Abstract
Autophagy is a highly conserved homeostatic pathway that plays an important role in tumor development and progression by acting on cancer cells in a cell-autonomous mechanism. However, the solid tumor is not an island, but rather an ensemble performance that includes nonmalignant stromal cells, such as macrophages. A growing body of evidence indicates that autophagy is a key component of the innate immune response. In this review, we discuss the role of autophagy in the control of macrophage production at different stages (including hematopoietic stem cell maintenance, monocyte/macrophage migration, and monocyte differentiation into macrophages) and polarization and discuss how modulating autophagy in tumor-associated macrophages (TAMs) may represent a promising strategy for limiting cancer growth and progression.
Keywords: autophagy, tumor-associated macrophages, cancer, macrophage production, macrophage polarization
Introduction
Autophagy is an evolutionarily conserved homeostatic pathway that is widely occurring in eukaryotic cells. 1 Induction of autophagy can generate lots of amino acids and other building blocks, which are required for cellular homeostasis. Moreover, autophagy is necessary for quality control for both organelles and proteins. 2 - 4 Autophagy is induced upon stimulation by various extrinsic and intrinsic cellular stress conditions, such as reactive oxygen species (ROS), endoplasmic reticulum stress, bacterial infection, and hypoxia, in order to clear damaged organelles, protein aggregates, and intracellular pathogens. Thus, autophagy is crucial for the maintenance of cellular homeostasis. 2 - 4 Based on different functions and mechanisms, 3 major forms of autophagy have been described. 5 , 6 Microautophagy allows for the degradation of portions of the cytoplasm, which are directly enwrapped by the lysosomal membrane. Macroautophagy (hereafter referred to as “autophagy”) is responsible for the degradation of bulk cytoplasm, long-lived proteins, and entire organelles, through the formation of a double-membrane compartment, called an autophagosome, which is subsequently targeted to lysosomal digestion. In contrast to these 2 types of autophagy, which mediate both selective and nonselective degradation, chaperone-mediated autophagy only degrades individual, unfolded soluble proteins in a selective manner. 7
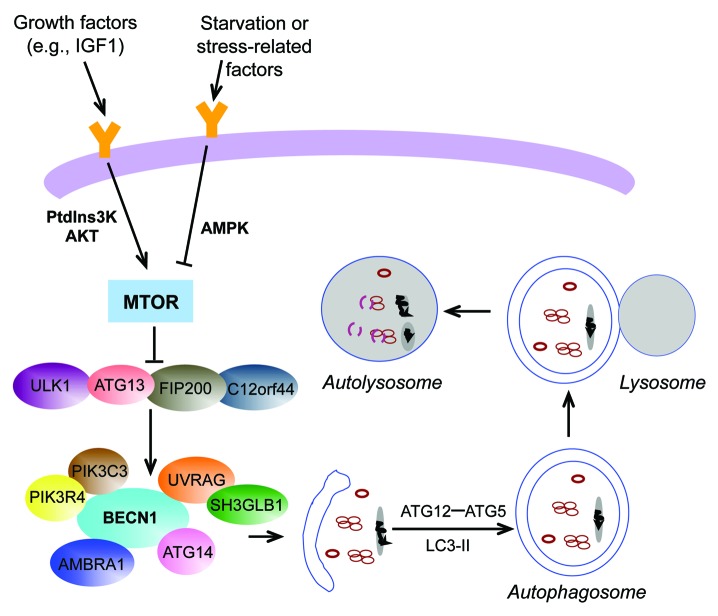
The autophagic process (Fig. 1) requires a set of evolutionarily conserved proteins, most of which are known as autophagy-related (ATG) proteins, functioning at different steps. 8 A kinase complex containing ULK1/ATG1, ATG13, RB1CC1/FIP200, and C12orf44/ATG101 is critical for autophagy induction. 9 A different set of complexes, which contain BECN1/Beclin 1, PIK3C3/VPS34, PIK3R4/VPS15, and either ATG14 and AMBRA1 (autophagy/Beclin 1 regulator 1), or UVRAG (UV radiation resistance associated) and SH3GLB1/Bif-1 (SH3-domain GRB2-like endophilin B1) is required for the nucleation and expansion of the phagophore, the initial sequestering compartment. 6 , 10 Autophagosome formation then requires 2 ubiquitin-like conjugation systems, ATG12–ATG5 and LC3/Atg8–phosphatidylethanolamine (LC3-II/Atg8–PE). In yeast, these proteins are involved in elongation and maturation of the phagophore. In mammals, there are 2 subfamilies of Atg8 proteins; the LC3 subfamily acts at the elongation stage, whereas the GABARAP proteins function later in autophagosome maturation. 11 Autophagy regulation is partly based on the phosphorylation and dephosphorylation of ATG proteins. The major upstream actor in these intracellular pathways is the MTOR (mechanistic target of rapamycin) kinase, which inhibits autophagy by regulating mRNA and protein levels of critical components, and also by direct phosphorylation of the autophagy machinery. For example, MTOR can phosphorylate ATG13, thus inhibiting the activity of ULK1 and autophagy. Given its critical role as a sensor for nutrients, MTOR is able to regulate translation by controlling the activity of some specific molecules involved in protein synthesis, such as EIF4EBP1/4E-BP1 (eukaryotic translation initiation factor 4E binding protein 1) and RPS6KB/p70S6K (ribosomal protein S6 kinase, 70 kDa, polypeptide 1). MTOR plays a major role in regulating switches between anabolic and catabolic metabolism, in order to stabilize cell viability in energy stress conditions. 12 MTOR is also regulated by some extracellular growth factors, such as IGF1 (insulin-like growth factor 1 [somatomedin C]), through class III phosphatidylinositol 3-kinase, AKT/PKB (v-akt murine thymoma viral oncogene homolog 1), and AMPK (AMP-activated protein kinase) pathways. 5
Figure 1. Schematic diagram summarizing the regulation of autophagy in mammalian cells. Following stimulation by growth factors, such as IGF1, MTOR is activated, whereas this pathway is inhibited upon stress conditions, such as starvation. MTOR inhibition is required to activate the ULK complex, since MTOR is able to induce the phosphorylation of ATG13, leading to reduction of ULK1 activity. By sensing the activation of the ULK complex, the BECN1 complex is activated leading to the nucleation of a phagophore. By means of 2 ubiquitin-like conjugation systems, generating ATG12–ATG5 and LC3-II, the membrane is elongated to form a double-membraned vesicle, the autophagosome. Finally, the autophagosome fuses with the lysosome, forming an autolysosome, where the cargo is digested by lysosomal enzymes and the degraded material released into the cytoplasm for recycling by the cell.
A large amount of experimental data demonstrates that autophagy functions as a key mechanism for the regulation of biological activities in both physiological conditions (e.g., cell/tissue homeostasis and development) and pathological conditions (e.g., cancer and neurodegenerative diseases), and autophagy dysfunction is associated with various diseases. 3 , 4 , 13 The role of autophagy in cancer is extremely complex. On the one hand, autophagy can act as a tumor suppressor by eliminating oncogenic protein substrates, unfolded proteins, and damaged organelles, and by preventing oxidative stress and genomic instability. On the other hand, autophagy can function as a tumor promoter in established cancers, by providing substrates that allow tumor cells to overcome nutrient limitation and hypoxia. 6 , 14 Most studies investigating the role of autophagy in tumors have sampled cancer cells. 6 , 14 , 15 However, the solid tumor also includes nonmalignant resident stromal cells, such as cancer-associated fibroblasts, endothelial cells, and bone marrow-derived cells, all of which extensively affect tumor growth and progression. 16 , 17 Recent studies have begun to unveil the significance of autophagy in the tumor microenvironment, a condition defined as the “autophagic tumor stroma.” 18 In established tumors, the autophagic tumor stroma is able to provide essential nutrients to cancer cells, remodel other components of the tumor microenvironment, and increase DNA damage and genetic instability of cancer cells, as well as decrease cancer cell apoptosis, thus representing an important regulator for tumor growth and progression. 18 Moreover, it was recently demonstrated that autophagy and inflammation work synergistically in the tumor microenvironment to facilitate tumor growth and metastasis. 19 These findings highlight the role of autophagy in inflammatory cells in affecting tumor progression, thus pointing at this process as a target for cancer therapies.
