Abstract
Aims
Heart failure (HF) is a multiorgan, pro‐inflammatory syndrome that impairs bone metabolism. Pro‐inflammatory cytokines and bone catabolism enhance periodontal disease, a local inflammatory, bacteria‐induced disease that causes bone loss and periodontal soft tissue destruction.
Methods and results
Medical and dental examinations were performed on patients with HF (n = 39), following heart transplantation (post‐HTx, n = 38) and controls (n = 32). Blood, saliva, and gingival crevicular fluid were analysed for bone metabolism and inflammation markers. HF average New York Heart Association classification was III. Average time since HTx was 1414 days. Pro‐inflammatory tumour necrosis factor‐alpha was higher in HF and HTx as compared with controls (P < 0.05). Both HF and HTx participants had higher levels of bone resorption marker C‐terminal telopeptide and parathyroid hormone with subjects in the HF group having the highest serum levels of all groups (P ≤ 0.05). In contrast, 25‐hydroxyvitamin D was lowest in HF. HF patients had greater clinical attachment loss, cumulative pockets depth (greater than 3 mm) and probing depth (P < 0.05) as compared with controls. Cumulative pockets depth correlated significantly with measures of the inflammatory burden, β‐glucuronidase in saliva (r = 0.4863, P < 0.01), interleukin‐1b in saliva (r = 0.5149, P < 0.01), and gingival crevicular fluid (r = 0.6056, P < 0.001) in HF. However, adjustment of periodontal results for measures of oral hygiene (plaque, bleeding on probing), systemic 25‐hydroxyvitamin D, and race attenuated significant differences between groups.
Conclusions
Patients with HF exhibit more severe periodontal disease associated with increased bone turnover markers when compared with control patients. However, local and systemic factors may account for this association and should be evaluated in future studies.
Keywords: Heart failure, Periodontal disease, Bone metabolism
Introduction
Heart failure (HF) is a pro‐inflammatory syndrome with multi‐organ involvement caused by the inability of the heart to meet the metabolic demands of the body.1, 2 An initial cardiac dysfunction such as ischemic, hypertensive, or genetic cardiomyopathy leads to the activation and increase of pro‐inflammatory cytokines as part of the disease process.3 Epidemiologically, it is a major cause of morbidity and mortality that affects around 4.8 million people in the USA every year.4 Currently, the only established definite surgical approach to treating advanced HF is cardiac transplantation, which improves haemodynamics and systemic metabolism.5 Clinical systemic manifestations of HF include exercise intolerance, muscle wasting, renal impairment, and abnormal bone metabolism.6 Previous studies have shown a compromised bone metabolism in HF,7 and as a result, patients with severe HF display increased serum bone resorption markers such as cross‐linked N‐telopeptides and C‐telopeptides of type I collagen (NTX and CTX) and parathyroid hormone (PTH).8
Pro‐inflammatory cytokines and bone catabolism can enhance periodontal disease, a local inflammatory, bacteria‐induced disease that causes bone loss and periodontal soft tissue destruction.9 It can be found in 47.2% of adults in the USA10 and ultimately leads to tooth loss if left untreated.11 Previously, it was assumed that periodontitis is primarily an infectious disease characterized by bacteria found in dental plaque.12, 13 However, later studies showed that systemic factors likely modify the individual host responses to bacteria and bacterial lipopolysaccharides and therefore control the amount of tissue destruction as a result of periodontitis.14, 15 Therefore, increasing interest now focuses on systemic conditions that are associated with the occurrence and progression of periodontal disease.9, 16, 17, 18
Despite HF being such a prevalent cardiac disease, only one retrospective radiographic study evaluated periodontal alveolar bone loss in a group of HF patients and described an increased rate of periodontal disease as compared with healthy controls.19 Overall, the impact of the periodontal disease experience on quality of life is considerable also from an epidemiological standpoint.20 Therefore, the purpose of this study was to investigate the prevalence of periodontal disease in patients with HF in comparison with patients after heart transplantation (HTx) and a non‐HF control population. We, furthermore, evaluated systemic measures of inflammation and bone metabolism as well as local measures of oral hygiene to determine to what extent they might account for an association between the two diseases.
Methods
We enrolled 109 patients between July 2010 and May 2012 in this cross‐sectional study. Patients with advanced HF before cardiac transplantation and patients with a prior diagnosis of advanced HF with at least 6 months post‐cardiac transplantation were included in the HF (mean age: 57 ± 11 years; 29 men and 10 women) and HTx (mean age: 54 ± 13 years; 30 men and eight women) group, respectively. Severity of HF was determined using the New York Heart Association classification.21 Non‐HF controls who do not differ from the other groups in age were included as control patients (mean age: 52 ± 9 years; 16 men and 16 women). Exclusion criteria for control patients included history of myocardial infarction, heart murmur, pregnancy, or HIV.
Subjects in all groups had to be dentulous with no drug‐induced gingival enlargement and older than 18 years. Because of the impact smoking might have on the periodontal condition, we included only subjects that were currently non‐smokers.22 Further, they should not have had invasive periodontal therapy in the past 6 months. Medical history, current medications, and history of smoking were recorded for all patients.
HF and HTx patients were recruited from the outpatient cardiac clinic, and control patients were recruited from the dental clinics at Columbia University Medical Center. Control subjects were recruited after presenting to the dental clinic for a routine general dental examination.
The Institutional Review Board at Columbia University approved the study protocol, and all patients provided written consent before inclusion in the study.
