Summary
Aims
Non‐cystic fibrosis bronchiectasis (NCFB) is a chronic, progressive respiratory disorder characterised by irreversibly and abnormally dilated airways, persistent cough, excessive sputum production and recurrent pulmonary infections. In the last several decades, its prevalence has increased, making it likely to be encountered in the primary care setting. The aim was to review the clinical presentation and diagnosis of NCFB, with an emphasis on the role of computed tomography (CT).
Methods
For this review, trials and reports were identified from PubMed/Medline and ClinicalTrials.gov from the US NIH and the Cochrane Register of Controlled Trials. The search used keywords: bronchiectasis, non‐cystic fibrosis bronchiectasis, chronic pulmonary infection and computed tomography. No date/language restrictions were used.
Results
Non‐cystic fibrosis bronchiectasis often coexists with other respiratory conditions, such as chronic obstructive pulmonary disease. The prevalence of NCFB is increasing, particularly in women and older individuals, possibly as a result of increased physician awareness and widespread use of CT, which is the gold standard for the diagnosis of NCFB. CT can assist in identifying an underlying cause of NCFB and determining the extent and severity of the disease.
Discussion
Non‐cystic fibrosis bronchiectasis should be suspected in the primary care setting in patients with chronic cough, purulent sputum and frequent respiratory infections that tend to resolve slowly or partially. Early diagnosis and determination of the extent and severity of the disease by CT and other tests are critical to establish therapy to improve quality of life and potentially slow progressive decline of lung function in patients with NCFB .
Review criteria
This non‐systematic review identified recent clinical and epidemiological studies identified from PubMed/Medline and ClinicalTrials.gov from the US NIH and the Cochrane Register of Controlled Trials using the search terms bronchiectasis, non‐cystic fibrosis bronchiectasis, chronic pulmonary infection and computed tomography. Case reports, editorials and other communications with a low evidence level were excluded.
Message for the clinic
The prevalence of non‐cystic fibrosis bronchiectasis (NCFB) has increased over the past decades possibly due to increased awareness and widespread use of computed tomography (CT). NCFB should be suspected in patients with persistent cough, excessive sputum production and recurrent pulmonary infections. CT confirms the diagnosis with findings characterised by abnormally dilated airways, and is useful in determining the extent and severity of the disease and detecting an underlying cause.
1. Introduction
Bronchiectasis is a chronic, progressive respiratory disorder characterised by irreversibly and abnormally dilated airways, persistent cough, excessive sputum production and recurrent pulmonary infections.1 The changes in the bronchial walls may be due to chronic inflammation secondary to recurrent or chronic infections in the lung, but often the exact cause is not identified.1 Symptoms vary from intermittent episodes of respiratory infections with excessive mucus production to chronic symptoms with persistent daily expectoration of purulent sputum.1 In patients without cystic fibrosis, the condition is known as non‐cystic fibrosis bronchiectasis (NCFB).1, 2, 3 Although NCFB was once a very uncommon diagnosis, in the last two decades its prevalence has been increasing, making it more likely that primary care clinicians will encounter these patients in their practice. The precise prevalence of NCFB is difficult to determine because estimates vary among populations, but studies report a prevalence ranging from 486 to 1106 per 100 000 persons with an incidence that appears to be rising, particularly in women and older individuals.4, 5, 6 Although not as common as asthma, which affects approximately 10% of the world population, NCFB has become far more common than other respiratory conditions such as idiopathic pulmonary fibrosis, which affects only 18.2 per 100 000 persons.7, 8 Hospitalisation rates owing to NCFB have also increased in the United States, particularly in the older population.9
Non‐cystic fibrosis bronchiectasis is associated with comorbidities such as anxiety, depression, fatigue, and has a significant impact on quality of life.10 Patients with NCFB require longer hospital stays and more outpatient visits compared with matched controls.4 Cough associated with NCFB can also impact the quality of life of family members.10 The effect of NCFB on mortality is not clear. Although reported mortality rates vary, evidence suggests that age‐adjusted mortality rates in patients with NCFB are at least twice that of the general population.5
The increased recognition of NCFB is likely a result of the use of high‐resolution computed tomography (HRCT), which has allowed for the identification of previously undiagnosed disease.11, 12, 13 HRCT, which is able to acquire 1‐mm thick images of the lung at 10‐mm intervals, is considered the gold standard for radiographic diagnosis of NCFB.1, 2 Other modalities of CT using different imaging algorithms have been frequently used to effectively diagnose bronchiectasis.12, 14 With enhanced diagnostic capabilities of CT for NCFB, it is important for primary care clinicians to have an understanding of the information that can be obtained with this procedure. Herein, we review key radiographic findings on CT in the context of an overview of the pathophysiology, clinical presentation, and the diagnosis of NCFB.
2. Methods
For this review, trials and reports were identified from the databases of PubMed/Medline and ClinicalTrials.gov from the US NIH and the Cochrane Register of Controlled Trials. The search was carried out using the keywords or combinations of keywords: bronchiectasis, non‐cystic fibrosis bronchiectasis, chronic pulmonary infection and computed tomography. The term chronic pulmonary infection was included to capture the articles that discussed this condition in association with CT as a possible precursor of the development of NCFB. A particular emphasis was made on publications that discussed the epidemiology, natural history, clinical presentation and diagnosis. In addition, there was a focus on the available studies and publications that examined the role of CT in the diagnosis of bronchiectasis and determination of severity. No date or language restrictions were used.