Among the inflammatory cells, macrophages are the most prominent cell type in the tumor microenvironment. Both experimental and clinical findings over the past decade demonstrated that tumor-associated macrophages (TAMs) favor malignant progression by suppressing antitumor immunity, by stimulating angiogenesis, and by enhancing tumor cell proliferation, migration, and invasion. 16 , 20 , 21 Autophagy is an important component of innate immunity by macrophages regulated by both toll-like receptors (TLRs) and intracellular pathogens. For example, lipopolysaccharide (LPS) can induce the formation of autophagosomes in macrophages, which is regulated through a TICAM1/TRIF (toll-like receptor adaptor molecule 1)-dependent TLR4 signaling pathway. 22 Listeria monocytogenes is an intracellular pathogen that can activate the autophagy pathway in macrophages via a MAPK/ERK (mitogen-activated protein kinase)-dependent TLR2 and Nucleotide Oligomerization domain 2 (NOD2) signaling pathways. 23 Increasing evidence demonstrates that autophagy can modulate the activity of macrophages and their response to different stimuli, 22 , 24 , 25 thus highlighting the connections among autophagy, macrophages, and cancer, and suggesting the potential to enhance an antitumor response by modulating autophagy in macrophages. Given the major roles of macrophages and autophagy in tumor progression and their correlation in biological activities, it is valuable to clarify the contribution and underlying molecular mechanisms of autophagy-mediated regulation of macrophages, and their implications for cancer. In the next sections we will summarize the significance of autophagy in regulating macrophage production and polarization, and discuss the value of autophagy modulation with regard to the protumoral functions of TAMs.
Role of Autophagy in Macrophage Production
TAMs are a type of cells that have a short half-life and cannot proliferate in tumor tissues. 26 In order to maintain high TAM levels, these cells require continuous replenishment throughout tumor growth and progression. TAMs are derived from bone marrow progenitor cells, a process that involves different steps, including the maintenance of hematopoietic stem cells (HSCs), the production of monocytes, the recruitment of monocytes into tumors, the differentiation of monocytes into macrophages, and the polarization of macrophages into TAMs. 20 , 27 In this section, we discuss experimental evidence demonstrating that autophagy is necessary for macrophage production (Fig. 2), even in the absence of cancer.
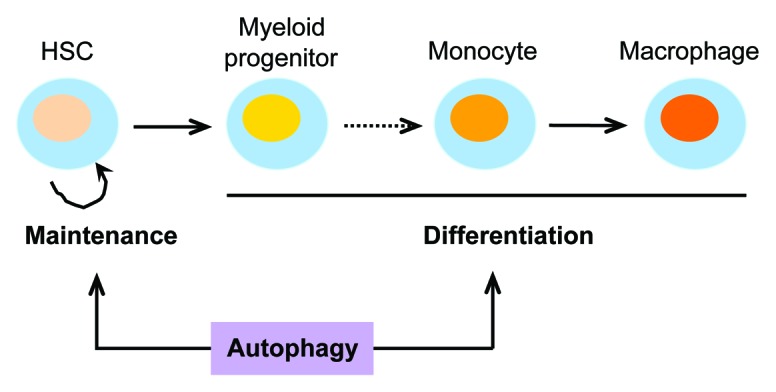
Figure 2. Schematic diagram of the roles of autophagy in macrophage production. Autophagy is involved in the maintenance and differentiation of HSCs, as well as in the differentiation of monocytes into macrophages. It is not yet known whether autophagy also plays a role in mediating the differentiation of HSCs into monocytes (dotted line).
Role of autophagy in hematopoietic stem cell maintenance
HSCs reside in the bone marrow niche and are generally in a quiescent state. 28 However, HSCs undergo distinct programs in response to stimulation, including self-renewal and differentiation. The balance of quiescence, self-renewal, and differentiation is tightly regulated in HSCs in order to maintain their physiological functions, and when this balance is not properly executed it may induce hematopoietic malignancies. 29 Bone marrow that hosts HSCs is usually a hypoxic environment, where a low-oxygen niche limits ROS production, thus providing long-term protection of HSCs from ROS stress. 30 ROS production and metabolic rate are increased when HSCs transit from a quiescent state to a proliferation/differentiation state, a process that is mediated by the MTOR pathway. 30 , 31 Activation of AKT, an upstream regulator of MTOR, decreases autophagy in HSCs and promotes myeloid proliferation, 32 whereas deletion of Rptor/Raptor (regulatory-associated protein of MTOR, complex 1), encoding a component of MTORC1 (MTOR complex 1), enhances autophagy and decreases this myeloid cell population (Table 1). 33 Moreover, HSC self-renewal can be restored by treatment with antioxidants or rapamycin. 31 , 39 RB1CC1 is an important regulator of autophagy, and conditional ablation of Rb1cc1 in HSCs causes perinatal lethality and severe anemia, with a marked increase of HSC proliferation, ROS levels, and mitochondrial mass (Table 1). 34 These findings provide indirect evidence that autophagy is potentially involved in the maintenance of HSCs.
Table 1. Summary of studies related to the roles of autophagy in macrophage polarization using mouse models deficient in autophagic components.
| Mice | Key findings | Refs. |
|---|---|---|
| Rptor knockout | Autophagy is enhanced, myeloid cell population is decreased | 33 |
| Rb1cc1 knockout in HSCs | Increase of HSC proliferation, ROS levels, and mitochondrial mass | 34 |
| Atg7 knockout in HSCs | Impaired production of lymphoid and myeloid progenitors | 35, 36 |
| Atg7 knockout | CSF1-induced differentiation of monocytes into macrophages is significantly hampered | 37, 38 |
| Atg5 knockout | M2-polarized macrophages are forced to produce a high level of M1-like cytokines | 61, 62 |
Notably, recent studies have provided direct evidence for the concept that autophagy functions as an important determinant for HSC fate. For example, a study demonstrated that autophagy is highly activated in HSCs in humans and that this process is required for the self-renewal and differentiation of HSCs. 40 Inhibition of autophagy by 3-methyladenine or by Atg5 siRNA-mediated knockdown results in a complete blockade of the differentiation and self-renewal of HSCs. 40 Inhibition of autophagy in HSCs by conditional ablation of Atg7 impairs the production of lymphoid and myeloid progenitors, thus suggesting that Atg7 is essential for HSC maintenance in a cell-autonomous fashion (Table 1). 35 , 36 The effect of autophagy in HSC maintenance is also displayed under metabolic stress conditions, when autophagy is robustly induced by a pathway regulated by FOXO3A to protect HSCs against apoptosis. Moreover, HSCs from aged mice have the ability to exhibit an intact FOXO3A-induced proautophagic gene program, and this ongoing autophagy is required for protecting HSCs against apoptosis and mitigating metabolic stress. 41 Altogether, these findings highlight autophagy as a key mechanism for the maintenance and for the proper function of HSCs. However, it is also very important to consider that targeting of autophagy in HSCs may lead to several side effects including loss of HSC function, anemia, myeloproliferation and, ultimately, development of leukemia. 29 , 35 , 36 Thus, manipulation of autophagy in HSCs should be treated with extreme caution.
