Abstract
Autophagy contributes to the pathogenesis of cancer, whereas toll-like receptors (TLRs) also play an important role in cancer development and immune escape. However, little is known about the potential interaction between TLR signaling and autophagy in cancer cells. Here we show that autophagy induced by TLR4 or TLR3 activation enhances various cytokine productions through promoting TRAF6 (TNF receptor-associated factor 6, E3 ubiquitin protein ligase) ubiquitination and thus facilitates migration and invasion of lung cancer cells. Stimulation of TLR4 and TLR3 with lipopolysaccharide (LPS) and polyinosinic-polycytidylic acid [poly(I:C)] respectively triggered autophagy in lung cancer cells. This was mediated by the adaptor protein, toll-like receptor adaptor molecule 1 (TICAM1/TRIF), and was required for TLR4- and TLR3-induced increases in the production of IL6, CCL2/MCP-1 [chemokine (C-C motif) ligand 2], CCL20/MIP-3α [chemokine (C-C motif) ligand 20], VEGFA (vascular endothelial growth factor A), and MMP2 [matrix metallopeptidase 2 (gelatinase A, 72 kDa gelatinase, 72 kDa type IV collagenase)]. These cytokines appeared to be necessary for enhanced migration and invasion of lung cancer cells upon TLR activation. Remarkably, inhibition of autophagy by chemical or genetic approaches blocked TLR4- or TLR3-induced Lys63 (K63)-linked ubiquitination of TRAF6 that was essential for activation of MAPK and NFKB (nuclear factor of kappa light polypeptide gene enhancer in B-cells) pathways, both of which were involved in the increased production of the cytokines. Collectively, these results identify induction of autophagy by TLR4 and TLR3 as an important mechanism that drives lung cancer progression, and indicate that inhibition of autophagy may be a useful strategy in the treatment of lung cancer.
Keywords: toll-like receptors, autophagy, migration, invasion, TRAF6, lung cancer cells
Introduction
Autophagy is an evolutionarily conserved process through which organelles and proteins are sequestered into autophagic vesicles (autophagosomes) within the cytosol. 1 These vesicles fuse with lysosomes to form autolysosomes leading to degradation of intracellular contents. 2 Although autophagy is regarded as a double-edged sword in cancer development, progression, and responses to treatment, 3 increasing evidence has shown that it promotes tumor cell survival and resistance to apoptosis. 3 - 5 However, whether autophagy plays a role in regulating cancer cell migration and invasion remains less understood.
Toll-like receptors (TLRs) are centrally involved in the initiation of innate immunity and induction of adaptive immune responses by recognizing distinct pathogen-associated molecular patterns. 6 , 7 They signal mainly through MYD88 (myeloid differentiation primary response 88) and/or TICAM1/TRIF (toll-like receptor adaptor molecule 1)-dependent pathways to activate NFKB (nuclear factor of kappa light polypeptide gene enhancer in B-cells) and MAPK pathways, leading to production of inflammatory cytokines. 6 , 8 Besides expression on immune cells such as macrophages and dendritic cells, TLRs are expressed on a wide variety of cancer cells. 9 Although TLR7 and TLR9 agonists produce antitumor effects, 9 , 10 emerging evidence indicates that activation of TLRs can promote cancer cell survival and proliferation. 11 - 13 Moreover, proinflammatory cytokines and immunosuppressive factors induced by TLR signaling in cancer cells inhibit immune cell functions and enhance resistance of tumor cells to cytotoxic lymphocyte-mediated killing, leading to immune evasion. 13 - 15
Pathogen-associated molecular patterns such as lipopolysaccharide (LPS), single-strand RNA, and bacteria are able to induce autophagy via different TLRs in innate immune cells. This plays an important role in elimination of intracellular pathogens. 16 - 18 However, it remains unknown whether TLRs can similarly trigger autophagy in cancer cells. In this report, we show that TLR4 and TLR3 activation induces autophagy via the TICAM1 adaptor in lung cancer cells, and that this in turn promotes ubiquitination of TRAF6 that is essential for TLR4- and TLR3-triggered increases in the production of multiple cytokines that enhance cell migration and invasion, including IL6, CCL2, CCL20, VEGFA, and MMP2. These results reveal that induction of autophagy contributes to TLR4- and TLR3-triggered progression of lung cancer cells, and suggest that inhibition of autophagy may be a useful strategy in the treatment of lung cancer.
Results
TLR4 and TLR3 activation induces autophagy in lung cancer cells
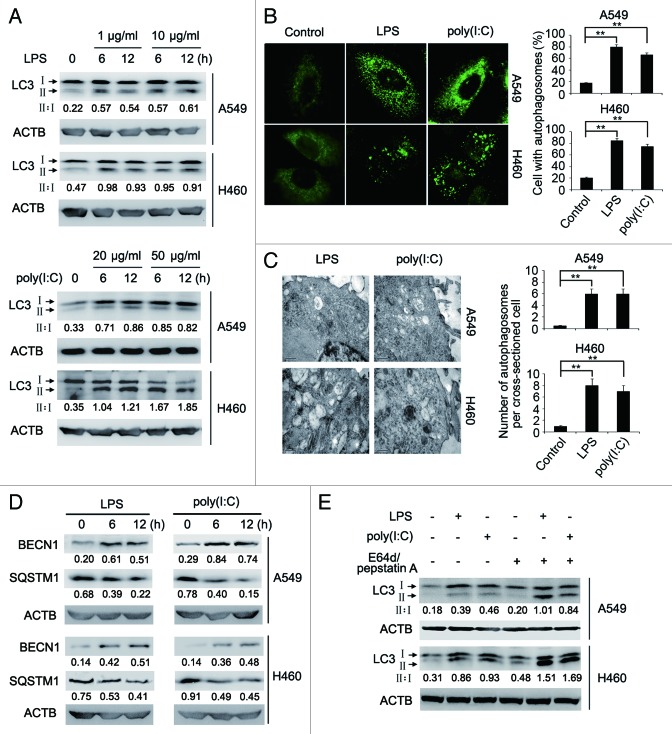
Consistent with previous reports that lung cancer cells express TLR4 and TLR3, 19 we found that TLR4 and TLR3 were expressed in A549 and H460 non-small cell lung cancer (NSCLC) cells (Fig. S1). Strikingly, we also found that the expression levels of TLR4 and TLR3 were upregulated by stimulation with the TLR4 ligand LPS and the TLR3 ligand polyinosinic-polycytidylic acid [poly(I:C)], respectively (Fig. S1). Moreover, exposure to LPS or poly(I:C) triggered autophagy in A549 and H460 cells. This was shown by conversion of microtubule-associated protein 1 light chain 3 α/β (MAP1LC3A/B, LC3A/B)-I into LC3-II, aggregation of LC3-II, formation of double-membraned autophagosomes, and increases in the expression of BECN1/Beclin 1 (Fig. 1A–D), and was further consolidated by degradation of sequestosome 1 (SQSTM1) (Fig. 1D) and increased accumulation of LC3-II when autophagy flux was blocked with the lysosomal protease inhibitors, E64d and pepstatin A (Fig. 1E), or by the autophagosome-lysosome fusion inhibitors, bafilomycin A1 (Fig. S2). 20
Figure 1. LPS or poly(I:C) induces autophagy in lung cancer cells. (A) Whole cell lysates from A549 and H460 cells treated with indicated doses of LPS or poly(I:C) for the indicated time were subjected for immunoblot analysis of LC3 and ACTB (as a loading control). The LC3-II to LC3-I ratio (II:I) is shown. (B) After stimulation with LPS (10 μg/ml) or poly(I:C) (20 μg/ml) for 12 h, A549 and H460 cells were immunolabeled with LC3 antibody followed by Alexa Fluor 488-conjugated secondary antibody (green). Images shown are representative fluorescence confocal microscopic photographs (left panel). Original magnifications: 630×. Quantification of the percentage of cells with autophagosomes is shown (right panel). (C) Ultrastructural features of A549 and H460 cells treated with LPS (10 μg/ml) or poly(I:C) (20 μg/ml) for 12 h, as revealed by transmission electron microscopy (left panel). Graph represents quantification of the number of autophagosomes per cross-sectioned cell (right panel). (D) Whole cell lysates from A549 and H460 cells treated with LPS (10 μg/ml) or poly(I:C) (20 μg/ml) for the indicated time were subjected for immunoblot analysis of BECN1, SQSTM1, and ACTB (as a loading control). Numbers below lanes indicate densitometry of the protein presented relative to ACTB expression in that same lane. (E) A549 and H460 cells cotreated with or without E64d (10 μg/ml) and pepstatin A (10 μg/ml) were stimulated with LPS (10 μg/ml) or poly(I:C) (20 μg/ml) for 12 h. Whole cell lysates were subjected for immunoblot analysis of LC3 and ACTB (as a loading control). The LC3-II to LC3-I ratio (II:I) is shown. Data are representative of 3 independent experiments with similar results (A, left panel in B–E) or are mean ± SEM from 3 independent experiments (right panel in B and C). **P < 0.01.