3. Pathophysiology of NCFB
Cole's ‘vicious cycle’ of infection, airway inflammation and lung damage15 is the most widely accepted mechanism of the development of NCFB (Figure 1). The cycle is triggered when the defence system of the lung is breached and mucociliary clearance is impaired. Potential causes of this breach include severe lower respiratory tract infections, gastric aspiration and/or inhalation of toxic gases. Other inflammatory processes may be associated with local and systemic inflammation that leads to changes in the architecture of bronchial airways. However, a clear trigger is not identified in up to 30%‐53% of patients.1 Regardless of the cause, when mucociliary clearance is impaired, mucous is retained in the airways.15 This in turn leads to microbial colonisation or infection with a subsequent development of an inflammatory response. As the host fails to eliminate the persistent infection, airway inflammation becomes chronic. Both host inflammatory responses and microbial cytotoxins cause additional structural damage to the lung and further impair mucus clearance, thus, the vicious cycle persists.15 Some microorganisms, such as Pseudomonas aeruginosa, may form biofilms in the bronchial airways. Biofilms are thin layers that form on colonised surfaces typically comprised of bacteria and a matrix of an extracellular polymeric substance that includes polysaccharides, proteins and DNA.16 This may facilitate the persistence of the “vicious cycle” of bronchiectasis because the biofilms protect bacteria from clearance by the host immune system and reduce the effects of antibiotics further potentiating airway inflammation.17 Disruption of the biofilms by some antibiotics (ie, macrolides) is thought to be one of the mechanisms by which chronic antibiotic therapy may improve outcomes in bronchiectasis.18, 19
Figure 1.
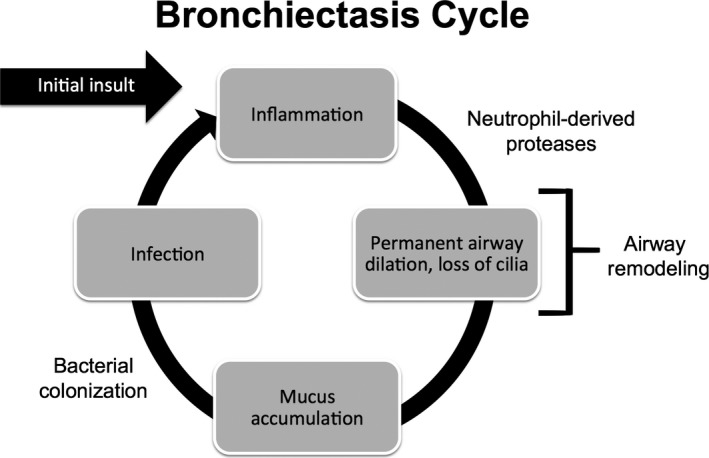
Representation of the cycle that leads to development of bronchiectasis, as described by Cole13
3.1. Conditions Associated with the Development of NCFB
The various aetiologies of NCFB can be conceptually divided into infectious and non‐infectious causes. Previous severe infections from bacteria, mycobacteria and viruses have been shown to cause parenchymal changes consistent with NCFB. Non‐infectious causes include various types of immune deficiencies, conditions with defects in mucociliary clearance, bronchial obstruction, autoimmune diseases, congenital disorders, postradiation treatment, postlung transplant, interstitial lung diseases, and graft‐versus‐host disease. Table 1 provides a comprehensive list of aetiologies.1, 20
Table 1.
| Categories | Examples |
|---|---|
| Infections |
|
| Immune deficiency |
|
| Mucociliary clearance defects |
|
| Bronchial obstruction |
|
| Autoimmune disease |
|
| Congenital disorders |
|
| Other |
|
COPD, chronic obstructive pulmonary disease; IgG, immunoglobulin G; MAC, mycobacterium avium complex; PCD, primary ciliary dyskinesia.
3.2. The Association Between COPD and Bronchiectasis
Chronic obstructive pulmonary disease (COPD) remains one of the most common pulmonary conditions worldwide, and patients with COPD are often seen in the primary care setting.21 Bronchiectasis and COPD often coexist with a varying prevalence amongst study populations (29%‐57%).22, 23, 24, 25 Both diseases share several pathophysiological features including increased airway inflammation, propensity for exacerbations, distortion of the architecture of the lung parenchyma and bacterial colonisation of the airways.
The presence of bronchiectasis identified on CT in patients with COPD has been associated with poor outcomes. A study by Martínez‐García demonstrated worse airflow limitation, increased isolation of potential pathogens, and more frequent hospital admissions in patients with COPD and bronchiectasis compared with patients with COPD alone.24 A follow‐up study from the same group showed that patients with bronchiectasis and COPD had an increased risk of all‐cause mortality.26 A meta‐analysis of 881 patients, including the above studies in addition to four others, revealed that the coexistence of COPD and bronchiectasis was associated with higher exacerbation rates, degree of inflammatory markers, and rates of Pseudomonas aeruginosa isolation.27 For these reasons, bronchiectasis is now considered a common comorbidity in patients with COPD.21 However, it is incompletely understood how these diseases exactly influence each other. The leading theory is that increased colonisation of the airways leads to increased inflammation, which in turn translates into increased risk for exacerbations.28 Future studies are needed to understand this complex association and to evaluate if identification of these patients early in their course may change outcomes.