Autophagy in the regulation of monocyte/macrophage recruitment
In most solid tumors, TAM density is significantly higher than in the surrounding normal tissues. Generally, TAMs first originate from monocytes that are recruited into tumors by chemoattractants, including chemokines and cytokines released from both tumor cells and stromal cells. Among these chemoattractants, CCL2 (chemokine [C–C motif] ligand 2) is the one exerting a prominent activity in recruiting monocytes into tumors. 16 Conversely, CCL2 is able to protect monocytes against apoptosis in the tumor microenvironment, by upregulating antiapoptotic proteins and inhibiting CASP8/caspase-8 cleavage, and it also induces hyperactivation of autophagy in these cells, 42 thus suggesting a role of autophagy in CCL2-induced monocyte recruitment. Cucurbitacin IIa (CuIIa), a member of the cucurbitacin family, exerts a wide spectrum of pharmacological activities including anticancer and anti-inflammatory activities, which can inhibit macrophage proliferation and migration as well as enhance LPS-induced autophagy in macrophages, suggesting that enhancement of autophagy may contribute to the antiinflammatory activity of CuIIa in vitro. 43 However, further studies are needed to investigate the potential role of autophagy in regulating the anticancer activity of CuIIa by inhibiting in vivo macrophage recruitment. Although these data suggest a possible role of autophagy in monocyte recruitment, the connections of autophagy with regulation of monocyte recruitment by CCL2 and antiinflammatory activity of CuIIa are still uncertain and represent an intriguing subject for future investigation.
Recombinant capsid viral protein 1 (rVP1) suppresses growth and metastasis of tumor cells by inducing apoptosis and by modulating CCL2 production. 44 Furthermore, rVP1 also acts on host immune cells and promotes macrophage migration by inducing autophagy in these cells. 45 Mechanistic studies suggested that the phosphorylation levels of MAPK3/ERK1 and MAPK1/ERK2 and the activity of MMP9 (matrix metalloproteinase 9 [gelatinase B, 92 kDa gelatinase, 92 kDa type IV collagenase]) are increased upon rVP1 treatment, leading to autophagy upregulation and macrophage migration by a mechanism dependent on WIPI1 (WD repeat domain, phosphoinositide interacting 1), WIPI2, ATG5, and ATG7, but not on BECN1. 45 In the larval wound model, autophagy is required for the recruitment of blood cells into wound sites and for the spreading of macrophages. 46 Taken together, these findings provide evidence demonstrating that autophagy is an important mechanism for mediating macrophage migration. However, further studies are needed to investigate how autophagy contributes to monocyte/macrophage recruitment in the tumor microenvironment.
Role of autophagy on monocyte differentiation into macrophages
The half-life of monocytes in blood is very short, with approximately 3 days in humans and 1 day in mice, where they are programmed to undergo apoptosis in the absence of stimulation. 47 However, when stimulated by inflammatory factors, they activate survival pathways, migrate into distinct tissues, and then differentiate into macrophages, dendritic cells, or osteoclasts. 48 CSF1 (colony stimulating factor 1 [macrophage]) is the main factor that can induce monocyte differentiation into macrophages and activate survival pathways. 49 Several lines of data imply the involvement of autophagy in monocyte differentiation into macrophages. When monocytes are stimulated to differentiate into macrophages, autophagy is induced via increased expression and phosphorylation of ULK1. 37 , 38 Studies involving the inhibition of autophagy by pharmacological agents, siRNA approaches or Atg7 knockout mice show that the CSF1-driven differentiation of monocytes into macrophages is significantly hampered (Table 1). 37 , 38 CSF2/GM-CSF (colony-stimulating factor 2 [granulocyte-macrophage]) is another important factor that can drive the differentiation of monocytes into macrophages, and it was demonstrated that autophagy is induced during monocyte differentiation into macrophages triggered by CSF2 in vitro and by thioglycolate in vivo. 47 Interestingly, CSF2 is able to promote monocyte survival and differentiation into macrophages by MAPK/JNK and by inducing the dissociation of BECN1 from BCL2 (B-cell CLL/lymphoma 2), thus stimulating autophagy, whereas blockade of autophagy has an inhibitory effect on CSF2-induced monocyte differentiation into macrophages. 47
CSF1-induced monocyte differentiation into macrophages is a process that requires the activation of CASP3/caspase-3 and CASP8 by modulating the AKT signaling pathway, 50 suggesting a role for properly regulated apoptosis in monocyte differentiation into macrophages. Even if CASP8 may cleave specific substrates required for monocyte differentiation, explaining the requirement for its limited activation during differentiation, CASP8 is considered as the upstream enzyme in the proteolytic caspase cascade whose activation is required for monocyte differentiation into macrophages. 51 Several studies have demonstrated that there is a crosstalk between apoptotic and autophagic pathways. For example, BCL2 can bind to BECN1 to inhibit BECN1-mediated autophagy, 52 whereas BECN1 can be cleaved by caspases, and its C-terminal fragment has the ability to amplify the apoptotic response. 53 , 54 Moreover, death stimuli can trigger calpain-mediated cleavage of ATG5 to promote mitochondrial-mediated apoptosis. 55 Altogether, these findings point at autophagy as an essential mechanism for monocyte differentiation, and they suggest that inhibition of autophagy may be a promising strategy for impairing macrophage production in tumors.
Autophagy-Mediated Control of Macrophage Polarization
Macrophages are heterogeneous and can display divergent phenotypes and functions dependent on distinct tissue microenvironments. 16 , 56 For instance, macrophages can be divided into classically activated (M1 phenotype) and alternatively activated (M2 phenotype), according to the T helper cell type (Th)1/Th2 dichotomy. 16 , 48 M1 macrophages stimulate a Th1 response against intracellular microorganisms and tumor cells by activating an immune response, whereas M2 macrophages are immunosuppressive cells, which promote angiogenesis as well as tissue repair and remodeling. 16 , 56 , 57
We have recently reviewed the findings suggesting that macrophage polarization is triggered by polarization-related factors in the tumor microenvironment. 16 By sensing the stimulation, several intracellular signaling pathways, such as NFKB (nuclear factor of kappa light polypeptide gene enhancer in B-cells) and MTOR, are thought to modulate this process. 16 NFKB is a transcriptional factor that can be regarded as a pivotal link between inflammation and cancer, 58 , 59 and it also plays a central role in the regulation of macrophage polarization. It has been shown that both M1 and M2 macrophage polarization in the tumor microenvironment require the NFKB pathway, 60 and isolated TAMs from various tumors exhibit low NFKB activity. 60 , 61 However, the molecular mechanisms by which NFKB is essential for M2 macrophage polarization and is downregulated in TAMs remain to be investigated experimentally. Interestingly, recent studies have demonstrated that hepatoma-derived TLR2-related ligands are able to polarize macrophages toward the M2 phenotype by controlling RELA/NFKB p65 (v-rel avian reticuloendotheliosis viral oncogene homolog A) through selective autophagy. 62 , 63 Hepatoma-derived TLR2 signals lead to the ubiquitination of RELA, thus forming aggresome-like structures in macrophages, which can be degraded by SQSTM1/p62-mediated autophagy. 62 , 63 Inhibition of autophagy through pharmacological and genetic approaches can rescue NFKB activity and force M2-polarized macrophages to produce a high level of M1-like cytokines (Table 1). 62 , 63 Furthermore, mechanistic studies demonstrated that TLR2 signals can promote the sustained phosphorylation of MAPK1 and MAPK3, thus stimulating autophagy-dependent NFKB regulation. 62 , 63 These studies highlight that the role of NFKB in macrophage polarization is regulated by SQSTM1/p62-mediated selective autophagy. However, it has been shown that the role of NFKB in regulating TAM polarization and function is complex, which is exhibited in context-and gene-dependent manners. 60 The specific role of NFKB with respect to the synthesis of tumor-promoting genes and M2 macrophage polarization remains to be fully investigated.
