Abstract
The progressive ankylosis protein (ANK) is a transmembrane protein that transports intracellular pyrophosphate (PPi) to the extracellular milieu. In this study we show increased fatty degeneration of the bone marrow of adult ank/ank mice, which lack a functional ANK protein. In addition, isolated bone marrow stromal cells (BMSCs) isolated from ank/ank mice showed a decreased proliferation rate and osteogenic differentiation potential, and an increased adipogenic differentiation potential compared to BMSCs isolated from wild type (WT) littermates. Wnt signaling pathway PCR array analysis revealed that Wnt ligands, Wnt receptors and Wnt signaling proteins that stimulate osteoblast differentiation were expressed at markedly lower levels in ank/ank BMSCs than in WT BMSCs. Lack of ANK function also resulted in impaired bone fracture healing, as indicated by a smaller callus formed and delayed bone formation in the callus site. Whereas 5 weeks after fracture, the fractured bone in WT mice was further remodeled and restored to original shape, the fractured bone in ank/ank mice was not fully restored and remodeled to original shape. In conclusion, our study provides evidence that ANK plays a critical role in the adipogenic/osteogenic fate decision of adult mesenchymal precursor cells. ANK functions in precursor cells are required for osteogenic differentiation of these cells during adult bone homeostasis and repair, whereas lack of ANK functions favors adipogenic differentiation.
Keywords: adipogenesis, bone fracture healing, osteogenesis, progressive ankylosis protein (ANK), Wnt/β-catenin signaling
1. INTRODUCTION
ANK is a transmembrane protein that transports intracellular pyrophosphate (PPi) to the extracellular milieu [1]. The progressive ankylosis allele (ank) is a spontaneous recessive mutation that causes the lack of a functional progressive ankylosis protein (ANK) in ank/ank mice [1]. We have previously shown that ank/ank mice show an osteopenic phenotype [2]. Several studies have now established that ANK is an important regulator of extracellular PPi homeostasis in skeletal tissues [1–5]. Previous findings from our laboratory have suggested that extracellular PPi directly affects osteogenic differentiation [2]. Other studies have suggested that ANK may play a role of mediating ATP efflux in musculoskeletal cells [6]. ATP and its hydrolysis products have been shown to affect osteoblast differentiation and mineralization [7–12]. Therefore, it is possible that ANK is an important regulator of osteoblast differentiation. However, it is not clear how ANK affects osteogenic differentiation of BMSCs.
Wnt/β-catenin signaling has been shown to induce osteogenic differentiation in osteoprogenitor cells [13]. Consequently, activating mutations in Wnt/β-catenin signaling pathway in humans causes high bone-mass phenotype such as van Buchem disease, whereas inactivating mutations cause osteopenic diseases such as osteoporosis-pseudoglioma syndrome [14]. In addition, loss of Wnt/β-catenin signaling is mainly responsible for the loss of osteogenic potential of aging bone marrow stromal cells (BMSCs), the osteogenic precursor cells in the bone marrow [15, 16]. These cells have the potential to differentiate into adipocytes, chondrocytes and osteoblasts [17]. As a consequence of loss of Wnt/β-catenin signaling, aging BMSCs decrease their ability to differentiate into osteoblasts and increase their ability to differentiate into adipocytes [18–20]. Little, however, is known about upstream factors that regulate Wnt/β-catenin signaling in aging BMSCs.
Since Wnt/β-catenin signaling in osteoprogenitor cells induces their differentiation, Wnt/β-catenin signaling is also essential during bone healing [21]. The declining Wnt/β-catenin signaling in aging osteogenic precursor cells has been suggested as a main reason for delayed fracture healing in elderly. In fact, a recent study has shown that treatment of a bone fracture with liposomal Wnt3a resulted in markedly faster and more robust bone healing with earlier onset of mineralization and new osteoid deposition than happens normally in young animals [15].
ank/ank mice have a limited life span of 4 to 6 months [22]. Previously we have shown that adult ank/ank BMSCs show reduced osteogenic differentiation [2], suggesting that these cells rapidly lose their osteogenic differentiation potential. The goal of this study was to determine how lack of ANK function affects the osteogenic differentiation potential of adult BMSCs. Specifically, we asked the question of whether ANK directly affects Wnt/β-catenin signaling and ultimately osteogenic differentiation of adult BMSCs. To accomplish this goal we analyzed the trabecular bone and bone marrow structure in adult ank/ank mice and WT littermates and analyzed the adipogenic and osteogenic differentiation potentials of BMSCs isolated from ank/ank mice and WT littermates. In addition, we analyzed how lack of ANK function affects bone fracture healing in mice.
2. MATERIALS AND METHODS
2.1. Animals
The ank/ank breeding colony used was originally on a hybrid background derived from crossing a C3H and C57BL/6 hybrid male with BALB/c female. Heterozygote breeders (C3FeB6 A/Aw-J-Ankank/J) were obtained from The Jackson Laboratory (Bar Harbor, ME). Heterozygote breeders were used to generate and study ank/ank and wild-type (WT) littermates, with genotypes analyzed by polymerase chain reaction (PCR) as previously described [1].
To analyze bone fracture healing, we utilized a well-established femur fracture model in 6-week-old ank/ank mice and WT littermates [23]. Briefly, a medial parapatellar incision was used to expose the femoral condyles. A hole was drilled into the femoral intramedullary canal at the intracondylar notch using a 30-gauge needle. A 0.01-inch diameter stainless steel pin was inserted into the intramedullary canal to stabilize the impending fracture. A closed diaphyseal femoral fracture was created using a blunt impact force in a three-point bending technique. Pain was managed postoperatively with subcutaneous doses of buprenorphine (0.05mg/kg). Mice were euthanized at 2 weeks and 5 weeks post fracture. Surgery was performed on approximately equivalent numbers of male and female mice. Each group consisted on 5 mice. Protocols were approved by the Institutional Animal Care and Use Committee at New York University School of Medicine in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
2.2. Histology
Hindlimbs from 2 month-old and 4-month-old female and male ank/ank mice and WT littermates were used. We analyzed the hindlimbs of 4 animals in each group (ank/ank and WT). Dissected femurs and tibias from ank/ank mice and WT littermates were fixed in 4% paraformaldehyde, decalcified in 0.2M EDTA pH 7.4 for 14 days, and embedded in paraffin. Five-micrometer sections were cut and stained with hematoxylin and eosin. For immunohistochemical staining, sections were pretreated with bovine testicular hyaluronidase (2mg/ml; Sigma) for 30 min at 37°C, blocked with goat serum for 20 min at room temperature, and incubated over night with a primary antibody specific for perilipin A (ab3526, Abcam, Cambridge, MA) followed by biotinylated secondary antibodies for 30 min at room temperature. After washing, sections were incubated with a streptavidin-peroxidase conjugate for 10 min at room temperature followed by a solution containing diaminobenzidine (chromogen) and 0.03% hydrogen peroxide for 5 min at room temperature. Control sections were incubated with nonimmune rabbit serum. Specimens were viewed under Evos FL Auto Cell Imaging System (Thermo Fisher Scientific). Quantitative analysis of the total area of the fat vacuoles in the bone marrow of the tibia was performed on sections from 2-month old and 4-month old tibiae from ank/ank mice (n = 3) and wild type littermates (n = 3). The analysis was performed in an area 100μm to 700μm distal to the growth plate using the Evos FL Auto Cell Imaging System software. In addition, the number of perilipin immune-positive cells in this area was counted using the Evos FL Auto Cell Imaging System software.
For histological evaluation of bone fracture healing, femurs were excised, the needle was removed, and the samples were fixed and decalcified as described above. Five-micrometer sections were cut and stained with hematoxylin and eosin or alcian blue.
2.3. MicroCT Evaluation
Specimens were fixed in 4% paraformaldehyde and analyzed by high-resolution microCT using a Skyscan 1172 (Bruker, Billerica, MA). To analyze the degree of bone fracture healing, the following parameters were determined: total callus volume (TCV), bone tissue volume in the callus (BV), bone tissue volume fraction of the total callus volume (BV/TCV).
2.4. Plain Radiography
Serial radiographs were taken directly after the creation of the fracture and 2 weeks after fracture surgery using the digital radiographic function of the IVIS Lumina III In Vivo Imaging System (PerkinElmer, Shelton, CA).