4. Clinical Presentation
Non‐cystic fibrosis bronchiectasis presents with a range of clinical manifestations from incidentally found bronchiectasis in asymptomatic individuals to massive haemoptysis and respiratory failure. With the widespread use of CT, clinicians increasingly encounter the ‘incidental’ finding of bronchiectasis in asymptomatic patients who undergo chest or abdominal imaging for unrelated purposes. However, the most common clinical presentation is between these extremes.
For those patients with symptomatic bronchiectasis, the most common complaint is chronic cough. In fact, many patients have a chronic productive cough for over 30 years before their diagnosis. In one study of 103 adults with NCFB, 98% reported a chronic cough, while daily sputum production (typically purulent and averaging greater than 30 mL in volume) occurred in 76% of patients.29 Dyspnoea (62%) and chronic fatigue (74%) are also common presenting symptoms.29, 30 Other common characteristics in these patients include chronic rhinosinusitis that ranges from intermittent nasal discharge to severe purulent sinusitis (present in 70% of patients) and a notable history of ear, nose and throat surgery for recurrent sinusitis (30% of patients).29 Mild, intermittent haemoptysis occurs in 26%‐51% of cases, but at times it can be severe and life‐threatening.29, 31 Many patients with bronchiectasis require frequent hospital admissions for recurrent pneumonia.29, 31 Depression and anxiety are common in patients with bronchiectasis, which may significantly affect their quality of life.32, 33
The natural history of NCFB is punctuated by periods of exacerbations, defined as acute worsening of respiratory symptoms that require management with antibiotic therapy.34 Patients with exacerbations experience change in one or more of the common symptoms of bronchiectasis, including increased sputum production or purulence, worsening cough and dyspnoea, and systemic symptoms.1 Usually, these episodes are triggered by a bronchial infection. In a study of 115 patients with NCFB, the most common signs of an acute exacerbation were increased frequency of cough (88%) and change in sputum characteristics (67%).35 Other symptoms included fever (28%), increase in sputum volume (42%) and increase in sputum purulence (35%).35 Exacerbations can range from a mild increase in respiratory symptoms to life‐threatening haemoptysis and respiratory failure.1
When should bronchiectasis be suspected in the primary care setting? The diagnosis should be suspected in patients with chronic cough (ie, >6 weeks) with variable production of purulent sputum, with or without recurrent haemoptysis, and frequent respiratory infections that tend to resolve slowly or partially with or without an underlying condition such as COPD. In some instances, bronchiectasis may be suggested on chest radiographs. This constellation of signs and symptoms should prompt the primary care provider to consider evaluating the patient for bronchiectasis.
5. Diagnostic Approach
5.1. Initial investigations
Physical exam and history may contribute to the diagnosis of bronchiectasis. The most common respiratory physical exam findings are crackles, occurring in up to 73% of patients.29, 31 Other common findings include rhonchi (44%) and wheezing (21%‐34%).29, 31 Clubbing of the digits is rare and occurs in 2%‐3% of cases.29, 31
Standard chest radiograph (CXR) is typically the first tool used to evaluate a patient with chronic respiratory symptoms. However, bronchiectasis may not be obvious with this imaging modality and is usually only apparent in severe cases.36, 37 Studies show that CXRs have moderate sensitivity (88%) and somewhat poor specificity (74%) for the detection of bronchiectasis,36, 37 and therefore is considered inadequate for the diagnosis/quantification of bronchiectasis.1 Despite this, CXRs may have some utility in NCFB. On close inspection, multiple areas of bronchial wall dilation may be seen in patients with moderate to severe bronchiectasis. Furthermore, other radiographic signs of chronic lower airway infection may be present in patients with NCFB such as calcifications or infiltrates.
5.2. Computed tomography
High‐resolution computed tomography of the chest is the current gold standard for the diagnosis/confirmation of bronchiectasis. It has a sensitivity of 96% and a specificity of 93%.13 Nevertheless, HRCT has some limitations, including respiratory and cardiac pulsation artefacts, false‐negative reports due to missed focal areas of bronchiectasis between section‐planes, and misinterpretation of bronchial‐artery ratio measurements.11 These limitations have been partially addressed using alternative CT modalities using different imaging algorithms. For example, a retrospective study evaluated the efficacy of HRCTs with 1‐mm slices every 10 mm, compared with multi‐detector CTs (MDCT) with contiguous 1‐mm slices for the diagnosis of bronchiectasis.12 MDCT was found to be superior in assessing the presence, extent and severity of bronchiectasis compared with HRCT.12 These findings were further supported by a prospective study, showing that MDCT indeed had greater accuracy for the identification and exclusion of bronchiectasis compared with HRCT.14 Although the utility of MDCT appears to be equivalent or even superior to HRCT, the radiation dose is up to five times higher with MDCT.38 Because of this concern, the use of low‐dose volumetric CT has been explored for the diagnosis of bronchiectasis. One such study by Jung and colleagues demonstrated no significant difference in the diagnostic yield of bronchiectasis, showing similar image quality and radiation exposures compared with HRCT.39
Despite these advances, regular volumetric CT is sufficient to make the diagnosis of NCFB. This imaging modality offers the same degree of accuracy for NCFB and may be more widely available.40 As imaging technology progresses, it is likely that newer tools will be developed to better assess the presence and severity of bronchiectasis. The development of automated image analysis is one exciting innovation that has the potential to advance this diagnostic area, making it easier to identify milder forms of the disease and to improve the reliability of the diagnosis.41, 42 By diagnosing patients with bronchiectasis earlier in their course, it would allow for earlier therapeutic intervention, with the potential to improve outcomes and alter the course of the disease.