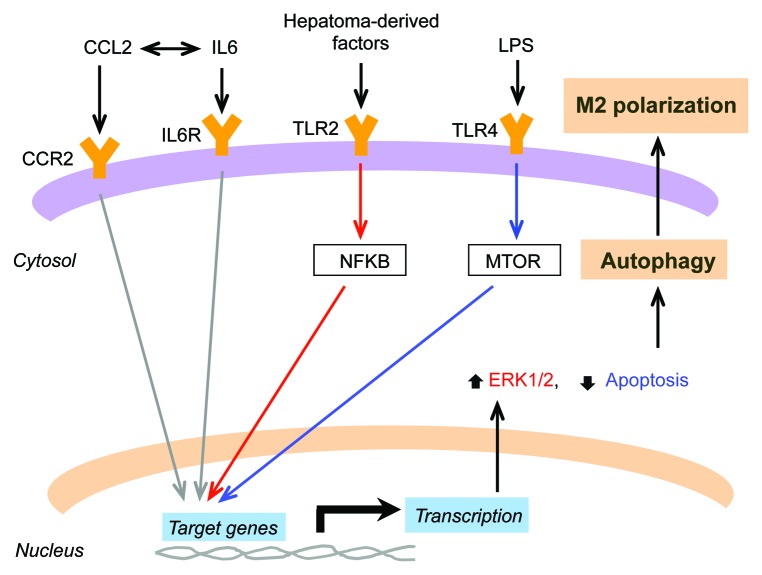
MTOR is an evolutionarily conserved protein kinase regulating autophagy, 64 , 65 which is also critical in the regulation of monocyte polarization into TAMs. In LPS-stimulated monocytes, inhibition of the MTOR pathway by rapamycin leads to polarization toward the M1 phenotype, whereas activation of this pathway by knockdown of the MTOR repressor TSC2 (tuberous sclerosis 2) exerts the opposite effect. 66 CCL2 and IL6 (interleukin 6 [interferon, β 2]) are 2 abundant cytokines in the tumor microenvironment, and their expression in myeloid cells is induced in a reciprocal manner. CCL2 and IL6 have a potent effect in inducing autophagy and inhibiting apoptosis in macrophages, as well as in stimulating macrophage polarization toward the M2 phenotype. Inhibition of CASP8 is able to promote autophagy in macrophages and increase M2 macrophage polarization. Inhibition of autophagy under these circumstances attenuates M2 macrophage polarization, which directly indicates that autophagy plays a key role in macrophage polarization. 67 Sorafenib is an antiangiogenic agent that has been approved for cancer treatment. However, some studies also demonstrated that antiangiogenic drugs may, in some conditions, accelerate cancer progression. 68 - 70 Moreover, TAMs can be recruited when sorafenib is administered, thus promoting the progression of hepatocellular carcinoma. 71 Interestingly, recent studies showed that sorafenib exerts a potent effect on macrophages by inducing autophagy and suppressing the expression of CD80, a marker of the M1 phenotype, suggesting the possible correlation between autophagy and macrophage polarization and highlighting the protumorigenic effect of sorafenib through modulation of macrophage polarization by autophagy. 72 Altogether, these findings support a key role of autophagy in the regulation of macrophage polarization in the tumor microenvironment (Fig. 3).
Figure 3. Schematic diagram of signaling pathways for tumor-derived factors contributing to macrophage polarization via induction of autophagy. Expression of IL6 and CCL2 in the tumor microenvironment is regulated in a reciprocal manner. Induction of autophagy triggered by binding of IL6 and CCL2 to IL6R (interleukin 6 receptor) and CCR2 (chemokine [C–C motif] receptor 2), respectively, is essential for macrophage polarizaton to the M2 phenotype. Following binding to TLR2, the hepatoma-derived factors are able to stimulate macrophage polarization to the M2 phenotype by controlling NFKB homeostasis through selective autophagy. Moreover, M2 macrophages can be induced by autophagy triggered by LPS or bacteria, which is modulated by the MTOR pathway via activation of TLR4.
The Significance of Macrophage Autophagy for Cancer
Our current understanding of the contribution of autophagy in controlling macrophage production, polarization, and function in cancer remains limited. Nonetheless, as we discussed above, it is now well established that autophagy plays a crucial role in macrophage production by regulating HSC maintenance, monocyte/macrophage recruitment, and monocyte differentiation into macrophages. Data obtained from patient biopsies indicate that TAM density is correlated with poor prognosis in most human cancers. 16 Tumor angiogenesis and progression are also affected by macrophage density in animal cancer models. For instance, inhibition or enhancement of macrophage density in tumors by genetic and pharmacological approaches, respectively, inhibits or promotes tumor angiogenesis, growth, and progression. 16 , 21 These findings highlight the significance of autophagy-mediated macrophage production in promoting cancer progression. The induction of autophagy in macrophages is triggered by TLR ligands, 22 , 63 , 73 suggesting the potential role of TLR signaling in modulating macrophage function by autophagy. Indeed, TLR2 deficiency induces a significant reduction of autophagy and macrophage infiltration in liver tissues, and promotes hepatocarcinogenesis, suggesting a potential role of TLR2 in tumorigenesis by modulation of autophagy in macrophages. 74 Future studies should aim at using genetic approaches that specifically inhibit or facilitate autophagy in macrophages or their precursors, thus helping to establish in detail the roles of autophagy in regulating macrophage production, tumor growth, and progression in vivo.
As discussed above, macrophages exhibit a spectrum of phenotypes including M1 and M2 phenotypes, which exert anticancer activity and favor tumor progression, respectively. 16 , 21 , 75 , 76 TAMs are mainly polarized toward the M2 phenotype, that promotes tumor angiogenesis, growth, and metastasis. 16 , 21 However, it should be noted that some exceptions to this pattern exist. For instance, TAMs are biased toward the M1 phenotype in nonprogressing, regressing, and early-stage tumors, 16 , 21 , 75 , 77 suggesting that the phenotype of TAMs can be polarized by the local tumor microenvironment. Interestingly, recent studies demonstrated that HRG (histidine-rich glycoprotein) inhibits tumor growth and metastasis by inducing TAM polarization toward the M1 phenotype through downregulation of PGF (placental growth factor). 77 ADM/adrenomedullin(22–52), an antagonist of ADM receptors, suppresses tumor growth by skewing TAMs polarization to the M1 phenotype through downregulation of ADM in an autocrine-dependent manner. 75 These findings suggest that the identification of potential targets that polarize TAMs toward the M1 phenotype should be a promising anticancer strategy. By sensing the stimulation from the tumor microenvironment, macrophages are polarized to specific phenotypes through different signaling pathways, including the induction of autophagy. 16 Several studies have shown that treatments targeting autophagy can modify the activation states of macrophages. 62 , 63 , 66 , 67 , 72 These findings should encourage studies to develop genetic and pharmacological approaches to skew TAM polarization to the M1 phenotype by targeting autophagy. For example, even if TLR2 deficiency causes a reduction of macrophage infiltration, this ablation also induces a significant suppression of autophagy and a reduction in the expression of TNF/TNFα (tumor necrosis factor), IFNG (interferon, gamma) and CXCL2 (chemokine [C–X–C motif] ligand 2) in liver tissues, indicating an increase of M2 macrophage polarization, which in turn promotes hepatocarcinogenesis. 74 Notably, recent findings highlight that activation of the MTOR-TSC2 pathway, a key regulator of autophagy, is critical for macrophage polarization toward the M2 phenotype to promote tumor angiogenesis and growth in mouse hepatocellular carcinoma models, whereas inhibition of this pathway exerts the opposite effects. 66 Thus, the polarization of macrophages regulated by autophagy may represent a promising and effective strategy for liver cancer therapies. In this respect, it is important to consider that the role of autophagy in cancer cells depends on different factors, such as tumor type, stage, and genetic context. Therefore, further studies are needed to assess whether and how these factors affect the function of autophagy modulation in macrophages.