2.5. Cell Cultures
Bone marrow stromal cells (BMSCs) were isolated from femurs of 8-week-old female and male ank/ank mice or WT littermates and cultured at 25 × 106 cells per 10-cm in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 15% fetal calf serum as described previously [24–26]. To obtain 25 × 106 cells for one experiment, we used 4 hindlimbs from 2 ank/ank mice and 4 hindlimbs from 2 WT littermates. For osteogenic differentiation, BMSCs were cultured in osteogenic differentiation medium (MEM supplemented with 10% fetal calf serum, 5mM β-glycerophosphate, 50μg/ml ascorbic acid, 10−7M dexamethasone) [2, 26, 27]. After 7, 14, and 21 days of culture, cells were stained for alkaline phosphatase (ALP) activity using Alkaline Phosphatase Magenta Immunohistochemical Substrate Solution (Sigma). To determine mineralization in these cultures, alizarin red S staining was performed on day 14 and day 21 as described previously [28]. For adipogenic differentiation, BMSCs were cultured in adipogenic differentiation medium (DMEM supplemented with 10% fetal calf serum, 0.5 mM 3-isobutyl-1-methylxanthine, 5 μg/ml insulin, and 1 μM dexamethasone) as described previously [26]. Oil red staining was performed after 7, 14, and 21 days of culture.
For Wnt PCR array using the RT2 Profiler PCR array for the Wnt signaling pathway (Qiagen) we cultured ank/ank and WT BMSCs after these cells have reached confluency for 2 days in osteogenic differentiation medium.
2.6. Luciferase Reporter Assays
For luciferase assays to determine β-catenin activity, cells were co-transfected with a firefly TCF/LEF-specific luciferase reporter vector (TOPFlash Reporter; EMD Millipore, Billerica, MA) and a constitutively expressed Renilla luciferase reporter, which served as an internal control for normalizing transfection efficiencies and monitoring cell viability. Luciferase activities from both reporters were measured using a dual luciferase assay kit (Promega Corp., Madison, WI). Luciferase activities were measured using the Tristar LB 941 luminometer (Berthold Technologies, Oak Ridge, TN). All experiments were performed in triplicate and repeated three times.
2.7. Real-time PCR analysis
Total RNA was isolated from cell cultures using the RNeasy Minikit (Qiagen). Gene expression was quantified by real-time PCR analysis as described previously [28]. Briefly, 1 μg of total RNA was reverse-transcribed using the Omniscript RT kit (Qiagen). A 1:100 dilution of the resulting cDNA was used as a template to quantify the relative content of mRNA by real-time PCR using SYBR Green. PCRs were performed with a SYBR Green PCR Master Mix kit (Applied Biosystems), with 95°C for 10 min followed by 40 cycles at 95°C for 15 s and 60°C for 1 min, and 1 cycle at 95°C for 15 s and 60°C for 1 min. The 18S rRNA was amplified at the same time and used as an internal control. The cycle threshold values for 18S rRNA and the samples were measured and calculated by computer software. Relative transcription levels were calculated as x = 2−ΔΔCT, in which ΔΔCT = ΔE − ΔC, ΔE = Ctexp Ct18S, and ΔC = Ctcrtl Ct18S. The gene-specific primers used are as follows: ank-forward, 5′-GCC CAT TGT CAA CCT CTT CGT-3′, and ank-reverse, 5′-GAA TGG CCA CTG CCT CTG TAG-3′; ALP-forward, 5′-AAC ACC AAT GTA GCC AAG-3′, and ALP-reverse, 5′-TCG GGC AGC GGT TAC TGT-3′; adipocyte protein 2 (AP2)-forward, 5′-CTT CAA ACT GGG CGT GGA A-3′, and AP2-reverse, 5′-CCA TCT AGG GTT ATG ATG CTC TTC A-3′; osteocalcin (OC)-forward, 5′-CCA GCG ACT CTG AGT CTG ACA A-3′, and OC-reverse, 5′-CCG GAG TCT ATT CAC CAC CTT ACT-3′; osterix-forward, 5′-TTC TGT CCC CTG CTC CTT CTA G-3′, and osterix-reverse, 5′-CGT CAA CGA CGT TAT GCT CTT C-3′; peroxisome proliferator-activated receptor gamma (PPARγ)-forward, 5′-CCC AAT GGT TGC TGA TTA CAA A-3′, and PPARγ-reverse, 5′-AAT AAT AAG GTG GAG ATG CAG GTT CT-3′; runx2-forward, 5′-AGT AGC CAG GTT CAA CGA TCT GA-3′, and runx2-reverse, 5′-GAC TGT TAT GGT CAA GGT GAA ACT CTT-3′.
To profile gene expression of Wnt proteins, Wnt receptors and Wnt target genes in ank/ank and WT BMSCs, we carried out a PCR array using the RT2 Profiler PCR array for the Wnt signaling pathway (Qiagen) following the manufacturer’s instructions. The average threshold cycle value (Ct value) was calculated and normalized to that of the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase. These experiments were repeated three times independently.
2.8. Cell Proliferation
For analysis of cell proliferation, 5000 ank/ank or WT BMSCs were dispensed in a 96-well plate. The plate was incubated for 12 h, 24 h or 48 h in a humidified incubator at 37°C followed by the addition of 10 μl Cell counting Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc., Rockville, MD) and incubation for 4 h at 37°C. After the incubation, the absorbance of the collected medium was measured at 450 nm. The absorbance of the collected medium after 12 h incubation of WT cells was set as 100%.
2.9. Statistical analysis
All measurements were performed at least in triplicates. Numerical data are presented as mean ± S.D. (n ≥ 3). Statistical analysis was performed by Student’s t-test to evaluate differences between the two groups. Analysis of variance was performed when the examined experimental groups exceeded three. Tukey’s multiple comparison test was applied as post hoc test. Statistical significance was defined as p < 0.05 (p-values are reported in the figure legends).
3. RESULTS
3.1. Lack of ANK function accelerates age-related fatty bone marrow
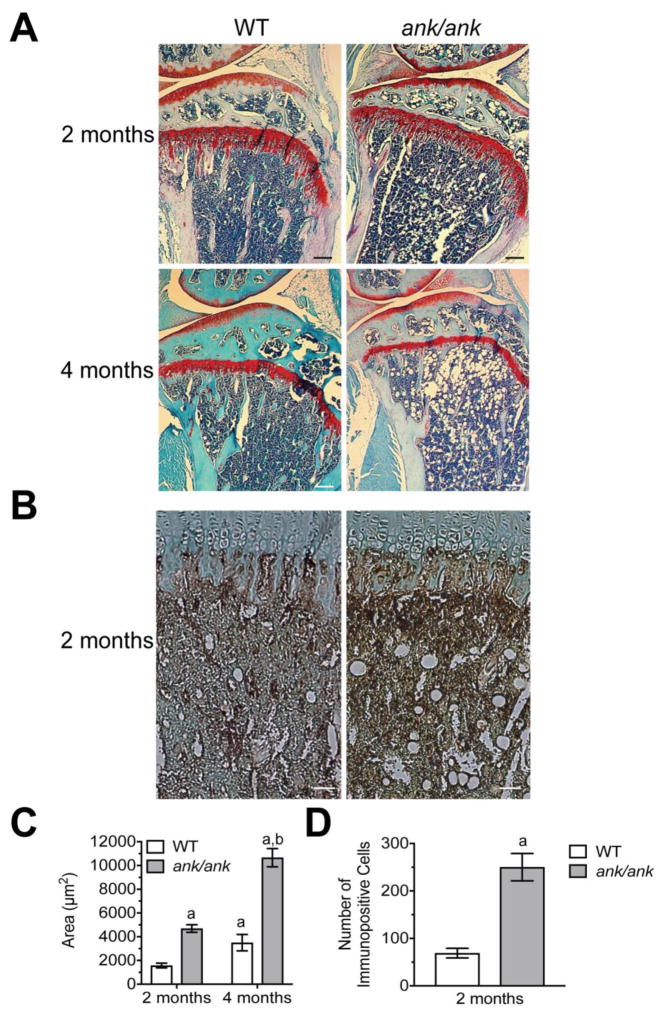
We have previously shown that ank/ank mice have an age-related osteoporotic phenotype, decreased osteoblast number and decreased bone formation compared to WT littermates [2]. Histological analysis of the femur and the tibia of 2-month old (n=4) and 4-month old (n=4) ank/ank mice revealed besides the osteoporotic phenotype increased fatty degeneration in the bone marrow compared to WT littermates (n=4 for 2-month old mice, and n=4 for 4-month old animals) as indicated by the increased number of fat vacuoles in the bone marrow (Fig 1A). Increased fatty degeneration of the bone marrow became evident in 2-month-old ank/ank mice (Fig. 1A), and markedly increased in 4-month old ank/ank mice (Fig. 1A). In addition, immunostaining with an antibody specific for perilipin, a protein that coats lipid droplets in adipocytes, revealed markedly increased immunostaining for this protein in the bone marrow of 2-month old ank/ank mice compared to WT littermates (Fig. 1B). Quantitative analysis revealed that the total area of fat vacuoles in the bone marrow of the tibia was increased in 2-month and 4-month-old ank/ank mice compared to wild type littermates (Fig. 1C). In addition, the total area of fat vacuoles in the bone marrow of the tibia increased in 4-month old ank/ank mice and wild type littermates compared to 2-month old ank/ank mice and wild type littermates, respectively (Fig. 1C). This increase in 4-month old mice, however, was more pronounced in ank/ank mice than wild type littermates (Fig. 1C). Furthermore, the number of perilipin immuno-positive cells in the bone marrow of 2-month old ank/ank tibiae was markedly higher than the number of immunopositive cells in the bone marrow of 2-month old wild type tibiae (Fig. 1D). These findings together with our previous findings suggest enhanced adipogenesis and decreased osteogenesis of precursor cells in vivo in adult ank/ank mice compared to WT littermates.
