Abstract
Aims
Hyponatraemia is an electrolyte disorder that occurs in advanced congestive heart failure (HF) and worsens prognosis. We explored the usefulness of tolvaptan, which has shown promising results in the treatment of this condition.
Methods and results
This study is based on a retrospective national registry (2011–15) of patients hospitalized with refractory HF and hyponatraemia who agreed to receive tolvaptan when standard treatment was ineffective. The benefit of tolvaptan was analysed according to the following criteria: normalization ([Na+] ≥ 135 mmol/L) or increased sodium levels [Na+] ≥ 4 mEq/L on completion of treatment, and increase in urine output by 300 or 500 mL at 48 h. Factors associated with tolvaptan benefit were explored. A total of 241 patients were included, 53.9% of whom had ejection fraction <40%. All patients received concomitant loop diuretics. Initial tolvaptan dose was 17.2 ± 6.1 mg, and end dose was 26.4 ± 23.2 mg (duration 7.8 ± 8.6 days). Serum sodium concentrations increased significantly at 24–48 h, from 126.5 ± 6.2 mEq/L at baseline to 134.1 ± 6.1 mEq/L at the end of treatment (P < 0.0001). Weight fell by ~5 kg before discharge (P < 0.0001) and urine output increased 1.3‐fold (P < 0.0001). Normal sodium levels and/or increases of 500 mL in urine output were achieved by 90.8% of patients (35.7% achieved both) and 94.8% increased to [Na+] ≥ 4 mEq/L and/or +300 mL in urine output (54.4% both).
Conclusions
An increase in sodium levels and/or improvement in urine output was observed in patients admitted for HF and refractory hyponatraemia under tolvaptan treatment. Tolvaptan may be useful in this setting, in which no effective proven alternatives are available.
Keywords: Hyponatraemia, Congestive heart failure, Diuresis, Tolvaptan, Vasopressin type 2 receptors
Introduction
The main physiopathological consequence of heart failure (HF) is sodium and water retention, which causes venopulmonary congestion.1 Diuretics constitute the therapeutic mainstay in this setting.1, 2 Patients with advanced HF often have hyponatraemia, caused by increased vasopressin concentrations or by overexcretion of sodium, if loop diuretics are used excessively or in combination with thiazides.3, 4 Moreover, resistance to diuretics is encountered relatively frequently, and has been reported in 10–30% of decompensated patients.5, 6 Resistance to diuretics is defined as persistent oedema despite adequate diuretic treatment and restricted salt intake. In practice, it can be suspected in a patient with a weight loss of <0.5–1 kg/day or urine output <500 mL/day, despite diuretic treatment. Published guidelines vary widely in their recommendations on the diagnosis and management of hyponatraemia,7, 8, 9, 10 particularly with regard to hypervolemic hyponatraemia associated with HF. Some guidelines propose the use of the vasopressin receptor antagonists, such as tolvaptan, if other treatment is ineffective.10
Tolvaptan competitively inhibits vasopressin type 2 (V2) receptors in the collecting duct of the kidney,9, 10, 11 effectively reducing water absorption. This leads to more dilute urine and an increase in intravascular sodium levels. The SALT11 and SALTWATER12 trials showed that treatment with tolvaptan increased serum sodium concentration in patients with hyponatraemia (serum sodium concentration [Na+] < 135 mEq/L) caused by different physiopathological and etiological mechanisms, including HF. Moreover, this drug has a good safety profile. A subanalysis of the EVEREST trial, which included patients with decompensated HF and reduced left ventricular function, found that tolvaptan reduced the morbidity and mortality of patients with serum [Na+] < 130 mEq/L.13, 14 Furthermore, a placebo‐controlled trial showed that hospitalized patients with HF who received tolvaptan in addition to the standard intravenous diuretic treatment for 7 days lost more weight and their congestive symptoms improved more than in the group who received conventional diuretics only.15 However, tolvaptan is only approved in Europe for the treatment of the syndrome of inappropriate antidiuretic hormone secretion (SIADH), while in the US, one of the approved indications is hyponatraemia in HF patients.10 In fact, the only European clinical practice guideline for hyponatraemia is positioned against the use of vaptans in this condition,16 in contrast to the American guideline, and contrary to current evidence.
In view of these findings, the aim of this study was to determine the outcomes in patients who received tolvaptan in addition to added to standard diuretic treatment in clinical practice, in terms of resolution of hyponatraemia and improvement of urine output in hyponatraemic patients with refractory congestive heart failure (HF) admitted to Spanish hospitals.
Methods
Patients and data source
We performed a retrospective analysis of patients aged 18 or older admitted to several Spanish hospitals with refractory, hyponatraemic HF, who received tolvaptan as inpatients, after the failure of other treatments with unconfirmed efficacy. This treatment was administered after tolvaptan was approved by the EMA for other therapeutic indications in 2011. Diuretic‐refractoriness was clinically defined as persistent oedema despite adequate diuretic treatment and restricted salt intake. Hyponatraemia was not an inclusion criterion, but fewer than 10% of study patients had normal serum sodium concentrations at the beginning of treatment.