The TICAM1 pathway is required for TLR4- and TLR3-induced autophagy in lung cancer cells
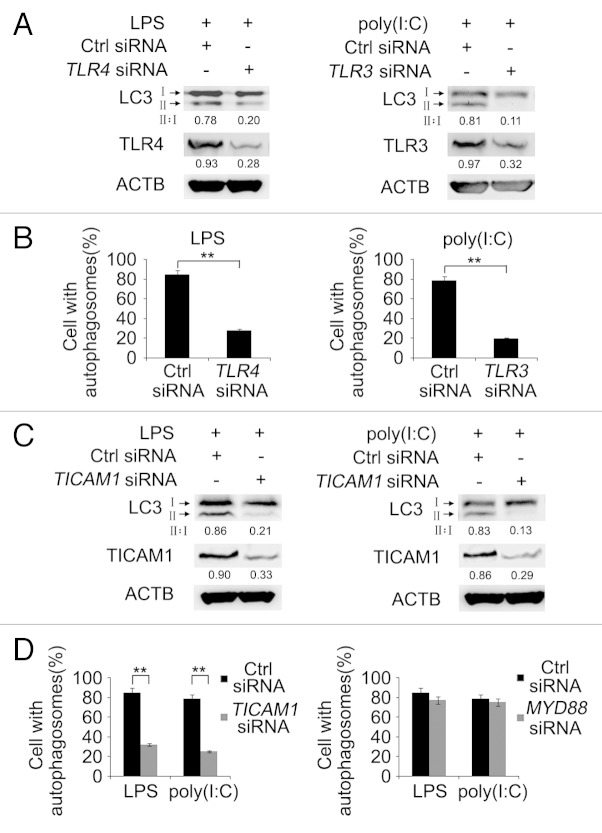
TLR4 initiates signaling through the adaptor proteins MYD88 and TICAM1, whereas TLR3 signals via TICAM1. 6 , 8 We investigated whether MYD88 and/or TICAM1 were involved in TLR-induced autophagy in lung cancer cells. siRNA knockdown of TLR4 inhibited conversion of LC3-I to LC3-II and LC3-II aggregation induced by LPS, whereas knockdown of TLR3 similarly inhibited LC3 conversion and LC3-II aggregation triggered by poly(I:C) in A549 cells (Fig. 2A and B), indicating that LPS and poly(I:C) induces autophagy via TLR4 and TLR3, respectively. Noticeably, inhibition of TICAM1 by siRNA recapitulated the effect of knockdown of TLR4 or TLR3 in A549 cells (Fig. 2C and D). In contrast, inhibition of MYD88 did not significantly impinge on LC3-II aggregation and LC3 conversion induced by LPS or poly(I:C) (Fig. 2D and data not shown). These results suggest that TICAM1 plays an essential role in TLR4- and TLR3-triggered autophagy in lung cancer cells.
Figure 2. LPS or poly(I:C) induces autophagy through the TLR receptor and TICAM1 adaptor protein. (A and B) A549 cells were transfected with control siRNA or siRNA specific for TLR4 (left panel) or TLR3 (right panel). Forty-eight hours later, cells were treated with LPS (10 μg/ml) or poly(I:C) (20 μg/ml) for 12 h. (A) Whole cell lysates were subjected for immunoblot analysis of LC3, TLR4, TLR3, and ACTB (as a loading control). The LC3-II to LC3-I ratio (II:I) is shown. (B) Cells were immunolabeled with LC3 antibody to visualize immunofluorescence images. Quantification of the percentage of cells with autophagosomes is shown. (C and D) A549 cells were transfected with control siRNA or siRNA specific for TICAM1 (C and D) or MYD88 (D). Forty-eight hours later, cells were treated with LPS (10 μg/ml) or poly(I:C) (20 μg/ml) for 12 h. (C) Whole cell lysates were subjected for immunoblot analysis of LC3, TICAM1, and ACTB (as a loading control). The LC3-II to LC3-I ratio (II:I) is shown. (D) Cells were immunolabeled with LC3 antibody to visualize immunofluorescence images. Quantification of the percentage of cells with autophagosomes is shown. Numbers below lanes indicate densitometry of TLR4, TLR3 or TICAM1 relative to ACTB expression in that same lane (A and C). Data are representative of 3 independent experiments with similar results (A and C) or are mean ± SEM from 3 independent experiments (B and D). **P < 0.01.
Autophagy facilitates induction of IL6, CCL2, CCL20, VEGFA, and MMP2 by TLR4 and TLR3 in lung cancer cells
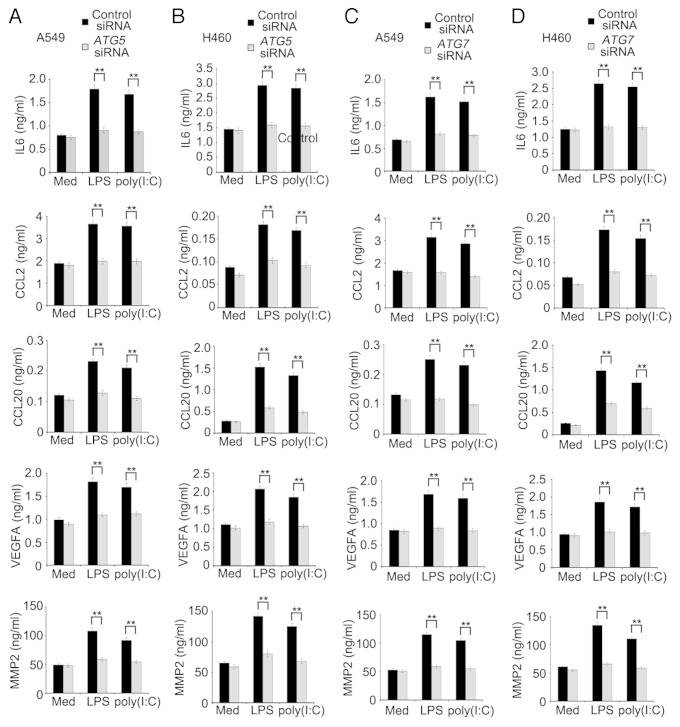
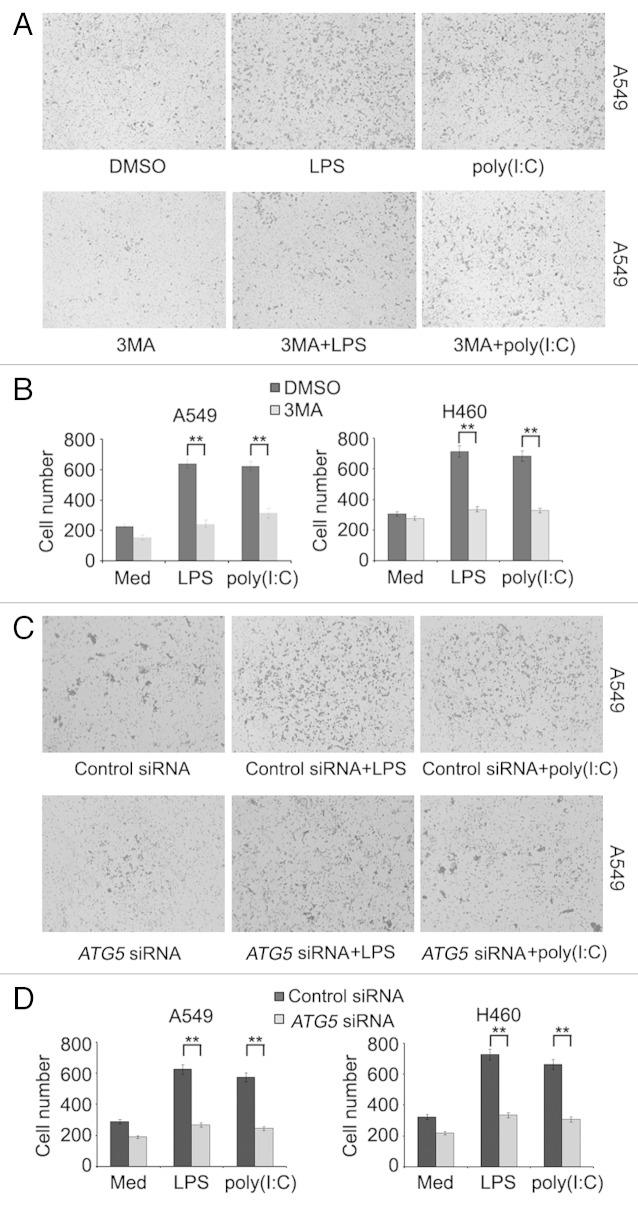
To examine whether induction of autophagy impacts on production of proinflammatory and immunosuppressive cytokines, chemokines, and MMPs triggered by TLR4 and TLR3 in lung cancer cells, we treated A549 and H460 cells with LPS or poly(I:C) in the presence or absence of 3-methyladenine (3MA). Remarkably, induction of IL6, CCL2, CCL20, VEGFA, and MMP2 by LPS or poly(I:C) was reduced when autophagy was blocked by 3MA (Figs. S3 and S4). In support, knockdown of autophagy-related 5 (ATG5) or autophagy-related 7 (ATG7) similarly inhibited the production of IL6, CCL2, CCL20, VEGFA, and MMP2 induced by LPS or poly(I:C) in A549 and H460 cells (Fig. 3A–D; Fig. S5). These results indicate that autophagy plays an important role in TLR-triggered production of the cytokines in lung cancer cells.