Periodontal examination
Clinical parameters were recorded at six sites per tooth using a UNC15 probe (mesio‐buccal, mid‐buccal, disto‐buccal, mesio‐lingual, mid‐lingual, and disto‐lingual) without including 3rd molars. We recorded probing depth (PD), recession (REC) measured as the distance between cemento‐enamel junction and gingival margin, clinical attachment loss (CAL). Bleeding on probing (BOP) determined as absence or presence of bleeding after 30 s, and plaque assessed dichotomously as absent or present were analysed as measures of oral hygiene.23 We further calculated cumulative pocket depth (CPD) by using the sum of all PDs greater than 3 mm as previously described.24 A panoramic radiograph was taken from every study subject to complement our clinical evaluation.
Blood, saliva, and gingival crevicular fluid samples
Samples were collected at study appointment. Serum levels of CTX, carboxylated osteocalcin, 25‐hydroxyvitamin D (25‐OHD), PTH, procollagen 1 N‐terminal peptide (P1NP), and tumour necrosis factor‐alpha (TNF‐α) were determined as previously described.8 β‐glucuronidase and interleukin‐1beta (IL‐1β) were analysed as previously described.25, 26
Statistical analysis
Because of skewed distributions, several of the primary variables were transformed before analysis. The natural log was taken for the variables CTX, PTH, TNF‐α, CPD, 25‐OHD, and osteocalcin. For the proportion variables quantifying plaque and BOP, an arcsine‐square root transformation was used. In order to detect differences in means across the three study groups, analysis of variance tests were conducted, separately for each of the primary outcome measures, with pairwise contrasts to further define significant differences. Additionally, all analyses were repeated, simultaneously adjusting for race, 25‐OHD, diabetes, plaque, and BOP. These parameters were included by being a risk factor for periodontal disease22 or being found to be significantly different during patient evaluation. Differences in the proportion of comorbidities, HF aetiology, and race were tested using Fisher's exact test. Pearson correlation coefficient and linear regression analysis were used to check for dependences. Analyses were performed using sas/9.3 or Prism 6/GraphPad.
Results
Patient characteristics
Body mass index and history of smoking were not found to be statistically significant between groups (Table 1). Average New York Heart Association classification was class III for the pre‐transplant group with 35.9% being classified as class III, while 20.51% were classified as class IV. Mean duration of HF was 8.5 ± 7.8 years, defined as the time from the patient's diagnosis of HF to the study enrolment date. On average, HTx patients had received a cardiac transplant 1414 ± 1198 days before study inclusion. As expected, measures associated with cardiac function such as ejection fraction (%) and resting heart rate were significantly lower in the HF group as compared with patients that had undergone HTx or the control group (Table 1). HF is a systemic disease that after the initial cardiac dysfunction can result in the impairment of multiple organs.4 Therefore, comorbidities analysed as part of the medical history and listed in Table 1 were in general significantly higher in HF and HTX subjects as compared with controls, whereas only history of hypertension was significantly different between HF and HTX.
Table 1.
Characteristics
| Control (n = 32) | Heart failure (n = 39) | Heart transplant (n = 38) | P‐value | |
|---|---|---|---|---|
| Age (years) | 52 ± 9 | 57 ± 11 | 54 ± 13 | 0.18 |
| Gender, male [# (%)] | 16 (50) | 29 (74) | 30 (79) | 0.0218 |
| BMI (kg/m2) | 27 ± 4 | 29 ± 5 | 28 ± 6 | 0.323 |
| Race [# (%)] | 0.0001 | |||
| Caucasian | 16 (50) | 14 (36) | 21 (55) | |
| Hispanic | 12 (38) | 7 (18) | 2 (5) | |
| African American | 2 (6) | 9 (23) | 12 (32) | |
| Asian | 0 (0) | 1 (3) | 2 (5) | |
| Other | 2 (6) | 8 (21) | 1 (3) | |
| Smoking (current) | 0 (0) | 0 (0) | 0 (0) | |
| Smoking (former) | 11 (34) | 18 (46) | 13 (34) | 0.47 |
| Systolic blood pressure (mmHg) | 123 ± 15 | 108 ± 17 | 133 ± 16b | |
| Diastolic blood pressure (mmHg) | 79 ± 10 | 67 ± 14 | 82 ± 12b | |
| Resting heart rate (b.p.m.) | – | 73 ± 11 | 92 ± 16b | |
| Ejection fraction (%) | >60 | 24 ± 11 | 59 ± 7b | |
| NYHA class | NA | 2.9 ± 0.8 | NA | |
| Heart failure duration (years) | NA | 8.5 ± 7.8 | NA | |
| Aetiology [# (%)] | 0.0204 | |||
| Ischemic cardiomyopathy | NA | 16 (41) | 10 (26) | |
| Dilated cardiomyopathy | NA | 8 (21) | 14 (37) | |
| Other | NA | 15 (38) | 14 (37) | |
| Comorbidities medical history [# (%)] | ||||
| Hypertension | 4 (13) | 29 (74)a | 16 (42)a , b | |
| Stroke | 0 (0) | 5 (13) | 8 (21)a | |
| Myocardial infarction | 0 (0) | 19 (49)a | 10 (26)a | |
| High cholesterol | 6 (19) | 26 (67)a | 18 (47)a | |
| Kidney disease | 0 (0) | 8 (21)a | 13 (43)a | |
| Liver disease | 0 (0) | 0 (0) | 2 (5) | |
| Diabetes | 1 (3) | 12 (31)a | 16 (42)a |
BMI, body mass index; NYHF, New York Heart Failure; SPO2, saturation of peripheral oxygen.
Values are expressed as mean ± standard deviation.
Significantly different from control.
Significantly different from heart failure.