Figure 1.
A: Histological analysis of the bone marrow of 2-month and 4-month old WT and ank/ank mice (tibia, safranin O staining). Note the increased fat fraction in the bone marrow of 2-month and 4-month old ank/ank mice compared to WT littermates. Bar, 200 μm. B: Immunohistological staining with antibodies specific for perilipin, a protein that coats lipid droplets in adipocytes, of sections from the bone marrow of 2 month-old WT and ank/ank mice. Bar, 200 μm. C: Quantitative analysis of the total area (μm2) of the fat vacuoles in the bone marrow of 2-month and 4-month old ank/ank and wild type tibiae D: Number of cells immunopositive for perilipin in an area 100μm to 700μm distal to the growth plate. Sections from three different ank/ank mice and three different wild type littermates were analyzed. Data are expressed as mean ± SD. (ap < 0.01 vs. 2-month old WT tibia; bp < 0.01 vs. 2-month old ank/ank tibia).
3.2. Lack of ANK function inhibits proliferation and osteogenic differentiation and accelerates adipogenic differentiation of BMSCs
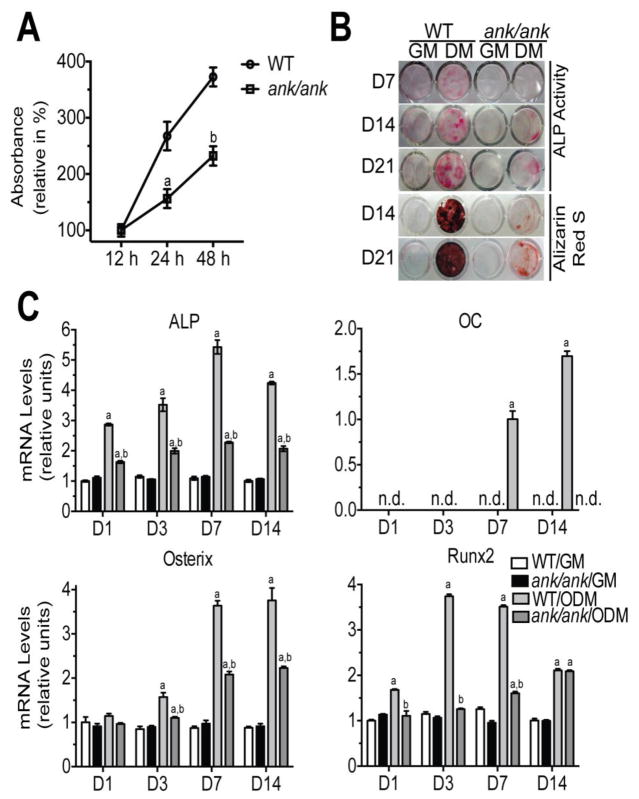
We analyzed proliferation of BMSCs isolated from ank/ank mice and WT littermates using the Cell Counting Kit-8 (CKK8). BMSCs isolated from ank/ank mice showed a markedly decreased rate of proliferation in culture at 1, 3 and 7 days compared to BMSCs isolated from WT littermates (Fig. 2A).
Figure 2.
A: Proliferation rate of WT and ank/ank BMSCs cultured for 12 h, 24 h and 48 h using the Cell counting Kit-8 (CCK-8). After the incubation with CKK-8 solution, the absorbance of the collected medium was measured at 450 nm at various time points. All the absorbance rates are expressed as percent of the absorbance rate of WT cells after 12 h culture period, which was set as 100%. Data were obtained from 3 different cultures and are expressed as mean ± S.D. (ap < 0.01 vs. WT cells cultured for 24 h; bp < 0.01 vs. WT cells cultured for 48 h). B: ALP activity and alizarin red S staining of WT and ank/ank BMSCs cultured for 7, 14 and 21 days in growth medium (GM) or osteogenic differentiation medium (ODM). C: mRNA levels of ALP (early osteogenic marker), osteocalcin (OC, late osteogenic marker) and the two osteogenic transcription factors osterix and runx2 in WT and ank/ank BMSCs cultured for 1d, 3d, 7d and 14d in GM or ODM. mRNA levels were determined by real time PCR using SYBR Green and normalized to the level of 18S RNA. The mRNA levels of ALP, osterix and runx2 are expressed as relative to the levels of WT BMSCs cultured in growth medium for 1 day, which were set as 1. mRNA levels of OC are expressed as relative to the levels of WT BMSCs cultured in ODM for 7 days, which was set as 1. mRNA levels of OC were not detectable (n.d.) in WT BMSCs cultured in GM, WT BMSCs cultured in ODM for 1 and 3 days, and ank/ank BMSCs cultured in GM or ODM. Data were obtained from triplicate PCRs using RNA from 3 different cultures. Data are expressed as mean ± S.D. (ap < 0.01 vs. WT cells cultured in GM; bp < 0.01 vs. WT cells cultured in ODM).
Next, we analyzed the osteogenic and adipogenic differentiation potential of BMSCs isolated from the bone marrow of 8-week old ank/ank mice and WT littermates in in vitro cell cultures. When BMSCs were cultured in osteogenic differentiation medium decreased ALP activity and bone nodule formation was evident in BMSCs obtained from ank/ank mice after 7, 14 and 21 days of culture in osteogenic differentiation medium compared to BMSCs isolated from WT littermates (Fig. 2B). In addition, markedly reduced mineralization as indicated by reduced alizarin red S staining was evident in ank/ank BMSCs cultured for 14 or 21 days in osteogenic differentiation medium compared to WT BMSCs cultured in osteogenic differentiation medium (Fig. 2B). The mRNA level of ALP, an early bone marker gene was markedly decreased in ank/ank BMSCs cultured in osteogenic differentiation medium during the 14-day culture period compared to the ALP mRNA levels of WT BMSCs cultured in osteogenic differentiation medium (Fig. 2C). The mRNA levels of osteocalcin (OC), a late osteogenic marker were only detectable in WT BMSCs cultured in osteogenic differentiation medium at day 7 and day 14, but were not detectable in ank/ank BMSCs cultured in osteogenic differentiation medium during the 14-day culture period (Fig. 2C). In addition, the mRNA levels for osterix and runx2, two major osteogenic transcription factors were markedly decreased in ank/ank BMSCs cultured in osteogenic differentiation medium over the 14-day culture period compared to WT BMSCs (Fig. 2C). The mRNA levels of ALP, osteocalcin, osterix and runx2 were low or not detectable in ank/ank and WT BMSCs cultured in growth medium and the mRNA levels of these bone markers did not change over the 14-day culture period in growth medium (Fig. 2C).
ANK mRNA levels in WT BMSCs cultured in growth medium did not change during the 14-day culture period (Fig. 3). In contrast, ANK mRNA levels increased during the first seven days of WT BMSCs cultured in osteogenic differentiation medium and reached the highest mRNA level on day 7 (Fig. 3). At day 14 the ANK mRNA levels in WT BMSCs cultured in osteogenic differentiation medium decreased to levels similar to the levels in WT BMSCs cultured in growth medium. ANK mRNA levels in WT BMSCs cultured in adipogenic differentiation medium decreased below the levels in WT BMSCs cultured in growth medium (Fig. 3).
Figure 3.