This was a national registry conducted with the collaboration of the Spanish Society of Internal Medicine (SEMI) and the Spanish Society of Cardiology (SEC) in eight hospitals distributed throughout Spain. The database was designed by representatives from SEMI and SEC, and was regularly audited to ensure data quality and integrity. The data included in this registry are fully anonymous, and data confidentiality is ensured in accordance with the Spanish Personal Data Protection Act 15/1999, 13 December 1999. All patients gave consent and were treated according to clinical practice guidelines, and this treatment was in most cases included in a protocol approved by the Pharmacy Commission of the participating hospitals. The decisions of the specialist regarding patient treatment and management were not influenced in any way, and the management of the participating hospitals was informed and authorized the registry project. Patients with incomplete data were excluded.
Variables
The SEMI‐SEC database collects a total of 63 study variables. These include patient clinical and demographic data (sex, age, HF aetiology, and drug treatments), details of diuretic treatment before tolvaptan (drugs, dose, duration), tolvaptan treatment data (initial and final dose and days of treatment), concomitant diuretic treatment data (drugs and doses), outcome variables related with the effect of tolvaptan, including baseline and post‐treatment laboratory results (electrolytes), kidney function parameters (urine output, glomerular filtration rate according to the Modification of Diet in Renal Disease [MDRD] Study equation,17 and weight) and follow‐up data (days of treatment, time to normal serum sodium concentrations,18 admission to intensive care unit, hospitalizations, death and cause, etc.).
Evaluation of the outcomes with tolvaptan and standard diuretic treatment
The criteria used to evaluate the outcomes were final values or changes in serum sodium and increased urine output, combined in four different premises reflecting benefit. For serum sodium concentration, benefit was classified as a final sodium value of ≥135 mEq/L or an increase from baseline at the end of treatment (Δ [Na+]) ≥ 4 mEq/L. These thresholds were established on the basis of a previously published hyponatraemia management algorithm, which defines normal serum sodium as [Na+] ≥ 135 mmol/L, and an appropriate response to treatment as an increase of 5–10 mEq/L.18 However, taking into account that response to treatment in HF is somewhat lower than that expected in other diseases (due to the multiple mechanisms involved in the physiopathology of the disease), we agreed, for the purposes of this study, to consider an increase of 4 mEq/L as indicative of response. With regard to urine output, two thresholds of increase, 300 or 500 mL, 24–48 h after starting treatment (Δ urine output), were selected as representative of the increase in urine output which defines resistance to diuretic treatment in clinical practice. Using these criteria, we established four premises reflecting benefit as follows: (i) final [Na+] ≥ 135 mEq/L, Δ urine output = +500 mL; (ii) Δ [Na+] ≥ 4 mEq/L, Δ urine output = +500 mL; (iii) final [Na+] ≥ 135 mEq/L, Δ urine output = +300 mL; and (iv) Δ [Na+] ≥ 4 mEq/L, Δ urine output = +300 mL. For each premise, four categories of benefit were determined, depending on whether one or the other, both or neither criterion were met.
Statistical analysis
A descriptive analysis was made of all variables. Continuous variables were expressed as mean and standard deviation (SD) and qualitative variables as frequencies and percentages. Comparisons between initial and final values of quantitative variables were made using the Student t‐test for paired samples, if the distribution of the variable was normal, and the Wilcoxon test if variables were not normally distributed. Variables determined at intermediate time points (24 h and 48 h) were analysed using ANCOVA methods for repeated measurements if distribution was normal, or if otherwise, using the Friedman test (serum sodium concentration and urine output).
A multinomial logistic regression was used to determine the relationship of each of the above‐mentioned premises of benefit with certain explanatory variables: sex, age, diastolic blood pressure, days of treatment (classified as T < 2 days; T = 2–10 days; T > 10 days), final blood potassium, and final glomerular filtration rate using the MDRD equation. Other variables which did not reach significance on a previous bivariate analysis, those with too high a proportion of missing data, and those not considered of clinical interest were excluded from this analysis. The reference level within each premise was that which met both criteria. The analysis was performed in the R environment.
Results
Baseline clinical characteristics and diuretic treatments
In total, 241 patients were included. Mean age was 67.5 ± 14.0 years and 58.5% were men. Patient demographic and clinical characteristics and HF aetiology are summarized in Table 1.
Table 1.