Results of laboratory examination are compared in Table 2. In summary, blood urea nitrogen was significantly higher in HF and HTx patients, whereas sodium, calcium, chloride, total protein, and albumin were significantly lower compared with controls. Total bilirubin and direct bilirubin as well as creatinine were highest in the HF group, and the difference to controls was significant in bilirubin measurements. The estimated glomerular filtration rate was significantly lower in HTx patients as compared with HF patients and controls. Alkaline phosphatase was significantly higher in HF subjects compared with HTx patients and controls. Glucose levels were non‐significantly different between groups (P = 0.09). Further, Hemoglobin A1c (HbA1c) analysis in HF and HTx patients revealed similar values that were mostly below 7, the therapeutic goal to limit diabetes‐associated complications (HF vs. HTx: 6.9 ± 1.7 vs. 6.9 ± 1.9, P = 0.56).27
Table 2.
Laboratory parameters
| Control (n = 32) | Heart failure (n=39) | Heart transplant (n = 38) | |
|---|---|---|---|
| WBC (×103/μL) | N/A | 6.9 ± 2.0 | 6.7 ± 2.4 |
| Haematocrit (%) | N/A | 39.1 ± 4.8 | 38.0 ± 4.7 |
| Platelet (×103/μL) | N/A | 206 ± 46 | 195 ± 68 |
| Glucose (mg/dL) | 98 ± 14 | 114 ± 42 | 122 ± 63 |
| HbA1c (%) | N/A | 6.9 ± 1.7 | 6.9 ± 1.9 |
| Sodium (mEq/L) | 147 ± 12 | 137 + 3a | 139 ± 3a |
| Potassium (mEq/L) | 4.5 ± 0.5 | 4.4 ± 0.5 | 4.3 ± 0.4 |
| Calcium (mg/dL) | 10.3 ± 1.0 | 9.3 ± 0.5a | 9.4 ± 0.4a |
| Chloride (mEq/L) | 110 ± 10 | 101 ± 4a | 104 ± 4a |
| Blood urea nitrogen (mg/dL) | 16.5 ± 4.7 | 28.8 ± 20.8a | 30.2 ± 11.2a |
| Creatinine (mg/dL) | 1.0 ± 0.2 | 1.6 ± 1.7 | 1.5 ± 0.5 |
| Total protein (g/dL) | 7.7 ± 0.8 | 7.3 ± 0.8a | 6.8 ± 0.6a , b |
| Albumin (mg/dL) | 4.9 ± 0.4 | 4.3 ± 0.5a | 4.2 ± 0.3a |
| Total bilirubin (mg/dL) | 0.49 ± 0.16 | 0.84 ± 0.59a | 0.70 ± 0.36 |
| Direct bilirubin (mg/dL) | 0.10 ± 0.02 | 0.24 ± 0.29a | 0.15 ± 0.09 |
| Aspartate aminotransferase (U/L) | 25.3 ± 19.9 | 25.1 ± 14.4 | 22.2 ± 9.4 |
| Alanine transaminase (U/L) | 25.6 ± 15.4 | 28.1 ± 29.4 | 20.5 ± 12.2 |
| Alkaline phosphatase (U/L) | 71.6 ± 19.1 | 92.3 ± 47.2a | 73.2 ± 24.9b |
| eGFR (mL/min/1.73 m2) | 75.7 ± 22.1 | 64.8 ± 23.2 | 53.1 ± 15.6a , b |
eGFR, estimated glomerular filtration rate; WBC, white blood cell.
Values are expressed as mean ± standard deviation.
Significantly different from control.
Significantly different from heart failure.
As shown in Table 3, all cardiac transplant patients received immunosuppressive medication to prevent transplant rejections. The majority of them took tacrolimus (82%), whereas only a small percentage took cyclosporine (16%). Further, 89% of the subjects in this group received prednisone therapy. A high percentage of patients in both the HF and HTx groups were either on aspirin (77% and 66%, respectively) or coumadin (44% in the HF group).
Table 3.
Medical therapy
| Control (n = 32) | Heart failure (n = 39) | Heart transplant (n = 38) | |
|---|---|---|---|
| Aspirin | 2 (6) | 30 (77) | 25 (66) |
| Statins | 3 (9) | 25 (64) | 26 (68) |
| Insulin | 0 (0) | 8 (21) | 10 (26) |
| Oral antidiabetic agents | 0 (0) | 5 (13) | 1 (3) |
| Coumadin | 0 (0) | 17 (44) | 0 (0) |
| Anti‐arrhythmics | 0 (0) | 14 (36) | 0 (0) |
| B‐blockers | 0 (0) | 33 (85) | 6 (16) |
| Diuretics | 0 (0) | 34 (87) | 9 (24) |
| Calcium channel blockers | 0 (0) | 2 (3) | 10 (26) |
| ACE inhibitor/ARBs | 0 (0) | 32 (79) | 7 (18) |
| Vasodilators | 0 (0) | 5 (13) | 0 (0) |
| Digoxin | 0 (0) | 17 (44) | 1 (3) |
| PPIs | 0 (0) | 11 (28) | 22 (58) |
| Immunosuppressive | |||
| Tacrolimus | 0 (0) | 0 (0) | 31 (82) |
| Mycophenolate | 0 (0) | 0 (0) | 25 (66) |
| Prednisone | 0 (0) | 0 (0) | 34 (89) |
| Cyclosporine | 0 (0) | 0 (0) | 6 (16) |
| Sirolimus | 0 (0) | 0 (0) | 4 (11) |
| Azathioprine | 0 (0) | 0 (0) | 3 (8) |
ACE, angiotensin converting enzyme; ARBs, angiotensin II receptor blocker; PPIs, proton pump inhibitor.
Values are expressed as number (percentage).