Concluding Remarks and Future Perspectives
A number of studies indicate that autophagy extensively regulates the response of macrophages to microenvironmental stimuli, and may modulate the function of TAMs in tumors. They also provide clear evidence that autophagy functions as a key determinant for macrophage production, by modulating HSC maintenance, monocyte differentiation into macrophages, and monocyte/macrophage recruitment, as well as for macrophage polarization. Macrophage production and polarization are 2 key events for the contribution of macrophages in promoting tumor growth and progression. Therefore, modulation of autophagy in macrophages by controlling these parameters represents a promising and effective strategy for anticancer therapies.
Although our knowledge of the role of autophagy in controlling macrophages has increased in the last few years, several open questions remain to be addressed regarding the molecular mechanisms underlying the effects of autophagy in macrophage production and activation, and the effects of macrophage autophagy in the context of tumors, as well as the value of macrophage autophagy as a target for anticancer therapies. Although autophagy is required for HSC maintenance and differentiation as discussed above, it remains to be defined whether autophagy is also required for HSC differentiation into monocytes, an important stage for macrophage production. The role of autophagy in the maturation of other types of hematopoetic cells has been clearly established. For instance, independent findings indicate that autophagy is essential for the maturation of red blood cells by the clearance of mitochondria, and functional studies demonstrated that the impairment of mitochondrial autophagy by elimination of BNIP3L/Nix, ULK1, or ATG7 causes serious defects in the maturation and function of red blood cells. 78 - 80 Although a number of experiments indicate that autophagy is essential for macrophage production and activation, further studies are needed to validate this concept in the context of tumors in vivo by specifically targeting autophagy in TAMs. In addition to increasing the understanding of the mechanisms regulating macrophage autophagy during cancer progression, prospective findings in this field may provide novel therapeutic targets for cancer therapy.
Acknowledgments
We apologize to the authors whose papers we could not cite because of space limitations. This work was supported by the Italian Ministry of Education, University and Research, the Cariparo Foundation, and the University of Padova.
Glossary
Abbreviations:
- ATG
autophagy-related
- Atg8–PE
Atg8–phosphatidylethanolamine
- CCL2
chemokine (C–C motif) ligand 2
- CSF1
colony stimulating factor 1 (macrophage)
- CSF2/GM-CSF
colony-stimulating factor 2 (granulocyte-macrophage)
- CuIIa
cucurbitacin IIa
- HSCs
hematopoietic stem cells
- IL6
interleukin 6 (interferon, beta 2)
- LPS
lipopolysaccharide
- MAPK
mitogen-activated protein kinase
- MTOR
mechanistic target of rapamycin
- NFKB
nuclear factor of kappa light polypeptide gene enhancer in B-cells
- RB1CC1
RB1-inducible coiled-coil 1
- ROS
reactive oxygen species
- rVP1
recombinant capsid viral protein 1
- TAMs
tumor-associated macrophages
- TLR
toll-like receptor
- ULK
unc-51 like autophagy activating kinase
Disclosure of Potential Conflicts of Interest
The authors declare no conflict of interest.
References
- 1. Amaravadi RK, Lippincott-Schwartz J, Yin XM, Weiss WA, Takebe N, Timmer W, DiPaola RS, Lotze MT, White E. . Principles and current strategies for targeting autophagy for cancer treatment. Clin Cancer Res 2011; 17:654 - 66; http://dx.doi.org/ 10.1158/1078-0432.CCR-10-2634; PMID: 21325294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mizushima N, Levine B. . Autophagy in mammalian development and differentiation. Nat Cell Biol 2010; 12:823 - 30; http://dx.doi.org/ 10.1038/ncb0910-823; PMID: 20811354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mizushima N, Komatsu M. . Autophagy: renovation of cells and tissues. Cell 2011; 147:728 - 41; http://dx.doi.org/ 10.1016/j.cell.2011.10.026; PMID: 22078875 [DOI] [PubMed] [Google Scholar]
- 4. Rubinsztein DC, Mariño G, Kroemer G. . Autophagy and aging. Cell 2011; 146:682 - 95; http://dx.doi.org/ 10.1016/j.cell.2011.07.030; PMID: 21884931 [DOI] [PubMed] [Google Scholar]
- 5. Ravikumar B, Futter M, Jahreiss L, Korolchuk VI, Lichtenberg M, Luo S, Massey DC, Menzies FM, Narayanan U, Renna M, et al. . Mammalian macroautophagy at a glance. J Cell Sci 2009; 122:1707 - 11; http://dx.doi.org/ 10.1242/jcs.031773; PMID: 19461070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Eskelinen EL. . The dual role of autophagy in cancer. Curr Opin Pharmacol 2011; 11:294 - 300; http://dx.doi.org/ 10.1016/j.coph.2011.03.009; PMID: 21498118 [DOI] [PubMed] [Google Scholar]
- 7. Mizushima N, Levine B, Cuervo AM, Klionsky DJ. . Autophagy fights disease through cellular self-digestion. Nature 2008; 451:1069 - 75; http://dx.doi.org/ 10.1038/nature06639; PMID: 18305538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xie Z, Klionsky DJ. . Autophagosome formation: core machinery and adaptations. Nat Cell Biol 2007; 9:1102 - 9; http://dx.doi.org/ 10.1038/ncb1007-1102; PMID: 17909521 [DOI] [PubMed] [Google Scholar]
- 9. Mizushima N. . The role of the Atg1/ULK1 complex in autophagy regulation. Curr Opin Cell Biol 2010; 22:132 - 9; http://dx.doi.org/ 10.1016/j.ceb.2009.12.004; PMID: 20056399 [DOI] [PubMed] [Google Scholar]
- 10. Choi AM, Ryter SW, Levine B. . Autophagy in human health and disease. N Engl J Med 2013; 368:651 - 62; http://dx.doi.org/ 10.1056/NEJMra1205406; PMID: 23406030 [DOI] [PubMed] [Google Scholar]
- 11. Ferraro E, Cecconi F. . Autophagic and apoptotic response to stress signals in mammalian cells. Arch Biochem Biophys 2007; 462:210 - 9; http://dx.doi.org/ 10.1016/j.abb.2007.02.006; PMID: 17374522 [DOI] [PubMed] [Google Scholar]
- 12. Di Bartolomeo S, Nazio F, Cecconi F. . The role of autophagy during development in higher eukaryotes. Traffic 2010; 11:1280 - 9; http://dx.doi.org/ 10.1111/j.1600-0854.2010.01103.x; PMID: 20633243 [DOI] [PubMed] [Google Scholar]