General patient characteristics
| n = 241 | Mean ± SD | n (%) |
|---|---|---|
| Age (years) | 67.5 ± 14.0 | |
| Sex (men) | 141 (58.5) | |
| Aetiology of heart failure | ||
| Idiopathic dilated cardiomyopathy | 44 (18.6) | |
| Arterial hypertension | 25 (10.5) | |
| Ischaemic heart disease | 72 (30.4) | |
| Valvular disease | 56 (26.6) | |
| Pulmonary hypertension | 9 (3.8) | |
| Other | 31 (13.1) | |
| Heart failure parameters (n = 232) | ||
| Ejection fraction (%) | 41.5 ± 18.7 | |
| Ejection fraction ≤40% | 125 (53.9) | |
| Ejection fraction >40% | 107 (46.1) | |
| Systolic blood pressure (mmHg) | 110.8 ± 19.6 | |
| Diastolic blood pressure (mmHg) | 62.7 ± 9.8 | |
| Serum sodium | ||
| Baseline [Na+] (mEq/L) | 126.5 ± 6.2 | |
| Patients with [Na+] ≥ 135 mEq/L | 16 (6.6) | |
| Admission to intermediate/intensive care units | 34 (14.4) | |
| No. of admissions in previous 12 months | 1.8 ± 2.3 |
SD, standard deviation.
Almost all patients were receiving loop diuretics before admission, and more than 60% were receiving mineralocorticoid receptor antagonists (MRA). These treatments were generally maintained after starting treatment with tolvaptan (Figure 1 A). The most common therapeutic strategy before the introduction of tolvaptan was a combination of loop diuretics and MRA (46.4%), and this continued to be the most frequently used combination during tolvaptan administration (39.0%) (Figure 1 B).
Figure 1.
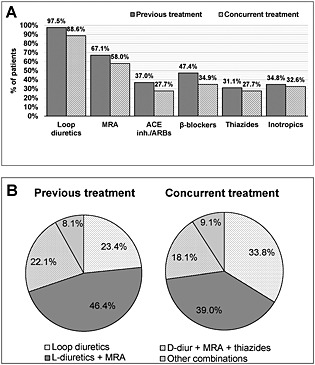
Patients' baseline treatment: (A) frequency of diuretic treatments and (B) most commonly used treatment combinations. ACE inhibitors, angiotensin converting enzyme inhibitors; ARBs, angiotensin II receptor blockers; L‐diuretics, loop diuretics; MRA, mineralocorticoid receptor antagonists.
Characteristics of treatment with tolvaptan and effects of overall treatment
Mean initial dose of tolvaptan was 17.2 ± 6.1 mg, which by the end of treatment had increased to 26.4 ± 23.2 mg. Mean treatment duration was 7.8 ± 8.6 days (19.9% <2 days, 55.1% 2–10 days, and 25.0% >10 days; up to a maximum of 40 days). In general, patients who achieved normal serum sodium concentrations ([Na+] ≥ 135 mEq/L) did so over a mean period of 4.3 ± 3.9 days, although 113 (47%) had not achieved normal levels at completion of treatment. Mean time to hospital discharge was 13.1 ± 11.9 days.
Figure 2 shows the effect of treatment on serum sodium, weight and urine output, at the start and end of treatment. A significant increase in serum sodium levels was observed, from 126.5 ± 6.2 mEq/L at baseline to 134.1 ± 6.1 mEq/L at the end of treatment (P < 0.0001). Moreover, the increase in serum sodium levels was already significant 24 h and 48 h after starting treatment (P < 0.0001). Mean weight had reduced by approximately 5 kg at the time of hospital discharge (P < 0.0001) and final urine output increased 1.3‐fold from baseline (P < 0.0001). Table 2 shows that plasma osmolarity also increased significantly. No significant changes were detected in glomerular filtration rate, blood creatinine or potassium levels.
Figure 2.
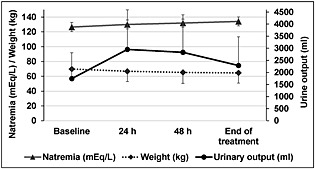
Comparison of serum sodium values (approximate 1:1 ratio of 1 mEq/L to 1 mmol/L), weight and urine output between start and end of tolvaptan treatment. Differences were significant for the 3 variables. Urine output was compared using the Friedman test (P < 0.0001 between time points; P = 0.900 between 24 and 48 h). Weight and serum sodium levels were compared using ANOVA for repeated measurements (P < 0.0001, also between all).
Table 2.
Comparison of laboratory and clinical parameters since the start of treatment with tolvaptan
| Mean ± SD | P‐value | |
|---|---|---|
| Blood creatinine (mg/dL) | ||
| Baseline | 1.61 ± 0.96 | 0.44a |
| End of treatment | 1.58 ± 0.89 | |
| Blood potassium (mEq/L) | ||
| Baseline | 4.31 ± 0.71 | 0.39b |
| End of treatment | 4.27 ± 0.75 | |
| Plasma osmolarityc (mOsm/L) | ||
| Baseline | 272.9 ± 19.6 | 0.0001b |
| End of treatment | 292.1 ± 19.8 | |
| Glomerular filtration rate (MDRD, mL/min/1.73m2) | ||
| Baseline | 47.7 ± 27.8 | 0.42a |
| End of treatment | 49.3 ± 31.3 |
Wilcoxon test.