The characteristic feature of NCFB demonstrated by CT is bronchial wall dilation with the luminal diameter greater than 1‐1.5 the size of the accompanying pulmonary artery branch. Other typical findings include absence of normal tapering of the bronchi, bronchial wall thickening, mucoid plugging of the airways and proximity of visible airways close to the pleura (<1 cm).1, 43
Bronchiectasis is classified by morphology. In cylindrical bronchiectasis, the bronchial walls are dilated, but maintain their cylindrical shape extending to the periphery of the lung (Figure 2A). The varicose type of bronchiectasis is characterised by airway dilation interspersed with areas of relative narrowing, resulting in a beaded appearance (Figure 2B). Cystic bronchiectasis represents the most advanced stage of airway remodelling, with the dilated airways appearing similar to cysts or analogous to grapes along a branch (Figure 2C).
Figure 2.
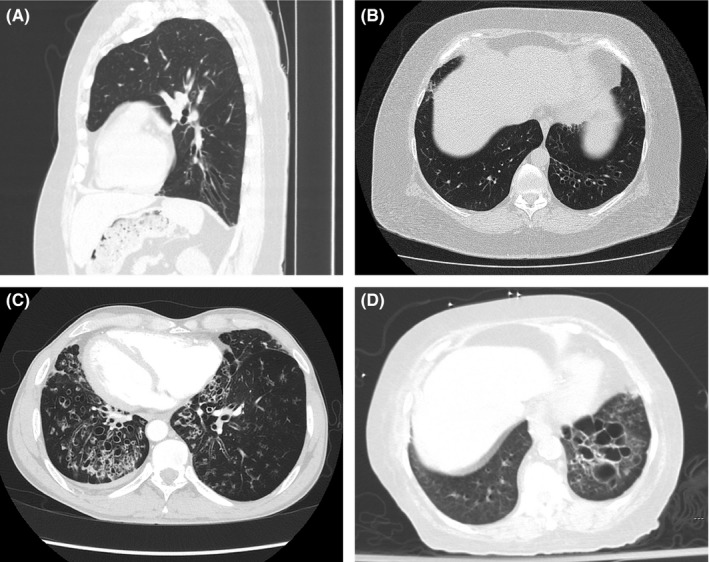
Images from chest CT illustrating bronchiectasis classification based on morphology: A and B, cylindrical bronchiectasis in a 63 year‐old man with rheumatoid arthritis; C, varicose bronchiectasis in a 49 year‐old man with Kartagener's syndrome (ciliary dyskinesia, chronic sinusitis and situs inversus); and D, cystic bronchiectasis in a 77 year‐old woman with a history of a left lower lobe pneumonia
The differential diagnosis is often narrowed based on imaging characteristics. For example, if a localised area of bronchiectasis is confirmed on CT, then referral to a pulmonologist for a bronchoscopy is required to evaluate for a foreign body, an endobronchial lesion, or an area of extrinsic compression that could potentially explain the focal finding.1 A localised area of bronchiectasis is rarely caused by a systemic/diffuse condition. If the area of bronchiectasis is diffuse on CT imaging, the differential diagnosis is broader.
Features or abnormalities demonstrated by CT may provide clues to the underlying cause of bronchiectasis.20, 44 In allergic bronchopulmonary aspergillosis, mucoid impaction of the large airways appears as tubular opacities referred to as the “finger‐in‐glove” sign. Similar to allergic bronchopulmonary aspergillosis, patients with CF have upper‐lobe predominant disease and may have the “finger‐in‐glove” sign. In patients with a foreign body, tumour or extrinsic compression, the bronchiectasis is evident in a focal area as opposed to more diffuse disease in other systemic conditions/aetiologies. Certain infections also may have a characteristic presentation. For example, atypical mycobacteria may favour the right middle lobe and the lingula.45 Despite identification of these key features on CT and extensive testing, no underlying aetiology is found in a large proportion of patients with bronchiectasis.46 The prevalence of idiopathic bronchiectasis varies amongst cohorts (32%‐66%) likely owing to geographic variations, diagnostic algorithms and other determinants.30, 47, 48, 49, 50, 51
Clinical symptoms, history of exacerbations and microbiological data are frequently used to stratify the severity of patients with NCFB; however, the incorporation of imaging abnormalities into these classifications may allow for a more complete picture of the impact of the disease.52, 53 Therefore, there is great interest in the evaluation of the extent and severity of bronchiectasis more objectively using CT.
A method to quantify the extent of the disease using CT was developed several decades ago.54 In this method, each lobe is scored by: (i) the number of segments involved, (ii) severity of bronchial dilation compared with the adjacent artery, (iii) bronchial wall thickening and (iv) morphological type of bronchiectasis.
While imaging may be useful in the objective determination of the extent of the disease, it may have limited value with regard to the prognosis of NCFB. Two comprehensive bronchiectasis indices have been created to address this limitation in hopes of better understanding the prognosis of patients with NCFB and improving treatment algorithms.55, 56 Both the Bronchiectasis Severity Index (BSI) and the FACED scores are effective tools that utilise multiple parameters, including extent of the disease based on CT, to predict future outcomes in patients with bronchiectasis.