Figure 3. LPS- or poly(I:C)-induced autophagy facilitates the production of IL6, CCL2, CCL20, VEGFA, and MMP2 in lung cancer cells. A549 (A and C) and H460 (B and D) cells were transfected with control siRNA, ATG5 siRNA (A and B) or ATG7 siRNA (C and D). Forty-eight hours later, cells were treated with LPS (10 μg/ml) or poly(I:C) (20 μg/ml) for 24 h, the levels of IL6, CCL2, CCL20, VEGFA, and MMP2 in the supernatant fraction were measured by ELISA. Data are mean ± SEM from 3 independent experiments. **P < 0.01.
TLR4- and TLR3-induced autophagy promotes migration of lung cancer cells
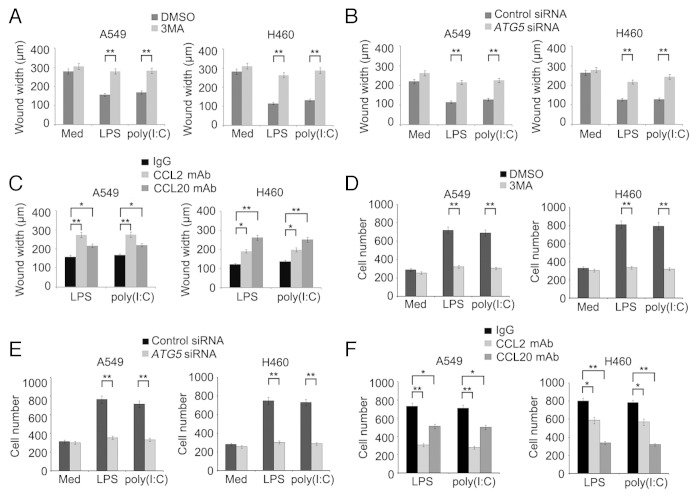
Tumor cell-derived CCL2 plays an important role in migration of the cells. 21 CCL20 has also been shown to be involved in metastasis of various cancers. 22 , 23 We therefore examined whether autophagy-mediated regulation of CCL2 and CCL20 resulting from activation of TLR4 and TLR3 impinges on migration of lung cancer cells. As anticipated, exposure to LPS or poly(I:C) enhanced migration of A549 and H460 cells as shown in wound healing and transwell assays. However, this increase was reversed by 3MA (Fig. 4A and D; Fig. S6A) and knockdown of ATG5 or ATG7 (Fig. 4B and E; Figs. S6B, S7A, and S7B), which was recapitulated by treatment with a neutralizing antibody against CCL2 or CCL20 (Fig. 4C and F; Fig. S6C). Therefore, TLR4- and TLR3-triggered autophagy promotes migration of lung cancer cells by promoting autocrine signaling of CCL2 and CCL20.
Figure 4. LPS- or poly(I:C)-induced autophagy promotes migration of lung cancer cells. (A–C) The confluent monolayer of A549 and H460 cells in 6-well plates was scratched with a yellow Gilson-pipette tip to inflict a 400-μm wound width, then incubated in FBS-free medium and treated with LPS (10 μg/ml) or poly(I:C) (20 μg/ml) in the presence or absence of 3MA (5 mM) (A) or neutralizing antibody against CCL2 or CCL20 (C) for 24 h. In (B), cells were transfected with control siRNA or siRNA specific for ATG5 48 h before scratch with pipette tip. Cells were photographed and the average wound widths were measured from 10 separate microscopy fields in each group. (D–F) A549 and H460 cells were placed in the upper chamber of transwell insert, then incubated with LPS (10 μg/ml) or poly(I:C) (20 μg/ml) in the presence or absence of 3MA (5 mM) (D) or neutralizing antibody against CCL2 or CCL20 (F) for 24 h. In (E), cells were transfected with control siRNA or siRNA specific for ATG5 48 h before placing in the upper chamber of transwell insert. The cells that migrated to another side of membrane were stained and cell numbers in 10 random fields were counted. Data are mean ± SEM of 3 independent experiments. *P < 0.05; **P < 0.01.
TLR4- and TLR3-induced autophagy promotes invasion of lung cancer cells
We also examined whether TLR4- and TLR3-triggered autophagy affects the invasive behavior of lung cancer cells using matrigel invasion assays. As shown in Figure 5A–D, cells treated with LPS or poly(I:C) displayed approximately a 3-fold increase in traversal of the matrigel membrane in comparison to those untreated, which was nevertheless markedly reversed by the addition of 3MA (Fig. 5A and B) or knockdown of ATG5 or ATG7 (Fig. 5C and D; Fig. S7C).
Figure 5. LPS- or poly(I:C)-induced autophagy promotes invasion of lung cancer cells. (A and B) A549 and H460 cells were placed in the upper chamber of matrigel invasion insert, then treated with LPS (10 μg/ml) or poly(I:C) (20 μg/ml) in the presence or absence of 3MA (5 mM) for 24 h. (C and D) A549 and H460cells were transfected with control siRNA or siRNA specific for ATG5 48 h before placing in the insert. Cells that penetrated through the matrigel to the lower surface of the filter were stained, photographed (A and C; A549 cells) and cell numbers in 10 random fields were counted (B and D). Data are representative of 3 independent experiments with similar results (A and C) or are mean ± SEM of 3 independent experiments (B and D). **P < 0.01.
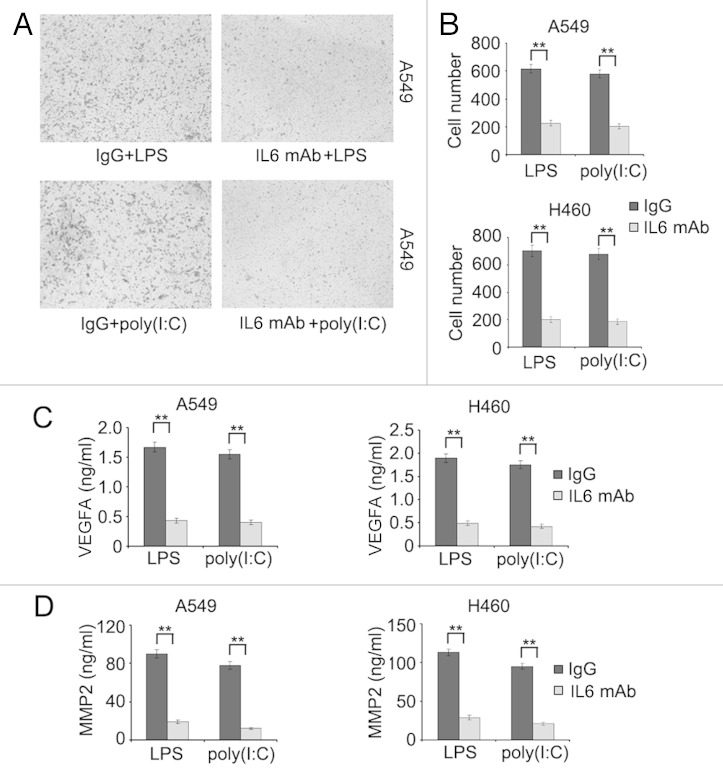
The pleiotropic cytokine IL6 induces autocrine release of VEGFA and MMPs that in turn mediate invasion of cancer cell. 24 - 26 We therefore examined whether IL6 similarly plays a role in invasion of lung cancer cells upon activation of TLR4 and TLR3. Cotreatment with a neutralizing antibody against IL6 markedly reversed the increase in invasion of A549 and H460 cells in response to LPS or poly(I:C) (Fig. 6A and B). This was associated with inhibition of release of VEGFA and MMP2 induced by LPS or poly(I:C) (Fig. 6C and D). These results suggest that IL6-mediated autocrine release of VEGFA and MMP2 plays an important role in invasion of lung cancer cells triggered by activation of TLR4 and TLR3.
