Abstract
Mitophagy, or the selective clearance of mitochondria by autophagy, plays a key role in mitochondrial quality control. Due to their postmitotic nature and metabolic dependence on mitochondria, either insufficient or unchecked mitophagy is detrimental to neurons. To better understand signals that regulate this process, we treated primary rat cortical neurons with the electron transport chain complex I inhibitor rotenone to elicit mitophagy. The lipidomic profiles of mitochondria from control or injured neurons were analyzed by mass spectrometry, revealing a significant redistribution of cardiolipin (CL) from the inner mitochondrial membrane to the outer mitochondrial surface. Direct liposome-binding studies, computational modeling, and site-directed mutagenesis indicate that microtubule-associated protein 1 light chain 3 (MAP1LC3/LC3), a defining protein of autophagic membranes, binds to CL. Preventing this interaction inhibits rotenone-induced mitochondrial delivery to autophagosomes and lysosomes and attenuates mitochondrial loss as assessed by western blot. The CL-LC3 interaction is also important for mitophagy induced by other stimuli including 6-hydroxydopamine, another chemical model of Parkinson disease. Given that a conserved LC3 phosphorylation site is adjacent to key residues involved in CL binding, signaling pathways could potentially modulate this interaction to fine-tune the mitochondrial recycling response.
Keywords: mitophagy, Parkinson, cardiolipin, rotenone, MAP1-LC3, neurons, 6-hydroxydopamine, cargo recognition, autophagy, neurodegenerative diseases
Mitochondrial quality control is centrally important to the function and well-being of neurons. Determining how mitochondria are selected for autophagic recycling represents a first step toward understanding what could go wrong in diseases, and whether this could be targeted therapeutically. Elevated mitophagy has been described in relation to an array of acute and chronic brain disorders, and dysfunction in this process can lead to accumulation of dysfunctional mitochondria. On the other hand, inhibitors of autophagy sometimes protect against axonal/dendritic degeneration or cell death associated with elevated mitophagy. These observations suggest the importance of tightly regulated mitophagy responses in neurons.
Mitophagy is engaged for the complete developmental clearance of mitochondria in reticulocytes and lens fiber cells. Likewise, hypoxic fibroblasts or HeLa cells treated with chemical mitochondrial uncouplers will degrade most of their mitochondria, whereas yeast cells grown on certain substrates exhibit partial clearance of this organelle. The ability to observe significant levels of relatively synchronized mitochondrial clearance in each of these systems has yielded important information on pathways that regulate selective mitophagy. Key discoveries include the Atg32 system in yeast cells, the involvement of LC3-interacting region (LIR) proteins such as BNIP3, BNIP3L/NIX, SQSTM1 or FUNDC1, and the PINK1 (PTEN-induced putative kinase 1)-PARK2/PARKIN pathway, which is defined by 2 proteins genetically linked to Parkinson disease. However, it has been more difficult to establish robust involvement of particular pathways in primary neurons. Not only are neurons prone to dying during strong mitophagy stimuli, but also they may exhibit a higher threshold for engaging mitophagy, consistent with the need to preserve some minimal level of mitochondrial function.
A Lipid Signal for Mitophagy Induced by Parkinsonian Toxins
We recently reported a novel mechanism by which damaged mitochondria signal their status to the autophagic machinery in primary cortical neurons and SH-SY5Y neuroblastoma cells. Rotenone is a systemic complex I inhibitor that selectively damages the nigrostriatal system. It is implicated in environmental contributions to Parkinson disease, and used to model parkinsonian mechanisms. 6-hydroxydopamine is another established model of parkinsonian neurodegeneration, which recapitulates oxidative stress due to handling of catecholamimergic neurotransmittors. Doses were titrated to elicit mitophagy, with no significant elevations in cell death observed at the times/doses studied.
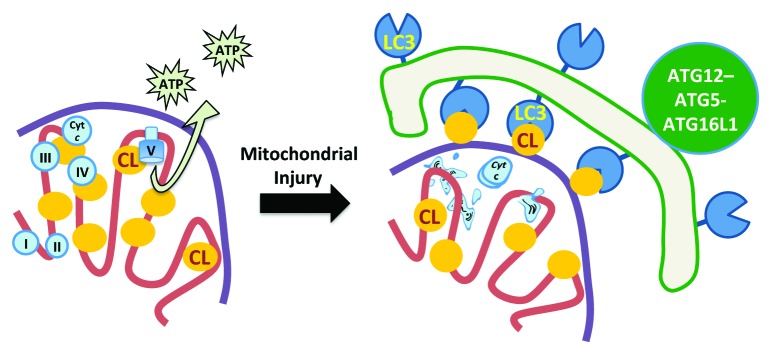
Mitophagy induced in either model involves an enzyme-dependent process by which CL, an inner mitochondrial membrane phospholipid that normally functions to stabilize respiratory complexes and cristae structures, is externalized to the outer surface of mitochondria (Fig. 1). CL is in turn recognized by LC3 via several clusters of basic amino acids on the LC3 surface. RNAi treatments that decrease mitochondrial CL content or that reduce expression of PLSCR3 (phospholipid scramblase 3) can inhibit rotenone- or 6-hydroxydopamine-induced mitophagy. Moreover, mutagenizing the amino acid residues at R10 and R11 reduces the ability of LC3 peptides to bind CL-containing liposomes in vitro, and attenuates the recruitment of GFP-LC3 to mitophagosomes in living cells.