5.3. Pulmonary function testing
Spirometry is frequently used to evaluate patients with respiratory symptoms. In bronchiectasis, the majority of patients present with mild to moderate airway obstruction, with a forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) ratio less than 70, along with an FEV1 >50% predicted.29, 31 Significant bronchodilator response (response of >200 mL and 12% predicted in FEV1 and FVC after administration of β2 agonists 57) occurs in only 22% of patients with NCFB.29 Interestingly, patients with a significant bronchodilator response tend to have more severe obstruction and poorer lung function as recently reported by Guan and colleagues.57 More severe obstruction (ie, FEV1<50% predicted) is also associated with a history of pseudomonas infection or colonisation, multi‐lobar involvement of disease, greater sputum volume, greater purulent sputum and those patients with at least four exacerbations over a 2‐year period.58 It is important to note that spirometry may not significantly differ between the stable and exacerbation states.35 Although one study showed a statistically significant decline of both FEV1 and FVC by 3% (P≤.01) between the stable and exacerbation states, this is unlikely to be clinically significant.59 Spirometry should be performed at least annually in patients with bronchiectasis to detect progression of the disease.1 Pulmonary function testing may help detect and determine the severity of other underlying conditions such as interstitial lung disease, asthma and pulmonary hypertension.
5.4. Microbiology
Sputum cultures should be obtained in all patients with bronchiectasis. The most common organisms initially isolated from the sputum of patients with NCFB are Gram‐negative bacteria including Haemophilus influenzae (47%), P. aeruginosa (12%) and Moraxella catarrhalis (8%).60 Over time, this microbiological distribution shifts slightly, with Pseudomonas increasing in frequency. Six years after diagnosis, the most commonly isolated organisms continue to be H. influenzae (40%), P. aeruginosa (18%) and M. catarrhalis (7%).60 Gram‐positive bacteria are rare. Streptococcus pneumoniae is isolated in 4% of samples and Staphylococcus aureus is found in 3% of samples.60
Isolation of P. aeruginosa is particularly important clinically. Patients with Pseudomonas have more severe disease, more rapid decline in lung function, more frequent exacerbations and reduced quality of life.60, 61, 62, 63 Thus, these patients need to be followed more carefully and referred to a pulmonologist for consideration of more aggressive therapeutic options.61
Non‐tuberculous mycobacteria (NTM) constitute another group of important microorganisms in patients with NCFB and account for 2%‐30% of pathogens isolated.64, 65, 66, 67 In patients with bronchiectasis, the incidence of lung infection with NTM has increased over the past decades.68, 69, 70 The most common NTM isolated in NCFB is Mycobacaterium avium complex.65, 66, 67 The diagnosis of NTM is particularly challenging in patients with NCFB because of existing abnormalities that may or may not be caused by NTM. Unlike Mycobaterium tuberculosis, NTM are harboured in the soil and water, and infection is acquired from these sources rather than person‐to‐person transmission. For these reasons, NTM isolated in the sputum may represent “contamination” or “colonization” but not necessarily infection. Consequently, the diagnosis of an NTM infection requires a careful evaluation of the clinical signs and symptoms, culture positivity and imaging abnormalities.71 Advanced age, low body‐mass index, female gender and low frequency of chronic P. aeruginosa infection have been independently linked to the presence of NTM in patients with NCFB.65, 66 The reasons for these observations are incompletely understood. In the primary care setting, when NTM is encountered in the sputum, repeat cultures should be obtained to further aid in the determination of the significance of these findings and refer the patient to a center that has experience in the treatment of these infections.
Patients with NCFB can become chronically colonised with bacteria that may result in recurrent episodes of pneumonia or exacerbations of bronchiectasis in the susceptible host.1, 72, 73 Dilated airways cause pooling of secretions in dependent regions of the lung, creating an ongoing reservoir for bacterial growth and re‐infection. Exacerbations may be characterised by an increased bacterial burden of these same organisms quantitatively, and not necessarily infection with a new organism.1 This is supported by observations that suggest that the lung microbiome remains stable during an exacerbation.74
5.5. Laboratory investigations
Once bronchiectasis is suspected based on clinical presentation and confirmed by radiographic imaging, determining the underlying cause is a key to effective therapeutic management. Initial laboratory tests should always include sputum culture, gram stain and three acid‐fast bacterial stains with culture to evaluate and treat an acute infectious process that may be ongoing.1 Microbiologic testing should be repeated in the patient presenting with worsening symptoms. There is no consensus on how often microbiologic testing should be performed in the “stable” patient, but experts advocate testing the sputum for pathogenic bacteria from every visit to every 6 months.1 Additional testing should include: a complete blood count, a chemistry panel (CHEM 10), liver function tests, an HIV screen, immunoglobulin panel, an alpha‐1 antitrypsin level, aspergillus serologies, serum protein electrophoresis, an erythrocyte sedimentation rate/C‐reactive protein level and a comprehensive autoimmune panel.1 These tests are recommended even in asymptomatic patients given that identifying an underlying cause of bronchiectasis may help establish therapy earlier and prevent the progression of the disease. More specific tests are required for patients with chronic sinus infections, those under the age of 40, or patients with symptoms of reflux. A diagnostic algorithm is shown in Figure 3.1
Figure 3.