- 13. Shoji-Kawata S, Sumpter R, Leveno M, Campbell GR, Zou Z, Kinch L, Wilkins AD, Sun Q, Pallauf K, MacDuff D, et al. . Identification of a candidate therapeutic autophagy-inducing peptide. Nature 2013; 494:201 - 6; http://dx.doi.org/ 10.1038/nature11866; PMID: 23364696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. White E. . Deconvoluting the context-dependent role for autophagy in cancer. Nat Rev Cancer 2012; 12:401 - 10; http://dx.doi.org/ 10.1038/nrc3262; PMID: 22534666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cheong H, Lu C, Lindsten T, Thompson CB. . Therapeutic targets in cancer cell metabolism and autophagy. Nat Biotechnol 2012; 30:671 - 8; http://dx.doi.org/ 10.1038/nbt.2285; PMID: 22781696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen P, Bonaldo P. . Role of macrophage polarization in tumor angiogenesis and vessel normalization: implications for new anticancer therapies. Int Rev Cell Mol Biol 2013; 301:1 - 35; http://dx.doi.org/ 10.1016/B978-0-12-407704-1.00001-4; PMID: 23317816 [DOI] [PubMed] [Google Scholar]
- 17. Hanahan D, Coussens LM. . Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 2012; 21:309 - 22; http://dx.doi.org/ 10.1016/j.ccr.2012.02.022; PMID: 22439926 [DOI] [PubMed] [Google Scholar]
- 18. Zhao X, He Y, Chen H. . Autophagic tumor stroma: mechanisms and roles in tumor growth and progression. Int J Cancer 2013; 132:1 - 8; http://dx.doi.org/ 10.1002/ijc.27664; PMID: 22684793 [DOI] [PubMed] [Google Scholar]
- 19. Martinez-Outschoorn UE, Whitaker-Menezes D, Lin Z, Flomenberg N, Howell A, Pestell RG, Lisanti MP, Sotgia F. . Cytokine production and inflammation drive autophagy in the tumor microenvironment: role of stromal caveolin-1 as a key regulator. Cell Cycle 2011; 10:1784 - 93; http://dx.doi.org/ 10.4161/cc.10.11.15674; PMID: 21566463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Squadrito ML, Etzrodt M, De Palma M, Pittet MJ. . MicroRNA-mediated control of macrophages and its implications for cancer. Trends Immunol 2013; 34:350 - 9; http://dx.doi.org/ 10.1016/j.it.2013.02.003; PMID: 23498847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Qian BZ, Pollard JW. . Macrophage diversity enhances tumor progression and metastasis. Cell 2010; 141:39 - 51; http://dx.doi.org/ 10.1016/j.cell.2010.03.014; PMID: 20371344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xu Y, Jagannath C, Liu XD, Sharafkhaneh A, Kolodziejska KE, Eissa NT. . Toll-like receptor 4 is a sensor for autophagy associated with innate immunity. Immunity 2007; 27:135 - 44; http://dx.doi.org/ 10.1016/j.immuni.2007.05.022; PMID: 17658277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Anand PK, Tait SW, Lamkanfi M, Amer AO, Nunez G, Pagès G, Pouysségur J, McGargill MA, Green DR, Kanneganti TD. . TLR2 and RIP2 pathways mediate autophagy of Listeria monocytogenes via extracellular signal-regulated kinase (ERK) activation. J Biol Chem 2011; 286:42981 - 91; http://dx.doi.org/ 10.1074/jbc.M111.310599; PMID: 22033934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liao X, Sluimer JC, Wang Y, Subramanian M, Brown K, Pattison JS, Robbins J, Martinez J, Tabas I. . Macrophage autophagy plays a protective role in advanced atherosclerosis. Cell Metab 2012; 15:545 - 53; http://dx.doi.org/ 10.1016/j.cmet.2012.01.022; PMID: 22445600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yuan K, Huang C, Fox J, Laturnus D, Carlson E, Zhang B, Yin Q, Gao H, Wu M. . Autophagy plays an essential role in the clearance of Pseudomonas aeruginosa by alveolar macrophages. J Cell Sci 2012; 125:507 - 15; http://dx.doi.org/ 10.1242/jcs.094573; PMID: 22302984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cortez-Retamozo V, Etzrodt M, Newton A, Rauch PJ, Chudnovskiy A, Berger C, Ryan RJ, Iwamoto Y, Marinelli B, Gorbatov R, et al. . Origins of tumor-associated macrophages and neutrophils. Proc Natl Acad Sci U S A 2012; 109:2491 - 6; http://dx.doi.org/ 10.1073/pnas.1113744109; PMID: 22308361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cortez-Retamozo V, Etzrodt M, Pittet MJ. . Regulation of macrophage and dendritic cell responses by their lineage precursors. J Innate Immun 2012; 4:411 - 23; http://dx.doi.org/ 10.1159/000335733; PMID: 22433183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Eliasson P, Jönsson JI. . The hematopoietic stem cell niche: low in oxygen but a nice place to be. J Cell Physiol 2010; 222:17 - 22; http://dx.doi.org/ 10.1002/jcp.21908; PMID: 19725055 [DOI] [PubMed] [Google Scholar]
- 29. Guan JL, Simon AK, Prescott M, Menendez JA, Liu F, Wang F, Wang C, Wolvetang E, Vazquez-Martin A, Zhang J. . Autophagy in stem cells. Autophagy 2013; 9:830 - 49; http://dx.doi.org/ 10.4161/auto.24132; PMID: 23486312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jang YY, Sharkis SJ. . A low level of reactive oxygen species selects for primitive hematopoietic stem cells that may reside in the low-oxygenic niche. Blood 2007; 110:3056 - 63; http://dx.doi.org/ 10.1182/blood-2007-05-087759; PMID: 17595331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen C, Liu Y, Liu R, Ikenoue T, Guan KL, Liu Y, Zheng P. . TSC-mTOR maintains quiescence and function of hematopoietic stem cells by repressing mitochondrial biogenesis and reactive oxygen species. J Exp Med 2008; 205:2397 - 408; http://dx.doi.org/ 10.1084/jem.20081297; PMID: 18809716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kharas MG, Okabe R, Ganis JJ, Gozo M, Khandan T, Paktinat M, Gilliland DG, Gritsman K. . Constitutively active AKT depletes hematopoietic stem cells and induces leukemia in mice. Blood 2010; 115:1406 - 15; http://dx.doi.org/ 10.1182/blood-2009-06-229443; PMID: 20008787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hoshii T, Tadokoro Y, Naka K, Ooshio T, Muraguchi T, Sugiyama N, Soga T, Araki K, Yamamura K, Hirao A. . mTORC1 is essential for leukemia propagation but not stem cell self-renewal. J Clin Invest 2012; 122:2114 - 29; http://dx.doi.org/ 10.1172/JCI62279; PMID: 22622041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liu F, Lee JY, Wei H, Tanabe O, Engel JD, Morrison SJ, Guan JL. . FIP200 is required for the cell-autonomous maintenance of fetal hematopoietic stem cells. Blood 2010; 116:4806 - 14; http://dx.doi.org/ 10.1182/blood-2010-06-288589; PMID: 20716775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mortensen M, Soilleux EJ, Djordjevic G, Tripp R, Lutteropp M, Sadighi-Akha E, Stranks AJ, Glanville J, Knight S, Jacobsen SE, et al. . The autophagy protein Atg7 is essential for hematopoietic stem cell maintenance. J Exp Med 2011; 208:455 - 67; http://dx.doi.org/ 10.1084/jem.20101145; PMID: 21339326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mortensen M, Watson AS, Simon AK. . Lack of autophagy in the hematopoietic system leads to loss of hematopoietic stem cell function and dysregulated myeloid proliferation. Autophagy 2011; 7:1069 - 70; http://dx.doi.org/ 10.4161/auto.7.9.15886; PMID: 21552009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jacquel A, Obba S, Boyer L, Dufies M, Robert G, Gounon P, Lemichez E, Luciano F, Solary E, Auberger P. . Autophagy is required for CSF-1-induced macrophagic differentiation and acquisition of phagocytic functions. Blood 2012; 119:4527 - 31; http://dx.doi.org/ 10.1182/blood-2011-11-392167; PMID: 22452982 [DOI] [PubMed] [Google Scholar]