Student's t‐test for paired samples.
Normal plasma osmolarity 280–295 mOsm/kg.
Mortality during admission was 20.7%, the main cause being HF (72.3%) followed by infections in 14.9% of cases, and other causes in 12.8%. A total of 22.4% of patients were readmitted within 30 days after discharge.
Premises of treatment benefit
Four efficacy premises were determined, based on final and initial sodium levels and changes in urine output in the first 48 h after starting treatment. Table 3 (first column) summarizes the percentage of patients who met the specific criteria of each premise. For the first premise (final [Na+] ≥ 135 mEq/L and Δ urine output = 500 mL), 90.8% of patients experienced one or other of the changes considered beneficial, and 35.7% met both conditions. The proportion of patients who achieved either of the benefit criteria of premise 2 (Δ [Na+] ≥ 4 mEq/L and Δ urine output = 500 mL) was 79.5%; 50.7% met both criteria. In premise 3, tolvaptan treatment was beneficial in 92.7% who had final [Na+] ≥ 135 mEq/L or Δ urine output = 300 mL or both at the end of treatment (38.6% met both criteria). Finally, 94.9% of patients showed a benefit according to the criteria of premise 4; 54.4% met both criteria (Δ [Na+] ≥ 4 mEq/L and Δ urine output = 300 mL).
Table 3.
Multivariate analysis of factors associated with achieving criteria for treatment benefit with tolvaptan according to the four premises
| % patients | Factor | B | P‐value | OR | 95% CI | |
|---|---|---|---|---|---|---|
| Premise 1. Final [Na+] ≥ 135 mEq/L, Δ Urine output = +500 mL | ||||||
| Final [Na+] ≥ 135 mEq/L | 23.2 | Final blood potassium | −1.02 | 0.036 | 0.36 | 0.14–0.94 |
| Final GFR | 0.05 | 0.006 | 1.05 | 1.02–1.09 | ||
| Δ Urine output = +500 mL | 31.9 | Final GFR | 0.05 | 0.007 | 1.05 | 1.01–1.09 |
| Final [Na+] ≥ 135 mEq/L and Δ Diuresis = +500 mL | 35.7 | Final blood potassium | −1.04 | 0.02 | 0.35 | 0.15–0.85 |
| No benefit | 9.2 | |||||
| Premise 2. Final [Na+] ≥ 4 mEq/L, Δ Diuresis = +500 mL | ||||||
| Δ [Na+] ≥ 4 mEq/L | 28.8 | Final GFR | 0.03 | 0.009 | 1.03 | 1.01–1.05 |
| T > 10 days | 1.61 | 0.035 | 4.98 | 1.12–22.15 | ||
| T = 2–10 days | 1.25 | 0.035 | 3.49 | 1.09–11.10 | ||
| Δ Urine output = +500 mL | – | |||||
| Δ [Na+] ≥ 4 mEq/L and Δ Urine output = +500 mL | 50.7 | Final GFR | 0.03 | 0.004 | 1.03 | 1.01–1.06 |
| T > 10 days | 1.79 | 0.022 | 6.00 | 1.30–27.79 | ||
| T = 2–10 days | 2.10 | 0.0001 | 8.17 | 2.55–26.18 | ||
| No benefit | 20.5 | |||||
| Premise 3. Final [Na+] ≥ 135 mEq/L, Δ Urine output = +300 mL | ||||||
| Final [Na+] ≥ 135 mEq/L | 20.3 | Final blood potassium | −1.14 | 0.021 | 0.32 | 0.12–0.88 |
| Final GFR | 0.04 | 0.024 | 1.05 | 1.01–1.09 | ||
| Δ Urine output = +300 mL | 33.8 | Final GFR | 0.04 | 0.021 | 1.05 | 1.01–1.09 |
| Final [Na+] ≥ 135 mEq/L and Δ Urine output = +300 mL | 38.6 | Final blood potassium | −1.00 | 0.033 | 0.37 | 0.15–0.92 |
| Final GFR | 0.04 | 0.054a | 1.04 | 1.00–1.08 | ||
| No benefit | 7.2 | |||||
| Premise 4. Δ [Na+] ≥ 4 mEq/L, Δ Urine output = +300 mL | ||||||
| Δ [Na+] ≥ 4 mEq/L | 25.1 | Final blood potassium | −1.17 | 0.044 | 0.31 | 0.10–0.97 |
| T = 2–10 days | 2.39 | <0.014 | 10.89 | 1.61–73.80 | ||
| Δ Urine output = +300 mL | 15.3 | DBP | 0.09 | 0.032 | 1.10 | 1.01–1.20 |
| Δ [Na+] ≥ 4 mEq/L and Δ Urine output = +300 mL | 54.4 | DBP | 0.10 | <0.012 | 1.11 | 1.02–1.20 |
| T = 2–10 days | 2.59 | <0.006 | 13.28 | 2.12–83.26 | ||
| No benefit | 5.1 | |||||
For clarity, this table generally includes only factors significantly associated with each criterion, but the P‐value marked
is given as it is indicative of a trend. Δ [Na+], increase of serum sodium between start and end of treatment; Δ Urine output, increase of urine output between start and end of treatment and at 24–48 h; GFR, glomerular filtration rate (MDRD), T: treatment time; DBP, diastolic blood pressure.