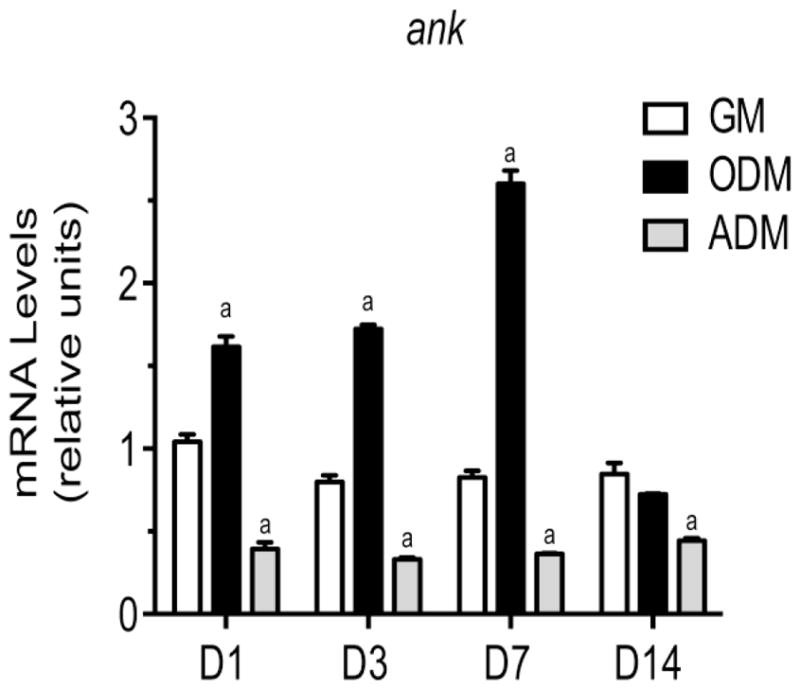
mRNA levels of ank in WT BMSCs cultured for 1, 3, 7 and 14 days in growth medium (GM), osteogenic differentiation medium (ODM), or adipogenic differentiation medium (ADM). mRNA levels were determined by real time PCR using SYBR Green and normalized to the level of 18S RNA. The mRNA levels are expressed as relative to the levels of WT BMSCs cultured in growth medium for 1 day, which were set as 1. Data were obtained from triplicate PCRs using RNA from 3 different cultures. Data are expressed as mean ± S.D. (ap < 0.01 vs. WT cells cultured in GM for 1 day).
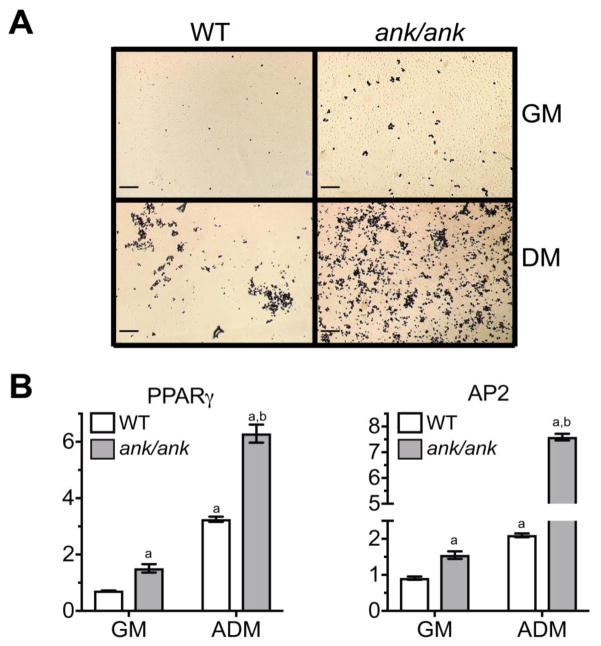
ank/ank BMSCs differentiated into oil-red O-positive adipocytes more efficiently than WT BMSCs. When cultured in growth medium for 21 days, the number of oil-red O-positive adipocytes in ank/ank BMSC cultures were more compared to oil-red O-positive WT cells (Fig. 4A). The number of oil-red O-positive adipocytes markedly increased when ank/ank BMSCs were culture in adipogenic differentiation medium compared to ank/ank BMSCs cultured in growth medium for 21 days (Fig. 4A). More importantly, notably more oil red O-positive adipocytes were evident in ank/ank BMSCs cultured for 21 days in adipogenic differentiation medium compared to WT BMSCs cultured for 21 days in adipogenic differentiation medium (Fig. 4A). Real time PCR analysis confirmed increased mRNA levels of PPARγ, a key transcription factor for adipogenesis, as well as acid binding protein AP2, a marker for the adipocyte phenotype, in ank/ank BMSCs cultured for 21 days in growth medium compared to the levels of these adipogenic markers in WT BMSCs cultured in growth medium (Fig. 4B). In addition, at day 21 the mRNA levels of PPARγ and AP2 were markedly higher in ank/ank BMSCs cultured in adipogenic differentiation medium compared to the levels in WT BMSCs cultured in adipogenic differentiation medium (Fig. 4B).
Figure 4.
A: Adipocyte formation in WT and ank/ank BMSCs cultured for 21 days in growth medium (GM) or adipogenic differentiation medium (ADM) (oil red O staining). Bar, 200μm. B: mRNA levels of PPARγ and AP2 in WT and ank/ank BMSCs cultured for 21d in growth medium (GM) or adipogenic differentiation medium (ADM). mRNA levels were determined by real time PCR using SYBR Green and normalized to the level of 18S RNA. The mRNA levels of PPARγ and AP2 are expressed as relative to the levels of WT BMSCs cultured in growth medium for 1 day, which were set as 1. Data were obtained from triplicate PCRs using RNA from 3 different cultures. Data are expressed as mean ± S.D. (ap < 0.01 vs. WT cells cultured in GM for 1 day, bp < 0.01 vs. WT cells cultured in ADM for 21 days).
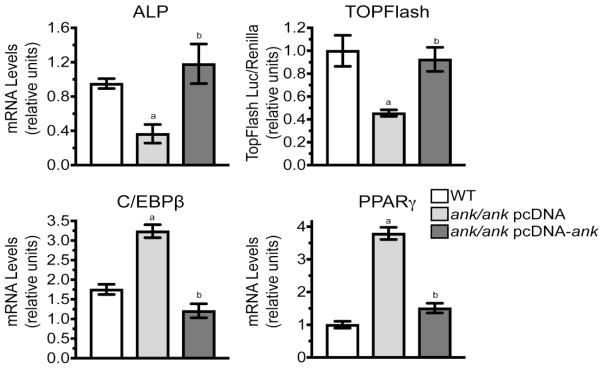
Next, we performed rescue experiments in which we transfected ank/ank BMSCs with empty pcDNA expression vector or pcDNA expression vector containing full-length ank. When cultured for 5 days in osteogenic differentiation medium, the mRNA levels of the early osteogenic marker ALP were lower in ank/ank BMSCs transfected with empty expression vector and cultured in osteogenic differentiation medium compared to the levels in WT cells (Fig. 5, ALP). Transfection of ank/ank BMSCs with expression vector containing ank increased mRNA levels of ALP to levels similar to WT cells cultured for 5 days in osteogenic differentiation medium (Fig. 5, ALP). In addition, transfection of ank/ank BMSCs with expression vector containing ank resulted in increased Wnt/β-catenin signaling as indicated by increased luciferase activity of the TOPFlash reporter compared to the levels in ank/ank BMSCs transfected with empty expression vector and cultured for 3 days in osteogenic differentiation medium (Fig. 5, TOPFlash). The luciferase activity from the TOPFlash reporter in ank/ank BMSCs transfected with expression vector containing ank was similar to the luciferase activity in WT BMSCs cultured for 3 days in osteogenic differentiation medium (Fig. 5, TOPFlash).
Figure 5.
Restoration of osteogenic and adipogenic differentiation potential of ank/ank BMSCs after transfection with expression vector containing ank. ank/ank BMSCs were transfected with empty expression vector (pcDNA) or expression vector containing ank (pcDNA-ank). In addition, ank/ank BMSCs and WT BMSCs were co-transfected with the TOPFlash luciferase reporter. A: Twenty-four hours after transfection cells were cultured in osteogenic differentiation medium for 5 days. mRNA levels of ALP were determined by real time PCR using SYBR Green and normalized to the level of 18S RNA. In addition, cell extracts were analyzed for luciferase activity from the TOPFlash reporter (TOPFlash). Transfection efficiency was monitored by co-transfection with Renilla luciferase vector, which provides constitutive expression of the Renilla luciferase reporter. B: Twenty-four hours after transfection cells were cultured in adipogenic differentiation medium for 5 days. mRNA levels of C/EBPβ and PPARγ were determined by real time PCR using SYBR Green and normalized to the level of 18S RNA. The mRNA levels of ALP, C/EBPβ and PPARγ are expressed as relative to the levels of WT BMSCs cultured in osteogenic or adipogenic differentiation medium for 5 days, which were set as 1. Data in A and B were obtained from three different experiments and are expressed as mean ± S.D. (ap < 0.01 vs. WT cells, bp < 0.01 vs. ank/ank transfected with empty pcDNA vector).