Figure 6. IL6 plays an important role in TLR-triggered invasion of lung cancer cells. (A and B) A549 and H460 cells were placed in the upper chamber of matrigel invasion insert, then treated with LPS (10 μg/ml) or poly(I:C) (20 μg/ml) in the presence or absence of neutralizing antibody against IL6 for 24 h. Cells that penetrated through the matrigel to the lower surface of the filter were stained, photographed (A, A549 cells) and cell numbers in 10 random fields were counted (B). (C and D) A549 and H460 cells were treated with LPS (10 μg/ml) or poly(I:C) (20 μg/ml) in the presence or absence of neutralizing antibody against IL6 for 24 h, the levels of VEGFA (C) and MMP2 (D) in the supernatant fraction were measured by ELISA. Data are representative of 3 independent experiments with similar results (A) or are mean ± SEM of 3 independent experiments (B–D). **P < 0.01.
Rapamycin induces autophagy in cancer cells. 20 However, it did not appear to induce the production of IL6, CCL2, CCL20, VEGFA, and MMP2 in A549 and H460 cells (Fig. S8A). Similarly, it did not impinge on the migration and invasion potential of A549 and H460 cells with or without ATG5 knocked down (Fig. S8B and S8C). These results suggest that autophagy-mediated promotion of lung cancer migration and invasion is highly context-dependent, and reiterate the importance of the interaction between TLR signaling and autophagy in lung cancer progression.
Inhibition of autophagy impairs TLR-triggered activation of the MAPK and NFKB pathways
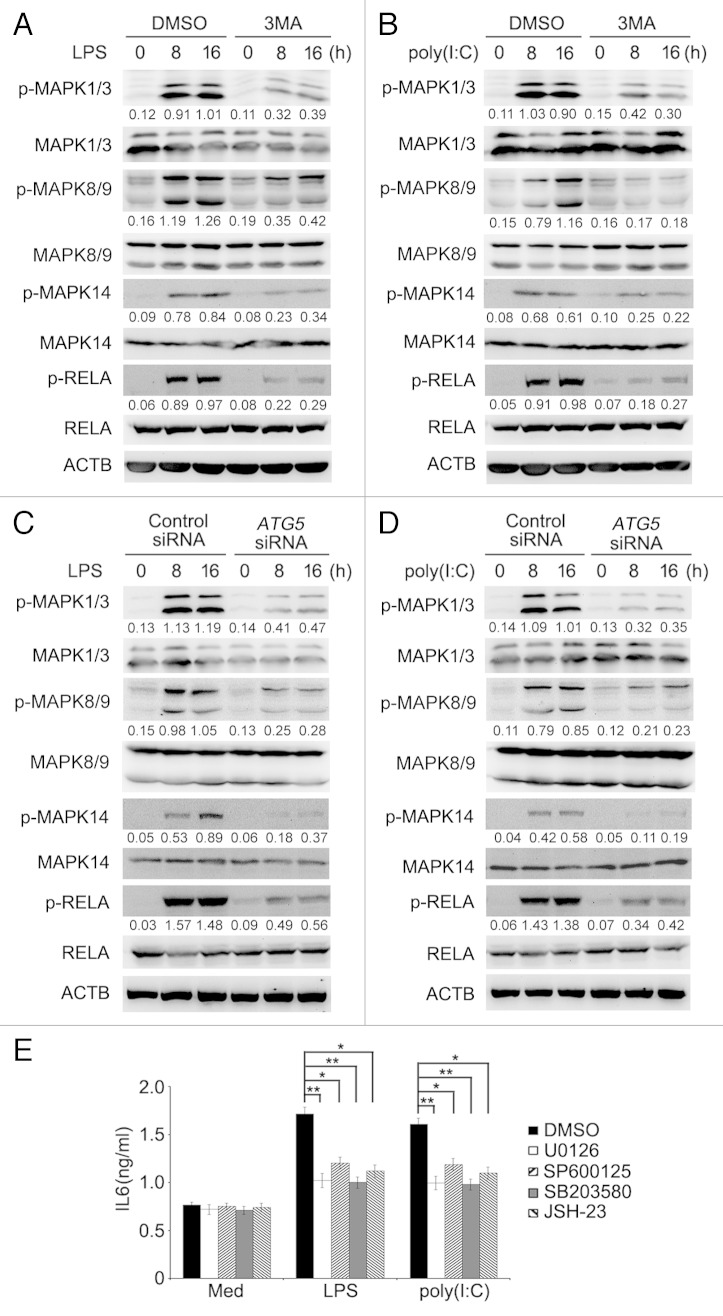
Similar to activation of survival signaling pathways in immune cells, stimulation of TLR4 and TLR3 induced activation of MAPK and NFKB signaling in lung cancer cells (Fig. 7A and B). However, activation of MAPK and NFKB by LPS or poly(I:C) was abolished when cells were cotreated with 3MA (Fig. 7A and B), suggesting that autophagy plays an important role in TLR4- and TLR3-induced activation of the MAPK and NFKB pathways. In support, phosphorylation of MAPK1/ERK2 and MAPK3/ERK1 (mitogen-activated protein kinase 1/3), MAPK8/JNK1 and MAPK9/JNK2 (mitogen-activated protein kinase 8/9), MAPK14/p38α (mitogen-activated protein kinase 14) and RELA/p65 (v-rel avian reticuloendotheliosis viral oncogene homolog A) by LPS or poly(I:C) was substantially attenuated in A549 cells with ATG5 knocked down (Fig. 7C and D).
Figure 7. Inhibition of autophagy attenuates TLR-triggered activation of MAPK and NFKB pathways. (A and B) Whole cell lysates from A549 cells treated with LPS (10 μg/ml) (A) or poly(I:C) (20 μg/ml) (B) in the presence or absence of 3MA (5 mM) for the indicated time were subjected for immunoblot analysis of phosphorylated (p-) or total level of MAPK1/3, MAPK8/9, MAPK14 or RELA. (C and D) A549 cells were transfected with control siRNA or ATG5 siRNA. Forty-eight hours later, cells were treated with LPS (10 μg/ml) (C) or poly(I:C) (20 μg/ml) (D) for the indicated time. Whole cell lysates were subjected for immunoblotanalysis as in (A). Numbers below lanes indicate densitometry of the phosphorylated protein relative to the total protein level in that same lane. (E) A549 cells were pretreated with inhibitors of MAPK1/3 (U0126), MAPK8/9 (SP600125), MAPK14 (SB203580) or RELA (JSH-23) for 30 min, then treated with LPS (10 μg/ml) or poly(I:C) (20 μg/ml) for 24 h. The level of IL6 in the supernatant fraction was measured by ELISA. Data are representative of 3 independent experiments with similar results (A–D), or are mean ± SEM of 3 independent experiments (E). *P < 0.05; **P < 0.01.
To clarify the role of MAPK and NFKB signaling in TLR-triggered, autophagy-mediated enhancement in cytokine production in lung cancer cells, we treated A549 cells with LPS or poly(I:C) in the presence of the specific inhibitor of MAPK1/3, MAPK8/9, MAPK14, or RELA. As shown in Figure 7E, the increase in IL6 production induced by LPS or poly(I:C) could be partially reversed by either the MAPK1/3, MAPK8/9, MAPK14, or RELA inhibitor. Collectively, these results suggest that autophagy is required for TLR4- and TLR3-triggered activation of MAPK and NFKB signaling that in turn plays an important role in the increased cytokine production in lung cancer cells.