Serum bone metabolism and inflammation markers
We analysed systemic bone metabolism and inflammation markers to determine the impact of HF and HTx. We investigated bone formation markers P1NP and osteocalcin, bone resorption marker CTX, and TNF‐α as a measure of systemic inflammation. Furthermore, we evaluated levels of calciotropic hormones 25‐OHD and PTH. Advanced HF is associated with renal insufficiency, resulting in abnormal vitamin D metabolism that can lead to secondary hyperparathyroidism and may contribute to bone loss in advanced HF.28, 29 In addition, an association between periodontal disease and lower 25‐OHD levels has been reported.30, 31
Pro‐inflammatory TNF‐α was significantly higher in serum of HF and HTx subjects compared with controls (Figure 1 A), while 25‐OHD was lowest in HF subjects (HF vs. HTx, P < 0.05, Figure 1 B) with similar values in controls and HTx patients. PTH, a measure of osteoclastic activity and bone resorption, typically varies inversely with 25‐OHD.32 In our study population, HF and HTx subjects had comparable values that were significantly higher than in controls (Figure 1 C).
Figure 1.
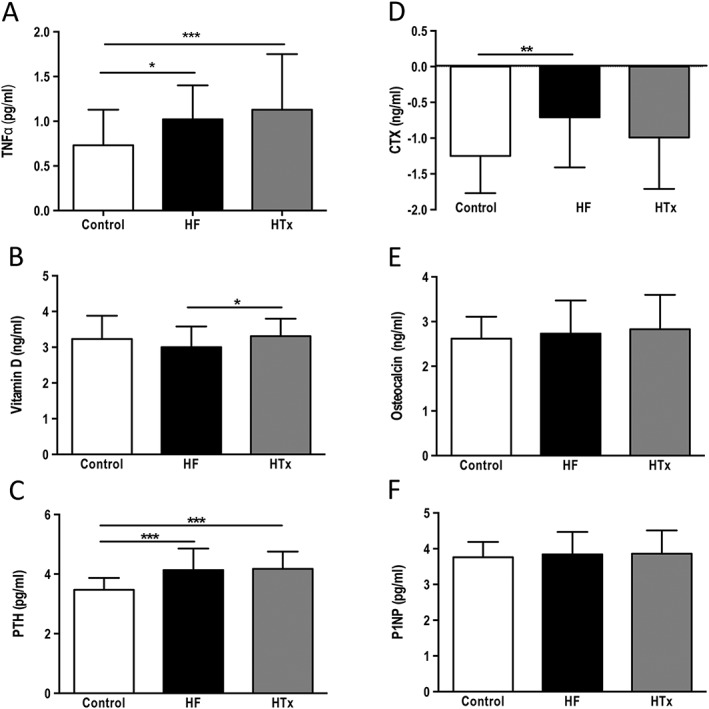
Comparison of bone metabolic and inflammatory markers between controls, patients with heart failure, and patients that had undergone cardiac transplantation. Data are presented as mean ± standard deviation. Transformed data are labeled as natural log data. (A) Natural log data: tumour necrosis factor‐alpha, (B) natural log data: 25‐hydroxyvitamin D, (C) natural log data: parathyroid hormone, (D) natural log data: cross‐linked C‐terminal telopeptide of type I collagen, (E) natural log data: osteocalcin, and (F) procollagen‐1 N‐terminal peptide. *P < 0.05, **P < 0.01, ***P < 0.001.
The bone resorption marker CTX was highest in HF subjects. When the natural log of the CTX values was analysed, statistically significant differences were found between HF and controls (Figure 1 D). Opposite to CTX, bone formation markers osteocalcin and P1NP did not differ much between groups (Figures 1 E/F).
Periodontal examination
During periodontal examination, no oral soft tissue lesions were detected. The mean number of missing teeth was comparable between all groups (HF: 6.7 ± 7.3, HTx: 4.8 ± 7.1, Control: 3.9 ± 4.2; P = 0.178).
As shown in Figure 2 A–C, subjects in the HF group show the highest measures of periodontal disease (PD, CAL, CPD) as compared with HTx and control subjects. CPD, a cumulative measure for periodontal pockets greater than 3 mm,24 was significantly higher in HF subjects as compared with controls and also HTx patients (Figure 2 C). It had been described in previous studies that saliva or gingival crevicular fluid (GCF) could be used to analyse inflammatory markers indicative of the host inflammatory response as a result of periodontal disease occurrence.25, 33, 34 In a sub‐analysis of our HF population, we analysed β‐glucuronidase in saliva and IL‐1β in saliva and GCF. CPD, a cumulative measure for periodontal pocketing, correlated significantly with β‐glucuronidase in saliva (r = 0.4863, P < 0.01), IL‐1β in saliva (r = 0.5149, P < 0.01), and GCF (r = 0.6056, P < 0.001) (Figure 3).
Figure 2.