Analysis of factors associated with treatment benefit
Reduction in blood potassium and increased glomerular filtration rate at the end of treatment were found to be directly associated with normalization of serum sodium levels; and final glomerular filtration rate was associated with increased urine output, although these parameters varied depending on the combination of premises (Table 3). Days of treatment were associated with an increase of 4 mEq/L, suggesting that this threshold is easier to achieve with longer treatments. In premise 4, a significant association was found between diastolic blood pressure and a 300 mL increase in urine output at 24–48 h, and also when urine output was combined with an increase of sodium in blood. No other variables showed a significant association with benefit.
Discussion
Our study sample, retrieved from the registry conducted in Spanish hospitals is, to our knowledge, the largest series in the literature on the use of tolvaptan in clinical practice in the treatment of hyponatraemia in patients hospitalized for acute HF. Hyponatraemia is a condition commonly associated with advanced HF, and is a prognostic marker of morbidity and mortality.3, 19, 20 Management of patients with HF and hyponatraemia is problematic,21 since water restriction is difficult to achieve. Moreover, this approach is largely ineffective, and outcomes are dismal.3, 22 Loop diuretics used to treat oedema are associated with the production of isotonic urine and do not help to increase serum sodium.3, 4, 23 Furosemide has a aquaretic effect and does contribute to increasing serum sodium, due to its effect on the renal medulla, but only in situations in which urinary osmolality is >350 mOsm/kg.18
This study offers real‐world data on the usefulness of tolvaptan, administered concomitantly with standard diuretic treatment, and shows that this drug contributes to increasing serum sodium levels and for increasing urine output in hospitalized HF patients, with no significant effects on renal function. It is interesting to note that over 50% of patients achieved normal serum sodium at the end of tolvaptan treatment administered in combination with standard diuretic drugs, and almost 80% experienced an increase in serum sodium of at least 4 mEq/L in the first 48 h. Moreover, more than half of the patients experienced an increase in urine output of over 500 mL.
The combination of diuretic treatment and tolvaptan was useful in 91% of patients who met the most demanding of the proposed premises of benefit, which was to achieve normal sodium levels or urine output >500 mL, or both (premise 1). The percentage of patients who experienced benefit rose to 95% when the criteria reflected a relative increase in serum sodium of ≥4 mEq/L and urine output of 300 mL.
Changes in sodium levels and urine output produced were accompanied by weight loss, a measurement directly associated with reduced oedema. Regarding improvement in sodium concentration, increased levels have been previously demonstrated in trials and in small clinical studies with HF patients.12, 24, 25, 26, 27 Therefore, our results contribute valuable data to the body of knowledge, by showing the contribution of tolvaptan also in other clinical aspects, namely increasing urine output and reducing oedema, two key factors in the clinical management of congestive symptoms and which indicate response to treatment and short‐term prognosis.
According to our data, in practice tolvaptan is initiated at low doses, and gradual dose increments are required to achieve normal sodium levels. This is usually reached in approximately 4 days, although some patients never reach this target. Tolvaptan is generally administered for a period of 2–10 days: treatment duration is important, as demonstrated by our observation of an association between duration and increased sodium levels (criteria for premise of benefit no. 4). A small number of patients received treatment for over 30 days, the limit stated by the Food and Drug Administration for this drug.28 However, this warning was not issued by the European Medicines Agency.29 Treatment may be extended for clinical reasons, and close monitoring of liver function is recommended. No liver complications were reported during the treatment period in any of the patients in the registry.
It is interesting to note that outcomes varied widely, as almost half of the patients did not achieve normal sodium levels during the treatment period. This figure is consistent with those of clinical trials of tolvaptan, in which 10–50% of patients did not respond,11, 12 but the reasons for this variation remain unclear. It has been previously reported that patient status at the beginning of treatment impacts on efficacy, and patients with severe hyponatraemia achieve better outcomes.13, 14, 26, 27 Circulating vasopressin levels can also affect outcome, as can the degree of success experienced with fluid restrictions.30 Moreover, possible differences in the approaches taken in the different hospitals (e.g. doses, treatment duration and concomitant management) may have been under‐reported, impacting on our analysis. In contrast to SIADH, in which hyponatraemia depends exclusively on antidiuretic hormone (ADH) action, the mechanism of hyponatraemia in HF is mixed. In HF, not only ADH, but also the renin angiotensin system and the adrenergic nervous system play a part, so response to blocking this hormone may be more heterogeneous. Finally, it has also been suggested that differences in response reflect V2 receptor mutations which affect tolvaptan pharmacokinetics (e.g. vasopressin‐independent SIADH).31
One of the objectives of this study was to determine the factors associated with achieving the different premises of benefit. Glomerular filtration at the end of treatment was related with an improvement in sodium levels and urine output, although overall increases in glomerular filtration did not reach statistical significance. Improved final glomerular filtration rate and reduced blood potassium were favourably associated with achieving normal sodium levels in the 2 benefit premises which included the criterion [Na+] ≥ 135 mEq/L. The significance of this association is difficult to ascertain, but it seems that patients who benefit more from the treatment, in terms of sodium levels and urine output, also tend to experience improvements in renal function.