Inhibition of autophagy impairs TLR-triggered TRAF6 ubiquitination and MAP3K7 activation
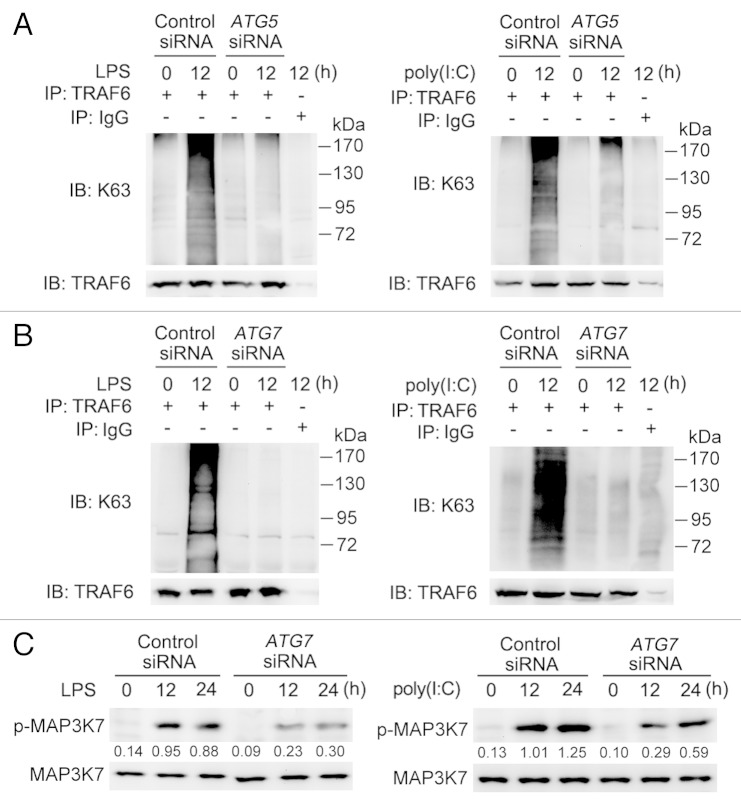
Since TLR4 and TLR3 activation causes Lys63 (K63)-linked ubiquitination of TRAF6, which in turn activates the downstream complex of MAP3K7/TAK1 (mitogen-activated protein kinase kinase kinase 7), and TAB1/2/3 (TGF-β activated kinase 1/MAP3K7 binding protein 1/2/3) leading to activation of MAPK and NFKB signaling and cytokine production, 8 we examined whether TRAF6 ubiquitination and MAP3K7 activation participates in autophagy-dependent activation of MAPK and NFKB signaling in lung cancer cells in response to stimulation with LPS or poly(I:C). Knockdown of ATG5 or ATG7 reduced K63-linked ubiquitination of TRAF6 in A549 cells treated with LPS or poly(I:C) (Fig. 8A and B). Similarly, blockade of autophagy by 3MA also downregulated TRAF6 ubiquitination (Fig. S9A). Moreover, phosphorylation of MAP3K7 induced by LPS or poly(I:C) was attenuated when ATG7 was knocked down or autophagy was inhibited by 3MA (Fig. 8C; Fig. S9B).These results indicate that autophagy induced by TLR activation maintains TRAF6 ubiquitination and subsequent MAP3K7 activation in lung cancer cells.
Figure 8. Blockade of autophagy impairs TLR-triggered TRAF6 ubiquitination and MAP3K7 activation. (A and B) A549 cells were transfected with control siRNA or siRNA specific for ATG5 (A) or ATG7 (B). Forty-eight hours later, cells were treated with LPS (10 μg/ml) or poly(I:C) (20 μg/ml) for 12 h or left untreated. Whole cell lysates were immunoprecipitated with anti-TRAF6 antibody and then immunoblotted with anti-K63 specific ubiquitin and anti-TRAF6 antibody. (C) A549 cells were transfected with control siRNA or siRNA specific for ATG7. Forty-eight hours later, cells were treated with LPS (10 μg/ml) or poly(I:C) (20 μg/ml) for the indicated time. Whole cell lysates were subjected for immunoblot analysis of phosphorylated (p-) or total level of MAP3K7. Numbers below lanes indicate densitometry of phospho-MAP3K7 relative to total MAP3K7 expression in that same lane. Data are representative of 3 independent experiments with similar results.
Discussion
In this report, we present evidence that TLR4 and TLR3 activation-induced, TICAM1 adaptor-dependent autophagy promotes migration and invasion of lung cancer cells by enhancing the production of chemokines and immunosuppressive factors including CCL2, CCL20, IL6, VEGFA, and MMP2. In addition, we show autophagy is critical for maintaining TLR4- and TLR3-triggered K63-linked ubiquitination of TRAF6 and subsequent activation of MAPK and NFKB signaling. Our results indicate that the crosstalk between autophagy and TLR signaling plays a key role in the pathogenesis of lung cancer.
TLR activation enhances cancer cell survival, proliferation, invasion, and metastasis. 27 - 29 Moreover, it triggers tumor cell release of a number of biological factors, including proinflammatory cytokines (i.e., IL6), chemokines, proangiogenic factor (i.e., VEGFA), immunosuppressive factors (i.e., PGE2 and TGFB1), and MMP, which promotes evasion of cancer cells from immune surveillance. 27 - 29 In particular, previous studies have shown that TLR4 signaling promotes proliferation of A549 lung cancer cells through PTGS2/COX-2 [prostaglandin-endoperoxide synthase 2 (prostaglandin G/H synthase and cyclooxygenase)] and EGFR (epidermal growth factor receptor) activation. 30 , 31 Moreover, TLR7 and TLR8 activation induces survival and chemoresistance of lung cancer cells. 32 However, little is known about the potential role of TLRs in migration and invasion of lung cancer cells. Our results showing that TLR4- and TLR3-induced autophagy increases the production of several cytokines leading to enhanced migration and invasion of lung cancer cells identify a new mechanism by which TLRs function to promote lung cancer progression.
Autophagy is required for cellular homeostasis and its deregulation is closely related to a variety of diseases, such as neurodegeneration and impaired immunity. 33 , 34 Although there have been conflicting reports in regard to the role of autophagy in cancer development and progression, 35 increasing evidence indicates that autophagy primarily promotes cancer cell survival. 2 , 5 In addition, autophagy plays a role in invasion of glioma cells. 36 These observations, along with our finding that autophagy resulting from TLR4 and TLR3 signaling is critical for TLR-triggered lung cancer cell migration and invasion, suggest that autophagy may have diverse roles in promoting the pathogenesis of cancer.
Autophagy has a role in regulating TLR-induced proinflammatory responses. For example, while several autophagy-related proteins including ATG16L1 [autophagy related 16-like 1 (S. cerevisiae)] and ATG5 negatively regulate inflammatory signaling activated by TLRs in macrophages, 37 , 38 autophagy appears to be necessary for TLR7-triggered induction of type I IFNs and other cytokines in plasmacytoid dendritic cells upon RNA virus infection. 39 Moreover, autophagy is required for TLR-mediated IL8 production in intestinal epithelial cells. 40 Similar to these observations, we found in this study that autophagy was required for TLR-triggered production of a number of chemokines and cytokines in lung cancer cells. It seems that autophagy may have distinct regulatory effects on TLR signaling in a cell type- and context-dependent manner.
It has been previously shown that TRAF6 plays an important role in tumorigenesis and invasion of lung cancer. 41 , 42 In this study, we found that TRAF6 was also critical for the crosstalk between TLR signaling and autophagy. Interestingly, TRAF6 activates autophagy induction after TLR activation or mechanistic target of rapamycin (MTOR) inhibition by acting as an E3 ligase to ubiquitinate BECN1 and ULK1 (unc-51 like autophagy activating kinase 1). 43 , 44 This, together with our data showing that TLR-induced autophagy maintains TRAF6 ubiquitination and activation, points to a positive feedback loop between TRAF6 and autophagy. Although the exact molecular mechanism(s) involved remains to be defined, some components of autophagosome or other proteins recruited by autophagy proteins may contribute to sustained ubiquitination of TRAF6 triggered by TLR activation. In this regard, we have found that ATG7, an E1 enzyme-like protein and a key component of ubiquitin-like systems essential for autophagosome formation, 45 , 46 interacted with TRAF6 when TLR signaling was activated (Fig. S10). Nevertheless, the functional significance of the interaction between ATG7 and TRAF6 remains a subject of further investigation.
A question that remains unaddressed is how TLR activation causes autophagy in lung cancer cells. Nevertheless, our results clearly showed that TICAM1-dependent signaling was essential for autophagy induced by activation of both TLR4 and TLR3 in lung cancer cells. Consistently, it has been reported that TLR4 stimulation triggers autophagy through TICAM1- but not MYD88-dependent signaling in human macrophages, 17 whereas TLR3-induced autophagy similarly requires TICAM1-mediated signaling in the mouse macrophage cell line RAW264.7. 47 However, MYD88 is involved in autophagy triggered by TLR4 activation in mouse macrophages. 47 It is therefore conceivable that the mechanisms by which TLRs trigger autophagy are cell type-dependent.