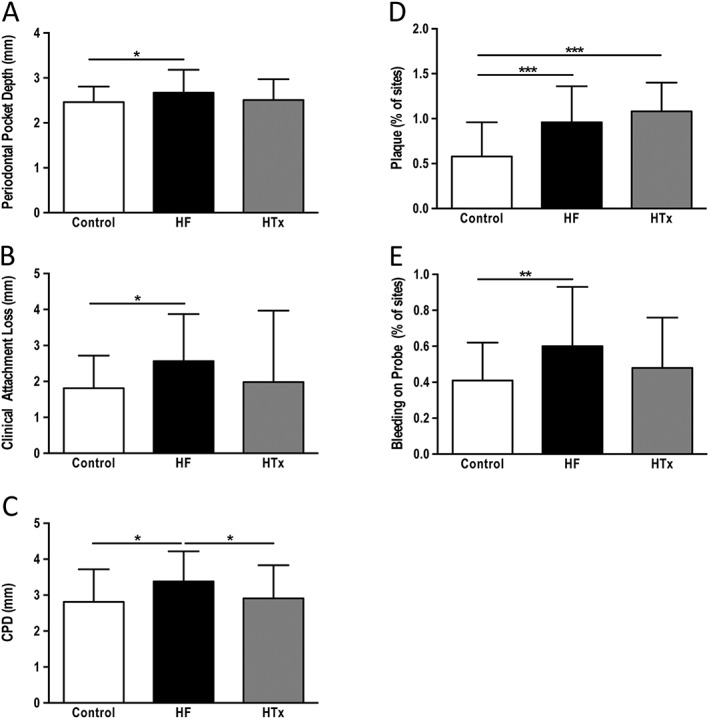
Comparison of periodontal markers between controls, patients with heart failure, and patients that had undergone cardiac transplantation. Data are presented as mean ± standard deviation. Transformed data are labeled as natural log data (A) periodontal pocket depth, (B) clinical attachment loss, (C) natural log data: cumulative probing depth, (D) % of sites with plaque, 1 = 100% (F) bleeding on probing, 1 = 100% *P < 0.05, **P < 0.01, ***P < 0.001.
Figure 3.

Saliva and gingival crevicular fluid analysis. Local inflammatory markers are increased and correlate with the presence of periodontal disease. β‐glucuronidase in saliva, interleukin‐1β in saliva, and gingival crevicular fluid were analysed in patients with heart failure.
Plaque accumulation causes an inflammatory reaction in the gingiva and, therefore, plaque and BOP are commonly used to determine oral hygiene status.23 Despite having similar plaque values that were significantly higher than in the control group (Figure 2 D), only subjects with HF presented with significantly higher BOP values when compared with controls (Figure 2 E).
Measures of periodontal disease and periodontal disease severity were adjusted to determine whether systemic and local factors might account for the association between HF and periodontal disease. PD, CAL, and CPD were adjusted for 25‐OHD serum levels, race, plaque, and BOP. These parameters were included by being a risk factor for periodontal disease22 or being found to be significantly different during patient evaluation. As a result, means were still different; however, significances between all three groups disappeared. Because of its potential impact on the periodontal condition, we further included diabetes in our adjusted model. This did not change the outcome of the analysis—significances between groups still disappeared.
Discussion
This is the first cross‐sectional study that examines the prevalence of periodontitis, systemic measures of inflammation and bone metabolism in patients with HF, patients after HTx, and a control population. Currently, there is only one study that described an increased rate of periodontal disease (76% for moderate to severe chronic periodontitis) in HF patients, which is comparable with the 69% we detected and considerably more than the 47.2% that had been described for the general population.10, 19 However, the study by Lessem et al.19 was a retrospective case‐record study that only used dental radiographs to categorize periodontal status, and findings were not adjusted for potential confounders. Our study involved actual clinical periodontal examination in HF patients before and after HTx and non‐HF controls and showed increased measures of periodontal disease severity in HF subjects; however, adjustment for local measures of oral hygiene, systemic 25‐OHD levels, and race attenuated significant differences between groups.
Periodontitis is a multifactorial disease in which the host response plays an important role,15 and recent studies have placed an emphasis on determining what systemic and local factors could influence periodontal disease. HF, a pro‐inflammatory debilitating disease, is associated with metabolic imbalances that impacts multiple organs and associated metabolic pathways.4 HF and HTX subjects in our study show a range of comorbidities associated with their medical history that distinguishes them from their non‐HF controls. They show a higher rate of diabetes, a known risk factor for periodontal disease,35 and we, therefore, corrected all our clinical and serological markers for diabetes to account for differences.
HF also negatively impacts bone metabolism,7 which is reflected in our current data showing that specifically bone resorption marker CTX is the highest in HF patients as compared with controls. Further, our data indicate that despite the improvement in cardiac function after HTx, CTX does not decrease substantially, and TNF‐α is significantly elevated in both HF and HTx subjects as compared with controls. This is opposite to previous work by Shane et al. 29 demonstrating that bone resorption markers are only increased during the first month post‐HTx and then return to baseline by 6 months. The authors had attributed their findings to the high rates of bone loss in the pre‐transplant population, a result of HF on skeletal bone remodelling, and also to post‐transplant medication such as prednisone.36 HTx subjects in our study group were on average transplanted 46 months ago and still displayed high markers of bone resorption. Perhaps, elevated TNF‐α levels in our HTx group are an indicator for a persistent pro‐inflammatory state despite an improved cardiac function that could negatively impact measures of bone metabolism. Our work and others' work demonstrated that increased levels of markers of bone resorption can be associated with increased periodontal disease severity and progression.17, 31 This is in line with our data showing that HF subjects present with more severe periodontal disease in addition to increased bone turnover markers.
Vitamin D status and bone metabolism are closely related, and it has been shown that low 25‐OHD levels can negatively impact biochemical markers of bone turnover. PTH (a measure of bone resorption) typically varies inversely with 25‐OHD,32 and increased CTX levels may be related to the relatively low 25‐OHD and higher PTH levels noted in our HF patient population. Further, an association between periodontal disease, gingival inflammation, and lower 25‐OHD levels has been reported.30, 31 Studies that evaluated thresholds for serum 25‐OHD in relation to bone mineral density, dental health, and fractures suggest that advantageous 25‐OHD serum concentrations begin at 30 ng/mL.37 When 25‐OHD levels were presented without the natural log as ng/mL, 25‐OHD values are below the 30 ng/mL threshold in our HF group (23.68 ng/mL ± 14.67), whereas HTx and controls were at 30.34 ng/mL ± 12.95 and 30.98 ng/mL ± 23.11, respectively.