When ank/ank BMSCs transfected with empty expression vector were cultured in adipogenic differentiation medium for 5 days, C/EBPβ, a transcription factor involved in early adipogenic differentiation [29], and PPARγ were increased compared to the levels in WT BMSCs cultured in adipogenic differentiation medium (Fig. 5 C/EBPβ, PPARγ). The mRNA levels of C/EBPβ and PPARγ were reduced to levels similar to the levels of WT BMSCs when ank/ank BMSCs were transfected with expression vector containing ank and cultured for 5 days in adipogenic differentiation medium (Fig. 5 C/EBPβ, PPARγ). These findings show that the transfection of ank/ank BMSCs with an expression vector that encodes functional ANK protein increases their osteogenic differentiation potential and decreases their adipogenic differentiation potential to levels similar to the osteogenic and adipogenic differentiation potential of WT BMSCs.
3.3. Lack of ANK Function Decreases the Expression of Wnt Ligands, Wnt Receptors and Wnt Signaling Proteins
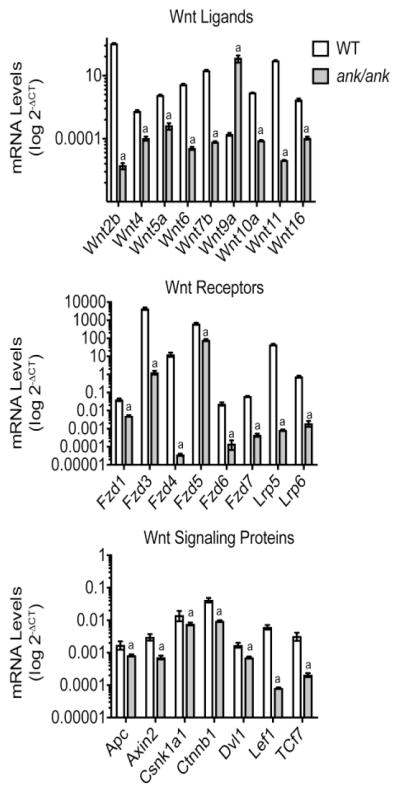
Wnt/β-catenin signaling plays a crucial role in osteogenic differentiation of precursor cells [30]. We performed a Wnt signaling pathway PCR array analysis to obtain insights of whether ANK affects the expression of Wnt ligands, Wnt receptors and Wnt signaling proteins. We used RNA isolated from ank/ank and WT BMSCs and cultured for 2 days in differentiation medium for the Wnt signaling pathway PCR array. PCR array analysis of the expression levels of the Wnt ligands revealed that Wnt2b, 4, 5a, 6, 7b, 9a, 10a, 11, and 16 were expressed in WT BMSCs cultured for two days in osteogenic differentiation medium with Wnt2b being expressed at the highest level (Fig. 6). Wnt2b stimulates Wnt/β-catenin signaling [31]. The expression levels of Wnt2b, 4, 5a, 6, 7b, 10a, 11 and 16 were markedly reduced in ank/ank BMSCs (Fig. 6). Wnt9a was the only Wnt protein that was expressed at markedly higher levels in ank/ank BMSCs compared to WT BMSCs (Fig. 6). Interestingly, Wnt9a expression was shown to be downregulated during the differentiation of precursor cells into mature osteoblasts, and knockdown of Wnt9a in osteoblasts increased expression of ALP, runx2, a key transcription factor for osteoblast differentiation, and osteocalcin, a late marker of osteoblast differentiation [32, 33]. In addition, knockdown of Wnt9a in osteoblasts increased their degree of mineralization [33], indicating that Wnt9a is an inhibitor of osteoblast differentiation. PCR array analysis revealed that the Wnt receptors Fzd1, 2, 3, 4, 5, 6, 7, and Lrp5 and Lrp6 were expressed in WT BMSCs cultured for 2 days in osteogenic differentiation medium. Fzd3, Fzd4, Fzd5 and Lrp5 were expressed at high levels in WT cells (Fig. 6). Fzd5 mediates canonical (β-catenin) and non-canonical Wnt signaling, and Lrp5 and Lrp6 are essential co-receptors for Wnt/β-catenin signaling in osteoblasts [30]. All these Wnt Fzd receptors and co-receptors were expressed at markedly lower levels in ank/ank BMSCs cultured for two days in osteogenic differentiation medium compared to WT cells (Fig. 6). Finally, the mRNA levels of Wnt signaling proteins, including members of the destruction complex (adenomatous polyposis coli (APC), axin 2, casein kinase I isoform alpha (CSNK1A1)), β-catenin (CTNNB1), disheveled segment polarity protein 1 (Dvl1), lymphoid enhancer-binding factor 1 (LEF1), and transcription factor 7 (TCF 7) were decreased in ank/ank BMSCs cultured for two days in osteogenic differentiation medium compared to WT BMSCs (Fig. 6). In summary, our findings revealed that most of the Wnt ligands, Wnt receptors, and Wnt signaling proteins that stimulate osteoblast differentiation were expressed at lower levels in ank/ank BMSCs cultured for two days in osteogenic differentiation medium than in WT BMSCs, whereas Wnt9a, an inhibitor of osteoblast differentiation, was expressed at higher levels in ank/ank BMSCs compared to WT BMSCs.
Figure 6.
PCR array analysis of Wnt proteins, Wnt receptors/co-receptors and Wnt signaling proteins in ank/ank and WT BMSCs cultured for two days in osteogenic differentiation medium. Total RNA was isolated from ank/ank and WT BMSCs cultured in osteogenic differentiation medium for 2 days. RNA was subjected to PCR array analysis of the expression of Wnt ligands, Wnt receptors and Wnt signaling proteins. Values represent expression levels of Wnt ligands, Wnt receptors/co-receptors and Wnt signaling proteins relative to glyceraldehyde-3-phosphate dehydrogenase. Values represent mean ± S.D. obtained from three independent samples.
3.4. Lack of ANK Function Delays Bone Fracture Healing
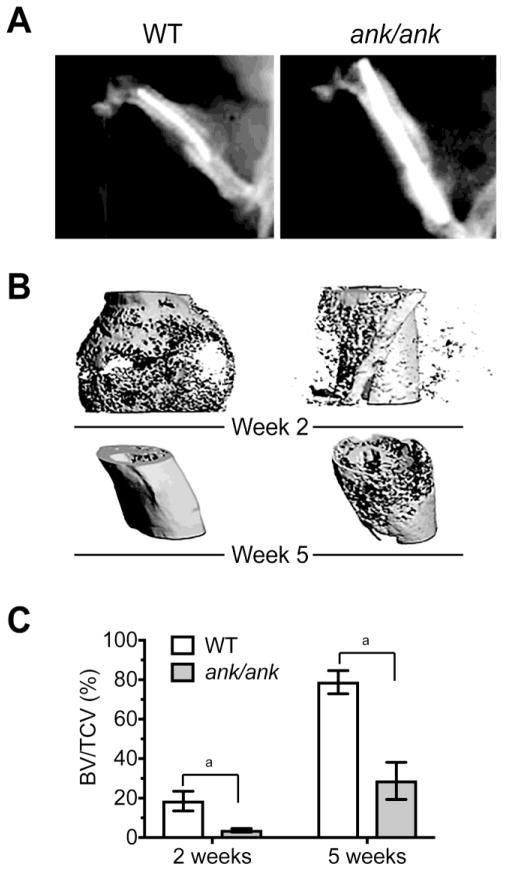
In the final set of experiments, we determined how lack of ANK function affects bone facture healing. We utilized a well-established femur fracture model in 6-week-old ank/ank mice and WT littermates [23]. X-ray analysis of the fractured hindlimb of ank/ank mice and WT littermates 2 weeks after the fracture revealed a markedly reduced callus size in ank/ank mice compared to WT littermates (Fig. 7A). Three-dimensional reconstructed microCT images of ank/ank mice and WT littermates 2 weeks after fracture confirmed the X-ray findings and quantitatively showed a markedly reduced size of the fracture callus in ank/ank mice compared to WT littermates (Fig. 7B). In addition, lower proportion of mineralized bone in the callus was observed in ank/ank mice at week 2 compared to WT littermates (Fig. 7B). At 5 weeks the fractured bone in WT mice was further remodeled and restored to original shape, whereas the fractured bone in ank/ank mice was not fully restored and remodeled to original shape (Fig. 7B). Average BV/TV in the callus of fractured bones from WT mice reached 20% on week 2 after fracture and 80% on week 5. In contrast, average BV/TV in the callus of fractured bones from ank/ank mice only reached 4% on week 2 and 30% on week 5 (Fig. 7C).
Figure 7.
Reduced callus size and less mineralized bone in the callus of fractured femurs from ank/ank mice compared to WT littermates. A: Representative radiographs of the fracture site from ank/ank mice and WT littermates two weeks after the fracture. B: Representative 3D images of fractured femur from ank/ank mice and WT littermates at week 2 and week 5 after fracture. C: Bone volume within the total callus volume (BV/TCV) in WT and ank/ank mice 2 weeks and 5 weeks after fracture (mean ± S.D., n=4 in each group, ap < 0.01).