In conclusion, we have shown in this study that autophagy induced by TLR signaling promotes TLR-triggered cytokine production, which is required for enhanced migration and invasion of lung cancer cells. These results provide new insights into the biological properties of autophagy and identify the crosstalk between autophagy and TLR signaling as an important mechanism that drives invasion and metastasis of lung cancer cells. These results reinforce that inhibition of autophagy may be a useful strategy in the treatment of lung cancer.
Materials and Methods
Cell lines
Human lung cancer cell lines A549 and H460 were from Type Culture Collection of the Chinese Academy of Sciences. A549 Cells were cultured in F-12K Medium (ATCC, 30-2004) supplemented with 10% fetal bovine serum (FBS) (Invitrogen, 26400044) and H460 cells were cultured in RPMI 1640 (Gibco, 11875-093) supplemented with 10% FBS in a humidified atmosphere of 5% CO2 at 37 °C.
Reagents
LPS (0111:B4, L3024), poly(I:C) (P1530), 3MA (M9281), E64d (E8640), pepstatin A (P4265) and bafilomycin A1 (B1793) was from Sigma. Inhibitors of MAPK1/3 (U0126, 662005), MAPK8/9 (SP600125, 420119), MAPK14 (SB203580, 559389) and RELA (JSH-23, 481408) were from Merck. Stock solutions were prepared in DMSO (Sigma, D8418). The final concentration of DMSO in culture medium is < 0.2% volume. Neutralizing antibodies against IL6 (AB-206-NA), CCL2 (AF-279-NA) and CCL20 (MAB360) were from R&D Systems. Antibodies were obtained from the following sources: TLR4 (ab13867), TLR3 (ab62566), Abcam; TICAM1 (4596), LC3A/B (4108), BECN1 (3495), ATG5 (8540), ATG7 (8558), phospho-MAPK1/3 (Thr202/Tyr204) (9106), phospho-MAPK8/9 (Thr183/Tyr185) (9255), phospho-MAPK14 (Thr180/Tyr182) (9211), phospho-RELA (Ser536) (3033), Phospho-MAP3K7 (Thr184/187) (4531), ACTB (3700), Cell Signaling Technology; Lys63-specific ubiquitin (05-1313), EMD Millipore; TRAF6 (sc-7221), SQSTM1 (sc-25575), Santa Cruz.
Cytokine detection
IL6 (D6050), CCL2 (DCP00), CCL20 (DM3A00), VEGFA (DVE00), and MMP2 (DMP2F0) levels in the supernatant fractions were measured by ELISA (R&D Systems) according to the manufacturer’s protocols.
RT-PCR and quantitative PCR
Total cellular RNA was exacted using TRIzol regent (Invitrogen, 15596-026) following the manufacturer’s instructions. Total RNA (1 μg) was used in a 20 ml reverse-transcription reaction using the First Strand cDNA Synthesis kit (Toyobo, FSK-101), and then the cDNA was diluted into 80 ml water as the template of real-time quantitative PCR (Q-PCR). Q-PCR analysis was performed with Light Cycler (Roche Diagnostic). Data were normalized by the level of ACTB expression in each sample. The Q-PCR primers for analysis of cytokine RNA levels are shown in Table S1.
RNA interference
Small interfering RNAs (siRNAs) targeting human ATG5 (6345), ATG7 (6604) and control siRNA (6568) were from Cell Signaling Technology. siRNAs targeting human TLR4 (L-008088-01), TLR3 (L-007745-00), TICAM1 (L-012833-00) and control siRNA (D-001810-10-05) were from Dharmacon. siRNA duplexes were transfected into cells using INTERFERin reagent (409-10, Polyplus-transfection) according to the standard protocol.
Immunoblot and immunoprecipitation analysis
Cells were washed twice with ice cold PBS and lysed with cell lysis buffer (Cell Signaling Technology, 9803) containing protease inhibitor mixture (Calbiochem, 539134). Protein concentrations of the extracts were measured with BCA assay (Pierce, 23225). Immunoprecipitation and immunoblot analysis were done as described previously. 48 The detection of LC3 conversion (LC3-I to LC3-II) by immunoblot analysis was performed as described previously. 20 , 49
Immunofluorescence microscopy
For detection of LC3 puncta, cells were grown on glass coverslips overnight. Then cells were fixed with 4% paraformaldehyde (Sigma, P-6148) and treated with 0.2% Triton X-100 to permeabilize for 30 min on ice. After blocking with 2% BSA for 1 h, cells were incubated with primary rabbit anti-LC3 antibody (1:200 dilution) for 2 h, then incubated with Alexa Fluor 488-conjugated donkey anti-rabbit IgG (Molecular Probes, A21206, 1:500 dilution) for 1 h at room temperature. After washing with PBS, slides were mounted in vecta shield mounting medium (Vector Laboratories, H-1000) and examined under a confocal laser microscope (Leica TCS SP5II STED). At least 200 cells on each slide were counted and the percentage of cells with LC3 puncta (autophagosomes) was calculated. 5 , 20
Transmission electron microscopy
Prepared cells were fixed with 2.5% glutaraldehyde in 0.1 M cacodylate buffer for 1 h, post fixed in 1% OsO4 for 30 min, rinsed with double-distilled water, and dehydrated in a graded series of ethanol. Then cells were embedded in EMbed 812 resin (Electron Microscopy Sciences, 14120), and sectioned at a thickness of 70 to 90 nm. The sections were mounted on mesh copper grids, stained with aqueous uranyl acetate and lead citrate, and then analyzed with transmission electron microscope (JEOL 1230). A minimum of 50 independent sectioned cells were examined and the number of autophagic vacuoles per cross-sectioned cell was calculated. 5 , 20
Wound-healing assay
Cells were plated in 6-well plates and grown to confluence. Subsequently the cell monolayer was gently scratched with a sterile yellow Gilson-pipette tip, thus resulting in the formation of an approximately 400-μm wide gap. Cells were then rinsed with medium to remove floating cells and debris. Prepared cells were incubated in FBS-free medium with other treatment for 24 h. Cells were photographed under phase-contrast microscopy (100×) (Leica, DMI3000 B). Gap width was measured using image software and measurements were taken from 10 individual microscopic fields in each group. 50
Transwell migration assay and matrigel invasion assays
For migration assay, transwell inserts (8-μm pore, Costar, 3422) were placed into the wells. Cells were kept for 24 h in serum-free medium, then were trypsinized and resuspended in serum-free medium and placed in the upper chamber of the transwell insert (5 × 104 cells/well). Full medium containing 10% FBS was added to the lower chamber. After culture with other treatment for 24 h, the nonmigrated cells in the upper chamber were mechanically removed with a cotton swab. The cells that migrated to another side of membrane were fixed with methanol, stained with Giemsa (Electron Microscopy Sciences, 26773-1A) and then photographed. Cell numbers were counted under light microscopy at 100× magnification (Leica, DMI3000 B). For invasion assay, cells were placed in the upper chamber of the Matrigel invasion chamber (BD Biocoat, 354480), and treated as described in transwell migration assay. 50
Statistical analysis
The statistical significance of comparisons between 2 groups was determined with the 2-tailed Student t test. P values of less than 0.05 were considered statistically significant
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest need to be disclosed.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (31100635, 31040024), Shanghai Leading Talents Program (2012053), Shanghai Rising-Star Program (13QA1403100), Key Disciplines Group Construction Project of Pudong Health Bureau of Shanghai (PKzxkq2010-01), Outstanding Leaders Training Program of Pudong Health Bureau of Shanghai (PKR2011-01), and the Fundamental Research Funds for the Central Universities (2012KJ043).