BOP, an indicator for local gingival inflammation, has been discussed as predictive measure that can foretell periodontal tissue breakdown.38 It can be influenced by local factors such as plaque accumulation but also by systemic medication.39 Our data show that BOP was higher in HF and HTx subjects as compared with controls. Interestingly, despite having similar plaque measures, only BOP in HF patients was significantly elevated as compared with controls. This could be related to the medication in HTx patients as 89% received prednisone that might have reduced or masked the local inflammatory response to plaque accumulation.39 At the same time, BOP in HF patients could have been more pronounced because of the high percentage of HF patients that were on aspirin (77%) or coumadin (44%). Elevated BOP is often observed in patients taking these medications.40 When adjusting periodontal measures for BOP, plaque, race, and 25‐OHD, not only did significant differences in periodontal measures disappear but BOP seems to be the determining factor that correlates with PD, CPD, and CAL (P < 0.001, P < 0.01, P < 0.05, respectively). As mentioned, BOP can be influenced not only by plaque accumulation but also by systemic medication.39 However, opposite to systemic medication or a complex medical status, local plaque status can be improved more easily during additional dental visits. This might then positively impact the periodontal condition and quality of life in HF patients.20
Our study is limited by the single‐centre nature of our patient cohort, and analysis of patients from different centres would be beneficial. Further, the control population might display a higher degree of periodontal disease than the average general population. Similar to a study by Pischon et al., subjects were recruited after presenting to the dental clinic where they were seeking dental care.41 A recent National Health and Nutrition Examination Survey (NHANES) study that utilized a full‐mouth periodontal examination described only a rate of periodontitis in 47% of subjects over 30 years old.10 Though, our study benefits from the inclusion of a HTx group that almost could be considered a true control group because of similar underlying medical condition.
We had performed full‐mouth periodontal evaluation and determined CPD.24 Opposite to CAL and PD, it only calculates periodontal pockets deeper than 3 mm. It has been shown that those pockets could potentially provide the anaerobic conditions that are necessary for periodontal pathogens to thrive putting these pockets at greater risk for further tissue breakdown.42 It has been suggested that the use of saliva may provide a more global measure of the oral inflammatory burden throughout the mouth.43 When analysing β‐glucuronidase, a cytoplasmic enzyme released by activated neutrophils that is responsible for degradation of proteoglycans and ground substance44, 45 and inflammatory IL‐1β, we found a positive correlation between the levels of IL‐1β and β‐glucuronidase in the saliva and GCF and CPD (PD > 3 mm). These findings support the trend observed in other studies that have demonstrated that the level of pro‐inflammatory cytokines in the saliva and GCF is associated with the level of periodontal disease as reflected by the clinical periodontal parameters.25, 33, 34, 46, 47
The cross‐sectional study design precludes us from identifying biological mechanism and can only describe association. Intervention trials that may show whether early treatment of periodontal disease can improve clinical parameters in patients with HF and following HTx and might help to distinguish between the impact of local versus systemic factors.
In conclusion, we have shown that patients with HF have slightly more periodontal disease with higher unadjusted CPD associated with abnormal serum biomarkers when compared with HTx and controls. Further, periodontal disease severity was detectable in saliva and GCF making it a potential useful screening tool for identifying the need for periodontal treatment in patients with advanced HF. As of yet, there are no guidelines describing the necessity and management of dental‐periodontal care in patients with HF and after HTx. Perhaps there is a need for a close interaction between physicians and dentists to improve patient care.
Conflict of interest
None declared.
Funding
The study was supported in part by Columbia University's CTSA (grant no. UL1RR024156) from NCATS‐NCRR/NIH. Dr. Schulze‐Späte was supported by the National Institute of Dental and Craniofacial Research (K08DE018968), and a Columbia University Professional Schools Diversity Fellowship. Dr. Schulze was supported by grants from the National Heart, Lung and Blood Institute (P30 HL101272, R01 HL114813, UL1 RR024156) and the Herbert and Florence Irving Scholar Award, Columbia University.
Schulze‐Späte, U. , Mizani, I. , Salaverry, K. R. , Chang, J. , Wu, C. , Jones, M. , Kennel, P. J. , Brunjes, D. L. , Choo, T.‐H. , Kato, T. S. , Mancini, D. , Grbic, J. , and Schulze, P. C. (2017) Periodontitis and bone metabolism in patients with advanced heart failure and after heart transplantation. ESC Heart Failure, 4: 169–177. doi: 10.1002/ehf2.12126.
Contributor Information
Ulrike Schulze‐Späte, Email: Ulrike.Schulze-Spaete@med.uni-jena.de.