Treatment duration (>2 days) had a strong and significant effect on achieving a relative increase of 4 mEq/L in serum sodium, so perhaps tolvaptan should be administered for more than 2 days before it is considered useless, and the dose should also be adjusted during the early days of administration, as recommended for other indications such as SIADH. Patient age (>65 years) has previously been reported as a factor impacting negatively on the efficacy of tolvaptan in terms of 24 h urine volume,32 but our analysis revealed no relationship between age and urine output. The mean age of our patients was 67 years, but we included patients aged from 18 to 95 years, so the age range was wide enough for assessment. No significant relationships between baseline patient variables were found that could be predictive of treatment efficacy.
Hyponatraemia occurring in advanced HF has been considered for many years as a poor prognostic factor in HF.19, 20 Recovery is associated with an improved prognosis, but the outlook is generally very poor in these patients, in view of their advanced disease. Tolvaptan may be useful as a decongestive aquaretic, but improving hyponatraemia may also improve the short‐term prognosis for morbidity and mortality in these patients.33, 34
The limitations of this study are those inherent to retrospective studies based on multicentre registries, in which only limited data on patient management are available, and in this study, no standardized treatment strategy was agreed among the hospitals. However, this database was specifically designed for the purposes of this study, and the data quality was ensured with appropriate periodical controls. Only clinical and laboratory data used in routine practice were recorded, and no extra sampling or determinations of possible biomarkers was not planned, as fast therapeutic decision‐making regarding treatment in these patients is crucial. Comparison with patients not refractory to diuretics was not planned. This study is valuable because it examines a national patient series, with the participation of different hospitals which followed their own clinical treatment protocols and included real patients with a wide range of baseline situations. Thus, the observation that tolvaptan added to these patients' treatment schedules provided overall clinical benefits can be considered to be robust. Nevertheless, various clinical and management factors which may have affected the outcome were not controlled, and these aspects should be explored in future studies.
In conclusion, most patients hospitalized for decompensated HF and hyponatraemia treated with tolvaptan added to conventional diuretic treatment presented an increase in serum sodium levels and/or urine output, and congestive symptoms were improved. While more studies are needed to examine in more depth the causes of lack of response and predictive factors for response, tolvaptan may be beneficial in patients for whom no alternative treatments with proven efficacy are available. In view of the data from the RCTs, the data presented here, and the general impossibility of treating hyponatraemia in severe HF without a vaptan, future updates of the European guidelines should consider the utility of V2 antagonists in these patients.
Conflict of interest
The authors state that they have imparted sessions sponsored by Otsuka Laboratories and collaborated as advisors.
Funding
None to declare.
Acknowledgements
Statistical analyses were performed by Medical Statistics Consulting (Valencia). Dr Blanca Piedrafita collaborated in the writing of the manuscript. All authors participated in the interpretation of the results and review of drafts, and approved the final version. Other physicians in the participating hospitals, apart from the study authors, also included patients in this study.
Pose, A. , Almenar, L. , Gavira, J. J. , López‐Granados, A. , Blasco, T. , Delgado, J. , Aramburu, O. , Rodríguez, A. , Manzano, L. , and Manito, N. (2017) Benefit of tolvaptan in the management of hyponatraemia in patients with diuretic‐refractory congestive heart failure: the SEMI‐SEC project. ESC Heart Failure, 4: 130–137. doi: 10.1002/ehf2.12124.