Figure 1. Cardiolipin redistribution acts as a signal for mitochondrial autophagy. Left: In healthy, respiring mitochondria, CL is localized almost exclusively to the inner mitochondrial membrane (pink line), where it supports the function of the respiratory chain. Right: Following mitochondrial injury, a significant portion of CL translocates to the outer surface of the outer mitochondrial membrane (purple line), where it interacts with LC3, one of the autophagy proteins present on the phagophore (green line), thereby mediating the selective enrichment of damaged mitochondria within the forming autophagosome. Cyt c, CYCS (cytochrome c, somatic).
Given that CL is normally distributed predominantly in the inner leaflet of the inner mitochondrial membrane, its exposure to the outer leaflet of the outer mitochondrial membrane probably requires the coordinated activity of more than one mechanism. Our data implicates PLSCR3 in at least one of the “flip-flop” translocations between leaflets, but mechanisms involved in crossing between the membranes remain to be delineated. This could occur at one of the inner-outer membrane contact sites, or involve multimeric enzymes that span the intermembrane space. A final question relates to the nature of the signal that initiates the process of CL translocation.
Fine-Tuning Mitophagy through Multiple Pathways
With regard to mitophagy, neurons may be caught between a rock and a hard place. Mitophagy is necessary for neuron health, but if unchecked, may prove harmful by removing too many mitochondria. Unlike cancer cells, in which most mitophagy studies have been done, neurons are both irreplaceable and dependent on mitochondria. This implies a need to set higher thresholds for apoptosis and mitophagy than in other cell types. For example, neurons may favor localized repair mechanisms or partial degradation mechanisms balanced against their capacity for mitochondrial biogenesis.
A key feature of the binding site that we discovered is that LC3 can be phosphorylated by PRKACA/PKA (protein kinase, cAMP-dependent, catalytic, α) at a site adjacent to a pair of arginines needed for mitophagy. We predict that by adding negative charges to this region, phosphorylation would serve to dampen the binding interaction with CL, thus raising the threshold for triggering mitophagy. Indeed we have found that LC3 phosphomimetic mutants can protect against dendrite retraction observed in the MPP+ and mutant LRRK2 models of Parkinson disease. That there is potentially a built-in braking mechanism allows a higher level of control through which signaling pathways can fine-tune the process.
Although the upstream mechanisms that trigger CL externalization in response to rotenone or 6-hydroxydopamine remain to be elucidated, they do not require collapse of mitochondrial membrane potential, stabilization of full-length PINK1, or the mitochondrial recruitment of PARK2 or SQSTM1. It is possible that by virtue of its intimate association with components of the respiratory chain, CL and its regulatory enzymes are well situated to sense less extreme impairments in mitochondrial function. While the upstream triggers appear distinct between the CL and the PARK2 pathways, whether or not PINK1 or PARK2 enzymatic activity may be involved remains to be determined. Moreover, the ability of CCCP to trigger both PINK1-PARK2-SQSTM1 recruitment and CL externalization suggests the possibility of pathway convergence further downstream. Alternatively, CCCP may simultaneously engage 2 distinct pathways with additive effects.
Notably, LC3-mediated recognition of CL does not rely on oxidation. In fact, externalization of nonoxidized CL as observed in these studies is sufficient to trigger mitophagy. It is logical to presume that CL peroxidation, resulting from persistent injuries or excessive ROS production, could serve to switch the CL-related mitochondrial signal from mitophagy to favor programmed cell death.
In conclusion, a growing number of recent studies indicate the existence of multiple mechanisms to target mitochondria into phagophores, further attesting to the importance of mitophagy. However, damaged mitochondria still build up during disease, so the processes are not completely redundant. Future studies include developing a better understanding of the relationship, if any, between the different mitophagy triggers and the molecular mechanisms elicited in specific cell types, determining what goes wrong with aspects of these pathways in disease, and figuring out how to selectively reverse or bypass the problem(s) based on this knowledge.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank the members of our laboratories and our collaborators for helpful discussions. Supported in part by funding from the National Institutes of Health (AG026389, NS065789, AG030821, NS061817, HL70755, OH008282, AIO68021, NS076511, ES020693, GM103712, U19AI068021).