Glossary
Abbreviations:
- 3MA
3-methyladenine
- ATG5
autophagy-related 5
- ATG7
autophagy-related 7
- BECN1
Beclin 1, autophagy related
- CCL2
chemokine (C-C motif) ligand 2
- CCL20
chemokine (C-C motif) ligand 20
- FBS
fetal bovine serum
- LPS
lipopolysaccharide
- MAP1LC3A/B (LC3A/B)
microtubule-associated protein 1 light chain 3 alpha/beta
- MAP3K7
mitogen-activated protein kinase kinase kinase 7
- MAPK1/3
mitogen-activated protein kinase 1/3
- MAPK8/9
mitogen-activated protein kinase 8/9
- MAPK14
mitogen-activated protein kinase 14
- MMP
matrix metallopeptidase
- MYD88
myeloid differentiation primary response 88
- NFKB
nuclear factor of kappa light polypeptide gene enhancer in B-cells
- poly(I:C)
polyinosinic-polycytidylic acid
- RELA
v-rel avian reticuloendotheliosis viral oncogene homolog A
- siRNA
small interfering RNA
- SQSTM1
sequestosome 1
- TICAM1
toll-like receptor adaptor molecule 1
- TLR
toll-like receptor
- TRAF6
TNF receptor-associated factor 6, E3 ubiquitin protein ligase
- VEGFA
vascular endothelial growth factor A
References
- 1. Rabinowitz JD, White E. . Autophagy and metabolism. Science 2010; 330:1344 - 8; http://dx.doi.org/ 10.1126/science.1193497; PMID: 21127245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kroemer G, Jäättelä M. . Lysosomes and autophagy in cell death control. Nat Rev Cancer 2005; 5:886 - 97; http://dx.doi.org/ 10.1038/nrc1738; PMID: 16239905 [DOI] [PubMed] [Google Scholar]
- 3. Kondo Y, Kanzawa T, Sawaya R, Kondo S. . The role of autophagy in cancer development and response to therapy. Nat Rev Cancer 2005; 5:726 - 34; http://dx.doi.org/ 10.1038/nrc1692; PMID: 16148885 [DOI] [PubMed] [Google Scholar]
- 4. Ravikumar B, Sarkar S, Davies JE, Futter M, Garcia-Arencibia M, Green-Thompson ZW, Jimenez-Sanchez M, Korolchuk VI, Lichtenberg M, Luo S, et al. . Regulation of mammalian autophagy in physiology and pathophysiology. Physiol Rev 2010; 90:1383 - 435; http://dx.doi.org/ 10.1152/physrev.00030.2009; PMID: 20959619 [DOI] [PubMed] [Google Scholar]
- 5. Zhan Z, Li Q, Wu P, Ye Y, Tseng HY, Zhang L, Zhang XD. . Autophagy-mediated HMGB1 release antagonizes apoptosis of gastric cancer cells induced by vincristine via transcriptional regulation of Mcl-1. Autophagy 2012; 8:109 - 21; http://dx.doi.org/ 10.4161/auto.8.1.18319; PMID: 22108005 [DOI] [PubMed] [Google Scholar]
- 6. Kawai T, Akira S. . The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol 2010; 11:373 - 84; http://dx.doi.org/ 10.1038/ni.1863; PMID: 20404851 [DOI] [PubMed] [Google Scholar]
- 7. Iwasaki A, Medzhitov R. . Regulation of adaptive immunity by the innate immune system. Science 2010; 327:291 - 5; http://dx.doi.org/ 10.1126/science.1183021; PMID: 20075244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. O’Neill LA, Bowie AG. . The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol 2007; 7:353 - 64; http://dx.doi.org/ 10.1038/nri2079; PMID: 17457343 [DOI] [PubMed] [Google Scholar]
- 9. Rakoff-Nahoum S, Medzhitov R. . Toll-like receptors and cancer. Nat Rev Cancer 2009; 9:57 - 63; http://dx.doi.org/ 10.1038/nrc2541; PMID: 19052556 [DOI] [PubMed] [Google Scholar]
- 10. Krieg AM. . Development of TLR9 agonists for cancer therapy. J Clin Invest 2007; 117:1184 - 94; http://dx.doi.org/ 10.1172/JCI31414; PMID: 17476348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Salaun B, Romero P, Lebecque S. . Toll-like receptors’ two-edged sword: when immunity meets apoptosis. Eur J Immunol 2007; 37:3311 - 8; http://dx.doi.org/ 10.1002/eji.200737744; PMID: 18034428 [DOI] [PubMed] [Google Scholar]
- 12. Huang B, Zhao J, Li H, He KL, Chen Y, Chen SH, Mayer L, Unkeless JC, Xiong H. . Toll-like receptors on tumor cells facilitate evasion of immune surveillance. Cancer Res 2005; 65:5009 - 14; http://dx.doi.org/ 10.1158/0008-5472.CAN-05-0784; PMID: 15958541 [DOI] [PubMed] [Google Scholar]
- 13. Basith S, Manavalan B, Yoo TH, Kim SG, Choi S. . Roles of toll-like receptors in cancer: a double-edged sword for defense and offense. Arch Pharm Res 2012; 35:1297 - 316; http://dx.doi.org/ 10.1007/s12272-012-0802-7; PMID: 22941474 [DOI] [PubMed] [Google Scholar]
- 14. Whiteside TL. . Tricks tumors use to escape from immune control. Oral Oncol 2009; 45:e119 - 23; http://dx.doi.org/ 10.1016/j.oraloncology.2009.03.006; PMID: 19467917 [DOI] [PubMed] [Google Scholar]
- 15. Finn OJ. . Immuno-oncology: understanding the function and dysfunction of the immune system in cancer. Ann Oncol 2012; 23:Suppl 8 i6 - 9; http://dx.doi.org/ 10.1093/annonc/mds256; PMID: 22918931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sanjuan MA, Dillon CP, Tait SW, Moshiach S, Dorsey F, Connell S, Komatsu M, Tanaka K, Cleveland JL, Withoff S, et al. . Toll-like receptor signalling in macrophages links the autophagy pathway to phagocytosis. Nature 2007; 450:1253 - 7; http://dx.doi.org/ 10.1038/nature06421; PMID: 18097414 [DOI] [PubMed] [Google Scholar]
- 17. Xu Y, Jagannath C, Liu XD, Sharafkhaneh A, Kolodziejska KE, Eissa NT. . Toll-like receptor 4 is a sensor for autophagy associated with innate immunity. Immunity 2007; 27:135 - 44; http://dx.doi.org/ 10.1016/j.immuni.2007.05.022; PMID: 17658277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Delgado MA, Elmaoued RA, Davis AS, Kyei G, Deretic V. . Toll-like receptors control autophagy. EMBO J 2008; 27:1110 - 21; http://dx.doi.org/ 10.1038/emboj.2008.31; PMID: 18337753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Samara KD, Antoniou KM, Karagiannis K, Margaritopoulos G, Lasithiotaki I, Koutala E, Siafakas NM. . Expression profiles of Toll-like receptors in non-small cell lung cancer and idiopathic pulmonary fibrosis. Int J Oncol 2012; 40:1397 - 404; PMID: 22344343 [DOI] [PubMed] [Google Scholar]
- 20. Klionsky DJ, Abdalla FC, Abeliovich H, Abraham RT, Acevedo-Arozena A, Adeli K, Agholme L, Agnello M, Agostinis P, Aguirre-Ghiso JA, et al. . Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy 2012; 8:445 - 544; http://dx.doi.org/ 10.4161/auto.19496; PMID: 22966490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Craig MJ, Loberg RD. . CCL2 (Monocyte Chemoattractant Protein-1) in cancer bone metastases. Cancer Metastasis Rev 2006; 25:611 - 9; http://dx.doi.org/ 10.1007/s10555-006-9027-x; PMID: 17160712 [DOI] [PubMed] [Google Scholar]
- 22. Ghadjar P, Rubie C, Aebersold DM, Keilholz U. . The chemokine CCL20 and its receptor CCR6 in human malignancy with focus on colorectal cancer. Int J Cancer 2009; 125:741 - 5; http://dx.doi.org/ 10.1002/ijc.24468; PMID: 19480006 [DOI] [PubMed] [Google Scholar]
- 23. Kirshberg S, Izhar U, Amir G, Demma J, Vernea F, Beider K, Shlomai Z, Wald H, Zamir G, Shapira OM, et al. . Involvement of CCR6/CCL20/IL-17 axis in NSCLC disease progression. PLoS One 2011; 6:e24856; http://dx.doi.org/ 10.1371/journal.pone.0024856; PMID: 21949768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kishimoto T. . IL-6: from its discovery to clinical applications. Int Immunol 2010; 22:347 - 52; http://dx.doi.org/ 10.1093/intimm/dxq030; PMID: 20410258 [DOI] [PubMed] [Google Scholar]