P. Christian Schulze, Email: Ulrike.Schulze-Spaete@med.uni-jena.de.
References
- 1. Braunwald E, Ross J, Jr. , Sonnenblick EH. Mechanisms of contraction of the normal and failing heart. N Engl J Med. 2009; 11: 28–38. [DOI] [PubMed] [Google Scholar]
- 2. Mann DL. Inflammatory mediators and the failing heart: past, present, and the foreseeable future. Circ Res 2002; 91: 988–998. [DOI] [PubMed] [Google Scholar]
- 3. Sharpe N, Doughty R. Epidemiology of heart failure and ventricular dysfunction. Lancet. 1998; 352: SI3–SI7. [DOI] [PubMed] [Google Scholar]
- 4. Neubauer S. The failing heart—an engine out of fuel. N Engl J Med 2007; 356: 1140–1151. [DOI] [PubMed] [Google Scholar]
- 5. Stehlik J, Edwards LB, Kucheryavaya AY, Aurora P, Christie JD, Kirk R, Dobbels F, Rahmel AO, Hertz MI. The Registry of the International Society for Heart and Lung Transplantation: twenty‐seventh official adult heart transplant report—2010. J Heart Lung Transplant: the official publication of the International Society for Heart Transplantation 1987; 277: 1012–1022. [Google Scholar]
- 6. Drexler H, Riede U, Munzel T, Konig H, Funke E, Just H. Alterations of skeletal muscle in chronic heart failure. Circulation 1992: 85: 1751–1759. [DOI] [PubMed] [Google Scholar]
- 7. Jankowska EA, Jakubaszko J, Cwynar A, Majda J, Ponikowska B, Kustrzycka‐Kratochwil D, Reczuch K, Borodulin‐Nadzieja L, Banasiak W, Poole‐Wilson PA, Ponikowski P. Bone mineral status and bone loss over time in men with chronic systolic heart failure and their clinical and hormonal determinants. Eur J Heart Fail 2009;11: 28–38. [DOI] [PubMed] [Google Scholar]
- 8. Wu C, Kato TS, Pronschinske K, Qiu S, Naka Y, Takayama H, Schulze‐Späte U, Cremers S, Shane E, Mancini D, Schulze PC. Dynamics of bone turnover markers in patients with heart failure and following haemodynamic improvement through ventricular assist device implantation. Eur J Heart Fail 2012; 14: 1356–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Graves DT, Li J, Cochran DL. Inflammation and uncoupling as mechanisms of periodontal bone loss. J Dent Res 2011; 90: 143–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Eke PI, Dye BA, Wei L, Thornton‐Evans GO, Genco RJ, CDC Periodontal Disease Surveillance workgroup: James Beck GDRP. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dent Res 2012; 91: 914–920. [DOI] [PubMed] [Google Scholar]
- 11. Papapanou PN, Wennstrom JL, Grondahl K. A 10‐year retrospective study of periodontal disease progression. J Clin Periodontol 1989; 16: 403–411. [DOI] [PubMed] [Google Scholar]
- 12. Theilade E. The non‐specific theory in microbial etiology of inflammatory periodontal diseases. J Clin Periodontol 1986; 13: 905–911. [DOI] [PubMed] [Google Scholar]
- 13. Loesche WJ. DNA probe and enzyme analysis in periodontal diagnostics. J Periodontol 1992; 63: 1102–1109. [DOI] [PubMed] [Google Scholar]
- 14. Schenkein HA. Host responses in maintaining periodontal health and determining periodontal disease. Periodontol 2000. 2006; 40: 77–93. [DOI] [PubMed] [Google Scholar]
- 15. Cekici A, Kantarci A, Hasturk H, Van Dyke TE. Inflammatory and immune pathways in the pathogenesis of periodontal disease. Periodontol 2000. 2014; 64: 57–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Takaishi Y, Okamoto Y, Ikeo T, Morii H, Takeda M, Hide K, Arai T, Nonaka K. Correlations between periodontitis and loss of mandibular bone in relation to systemic bone changes in postmenopausal Japanese women. Osteoporos Int 2005; 16: 1875–1882. [DOI] [PubMed] [Google Scholar]
- 17. Yoshihara A, Deguchi T, Hanada N, Miyazaki H. Relation of bone turnover markers to periodontal disease and jaw bone morphology in elderly Japanese subjects. Oral Dis 2009; 15: 176–181. [DOI] [PubMed] [Google Scholar]
- 18. Hasturk H, Kantarci A, Van Dyke TE. Oral inflammatory diseases and systemic inflammation: role of the macrophage. Front Immunol 2012; 3: 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lessem J, Drisko C, Greenwell H, Persson R, Newman H, Smart G, Hopkins L, Parameshwar J, Fishbein D, Partridge C, Bhat G, Goldsmith J. Are cardiac transplant patients more likely to have periodontitis? A case record study. J Int Acad Periodontol 2002; 4: 95–100. [PubMed] [Google Scholar]
- 20. Jansson H, Wahlin A, Johansson V, Akerman S, Lundegren N, Isberg PE, Norderyd O. Impact of periodontal disease experience on oral health‐related quality of life. J Periodontol 2014; 85: 438–445. [DOI] [PubMed] [Google Scholar]
- 21. Dolgin M, New York Heart Association. Criteria Committee Nomenclature and Criteria for Diagnosis of Diseases of the Heart and Great Vessels. 9th ed. New York: Boston Little, Brown, 1994. [Google Scholar]
- 22. Genco RJ, Borgnakke WS. Risk factors for periodontal disease. Periodontol 2000. 2013; 62: 59–94. [DOI] [PubMed] [Google Scholar]
- 23. Ainamo J, Bay I. Problems and proposals for recording gingivitis and plaque. Int Dent J 1975; 25: 229–235. [PubMed] [Google Scholar]
- 24. Dietrich T, Jimenez M, Krall Kaye EA, Vokonas PS, Garcia RI. Age‐dependent associations between chronic periodontitis/edentulism and risk of coronary heart disease. Circulation. 2008 April 1, 2008; 117: 1668–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lamster IB, Kaufman E, Grbic JT, Winston LJ, Singer RE. Beta‐glucuronidase activity in saliva: relationship to clinical periodontal parameters. J Periodontol 2003; 74: 353–359. [DOI] [PubMed] [Google Scholar]