References
- 1. McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez‐Sanchez MA, Jaarsma T, Kober L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 2012; 33: 1787–1847. [DOI] [PubMed] [Google Scholar]
- 2. Trullàs JC, Morales‐Rull JL, Formiga F. Tratamiento diurético en la insuficiencia cardiaca. Med Clin 2014; 142: 163–170. [DOI] [PubMed] [Google Scholar]
- 3. Filippatos TD, Elisaf MS. Hyponatremia in patients with heart failure. World J Cardiol 2013; 5: 317–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Oren RM. Hyponatremia in congestive heart failure. Am J Cardiol 2005; 95: 2B‐7B. [DOI] [PubMed] [Google Scholar]
- 5. Iqbal J, Javaid MM. Diuretic resistance and its management. Br J Hosp Med (Lond) 2014; 75: C103–107. [DOI] [PubMed] [Google Scholar]
- 6. Lin TE, Adams KF, Jr. , Patterson JH. Potential roles of vaptans in heart failure: experience from clinical trials and considerations for optimizing therapy in target patients. Heart Fail Clin 2014; 10: 607–620. [DOI] [PubMed] [Google Scholar]
- 7. Nagler EV, Vanmassenhove J, van der Veer SN, Nistor I, Van Biesen W, Webster AC, Vanholder R. Diagnosis and treatment of hyponatremia: a systematic review of clinical practice guidelines and consensus statements. BMC Med 2014; 12: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Verbalis JG, Grossman A, Hoybye C, Runkle I. Review and analysis of differing regulatory indications and expert panel guidelines for the treatment of hyponatremia. Curr Med Res Opin 2014; 30: 1201–1207. [DOI] [PubMed] [Google Scholar]
- 9. Schrier RW, Gross P, Gheorghiade M, Berl T, Verbalis JG, Czerwiec FS, Orlandi C. Tolvaptan, a selective oral vasopressin V2‐receptor antagonist, for hyponatremia. N Engl J Med 2006; 355: 2099–2112. [DOI] [PubMed] [Google Scholar]
- 10. Verbalis JG, Goldsmith SR, Greenberg A, Korzelius C, Schrier RW, Sterns RH, Thompson CJ. Diagnosis, evaluation, and treatment of hyponatremia: expert panel recommendations. Am J Med 2013; 126: S1–42. [DOI] [PubMed] [Google Scholar]
- 11. Matsuzaki M, Hori M, Izumi T, Asanoi H, Tsutamoto T. Effects of tolvaptan on volume overload in Japanese patients with heart failure: results of a phase II, multicenter, randomized, double‐blind, placebo‐controlled, parallel‐group study. Cardiovasc Drugs Ther 2011; 25 Suppl 1:S19–31. [DOI] [PubMed] [Google Scholar]
- 12. Berl T, Quittnat‐Pelletier F, Verbalis JG, Schrier RW, Bichet DG, Ouyang J, Czerwiec FS. Oral tolvaptan is safe and effective in chronic hyponatremia. J Am Soc Nephrol 2010; 21: 705–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gheorghiade M, Konstam MA, Burnett JC, Jr. , Grinfeld L, Maggioni AP, Swedberg K, Udelson JE, Zannad F, Cook T, Ouyang J, Zimmer C, Orlandi C. Short‐term clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: the EVEREST Clinical Status Trials. JAMA 2007; 297: 1332–1343. [DOI] [PubMed] [Google Scholar]
- 14. Konstam MA, Gheorghiade M, Burnett JC, Jr. , Grinfeld L, Maggioni AP, Swedberg K, Udelson JE, Zannad F, Cook T, Ouyang J, Zimmer C, Orlandi C. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. JAMA 2007; 297: 1319–1331. [DOI] [PubMed] [Google Scholar]
- 15. Matsuzaki M, Hori M, Izumi T, Fukunami M. Efficacy and safety of tolvaptan in heart failure patients with volume overload despite the standard treatment with conventional diuretics: a phase III, randomized, double‐blind, placebo‐controlled study (QUEST study). Cardiovasc Drugs Ther 2011; 25 Suppl 1:S33–45. [DOI] [PubMed] [Google Scholar]
- 16. Spasovski G, Vanholder R, Allolio B, Annane D, Ball S, Bichet D, Decaux G, Fenske W, Hoorn EJ, Ichai C, Joannidis M, Soupart A, Zietse R, Haller M, van der Veer S, Van Biesen W, Nagler E. Clinical practice guideline on diagnosis and treatment of hyponatraemia. Intensive Care Med 2014; 40: 320–331. [DOI] [PubMed] [Google Scholar]
- 17. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999; 130: 461–470. [DOI] [PubMed] [Google Scholar]
- 18. Runkle I, Villabona C, Navarro A, Pose A, Formiga F, Tejedor A, Poch E. Treatment of hyponatremia induced by the syndrome of inappropriate antidiuretic hormone secretion: a multidisciplinary spanish algorithm. Nefrologia 2014; 34: 439–450. [DOI] [PubMed] [Google Scholar]
