Abstract
Most current gene transfer methods function satisfactorily in specialized systems involving established cell lines but are often not applicable with nonadherent, primary hematopoietic cells, which are notoriously difficult to transfect. To approach this problem, we have investigated an alternative method of gene transfer, "transferrinfection," in which DNA complexed to transferrin-polycation conjugates is introduced into cells by receptor-mediated endocytosis [Wagner, E., Zenke, M., Cotten, M., Beug, H. & Birnstiel, M. L. (1990) Proc. Natl. Acad. Sci. USA 87, 3410-3414]. We show here that transferrin-polylysine and transferrin-protamine, when complexed to plasmid DNA containing a luciferase reporter gene, is efficiently bound and moved into avian erythroblasts by endocytosis. Successful transfer and expression of the luciferase reporter gene depends on specific interaction of the transferrin-polylysine-DNA complex with the transferrin receptor and occurs in a significant fraction (greater than 95%) of the cells. Gene transfer efficiency by transferrinfection is lower than with an optimized DEAE-dextran transfection method but reaches similar efficiencies when the cells are treated with chloroquine. Because the procedure in the absence of chloroquine is completely nontoxic to cells, a constant expression level of transferred genes may be maintained by repeated additions of transferrin-polylysine-DNA complex. In addition, the usefulness of transferrinfection for gene transfer into primary hematopoietic cells is demonstrated.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adkins B., Leutz A., Graf T. Autocrine growth induced by src-related oncogenes in transformed chicken myeloid cells. Cell. 1984 Dec;39(3 Pt 2):439–445. doi: 10.1016/0092-8674(84)90451-3. [DOI] [PubMed] [Google Scholar]
- Ascoli M., Puett D. Tritium labeling of luteinizing hormone by reductive methylation. Biochim Biophys Acta. 1974 Nov 5;371(1):203–210. doi: 10.1016/0005-2795(74)90169-x. [DOI] [PubMed] [Google Scholar]
- Beug H., Doederlein G., Freudenstein C., Graf T. Erythroblast cell lines transformed by a temperature-sensitive mutant of avian erythroblastosis virus: a model system to study erythroid differentiation in vitro. J Cell Physiol Suppl. 1982;1:195–207. doi: 10.1002/jcp.1041130427. [DOI] [PubMed] [Google Scholar]
- Choi O. R., Engel J. D. Developmental regulation of beta-globin gene switching. Cell. 1988 Oct 7;55(1):17–26. doi: 10.1016/0092-8674(88)90005-0. [DOI] [PubMed] [Google Scholar]
- Dean R. T., Jessup W., Roberts C. R. Effects of exogenous amines on mammalian cells, with particular reference to membrane flow. Biochem J. 1984 Jan 1;217(1):27–40. doi: 10.1042/bj2170027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felgner P. L., Gadek T. R., Holm M., Roman R., Chan H. W., Wenz M., Northrop J. P., Ringold G. M., Danielsen M. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf T. Two types of target cells for transformation with avian myelocytomatosis virus. Virology. 1973 Aug;54(2):398–413. doi: 10.1016/0042-6822(73)90152-9. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Huebers H. A., Finch C. A. The physiology of transferrin and transferrin receptors. Physiol Rev. 1987 Apr;67(2):520–582. doi: 10.1152/physrev.1987.67.2.520. [DOI] [PubMed] [Google Scholar]