- 26. Engebretson SP, Hey‐Hadavi J, Ehrhardt FJ, Hsu D, Celenti RS, Grbic JT, Lamster IB. Gingival crevicular fluid levels of interleukin‐1beta and glycemic control in patients with chronic periodontitis and type 2 diabetes. J Periodontol 2004; 75: 1203–1208. [DOI] [PubMed] [Google Scholar]
- 27. American Diabetes A . Standards of medical care in diabetes—2012. Diabetes Care 2012; 35: S11–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zittermann A, Schleithoff SS, Koerfer R. Markers of bone metabolism in congestive heart failure. Clin Chim Acta; international journal of clinical chemistry. 2006; 366: 27–36. [DOI] [PubMed] [Google Scholar]
- 29. Shane E, Mancini D, Aaronson K, Silverberg SJ, Seibel MJ, Addesso V, McMahon DJ. Bone mass, vitamin D deficiency, and hyperparathyroidism in congestive heart failure. Am J Med 1997; 103: 197–207. [DOI] [PubMed] [Google Scholar]
- 30. Dietrich T, Joshipura KJ, Dawson‐Hughes B, Bischoff‐Ferrari HA. Association between serum concentrations of 25‐hydroxyvitamin D3 and periodontal disease in the US population. Am J Clin Nutr 2004; 80: 108–113. [DOI] [PubMed] [Google Scholar]
- 31. Schulze‐Spate U, Turner R, Wang Y, Chao R, Christian Schulze P, Phipps K, Orwoll E, Dam, TT , Osteoporotic Fractures in Men Research G. Relationship of Bone Metabolism Biomarkers and Periodontal Disease: The Osteoporotic Fractures in Men (MrOS) Study. J Clin Endocrinol Metab. 2015. Apr 9:jc20144180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Steingrimsdottir L, Gunnarsson O, Indridason OS, Franzson L, Sigurdsson G. Relationship between serum parathyroid hormone levels, vitamin D sufficiency, and calcium intake. JAMA 2005; 294: 2336–2341. [DOI] [PubMed] [Google Scholar]
- 33. Yoon AJ, Cheng B, Philipone E, Turner R, Lamster IB. Inflammatory biomarkers in saliva: assessing the strength of association of diabetes mellitus and periodontal status with the oral inflammatory burden. J Clin Periodontol 2012; 39: 434–440. [DOI] [PubMed] [Google Scholar]
- 34. Engebretson SP, Grbic JT, Singer R, Lamster IB. GCF IL‐1beta profiles in periodontal disease. J Clin Periodontol 2002; 29: 48–53. [DOI] [PubMed] [Google Scholar]
- 35. Tsai C, Hayes C, Taylor GW. Glycemic control of type 2 diabetes and severe periodontal disease in the US adult population. Community Dent Oral Epidemiol 2002; 30: 182–192. [DOI] [PubMed] [Google Scholar]
- 36. Shane E, Rivas M, McMahon DJ, Staron RB, Silverberg SJ, Seibel MJ, Mancini D, Michler RE, Aaronson K, Addesso V, Lo SH. Bone loss and turnover after cardiac transplantation. J Clin Endocrinol Metab 1997; 82: 1497–1506. [DOI] [PubMed] [Google Scholar]
- 37. Bischoff‐Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson‐Hughes B. Estimation of optimal serum concentrations of 25‐hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. [Meta‐Analysis Research Support, Non‐U.S. Gov't Review]. 2006; 84: 18–28. [DOI] [PubMed] [Google Scholar]
- 38. Lang NP, Joss A, Orsanic T, Gusberti FA, Siegrist BE. Bleeding on probing. A predictor for the progression of periodontal disease? J Clin Periodontol 1986; 13: 590–596. [DOI] [PubMed] [Google Scholar]
- 39. Seymour RA. Effects of medications on the periodontal tissues in health and disease. Periodontol 2000. 2006; 40: 120–129. [DOI] [PubMed] [Google Scholar]
- 40. Royzman D, Recio L, Badovinac RL, Fiorellini J, Goodson M, Howell H, Karimbux N. The effect of aspirin intake on bleeding on probing in patients with gingivitis. J Periodontol 2004; 75: 679–684. [DOI] [PubMed] [Google Scholar]
- 41. Pischon N, Pischon T, Kroger J, Gulmez E, Kleber BM, Bernimoulin JP, Landau H, Brinkmann PG, Schlattmann P, Zernicke J, Buttgereit F, Detert J. Association among rheumatoid arthritis, oral hygiene, and periodontitis. J Periodontol 2008; 79: 979–986. [DOI] [PubMed] [Google Scholar]
- 42. Machtei EE, Dunford R, Hausmann E, Grossi SG, Powell J, Cummins D, Zambon JJ, Genco RJ. Longitudinal study of prognostic factors in established periodontitis patients. J Clin Periodontol 1997; 24: 102–109. [DOI] [PubMed] [Google Scholar]
- 43. Kaufman E, Lamster IB. The diagnostic applications of saliva—a review. Crit Rev Oral Biol Med : an official publication of the American Association of Oral Biologists 2002; 13: 197–212. [DOI] [PubMed] [Google Scholar]
- 44. Boxer LA, Smolen JE. Neutrophil granule constituents and their release in health and disease. Hematol Oncol Clin North Am 1988; 2: 101–134. [PubMed] [Google Scholar]
- 45. Falloon J, Gallin JI. Neutrophil granules in health and disease. J Allergy Clin Immunol 1986; 77: 653–662. [DOI] [PubMed] [Google Scholar]
- 46. Lamster IB, Holmes LG, Gross KB, Oshrain RL, Cohen DW, Rose LF, Peters LM, Pope MR. The relationship of beta‐glucuronidase activity in crevicular fluid to clinical parameters of periodontal disease. Findings from a multicenter study. J Clin Periodontol 1994; 21: 118–127. [DOI] [PubMed] [Google Scholar]
- 47. Lamster IB, Holmes LG, Gross KB, Oshrain RL, Cohen DW, Rose LF, Peters LM, Pope MR. The relationship of beta‐glucuronidase activity in crevicular fluid to probing attachment loss in patients with adult periodontitis. Findings from a multicenter study. J Clin Periodontol 1995; 22: 36–44. [DOI] [PubMed] [Google Scholar]


