐stage congestive heart failure refractory to optimal treatment. Int J Cardiol 1990; 28(Suppl 1): S33–S42; discussion S43. [DOI] [PubMed] [Google Scholar]
- 22. Feldman AM, Bristow MR, Parmley WW, Carson PE, Pepine CJ, Gilbert EM, Strobeck JE, Hendrix GH, Powers ER, Bain RP, White BG. Effects of vesnarinone on morbidity and mortality in patients with heart failure. Vesnarinone study group. New Engl J Med 1993; 329: 149–155. [DOI] [PubMed] [Google Scholar]
- 23. Likoff MJ, Weber KT, Andrews V, Janicki JS, Sutton MS, Wilson H, Rocci ML Jr. Amrinone in the treatment of chronic cardiac failure. J Am Coll Cardiol 1984; 3: 1282–1290. [DOI] [PubMed] [Google Scholar]
- 24. Khalife K, Zannad F, Brunotte F, Belhadj K, Juilliere Y, Iannascoli F, Gilgenkrantz JM. Placebo‐controlled study of oral enoximone in congestive heart failure with initial and final intravenous hemodynamic evaluation. Am J Cardiol 1987; 60: 75C–79C. [DOI] [PubMed] [Google Scholar]
- 25. Guglin M, Kaufman M. Inotropes do not increase mortality in advanced heart failure. Int J Gen Med 2014; 7: 237–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Altenberger J, Parissis JT, Costard‐Jaeckle A, Winter A, Ebner C, Karavidas A, Sihorsch K, Avgeropoulou E, Weber T, Dimopoulos L, Ulmer H, Poelzl G. Efficacy and safety of the pulsed infusions of levosimendan in outpatients with advanced heart failure (LevoRep) study: a multicentre randomized trial. Eur J Heart Fail 2014; 16: 898–906. [DOI] [PubMed] [Google Scholar]
- 27. Unverferth DV, Magorien RD, Lewis RP, Leier CV. Long‐term benefit of dobutamine in patients with congestive cardiomyopathy. Am Heart J 1980; 100: 622–630. [DOI] [PubMed] [Google Scholar]
- 28. Cesario D, Clark J, Maisel A. Beneficial effects of intermittent home administration of the inotrope/vasodilator milrinone in patients with end‐stage congestive heart failure: a preliminary study. Am Heart J 1998; 135: 121–129. [DOI] [PubMed] [Google Scholar]
- 29. Roffman DS, Applefeld MM, Grove WR, Talesnick BS, Sutton FJ, Newman KA, Reed WP. Intermittent dobutamine hydrochloride infusions in outpatients with chronic congestive heart failure. Clin Pharm 1985; 4: 195–199. [PubMed] [Google Scholar]
- 30. López‐Candales A, Carron C, Schwartz J. Need for hospice and palliative care services in patients with end‐stage heart failure treated with intermittent infusion of inotropes. Clin Cardiol 2004; 27: 23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. López‐Candales A, Vora T, Gibbons W, Carron C, Simmons P, Schwartz J. Symptomatic improvement in patients treated with intermittent infusion of inotropes: a double‐blind placebo controlled pilot study. J Med 2002; 33: 129–146. [PubMed] [Google Scholar]
- 32. Owan TE, Odge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 2006; 355: 251. [DOI] [PubMed] [Google Scholar]
- 33. Smith GL, Masoudi FA, Vaccarino V, Radford MJ, Kurmholz HM. Outcomes in heart failure patients with preserved ejection fraction: mortality, readmission and functional decline. J Am Coll Cardiol 2003; 41: 1510. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Baseline characteristics of study population receiving at least 1 year of treatment (N = 137) at the beginning of treatment comparing patients according to their change in QOL.
Supporting info item