- 19. Lee SE, Choi DJ, Yoon CH, Oh IY, Jeon ES, Kim JJ, Cho MC, Chae SC, Ryu KH, Oh BH. Improvement of hyponatraemia during hospitalisation for acute heart failure is not associated with improvement of prognosis: an analysis from the Korean Heart Failure (KorHF) registry. Heart 2012; 98: 1798–1804. [DOI] [PubMed] [Google Scholar]
- 20. Gheorghiade M, Rossi JS, Cotts W, Shin DD, Hellkamp AS, Pina IL, Fonarow GC, DeMarco T, Pauly DF, Rogers J, DiSalvo TG, Butler J, Hare JM, Francis GS, Stough WG, O'Connor CM. Characterization and prognostic value of persistent hyponatremia in patients with severe heart failure in the ESCAPE Trial. Arch Intern Med 2007; 167: 1998–2005. [DOI] [PubMed] [Google Scholar]
- 21. Hauptman PJ, Burnett J, Gheorghiade M, Grinfeld L, Konstam MA, Kostic D, Krasa HB, Maggioni A, Ouyang J, Swedberg K, Zannad F, Zimmer C, Udelson JE. Clinical course of patients with hyponatremia and decompensated systolic heart failure and the effect of vasopressin receptor antagonism with tolvaptan. J Card Fail 2013; 19: 390–397. [DOI] [PubMed] [Google Scholar]
- 22. Fraser CL, Arieff AI. Epidemiology, pathophysiology, and management of hyponatremic encephalopathy. Am J Med 1997; 102: 67–77. [DOI] [PubMed] [Google Scholar]
- 23. Greenberg A. Diuretic complications. Am J Med Sci 2000; 319: 10–24. [PubMed] [Google Scholar]
- 24. Edo‐Solsona MD, Ruiz‐Ramos J, Montero‐Hernández M, Font‐Noguera I, Poveda‐Andrés JL. Effectiveness and adequacy of tolvaptan prescription in hospitalized patients. Farm Hosp 2013; 37: 178–181. [DOI] [PubMed] [Google Scholar]
- 25. Rodríguez‐de Muñoz YM, Sánchez‐Lázaro IJ, Almenar‐Bonet L, Martínez‐Dolz L, Rodríguez‐Serrano M, Salvador‐Sanz A. Use of tolvaptan in patients with hyponatremia due to heart failure: initial experience. Rev Esp Cardiol (Engl Ed) 2013; 66: 319–321. [DOI] [PubMed] [Google Scholar]
- 26. Salterain‐González N, Esteban‐Fernández A, García‐López M, Lavilla‐Royo FJ, Gavira‐Gómez JJ. Efficacy of tolvaptan in patients hospitalized for heart failure with refractory hyponatremia. Clinical experience in daily practice. Rev Esp Cardiol (Engl Ed) 2013; 66: 503–504. [DOI] [PubMed] [Google Scholar]
- 27. Martínez‐Dolz L, Rodríguez‐Pichardo Y, Sánchez‐Lázaro IJ, Ruiz‐Ramos J, Montero‐Hernández M, Portoles‐Sanz M, Rivera‐Otero M, Marqués‐Sule E, Salvador‐Sanz A, Almenar‐Bonet L. Efectiveness and safety of tolvaptan in hyponatremic patients with heart failure [Abstract P1368]. Eur J Heart Fail 2014; Abstracts Supplement (2014) 16: 263. [Google Scholar]
- 28. US Food and Drug Administration . FDA Drug Safety Communication: FDA limits duration and usage of Samsca (tolvaptan) due to possible liver injury leading to organ transplant or death. Safety Announcement. 2013. http://www.fda.gov/downloads/Drugs/DrugSafety/UCM350084.pdf.
- 29. European Medicines Agency . Summary of product characteristics for samsca tablets. EMEA/H/C/000980 ‐R/0016. 2014. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Product_Information/human/000980/WC500048716.pdf.
- 30. Berl T. Vasopressin Antagonists. N Engl J Med 2015; 373: 981. [DOI] [PubMed] [Google Scholar]
- 31. Aditya S, Rattan A. Vaptans: a new option in the management of hyponatremia. International Journal of Applied and Basic Medical Research 2012; 2: 77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Imamura T, Kinugawa K, Shiga T, Kato N, Muraoka H, Minatsuki S, Inaba T, Maki H, Hatano M, Yao A, Kyo S, Nagai R. Novel criteria of urine osmolality effectively predict response to tolvaptan in decompensated heart failure patients—association between non‐responders and chronic kidney disease. Circ J 2013; 77: 397–404. [DOI] [PubMed] [Google Scholar]
- 33. Rossi J, Bayram M, Udelson JE, Lloyd‐Jones D, Adams KF, Oconnor CM, Stough WG, Ouyang J, Shin DD, Orlandi C, Gheorghiade M. Improvement in hyponatremia during hospitalization for worsening heart failure is associated with improved outcomes: insights from the Acute and Chronic Therapeutic Impact of a Vasopressin Antagonist in Chronic Heart Failure (ACTIV in CHF) trial. Acute Card Care 2007; 9: 82–86. [DOI] [PubMed] [Google Scholar]
- 34. Udelson JE, McGrew FA, Flores E, Ibrahim H, Katz S, Koshkarian G, O'Brien T, Kronenberg MW, Zimmer C, Orlandi C, Konstam MA. Multicenter, randomized, double‐blind, placebo‐controlled study on the effect of oral tolvaptan on left ventricular dilation and function in patients with heart failure and systolic dysfunction. J Am Coll Cardiol 2007; 49: 2151–2159. [DOI] [PubMed] [Google Scholar]