- 38. Jacquel A, Obba S, Solary E, Auberger P. . Proper macrophagic differentiation requires both autophagy and caspase activation. Autophagy 2012; 8:1141 - 3; http://dx.doi.org/ 10.4161/auto.20367; PMID: 22751215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chen C, Liu Y, Liu Y, Zheng P. . mTOR regulation and therapeutic rejuvenation of aging hematopoietic stem cells. Sci Signal 2009; 2:ra75; http://dx.doi.org/ 10.1126/scisignal.2000559; PMID: 19934433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Salemi S, Yousefi S, Constantinescu MA, Fey MF, Simon HU. . Autophagy is required for self-renewal and differentiation of adult human stem cells. Cell Res 2012; 22:432 - 5; http://dx.doi.org/ 10.1038/cr.2011.200; PMID: 22184008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Warr MR, Binnewies M, Flach J, Reynaud D, Garg T, Malhotra R, Debnath J, Passegué E. . FOXO3A directs a protective autophagy program in haematopoietic stem cells. Nature 2013; 494:323 - 7; http://dx.doi.org/ 10.1038/nature11895; PMID: 23389440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Roca H, Varsos ZS, Sud S, Craig MJ, Ying C, Pienta KJ. . CCL2 and interleukin-6 promote survival of human CD11b+ peripheral blood mononuclear cells and induce M2-type macrophage polarization. J Biol Chem 2009; 284:34342 - 54; http://dx.doi.org/ 10.1074/jbc.M109.042671; PMID: 19833726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. He J, Wang Y, Xu LH, Qiao J, Ouyang DY, He XH. . Cucurbitacin IIa induces caspase-3-dependent apoptosis and enhances autophagy in lipopolysaccharide-stimulated RAW 264.7 macrophages. Int Immunopharmacol 2013; 16:27 - 34; http://dx.doi.org/ 10.1016/j.intimp.2013.03.013; PMID: 23541744 [DOI] [PubMed] [Google Scholar]
- 44. Peng JM, Chen YH, Hung SW, Chiu CF, Ho MY, Lee YJ, Lai TC, Hsiao M, Liang CM, Liang SM. . Recombinant viral protein promotes apoptosis and suppresses invasion of ovarian adenocarcinoma cells by targeting α5β1 integrin to down-regulate Akt and MMP-2. Br J Pharmacol 2012; 165:479 - 93; http://dx.doi.org/ 10.1111/j.1476-5381.2011.01581.x; PMID: 21740408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Liao CC, Ho MY, Liang SM, Liang CM. . Recombinant protein rVP1 upregulates BECN1-independent autophagy, MAPK1/3 phosphorylation and MMP9 activity via WIPI1/WIPI2 to promote macrophage migration. Autophagy 2013; 9:5 - 19; http://dx.doi.org/ 10.4161/auto.22379; PMID: 23051912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kadandale P, Stender JD, Glass CK, Kiger AA. . Conserved role for autophagy in Rho1-mediated cortical remodeling and blood cell recruitment. Proc Natl Acad Sci U S A 2010; 107:10502 - 7; http://dx.doi.org/ 10.1073/pnas.0914168107; PMID: 20498061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhang Y, Morgan MJ, Chen K, Choksi S, Liu ZG. . Induction of autophagy is essential for monocyte-macrophage differentiation. Blood 2012; 119:2895 - 905; http://dx.doi.org/ 10.1182/blood-2011-08-372383; PMID: 22223827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gordon S, Taylor PR. . Monocyte and macrophage heterogeneity. Nat Rev Immunol 2005; 5:953 - 64; http://dx.doi.org/ 10.1038/nri1733; PMID: 16322748 [DOI] [PubMed] [Google Scholar]
- 49. Hume DA, MacDonald KP. . Therapeutic applications of macrophage colony-stimulating factor-1 (CSF-1) and antagonists of CSF-1 receptor (CSF-1R) signaling. Blood 2012; 119:1810 - 20; http://dx.doi.org/ 10.1182/blood-2011-09-379214; PMID: 22186992 [DOI] [PubMed] [Google Scholar]
- 50. Jacquel A, Benikhlef N, Paggetti J, Lalaoui N, Guery L, Dufour EK, Ciudad M, Racoeur C, Micheau O, Delva L, et al. . Colony-stimulating factor-1-induced oscillations in phosphatidylinositol-3 kinase/AKT are required for caspase activation in monocytes undergoing differentiation into macrophages. Blood 2009; 114:3633 - 41; http://dx.doi.org/ 10.1182/blood-2009-03-208843; PMID: 19721010 [DOI] [PubMed] [Google Scholar]
- 51. Rébé C, Cathelin S, Launay S, Filomenko R, Prévotat L, L’Ollivier C, Gyan E, Micheau O, Grant S, Dubart-Kupperschmitt A, et al. . Caspase-8 prevents sustained activation of NF-kappaB in monocytes undergoing macrophagic differentiation. Blood 2007; 109:1442 - 50; http://dx.doi.org/ 10.1182/blood-2006-03-011585; PMID: 17047155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Levine B, Sinha S, Kroemer G. . Bcl-2 family members: dual regulators of apoptosis and autophagy. Autophagy 2008; 4:600 - 6; PMID: 18497563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Djavaheri-Mergny M, Maiuri MC, Kroemer G. . Cross talk between apoptosis and autophagy by caspase-mediated cleavage of Beclin 1. Oncogene 2010; 29:1717 - 9; http://dx.doi.org/ 10.1038/onc.2009.519; PMID: 20101204 [DOI] [PubMed] [Google Scholar]
- 54. Wirawan E, Vande Walle L, Kersse K, Cornelis S, Claerhout S, Vanoverberghe I, Roelandt R, De Rycke R, Verspurten J, Declercq W, et al. . Caspase-mediated cleavage of Beclin-1 inactivates Beclin-1-induced autophagy and enhances apoptosis by promoting the release of proapoptotic factors from mitochondria. Cell Death Dis 2010; 1:e18; http://dx.doi.org/ 10.1038/cddis.2009.16; PMID: 21364619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yousefi S, Perozzo R, Schmid I, Ziemiecki A, Schaffner T, Scapozza L, Brunner T, Simon HU. . Calpain-mediated cleavage of Atg5 switches autophagy to apoptosis. Nat Cell Biol 2006; 8:1124 - 32; http://dx.doi.org/ 10.1038/ncb1482; PMID: 16998475 [DOI] [PubMed] [Google Scholar]
- 56. Mantovani A, Biswas SK, Galdiero MR, Sica A, Locati M. . Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol 2013; 229:176 - 85; http://dx.doi.org/ 10.1002/path.4133; PMID: 23096265 [DOI] [PubMed] [Google Scholar]
- 57. Gordon S, Martinez FO. . Alternative activation of macrophages: mechanism and functions. Immunity 2010; 32:593 - 604; http://dx.doi.org/ 10.1016/j.immuni.2010.05.007; PMID: 20510870 [DOI] [PubMed] [Google Scholar]