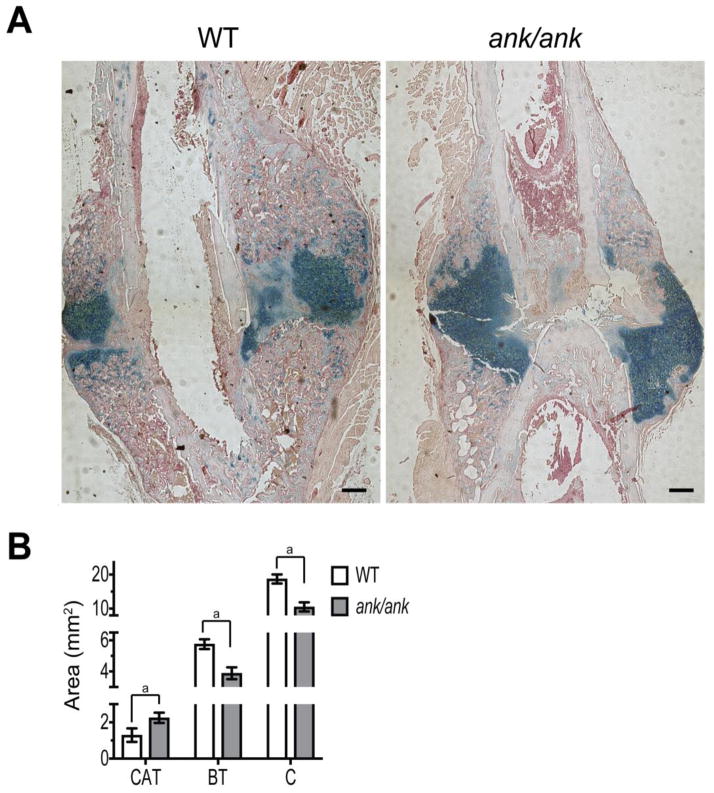
Histological analysis of sections from the callus formed 2 weeks after fracture surgery confirmed microCT analysis and revealed a smaller size callus (C) in the fracture site of ank/ank mice compared to WT littermates (Fig. 8A, B). However, alcian blue staining revealed that the ank/ank callus contained more cartilaginous tissue (CT) and less bony tissue than the WT callus (Fig. 8A, B). In summary, these experiments reveal delayed fracture healing in ank/ank mice compared to WT littermates.
Figure 8.
A: Representative histological sections of the fracture callus from ank/ank mice and WT littermates 2 weeks after fracture stained with alcian blue (cartilage) and H & E. Bar, 200 μm. B: Area of the total callus (C), cartilage tissue (CAT, blue), and bone tissue (BT) within the callus. Data are expressed as mean ± S.D. (n = 4 in each group; ap < 0.01)
4. DISCUSSION
In this study we demonstrate increased fatty degeneration of the bone marrow in femur and tibia of 2-month and 4-month old ank/ank mice similar to the age-related fatty degeneration of the bone marrow in human patients [15, 18, 20, 34]. Fatty degeneration of the bone marrow in the ank/ank mice was observed in 2-month old mice and severely increased in 4-month old mice. In addition, we show that lack of ANK function in BMSCs delays their osteogenic differentiation and promotes adipogenic differentiation of these cells. Expression of functional ANK in ank/ank BMSCs was able to rescue the phenotype resulting in increased osteogenesis and decreased adipogenesis. Finally, we demonstrate delayed bone fracture healing with delayed formation of bony tissue in ank/ank mice. Previous findings have shown that adipogenic-osteogenic fate decision plays a key role in bone remodeling and bone healing and that this fate decision alters during aging with a decline in the osteogenic differentiation potential of aging BMSCs [34–36]. Interestingly, the current study reveals that ANK expression is upregulated during the initial stages of osteogenic differentiation of precursor cells, whereas ANK expression is downregulated in these cells when they undergo adipogenic differentiation. Therefore, our data suggest that ANK plays a key role in the osteogenic fate decision of adult precursor cells.
Recent studies have revealed that Wnt/β-catenin signaling is a key signaling pathway that regulates osteogenic fate decision. Abrogation of Wnt/β-catenin signaling leads to adipogenic differentiation, whereas potentiation of Wnt/β-catenin signaling leads to osteogenic differentiation of mesenchymal stem cells [15, 16]. In addition, other studies have shown that Wnt ligands, Wnt receptors, Wnt signaling proteins and ultimately Wnt/β-catenin signaling is increased during bone fracture healing and plays a major role in the repair process [37]. Specifically, Wnt/β-catenin signaling promotes osteoblast differentiation and thereby stimulates bone fracture healing [37]. Our findings show that lack of ANK function results in decreased expression of several Wnt ligands, Wnt receptors and Wnt signaling proteins in BMSCs when cultured in osteogenic differentiation medium that have been shown to stimulate Wnt/β-catenin signaling in these cells and ultimately osteogenesis [14]. In contrast, Wnt9a, a Wnt ligand that has been shown to inhibit osteogenesis was increased in ank/ank BMSCs [33]. These findings suggest that the increased expression of ANK during early osteogenesis is a key mediator of stimulating the expression of Wnt ligands and receptors, including Lrp5 and Lrp6, key co-receptors for the activation of Wnt/β-catenin signaling, and Wnt signaling proteins that are required for osteogenic fate decision of adult mesenchymal stem cells [15]. In addition, our findings suggest that the reduced expression of Wnt ligands, receptors and signaling proteins in ank/ank osteogenic precursor cells may be a reason for the delayed bone fracture healing in ank/ank mice.
At this time we can only speculate how ANK mediates the stimulation of the expression of Wnt ligands, receptors and signaling proteins, and ultimately is required for osteogenic differentiation of precursor cells. ANK was shown to be a transmembrane protein that transports intracellular PPi to the extracellular milieu [1, 3]. Extracellular PPi has been shown to act as a mineralization inhibitor by preventing the growth of hydroxyapatite crystals [38]. Extracellular PPi, however, can easily being hydrolyzed by enzymes, such as ALP, into inorganic phosphate (Pi) [39]. Previous studies from our and other laboratories have shown that extracellular Pi stimulates osteogenic differentiation [2, 40, 41]. Interestingly, a recent study has shown that high extracellular Pi induced phenotypic transformation of vascular smooth muscle cells into osteogenic-like cells via the activation of Wnt/β-catenin signaling in these cells [42]. Our previous study, however, also suggested that extracellular PPi directly stimulates osteogenesis [2, 43]. Therefore, it is possible that extracellular PPi resulting from ANK transport directly or via its hydrolysis to extracellular Pi may affect Wnt/β-catenin signaling in osteogenic precursor cells. This possibility is supported by previous findings showing that extracellular PPi affects Wnt ligand expression and Wnt/β-catenin signaling in articular chondrocytes [44].
We cannot, however, rule out the possibility that ANK uses other mechanisms than the regulation of PPi homeostasis to control osteogenic differentiation of precursor cells. For example a recent study has shown that ANK regulates ATP efflux in articular chondrocytes [6]. Extracellular ATP has been shown to stimulate osteogenic differentiation of mesenchymal stem cells via purinergic receptors [7]. In addition, we and others have shown that ANK interacts with other intracellular proteins [45, 46]. For example, we have shown that ANK interacts with sphingosine kinase 1 (SPHK1) in chondrocytes [46]. This interaction resulted in increased SPHK1 activity and as a consequence increased sphingosine-1 phosphate (S1P) as the actual modulator of SPHK1 activity affecting cellular function levels in chondrocytes [46]. Recent studies have suggested that S1P may play an important role in the regulation of osteogenesis. Specifically, these studies have shown that S1P promotes osteogenic differentiation of precursor cells and inhibits their adipogenic differentiation [47–49]. Future studies have to determine the exact mechanisms of how ANK affects osteogenic and adipogenic differentiation of precursor cells.
5. CONCLUSION
Our study provides evidence that ANK plays a critical role in the adipogenic/osteogenic fate decision of adult mesenchymal precursor cells. ANK functions in precursor cells are required for osteogenic differentiation of precursor cells, whereas lack of ANK function favors adipogenic differentiation of mesenchymal precursor cells. In addition, lack of ANK function resulted in delayed bone fracture healing in mice as a consequence of delayed osteogenesis. With age, mesenchymal stem cells in the bone marrow become inclined to undergo adipogenic differentiation rather than osteogenic differentiation, resulting in increased adipocytes and fat deposition in the bone marrow and a decreased number of osteoblasts causing osteoporosis. Our findings suggest that ANK may play a key role in the regulation of bone formation during bone homeostasis and bone fracture healing during adulthood and that a detailed understanding of the mechanisms of how ANK regulates osteogenic fate decision in precursor cells may provide novel anti-aging therapeutic targets in the skeletal system.