Algorithm outlining the diagnostic steps for determining the underlying cause of bronchiectasis in symptomatic adults. A1AT, α1‐antitrypsin; ABPA, allergic bronchopulmonary aspergillosis; AFB, acid‐fast bacilli; CBC, complete blood count; CCP, cyclic citrullinated peptide; CF, cystic fibrosis; CRP, C‐reactive protein; CVID, common variable immune deficiency; EGD, esophagogastroduodenoscopy; ESR, erythrocyte sedimentation rate; GERD, gastroesophageal reflux disease; GS, gram stain; IgG, immunoglobulin G; LFT, liver function test; NTM, nontuberculous mycobacteria; PCD, primary ciliary dyskinesia; RAST, radioallergosorbent test; RF, rheumatoid factor; SSA, Sjögren's syndrome A antibody; SSB, Sjogren's syndrome B antibody; UACS, Upper Airway Cough Syndrome
5.6. When should primary care consult a specialist?
Because bronchiectasis may be associated with progressive lung function decline and recurrent pulmonary infections, it is important for this disease to be recognised early so appropriate interventions can be instituted. The first step is to attempt to identify underlying causes of bronchiectasis (as discussed above and shown in Figure 3) and treat them when indicated. In the primary care setting, patients with bronchiectasis should be followed periodically for an assessment of their symptoms and lung function. A careful history of their respiratory symptoms, previous infections and imaging abnormalities should be documented. Patients with bronchiectasis should be referred to a respiratory specialist if they have haemoptysis, recurrent infections/exacerbations or a rapid decline in the exercise tolerance or pulmonary function testing.1, 10 Patients with bronchiectasis limited to one pulmonary lobe may require a referral to a pulmonologist for a bronchoscopy to evaluate for the presence of a local endobronchial obstruction or narrowing. A respiratory therapist should be consulted if the patient has production of copious secretions or evidence of mucus plugging on CT to establish airway clearance techniques (ACT).
After the underlying cause of bronchiectasis is treated (if identified), the goals of therapy should be directed towards disrupting the “vicious cycle”. ACT may prevent mucous retention in the airways and have been shown to improve sputum expectoration, pulmonary function and quality of life.75 ACT in clinical practice comprise of various manoeuvres such as drainage assisted by gravity, breathing exercises, directed coughing (“huff‐cough”), expiratory oscillating devices (ie, Acapella®, Aerobika®) and positive expiratory pressure devices. Patient preference, tolerability and severity of the disease should be considered when respiratory therapists select the most appropriate ACT for a patient with bronchiectasis. In the primary care setting, once a specific type of ACT has been selected, the patients should be questioned about the regular use of this therapy and encouraged to be as compliant as possible. Chronic use of antibiotics, both oral and inhaled, has been used as suppressive therapy in selected patients with additional improvements in exacerbation rates, symptoms scores and quality of life markers.76, 77 These therapies should be carefully tailored based on the extent of the disease, daily symptoms and culture results.
6. Conclusion
The incidence of NCFB has risen over the past decades. The reasons for this increase is multifactorial and may be related to improved physician awareness, improved testing and widespread availability of CT. Other, more complex, explanations such as the rise in the incidence of infections, antibiotic resistance and increased incidence of autoimmune conditions likely play a part in the increased rate in which NCFB is encountered in clinical practice. The conditions associated with the development of NCFB are varied and the diagnostic workup should target these when clinically indicated. In the primary care setting, it is important to consider the diagnosis of bronchiectasis in patients with chronic respiratory symptoms that cannot be explained by another diagnosis. CT of the chest is the gold standard for the diagnosis of NCFB. In addition, this tool provides a wealth of information that may be used to identify an underlying condition and determine the extent and severity of the disease. With early and improved diagnosis of NCFB, it is more likely that therapeutic regimens will be effective and prevent the progression of the disease.
Author Contributions
All authors participated in the interpretation of collected literature, and in the drafting, critical revision and approval of the final version of the manuscript.
Disclosures
The authors have nothing to disclose.
Supporting information
Acknowledgements
The authors thank Grifols for sponsoring editorial assistance, which was thoroughly provided by Susan Sutch, PharmD, CMPP, of Evidence Scientific Solutions, Philadelphia, PA, USA.