- Joyner A. L., Skarnes W. C., Rossant J. Production of a mutation in mouse En-2 gene by homologous recombination in embryonic stem cells. Nature. 1989 Mar 9;338(6211):153–156. doi: 10.1038/338153a0. [DOI] [PubMed] [Google Scholar]
- Khazaie K., Dull T. J., Graf T., Schlessinger J., Ullrich A., Beug H., Vennström B. Truncation of the human EGF receptor leads to differential transforming potentials in primary avian fibroblasts and erythroblasts. EMBO J. 1988 Oct;7(10):3061–3071. doi: 10.1002/j.1460-2075.1988.tb03171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolau C., Legrand A., Grosse E. Liposomes as carriers for in vivo gene transfer and expression. Methods Enzymol. 1987;149:157–176. doi: 10.1016/0076-6879(87)49054-x. [DOI] [PubMed] [Google Scholar]
- Nolan G. P., Fiering S., Nicolas J. F., Herzenberg L. A. Fluorescence-activated cell analysis and sorting of viable mammalian cells based on beta-D-galactosidase activity after transduction of Escherichia coli lacZ. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2603–2607. doi: 10.1073/pnas.85.8.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radke K., Beug H., Kornfeld S., Graf T. Transformation of both erythroid and myeloid cells by E26, an avian leukemia virus that contains the myb gene. Cell. 1982 Dec;31(3 Pt 2):643–653. doi: 10.1016/0092-8674(82)90320-8. [DOI] [PubMed] [Google Scholar]
- Royer-Pokora B., Beug H., Claviez M., Winkhardt H. J., Friis R. R., Graf T. Transformation parameters in chicken fibroblasts transformed by AEV and MC29 avian leukemia viruses. Cell. 1978 Apr;13(4):751–760. doi: 10.1016/0092-8674(78)90225-8. [DOI] [PubMed] [Google Scholar]
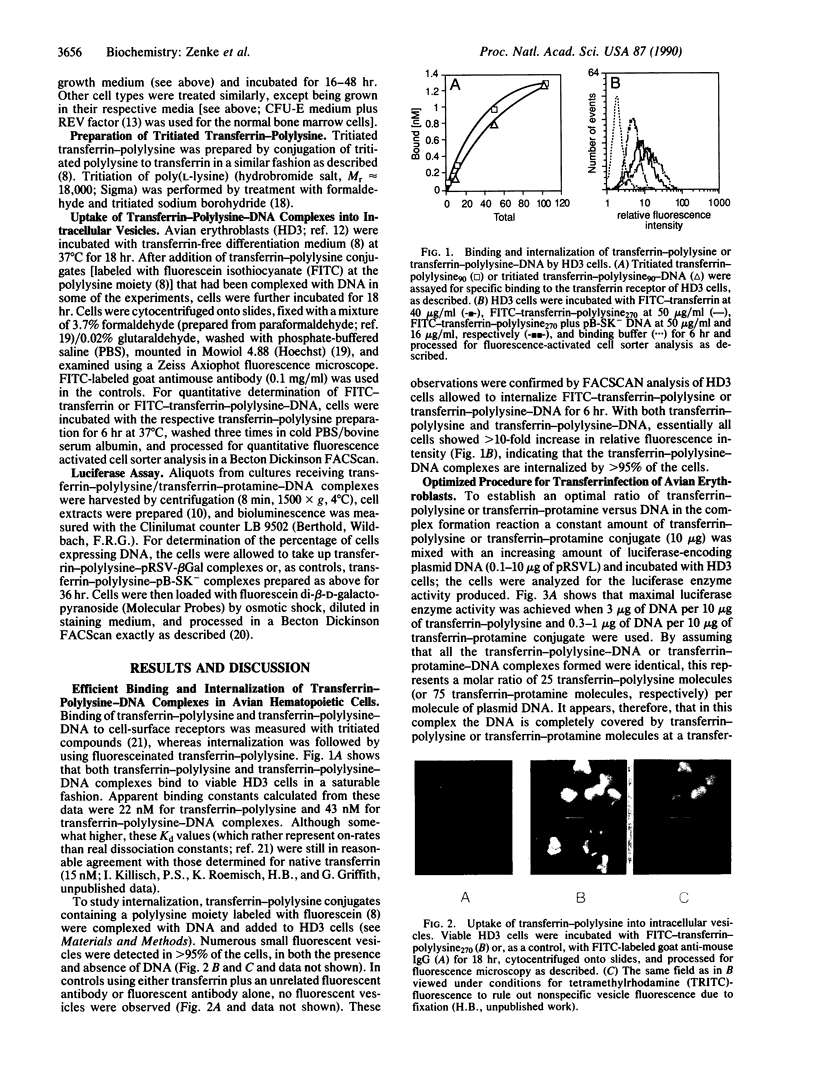
- Schaffner W. Direct transfer of cloned genes from bacteria to mammalian cells. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2163–2167. doi: 10.1073/pnas.77.4.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt J. A., Marshall J., Hayman M. J., Doderlein G., Beug H. Monoclonal antibodies to novel erythroid differentiation antigens reveal specific effects of oncogenes on the leukaemic cell phenotype. Leuk Res. 1986;10(3):257–272. doi: 10.1016/0145-2126(86)90023-8. [DOI] [PubMed] [Google Scholar]
- Stein B. S., Bensch K. G., Sussman H. H. Complete inhibition of transferrin recycling by monensin in K562 cells. J Biol Chem. 1984 Dec 10;259(23):14762–14772. [PubMed] [Google Scholar]
- Wagner E., Zenke M., Cotten M., Beug H., Birnstiel M. L. Transferrin-polycation conjugates as carriers for DNA uptake into cells. Proc Natl Acad Sci U S A. 1990 May;87(9):3410–3414. doi: 10.1073/pnas.87.9.3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G. Y., Wu C. H. Receptor-mediated in vitro gene transformation by a soluble DNA carrier system. J Biol Chem. 1987 Apr 5;262(10):4429–4432. [PubMed] [Google Scholar]
- Zenke M., Kahn P., Disela C., Vennström B., Leutz A., Keegan K., Hayman M. J., Choi H. R., Yew N., Engel J. D. v-erbA specifically suppresses transcription of the avian erythrocyte anion transporter (band 3) gene. Cell. 1988 Jan 15;52(1):107–119. doi: 10.1016/0092-8674(88)90535-1. [DOI] [PubMed] [Google Scholar]
- de Wet J. R., Wood K. V., DeLuca M., Helinski D. R., Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987 Feb;7(2):725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]