Highlights.
Lack of functional ANK results in decreased proliferation and osteogenic differentiation of precursor cells.
Lack of functional ANK results in increased adipogenic differentiation and increased fatty degeneration in the bone marrow of aging mice.
Lack of functional ANK results in decreased expression of Wnt ligands, receptors and signaling proteins in osteogenic precursor cells.
Lack of functional ANK results in impaired bone fracture healing.
Acknowledgments
This work was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (R01-AR064186, 2019 to TK).
Abbreviations
- ADM
adipogenic differentiation medium
- ALP
alkaline phosphatase
- ANK
progressive ankylosis protein
- ank
progressive ankylosis gene
- AP2
adipocyte protein 2
- APC
adenomatous polyposis coli
- beta-catenin
CTNNB1
- BMSCs
bone marrow stromal cells
- BV
bone tissue volume in callus
- BV/TCV
bone tissue volume fraction of the total callus volume
- C
callus
- CAT
cartilaginous tissue
- C/EBPβ
CCAAT-enhancer-binding protein beta
- CKK-8
cell counting kit-8
- CSNK1A1
casein kinase I isoform alpha
- CT
cycle value
- DMEM
Dulbecco’s Modified Eagle Medium
- Dvl1
disheveled segment polarity protein 1
- GM
growth medium
- LEF1
lymphoid enhancer-binding factor 1
- n.d
not detectable
- OC
osteocalcin
- ODM
osteogenic differentiation medium
- PCR
polymerase chain reaction
- Pi
inorganic phosphate
- PPARγ
peroxisome proliferator-activated receptor gamma
- PPi
pyrophosphate
- S1P
sphingosine-1 phosphate
- SPHK1
sphingosine kinase 1
- TCF 7
transcription factor 7
- TCV
total callus volume
- WT
wild type
Footnotes
Conflict of Interest
None of the authors have a conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ho AM, Johnson MD, Kingsley DM. Role of the mouse ank gene in control of tissue calcification and arthritis. Science. 2000;289(5477):265–70. doi: 10.1126/science.289.5477.265. [DOI] [PubMed] [Google Scholar]
- 2.Kim HJ, Minashima T, McCarthy EF, Winkles JA, Kirsch T. Progressive ankylosis protein (ANK) in osteoblasts and osteoclasts controls bone formation and bone remodeling. J Bone Miner Res. 2010;25(8):1771–83. doi: 10.1002/jbmr.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gurley KA, Reimer RJ, Kingsley DM. Biochemical and genetic analysis of ANK in arthritis and bone disease. Am J Hum Genet. 2006;79(6):1017–29. doi: 10.1086/509881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pendleton A, Johnson MD, Hughes A, Gurley KA, Ho AM, Doherty M, Dixey J, Gillet P, Loeuille D, McGrath R, Reginato A, Shiang R, Wright G, Netter P, Williams C, Kingsley DM. Mutations in ANKH cause chondrocalcinosis. Am J Hum Genet. 2002;71(4):933–40. doi: 10.1086/343054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nurnberg P, Thiele H, Chandler D, Hohne W, Cunningham ML, Ritter H, Leschik G, Uhlmann K, Mischung C, Harrop K, Goldblatt J, Borochowitz ZU, Kotzot D, Westermann F, Mundlos S, Braun HS, Laing N, Tinschert S. Heterozygous mutations in ANKH, the human ortholog of the mouse progressive ankylosis gene, result in craniometaphyseal dysplasia. Nat Genet. 2001;28(1):37–41. doi: 10.1038/ng0501-37. [DOI] [PubMed] [Google Scholar]
- 6.Rosenthal AK, Gohr CM, Mitton-Fitzgerald E, Lutz MK, Dubyak GR, Ryan LM. The progressive ankylosis gene product ANK regulates extracellular ATP levels in primary articular chondrocytes. Arthritis Res Ther. 2013;15(5):R154. doi: 10.1186/ar4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lenertz LY, Baughman CJ, Waldschmidt NV, Thaler R, van Wijnen AJ. Control of bone development by P2X and P2Y receptors expressed in mesenchymal and hematopoietic cells. Gene. 2015;570(1):1–7. doi: 10.1016/j.gene.2015.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manaka S, Tanabe N, Kariya T, Naito M, Takayama T, Nagao M, Liu D, Ito K, Maeno M, Suzuki N, Miyazaki M. Low-intensity pulsed ultrasound-induced ATP increases bone formation via the P2X7 receptor in osteoblast-like MC3T3-E1 cells. FEBS Lett. 2015;589(3):310–8. doi: 10.1016/j.febslet.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 9.Laiuppa JA, Santillan GE. Effect of Combined Action of Extracellular ATP and Elevated Calcium on Osteogenic Differentiation of Primary Cultures From Rat Calvaria. J Cell Biochem. 2016;117(11):2658–68. doi: 10.1002/jcb.25565. [DOI] [PubMed] [Google Scholar]
- 10.Kariya T, Tanabe N, Shionome C, Manaka S, Kawato T, Zhao N, Maeno M, Suzuki N, Shimizu N. Tension force-induced ATP promotes osteogenesis through P2X7 receptor in osteoblasts. J Cell Biochem. 2015;116(1):12–21. doi: 10.1002/jcb.24863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang N, Rumney RM, Yang L, Robaye B, Boeynaems JM, Skerry TM, Gartland A. The P2Y13 receptor regulates extracellular ATP metabolism and the osteogenic response to mechanical loading. J Bone Miner Res. 2013;28(6):1446–56. doi: 10.1002/jbmr.1877. [DOI] [PubMed] [Google Scholar]
- 12.Ciciarello M, Zini R, Rossi L, Salvestrini V, Ferrari D, Manfredini R, Lemoli RM. Extracellular purines promote the differentiation of human bone marrow-derived mesenchymal stem cells to the osteogenic and adipogenic lineages. Stem Cells Dev. 2013;22(7):1097–111. doi: 10.1089/scd.2012.0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu C, Wang J, Zhu T, Shen Y, Tang X, Fang L, Xu Y. Cross-talking between PPAR and WNT signaling and its regulation in mesenchymal stem cell differentiation. Curr Stem Cell Res Ther. 2016;11(3):247–54. doi: 10.2174/1574888x10666150723145707. [DOI] [PubMed] [Google Scholar]
- 14.Baron R, Kneissel M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nature medicine. 2013;19(2):179–92. doi: 10.1038/nm.3074. [DOI] [PubMed] [Google Scholar]
- 15.Leucht P, Jiang J, Cheng D, Liu B, Dhamdhere G, Fang MY, Monica SD, Urena JJ, Cole W, Smith LR, Castillo AB, Longaker MT, Helms JA. Wnt3a reestablishes osteogenic capacity to bone grafts from aged animals. J Bone Joint Surg Am. 2013;95(14):1278–88. doi: 10.2106/JBJS.L.01502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Longo KA, Wright WS, Kang S, Gerin I, Chiang SH, Lucas PC, Opp MR, MacDougald OA. Wnt10b inhibits development of white and brown adipose tissues. J Biol Chem. 2004;279(34):35503–9. doi: 10.1074/jbc.M402937200. [DOI] [PubMed] [Google Scholar]
- 17.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 18.Nishikawa K, Nakashima T, Takeda S, Isogai M, Hamada M, Kimura A, Kodama T, Yamaguchi A, Owen MJ, Takahashi S, Takayanagi H. Maf promotes osteoblast differentiation in mice by mediating the age-related switch in mesenchymal cell differentiation. J Clin Invest. 2010;120(10):3455–65. doi: 10.1172/JCI42528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Asumda FZ, Chase PB. Age-related changes in rat bone-marrow mesenchymal stem cell plasticity. BMC Cell Biol. 2011;12:44. doi: 10.1186/1471-2121-12-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou S, Greenberger JS, Epperly MW, Goff JP, Adler C, Leboff MS, Glowacki J. Age-related intrinsic changes in human bone-marrow-derived mesenchymal stem cells and their differentiation to osteoblasts. Aging Cell. 2008;7(3):335–43. doi: 10.1111/j.1474-9726.2008.00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hankenson KD, Gagne K, Shaughnessy M. Extracellular signaling molecules to promote fracture healing and bone regeneration. Adv Drug Deliv Rev. 2015;94:3–12. doi: 10.1016/j.addr.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 22.Murshed M, Harmey D, Millan JL, McKee MD, Karsenty G. Unique coexpression in osteoblasts of broadly expressed genes accounts for the spatial restriction of ECM mineralization to bone. Genes Dev. 2005;19(9):1093–104. doi: 10.1101/gad.1276205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gerstenfeld LC, Cullinane DM, Barnes GL, Graves DT, Einhorn TA. Fracture healing as a post-natal developmental process: molecular, spatial, and temporal aspects of its regulation. J Cell Biochem. 2003;88(5):873–84. doi: 10.1002/jcb.10435. [DOI] [PubMed] [Google Scholar]