- 25. Kossakowska AE, Edwards DR, Prusinkiewicz C, Zhang MC, Guo D, Urbanski SJ, Grogan T, Marquez LA, Janowska-Wieczorek A. . Interleukin-6 regulation of matrix metalloproteinase (MMP-2 and MMP-9) and tissue inhibitor of metalloproteinase (TIMP-1) expression in malignant non-Hodgkin’s lymphomas. Blood 1999; 94:2080 - 9; PMID: 10477738 [PubMed] [Google Scholar]
- 26. Tzeng HE, Tsai CH, Chang ZL, Su CM, Wang SW, Hwang WL, Tang CH. . Interleukin-6 induces vascular endothelial growth factor expression and promotes angiogenesis through apoptosis signal-regulating kinase 1 in human osteosarcoma. Biochem Pharmacol 2013; 85:531 - 40; http://dx.doi.org/ 10.1016/j.bcp.2012.11.021; PMID: 23219526 [DOI] [PubMed] [Google Scholar]
- 27. Chen R, Alvero AB, Silasi DA, Steffensen KD, Mor G. . Cancers take their Toll--the function and regulation of Toll-like receptors in cancer cells. Oncogene 2008; 27:225 - 33; http://dx.doi.org/ 10.1038/sj.onc.1210907; PMID: 18176604 [DOI] [PubMed] [Google Scholar]
- 28. Pinto A, Morello S, Sorrentino R. . Lung cancer and Toll-like receptors. Cancer Immunol Immunother 2011; 60:1211 - 20; http://dx.doi.org/ 10.1007/s00262-011-1057-8; PMID: 21789594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Huang B, Zhao J, Unkeless JC, Feng ZH, Xiong H. . TLR signaling by tumor and immune cells: a double-edged sword. Oncogene 2008; 27:218 - 24; http://dx.doi.org/ 10.1038/sj.onc.1210904; PMID: 18176603 [DOI] [PubMed] [Google Scholar]
- 30. Kelly MG, Alvero AB, Chen R, Silasi DA, Abrahams VM, Chan S, Visintin I, Rutherford T, Mor G. . TLR-4 signaling promotes tumor growth and paclitaxel chemoresistance in ovarian cancer. Cancer Res 2006; 66:3859 - 68; http://dx.doi.org/ 10.1158/0008-5472.CAN-05-3948; PMID: 16585214 [DOI] [PubMed] [Google Scholar]
- 31. Hattar K, Savai R, Subtil FS, Wilhelm J, Schmall A, Lang DS, Goldmann T, Eul B, Dahlem G, Fink L, et al. . Endotoxin induces proliferation of NSCLC in vitro and in vivo: role of COX-2 and EGFR activation. Cancer Immunol Immunother 2013; 62:309 - 20; http://dx.doi.org/ 10.1007/s00262-012-1341-2; PMID: 22923191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cherfils-Vicini J, Platonova S, Gillard M, Laurans L, Validire P, Caliandro R, Magdeleinat P, Mami-Chouaib F, Dieu-Nosjean MC, Fridman WH, et al. . Triggering of TLR7 and TLR8 expressed by human lung cancer cells induces cell survival and chemoresistance. J Clin Invest 2010; 120:1285 - 97; http://dx.doi.org/ 10.1172/JCI36551; PMID: 20237413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wong E, Cuervo AM. . Autophagy gone awry in neurodegenerative diseases. Nat Neurosci 2010; 13:805 - 11; http://dx.doi.org/ 10.1038/nn.2575; PMID: 20581817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Deretic V. . Autophagy in innate and adaptive immunity. Trends Immunol 2005; 26:523 - 8; http://dx.doi.org/ 10.1016/j.it.2005.08.003; PMID: 16099218 [DOI] [PubMed] [Google Scholar]
- 35. Rosenfeldt MT, Ryan KM. . The multiple roles of autophagy in cancer. Carcinogenesis 2011; 32:955 - 63; http://dx.doi.org/ 10.1093/carcin/bgr031; PMID: 21317301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Macintosh RL, Timpson P, Thorburn J, Anderson KI, Thorburn A, Ryan KM. . Inhibition of autophagy impairs tumor cell invasion in an organotypic model. Cell Cycle 2012; 11:2022 - 9; http://dx.doi.org/ 10.4161/cc.20424; PMID: 22580450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Saitoh T, Akira S. . Regulation of innate immune responses by autophagy-related proteins. J Cell Biol 2010; 189:925 - 35; http://dx.doi.org/ 10.1083/jcb.201002021; PMID: 20548099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jounai N, Takeshita F, Kobiyama K, Sawano A, Miyawaki A, Xin KQ, Ishii KJ, Kawai T, Akira S, Suzuki K, et al. . The Atg5 Atg12 conjugate associates with innate antiviral immune responses. Proc Natl Acad Sci U S A 2007; 104:14050 - 5; http://dx.doi.org/ 10.1073/pnas.0704014104; PMID: 17709747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lee HK, Lund JM, Ramanathan B, Mizushima N, Iwasaki A. . Autophagy-dependent viral recognition by plasmacytoid dendritic cells. Science 2007; 315:1398 - 401; http://dx.doi.org/ 10.1126/science.1136880; PMID: 17272685 [DOI] [PubMed] [Google Scholar]
- 40. Li YY, Ishihara S, Aziz MM, Oka A, Kusunoki R, Tada Y, Yuki T, Amano Y, Ansary MU, Kinoshita Y. . Autophagy is required for toll-like receptor-mediated interleukin-8 production in intestinal epithelial cells. Int J Mol Med 2011; 27:337 - 44; PMID: 21225224 [DOI] [PubMed] [Google Scholar]
- 41. Zhong L, Cao F, You Q. . Effect of TRAF6 on the biological behavior of human lung adenocarcinoma cell. Tumour Biol 2013; 34:231 - 9; http://dx.doi.org/ 10.1007/s13277-012-0543-8; PMID: 23055197 [DOI] [PubMed] [Google Scholar]
- 42. Liu H, Zhang T, Ye J, Li H, Huang J, Li X, Wu B, Huang X, Hou J. . TNF receptor-associated factor 6 in advanced non-small cell lung cancer: clinical and prognostic implications. J Cancer Res Clin Oncol 2012; 138:1853 - 63; http://dx.doi.org/ 10.1007/s00432-012-1255-6; PMID: 22736025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shi CS, Kehrl JH. . TRAF6 and A20 regulate lysine 63-linked ubiquitination of Beclin-1 to control TLR4-induced autophagy. Sci Signal 2010; 3:ra42; http://dx.doi.org/ 10.1126/scisignal.2000751; PMID: 20501938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nazio F, Strappazzon F, Antonioli M, Bielli P, Cianfanelli V, Bordi M, Gretzmeier C, Dengjel J, Piacentini M, Fimia GM, et al. . mTOR inhibits autophagy by controlling ULK1 ubiquitylation, self-association and function through AMBRA1 and TRAF6. Nat Cell Biol 2013; 15:406 - 16; http://dx.doi.org/ 10.1038/ncb2708; PMID: 23524951 [DOI] [PubMed] [Google Scholar]
- 45. Tanaka K, Suzuki T, Chiba T. . The ligation systems for ubiquitin and ubiquitin-like proteins. Mol Cells 1998; 8:503 - 12; PMID: 9856335 [PubMed] [Google Scholar]
- 46. van der Veen AG, Ploegh HL. . Ubiquitin-like proteins. Annu Rev Biochem 2012; 81:323 - 57; http://dx.doi.org/ 10.1146/annurev-biochem-093010-153308; PMID: 22404627 [DOI] [PubMed] [Google Scholar]
- 47. Shi CS, Kehrl JH. . MyD88 and Trif target Beclin 1 to trigger autophagy in macrophages. J Biol Chem 2008; 283:33175 - 82; http://dx.doi.org/ 10.1074/jbc.M804478200; PMID: 18772134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Liu X, Zhan Z, Li D, Xu L, Ma F, Zhang P, Yao H, Cao X. . Intracellular MHC class II molecules promote TLR-triggered innate immune responses by maintaining activation of the kinase Btk. Nat Immunol 2011; 12:416 - 24; http://dx.doi.org/ 10.1038/ni.2015; PMID: 21441935 [DOI] [PubMed] [Google Scholar]
- 49. Mizushima N, Yoshimori T. . How to interpret LC3 immunoblotting. Autophagy 2007; 3:542 - 5; PMID: 17611390 [DOI] [PubMed] [Google Scholar]
- 50. Lin SS, Lai KC, Hsu SC, Yang JS, Kuo CL, Lin JP, Ma YS, Wu CC, Chung JG. . Curcumin inhibits the migration and invasion of human A549 lung cancer cells through the inhibition of matrix metalloproteinase-2 and -9 and Vascular Endothelial Growth Factor (VEGF). Cancer Lett 2009; 285:127 - 33; http://dx.doi.org/ 10.1016/j.canlet.2009.04.037; PMID: 19477063 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.