Maselli DJ, Amalakuhan B, Keyt H, Diaz AA. Suspecting non‐cystic fibrosis bronchiectasis: What the busy primary care clinician needs to know. Int J Clin Pract. 2017;71:e12924. https://doi.org/10.1111/ijcp.12924
References
- 1. Pasteur MC, Bilton D, Hill AT; on behalf of the British Thoracic Society Bronchiectasis (non‐CF) Guideline Group . British Thoracic Society guideline for non‐CF bronchiectasis. Thorax. 2010; 65 (Suppl. 1): i1–i58. [DOI] [PubMed] [Google Scholar]
- 2. Goeminne P, Dupont L. Non‐cystic fibrosis bronchiectasis: diagnosis and management in 21st century. Postgrad Med J. 2010;86:493–501. [DOI] [PubMed] [Google Scholar]
- 3. Altenburg J, Wortel K, van der Werf TS, Boersma WG. Non‐cystic fibrosis bronchiectasis: clinical presentation, diagnosis and treatment, illustrated by data from a Dutch Teaching Hospital. Neth J Med. 2015;73:147–154. [PubMed] [Google Scholar]
- 4. Weycker D, Edelsberg J, Oster G, Tino G. Prevalence and economic burden of bronchiectasis. Clin Pulm Med. 2005;12:205–209. [Google Scholar]
- 5. Quint JK, Millett ERC, Joshi M, et al. Changes in the incidence, prevalence and mortality of bronchiectasis in the UK from 2004 to 2013: a population‐based cohort study. Eur Respir J. 2016;47:186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Seitz AE, Olivier KN, Adjemian J, et al. Trends in bronchiectasis among Medicare beneficiaries in the United States, 2000 to 2007. Chest. 2012;142:432–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lai CK, Beasley R, Crane J, et al. Global variation in the prevalence and severity of asthma symptoms: phase three of the International Study of Asthma and Allergies in Childhood (ISAAC). Thorax. 2009;64:476–483. [DOI] [PubMed] [Google Scholar]
- 8. Raghu G, Chen SY, Hou Q, Yeh WS, Collard HR. Incidence and prevalence of idiopathic pulmonary fibrosis in US adults 18‐64 years old. Eur Respir J. 2016;48:179–186. [DOI] [PubMed] [Google Scholar]
- 9. Seitz AE, Olivier KN, Steiner CA, et al. Trends and burden of bronchiectasis‐associated hospitalizations in the United States, 1993‐2006. Chest. 2010;138:944–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hill AT, Pasteur M, Cornford C, et al. Primary care summary of the British Thoracic Society Guideline on the management of non‐cystic fibrosis bronchiectasis. Prim Care Respir J. 2011;20:135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kang EY, Miller RR, Müller NL. Bronchiectasis: comparison of preoperative thin‐section CT and pathologic findings in resected specimens. Radiology. 1995;195:649–654. [DOI] [PubMed] [Google Scholar]
- 12. Dodd JD, Souza CA, Müller NL. Conventional high‐resolution CT versus helical high‐resolution MDCT in the detection of bronchiectasis. AJR Am J Roentgenol. 2006;187:414–420. [DOI] [PubMed] [Google Scholar]
- 13. Grenier P, Maurice F, Musset D, et al. Bronchiectasis: assessment by thin‐section CT. Radiology. 1986;161:95–99. [DOI] [PubMed] [Google Scholar]
- 14. Hill LE, Ritchie G, Wightman AJ, et al. Comparison between conventional interrupted high‐resolution CT and volume multidetector CT acquisition in the assessment of bronchiectasis. Br J Radiol. 2010;83:67–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cole P. The damaging role of bacteria in chronic lung infection. J Antimicrob Chemother. 1997;40(Suppl. A):5–10. [DOI] [PubMed] [Google Scholar]
- 16. Hall‐Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol. 2004;2:95–108. [DOI] [PubMed] [Google Scholar]
- 17. Chalmers JD, Hill AT. Mechanisms of immune dysfunction and bacterial persistence in non‐cystic fibrosis bronchiectasis. Mol Immunol. 2013;55:27–34. [DOI] [PubMed] [Google Scholar]
- 18. Yanagihara K, Tomono K, Imamura Y, et al. Effect of clarithromycin on chronic respiratory infection caused by Pseudomonas aeruginosa with biofilm formation in an experimental murine model. J Antimicrob Chemother. 2002;49:867–870. [DOI] [PubMed] [Google Scholar]
- 19. Wozniak DJ, Keyser R. Effects of subinhibitory concentrations of macrolide antibiotics on Pseudomonas aeruginosa. Chest. 2004;125(2 Suppl):62S–69S. [DOI] [PubMed] [Google Scholar]
- 20. Barker AF. Bronchiectasis. N Engl J Med. 2002;346:1383–1393. [DOI] [PubMed] [Google Scholar]
- 21. Global Strategy for the Diagnosis, Management and Prevention of COPD . Global Initiative for Chronic Obstructive Lung Disease (GOLD), 2016. http://www.goldcopd.org/. Accessed March 21, 2016.