- 24.Tashiro K, Kondo A, Kawabata K, Sakurai H, Sakurai F, Yamanishi K, Hayakawa T, Mizuguchi H. Efficient osteoblast differentiation from mouse bone marrow stromal cells with polylysin-modified adenovirus vectors. Biochem Biophys Res Commun. 2009;379(1):127–32. doi: 10.1016/j.bbrc.2008.12.055. [DOI] [PubMed] [Google Scholar]
- 25.Nadri S, Soleimani M, Hosseni RH, Massumi M, Atashi A, Izadpanah R. An efficient method for isolation of murine bone marrow mesenchymal stem cells. Int J Dev Biol. 2007;51(8):723–9. doi: 10.1387/ijdb.072352ns. [DOI] [PubMed] [Google Scholar]
- 26.Soleimani M, Nadri S. A protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. Nat Protoc. 2009;4(1):102–6. doi: 10.1038/nprot.2008.221. [DOI] [PubMed] [Google Scholar]
- 27.Taipaleenmaki H, Suomi S, Hentunen T, Laitala-Leinonen T, Saamanen AM. Impact of stromal cell composition on BMP-induced chondrogenic differentiation of mouse bone marrow derived mesenchymal cells. Exp Cell Res. 2008;314(13):2400–10. doi: 10.1016/j.yexcr.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 28.Wang W, Kirsch T. Retinoic acid stimulates annexin-mediated growth plate chondrocyte mineralization. J Cell Biol. 2002;157(6):1061–9. doi: 10.1083/jcb.200203014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yeh WC, Cao Z, Classon M, McKnight SL. Cascade regulation of terminal adipocyte differentiation by three members of the C/EBP family of leucine zipper proteins. Genes Dev. 1995;9(2):168–81. doi: 10.1101/gad.9.2.168. [DOI] [PubMed] [Google Scholar]
- 30.Yavropoulou MP, Yovos JG. The role of the Wnt signaling pathway in osteoblast commitment and differentiation. Hormones. 2007;6(4):279–94. doi: 10.14310/horm.2002.1111024. [DOI] [PubMed] [Google Scholar]
- 31.Kubo F, Takeichi M, Nakagawa S. Wnt2b controls retinal cell differentiation at the ciliary marginal zone. Development. 2003;130(3):587–98. doi: 10.1242/dev.00244. [DOI] [PubMed] [Google Scholar]
- 32.Boland GM, Perkins G, Hall DJ, Tuan RS. Wnt 3a promotes proliferation and suppresses osteogenic differentiation of adult human mesenchymal stem cells. J Cell Biochem. 2004;93(6):1210–30. doi: 10.1002/jcb.20284. [DOI] [PubMed] [Google Scholar]
- 33.Singhatanadgit W, Varodomrujiranon M. Osteogenic potency of stem cell-based genetic engineering targeting Wnt3a and Wnt9a. Cent Eur J Biol. 2011;6(6):963–72. [Google Scholar]
- 34.Mueller SM, Glowacki J. Age-related decline in the osteogenic potential of human bone marrow cells cultured in three-dimensional collagen sponges. J Cell Biochem. 2001;82(4):583–90. doi: 10.1002/jcb.1174. [DOI] [PubMed] [Google Scholar]
- 35.Meunier P, Aaron J, Edouard C, Vignon G. Osteoporosis and the replacement of cell populations of the marrow by adipose tissue. A quantitative study of 84 iliac bone biopsies. Clin Orthop Relat Res. 1971;80:147–54. doi: 10.1097/00003086-197110000-00021. [DOI] [PubMed] [Google Scholar]
- 36.Moerman EJ, Teng K, Lipschitz DA, Lecka-Czernik B. Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: the role of PPAR-gamma2 transcription factor and TGF-beta/BMP signaling pathways. Aging Cell. 2004;3(6):379–89. doi: 10.1111/j.1474-9728.2004.00127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Secreto FJ, Hoeppner LH, Westendorf JJ. Wnt signaling during fracture repair. Curr Osteoporos Rep. 2009;7(2):64–9. doi: 10.1007/s11914-009-0012-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Addison WN, Azari F, Sorensen ES, Kaartinen MT, McKee MD. Pyrophosphate inhibits mineralization of osteoblast cultures by binding to mineral, up-regulating osteopontin, and inhibiting alkaline phosphatase activity. J Biol Chemi. 2007;282(21):15872–83. doi: 10.1074/jbc.M701116200. [DOI] [PubMed] [Google Scholar]
- 39.Hessle L, Johnson KA, Anderson HC, Narisawa S, Sali A, Goding JW, Terkeltaub R, Millan JL. Tissue-nonspecific alkaline phosphatase and plasma cell membrane glycoprotein-1 are central antagonistic regulators of bone mineralization. Proc Natl Acad Sci U S A. 2002 Jul 9;99(14):9445–9. doi: 10.1073/pnas.142063399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beck GR., Jr Inorganic phosphate as a signaling molecule in osteoblast differentiation. J Cell Biochem. 2003;90(2):234–43. doi: 10.1002/jcb.10622. [DOI] [PubMed] [Google Scholar]
- 41.Beck GR, Jr, Moran E, Knecht N. Inorganic phosphate regulates multiple genes during osteoblast differentiation, including Nrf2. Exp Cell Res. 2003;288(2):288–300. doi: 10.1016/s0014-4827(03)00213-1. [DOI] [PubMed] [Google Scholar]
- 42.Guerrero F, Herencia C, Almaden Y, Martinez-Moreno JM, Montes de Oca A, Rodriguez-Ortiz ME, Diaz-Tocados JM, Canalejo A, Florio M, Lopez I, Richards WG, Rodriguez M, Aguilera-Tejero E, Munoz-Castaneda JR. TGF-beta prevents phosphate-induced osteogenesis through inhibition of BMP and Wnt/beta-catenin pathways. PloS One. 2014;9(2):e89179. doi: 10.1371/journal.pone.0089179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pujari-Palmer M, Pujari-Palmer S, Lu X, Lind T, Melhus H, Engstrand T, Karlsson-Ott M, Engqvist H. Pyrophosphate Stimulates Differentiation, Matrix Gene Expression and Alkaline Phosphatase Activity in Osteoblasts. PloS one. 2016;11(10):e0163530. doi: 10.1371/journal.pone.0163530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cailotto F, Sebillaud S, Netter P, Jouzeau JY, Bianchi A. The inorganic pyrophosphate transporter ANK preserves the differentiated phenotype of articular chondrocyte. J Biol Chem. 2010;285(14):10572–82. doi: 10.1074/jbc.M109.050534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang J, Tsui HW, Beier F, Tsui FW. The CPPDD-associated ANKH M48T mutation interrupts the interaction of ANKH with the sodium/phosphate cotransporter PiT-1. J Rheumatol. 2009;36(6):1265–72. doi: 10.3899/jrheum.081118. [DOI] [PubMed] [Google Scholar]
- 46.Minashima T, Campbell KA, Hadley SR, Zhang Y, Kirsch T. The role of ANK interactions with MYBBP1a and SPHK1 in catabolic events of articular chondrocytes. Osteoarthritis Cartilage. 2014;22(6):852–61. doi: 10.1016/j.joca.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 47.Sato C, Iwasaki T, Kitano S, Tsunemi S, Sano H. Sphingosine 1-phosphate receptor activation enhances BMP-2-induced osteoblast differentiation. Biochem Biophys Res Commun. 2012;423(1):200–5. doi: 10.1016/j.bbrc.2012.05.130. [DOI] [PubMed] [Google Scholar]
- 48.Lotinun S, Kiviranta R, Matsubara T, Alzate JA, Neff L, Luth A, Koskivirta I, Kleuser B, Vacher J, Vuorio E, Horne WC, Baron R. Osteoclast-specific cathepsin K deletion stimulates S1P-dependent bone formation. J Clin Invest. 2013;123(2):666–81. doi: 10.1172/JCI64840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hashimoto Y, Matsuzaki E, Higashi K, Takahashi-Yanaga F, Takano A, Hirata M, Nishimura F. Sphingosine-1-phosphate inhibits differentiation of C3H10T1/2 cells into adipocyte. Mol Cell Biochem. 2015;401(1–2):39–47. doi: 10.1007/s11010-014-2290-1. [DOI] [PubMed] [Google Scholar]