- 58. Ben-Neriah Y, Karin M. . Inflammation meets cancer, with NF-κB as the matchmaker. Nat Immunol 2011; 12:715 - 23; http://dx.doi.org/ 10.1038/ni.2060; PMID: 21772280 [DOI] [PubMed] [Google Scholar]
- 59. Grivennikov SI, Greten FR, Karin M. . Immunity, inflammation, and cancer. Cell 2010; 140:883 - 99; http://dx.doi.org/ 10.1016/j.cell.2010.01.025; PMID: 20303878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Biswas SK, Lewis CE. . NF-κB as a central regulator of macrophage function in tumors. J Leukoc Biol 2010; 88:877 - 84; http://dx.doi.org/ 10.1189/jlb.0310153; PMID: 20573802 [DOI] [PubMed] [Google Scholar]
- 61. Mancino A, Lawrence T. . Nuclear factor-kappaB and tumor-associated macrophages. Clin Cancer Res 2010; 16:784 - 9; http://dx.doi.org/ 10.1158/1078-0432.CCR-09-1015; PMID: 20103670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Chang CP, Su YC, Lee PH, Lei HY. . Targeting NFKB by autophagy to polarize hepatoma-associated macrophage differentiation. Autophagy 2013; 9:619 - 21; http://dx.doi.org/ 10.4161/auto.23546; PMID: 23360732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Chang CP, Su YC, Hu CW, Lei HY. . TLR2-dependent selective autophagy regulates NF-κB lysosomal degradation in hepatoma-derived M2 macrophage differentiation. Cell Death Differ 2013; 20:515 - 23; http://dx.doi.org/ 10.1038/cdd.2012.146; PMID: 23175187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Alers S, Löffler AS, Wesselborg S, Stork B. . Role of AMPK-mTOR-Ulk1/2 in the regulation of autophagy: cross talk, shortcuts, and feedbacks. Mol Cell Biol 2012; 32:2 - 11; http://dx.doi.org/ 10.1128/MCB.06159-11; PMID: 22025673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Jung CH, Ro SH, Cao J, Otto NM, Kim DH. . mTOR regulation of autophagy. FEBS Lett 2010; 584:1287 - 95; http://dx.doi.org/ 10.1016/j.febslet.2010.01.017; PMID: 20083114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Chen W, Ma T, Shen XN, Xia XF, Xu GD, Bai XL, Liang TB. . Macrophage-induced tumor angiogenesis is regulated by the TSC2-mTOR pathway. Cancer Res 2012; 72:1363 - 72; http://dx.doi.org/ 10.1158/0008-5472.CAN-11-2684; PMID: 22287548 [DOI] [PubMed] [Google Scholar]
- 67. Chen TA, Wang JL, Hung SW, Chu CL, Cheng YC, Liang SM. . Recombinant VP1, an Akt inhibitor, suppresses progression of hepatocellular carcinoma by inducing apoptosis and modulation of CCL2 production. PLoS One 2011; 6:e23317; http://dx.doi.org/ 10.1371/journal.pone.0023317; PMID: 21826248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zhang W, Sun HC, Wang WQ, Zhang QB, Zhuang PY, Xiong YQ, Zhu XD, Xu HX, Kong LQ, Wu WZ, et al. . Sorafenib down-regulates expression of HTATIP2 to promote invasiveness and metastasis of orthotopic hepatocellular carcinoma tumors in mice. Gastroenterology 2012; 143:1641 - 9, e5; http://dx.doi.org/ 10.1053/j.gastro.2012.08.032; PMID: 22922424 [DOI] [PubMed] [Google Scholar]
- 69. Ebos JM, Lee CR, Cruz-Munoz W, Bjarnason GA, Christensen JG, Kerbel RS. . Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell 2009; 15:232 - 9; http://dx.doi.org/ 10.1016/j.ccr.2009.01.021; PMID: 19249681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Pàez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Viñals F, Inoue M, Bergers G, Hanahan D, Casanovas O. . Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell 2009; 15:220 - 31; http://dx.doi.org/ 10.1016/j.ccr.2009.01.027; PMID: 19249680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zhang W, Zhu XD, Sun HC, Xiong YQ, Zhuang PY, Xu HX, Kong LQ, Wang L, Wu WZ, Tang ZY. . Depletion of tumor-associated macrophages enhances the effect of sorafenib in metastatic liver cancer models by antimetastatic and antiangiogenic effects. Clin Cancer Res 2010; 16:3420 - 30; http://dx.doi.org/ 10.1158/1078-0432.CCR-09-2904; PMID: 20570927 [DOI] [PubMed] [Google Scholar]
- 72. Lin JC, Liu CL, Lee JJ, Liu TP, Ko WC, Huang YC, Wu CH, Chen YJ. . Sorafenib induces autophagy and suppresses activation of human macrophage. Int Immunopharmacol 2013; 15:333 - 9; http://dx.doi.org/ 10.1016/j.intimp.2013.01.006; PMID: 23337882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Bortoluci KR, Medzhitov R. . Control of infection by pyroptosis and autophagy: role of TLR and NLR. Cell Mol Life Sci 2010; 67:1643 - 51; http://dx.doi.org/ 10.1007/s00018-010-0335-5; PMID: 20229126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Lin H, Yan J, Wang Z, Hua F, Yu J, Sun W, Li K, Liu H, Yang H, Lv Q, et al. . Loss of immunity-supported senescence enhances susceptibility to hepatocellular carcinogenesis and progression in Toll-like receptor 2-deficient mice. Hepatology 2013; 57:171 - 82; http://dx.doi.org/ 10.1002/hep.25991; PMID: 22859216 [DOI] [PubMed] [Google Scholar]
- 75. Chen P, Huang Y, Bong R, Ding Y, Song N, Wang X, Song X, Luo Y. . Tumor-associated macrophages promote angiogenesis and melanoma growth via adrenomedullin in a paracrine and autocrine manner. Clin Cancer Res 2011; 17:7230 - 9; http://dx.doi.org/ 10.1158/1078-0432.CCR-11-1354; PMID: 21994414 [DOI] [PubMed] [Google Scholar]
- 76. Chen P, Cescon M, Bonaldo P. . Collagen VI in cancer and its biological mechanisms. Trends Mol Med 2013; 19:410 - 7; http://dx.doi.org/ 10.1016/j.molmed.2013.04.001; PMID: 23639582 [DOI] [PubMed] [Google Scholar]
- 77. Rolny C, Mazzone M, Tugues S, Laoui D, Johansson I, Coulon C, Squadrito ML, Segura I, Li X, Knevels E, et al. . HRG inhibits tumor growth and metastasis by inducing macrophage polarization and vessel normalization through downregulation of PlGF. Cancer Cell 2011; 19:31 - 44; http://dx.doi.org/ 10.1016/j.ccr.2010.11.009; PMID: 21215706 [DOI] [PubMed] [Google Scholar]
- 78. Sandoval H, Thiagarajan P, Dasgupta SK, Schumacher A, Prchal JT, Chen M, Wang J. . Essential role for Nix in autophagic maturation of erythroid cells. Nature 2008; 454:232 - 5; http://dx.doi.org/ 10.1038/nature07006; PMID: 18454133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Zhang J, Randall MS, Loyd MR, Dorsey FC, Kundu M, Cleveland JL, Ney PA. . Mitochondrial clearance is regulated by Atg7-dependent and -independent mechanisms during reticulocyte maturation. Blood 2009; 114:157 - 64; PMID: 19417210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Mortensen M, Ferguson DJ, Edelmann M, Kessler B, Morten KJ, Komatsu M, Simon AK. . Loss of autophagy in erythroid cells leads to defective removal of mitochondria and severe anemia in vivo. Proc Natl Acad Sci U S A 2010; 107:832 - 7; http://dx.doi.org/ 10.1073/pnas.0913170107; PMID: 20080761 [DOI] [PMC free article] [PubMed] [Google Scholar]