- 22. O'Brien C, Guest PJ, Hill SL, Stockley RA. Physiological and radiological characterisation of patients diagnosed with chronic obstructive pulmonary disease in primary care. Thorax. 2000;55:635–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rezende Gonçalves J, Corso Pereira M, Figueiras Pedreira De Cerqueira EM, et al. Severe obstructive disease: similarities and differences between smoker and non‐smoker patients with COPD and/or bronchiectasis. Rev Port Pneumol. 2013;19:13–18. [DOI] [PubMed] [Google Scholar]
- 24. Martínez‐García MA, de la Rosa Carrillo D, Soler‐Cataluña JJ, et al. Prognostic value of bronchiectasis in patients with moderate‐to‐severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187:823–831. [DOI] [PubMed] [Google Scholar]
- 25. Mao B, Lu HW, Li MH, et al. The existence of bronchiectasis predicts worse prognosis in patients with COPD. Sci Rep. 2015;5:10961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Martínez‐García MÁ, Soler‐Cataluña JJ, Donat Sanz Y, et al. Factors associated with bronchiectasis in patients with COPD. Chest. 2011;140:1130–1137. [DOI] [PubMed] [Google Scholar]
- 27. Ni Y, Shi G, Yu Y, et al. Clinical characteristics of patients with chronic obstructive pulmonary disease with comorbid bronchiectasis: a systemic review and meta‐analysis. Int J Chron Obstruct Pulmon Dis. 2015;10:1465–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stockley RA. Bronchiectasis with chronic obstructive pulmonary disease: association or a further phenotype? Am J Respir Crit Care Med. 2013;187:786–788. [DOI] [PubMed] [Google Scholar]
- 29. King PT, Holdsworth SR, Freezer NJ, et al. Characterisation of the onset and presenting clinical features of adult bronchiectasis. Respir Med. 2006;100:2183–2189. [DOI] [PubMed] [Google Scholar]
- 30. Qi Q, Wang W, Li T, et al. Aetiology and clinical characteristics of patients with bronchiectasis in a Chinese Han population: a prospective study. Respirology. 2015;20:917–924. [DOI] [PubMed] [Google Scholar]
- 31. Nicotra MB, Rivera M, Dale AM, et al. Clinical, pathophysiologic, and microbiologic characterization of bronchiectasis in an aging cohort. Chest. 1995;108:955–961. [DOI] [PubMed] [Google Scholar]
- 32. Olveira C, Olveira G, Gaspar I, et al. Depression and anxiety symptoms in bronchiectasis: associations with health‐related quality of life. Qual Life Res. 2013;22:597–605. [DOI] [PubMed] [Google Scholar]
- 33. Girón Moreno RM, Fernandes Vasconcelos G, Cisneros C, et al. Presence of anxiety and depression in patients with bronchiectasis unrelated to cystic fibrosis. Arch Bronconeumol. 2013;49:415–420. [DOI] [PubMed] [Google Scholar]
- 34. Brill SE, Patel AR, Singh R, et al. Lung function, symptoms and inflammation during exacerbations of non‐cystic fibrosis bronchiectasis: a prospective observational cohort study. Respir Res. 2015;16:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kapur N, Masters IB, Chang AB. Exacerbations in noncystic fibrosis bronchiectasis: clinical features and investigations. Respir Med. 2009;103:1681–1687. [DOI] [PubMed] [Google Scholar]
- 36. Munro NC, Han LY, Currie DC, et al. Radiological evidence of progression of bronchiectasis. Respir Med. 1992;86:397–401. [DOI] [PubMed] [Google Scholar]
- 37. van der Bruggen‐Bogaarts BA, van der Bruggen HM, van Waes PF, Lammers JW. Screening for bronchiectasis. A comparative study between chest radiography and high‐resolution CT. Chest. 1996;109:608–611. [DOI] [PubMed] [Google Scholar]
- 38. Yi CA, Lee KS, Kim TS, et al. Multidetector CT of bronchiectasis: effect of radiation dose on image quality. AJR Am J Roentgenol. 2003;181:501–505. [DOI] [PubMed] [Google Scholar]
- 39. Jung KJ, Lee KS, Kim SY, et al. Low‐dose, volumetric helical CT: image quality, radiation dose, and usefulness for evaluation of bronchiectasis. Invest Radiol. 2000;35:557–563. [DOI] [PubMed] [Google Scholar]
- 40. Dodd JD, Lavelle LP, Fabre A, Brady D. Imaging in cystic fibrosis and non‐cystic fibrosis bronchiectasis. Semin Respir Crit Care Med. 2015;36:194–206. [DOI] [PubMed] [Google Scholar]
- 41. Prasad M, Sowmya A, Wilson P. Automatic detection of bronchial dilatation in HRCT lung images. J Digit Imaging. 2008;21(Suppl. 1):S148–S163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. DeBoer EM, Swiercz W, Heltshe SL, et al. Automated CT scan scores of bronchiectasis and air trapping in cystic fibrosis. Chest. 2014;145:593–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bonavita J, Naidich DP. Imaging of bronchiectasis. Clin Chest Med. 2012;33:233–248. [DOI] [PubMed] [Google Scholar]
- 44. Westcott JL, Cole SR. Traction bronchiectasis in end‐stage pulmonary fibrosis. Radiology. 1986;161:665–669. [DOI] [PubMed] [Google Scholar]
- 45. Park IK, Olivier KN. Nontuberculous mycobacteria in cystic fibrosis and non‐cystic fibrosis bronchiectasis. Semin Respir Crit Care Med. 2015;36:217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Reiff DB, Wells AU, Carr DH, et al. CT findings in bronchiectasis: limited value in distinguishing between idiopathic and specific types. AJR Am J Roentgenol. 1995;165:261–267. [DOI] [PubMed] [Google Scholar]
- 47. Kadowaki T, Yano S, Wakabayashi K, et al. An analysis of etiology, causal pathogens, imaging patterns, and treatment of Japanese patients with bronchiectasis. Respir Investig. 2015;53:37–44. [DOI] [PubMed] [Google Scholar]
- 48. Guan WJ, Gao YH, Xu G, et al. Aetiology of bronchiectasis in Guangzhou, southern China. Respirology. 2015;20:739–748. [DOI] [PubMed] [Google Scholar]
- 49. Lonni S, Chalmers JD, Goeminne PC, et al. Etiology of non‐cystic fibrosis bronchiectasis in adults and its correlation to disease severity. Ann Am Thorac Soc. 2015;12:1764–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Amorim A, Bento J, Vaz AP, et al. Bronchiectasis: a retrospective study of clinical and aetiological investigation in a general respiratory department. Rev Port Pneumol. 2015;21:5–10. [DOI] [PubMed] [Google Scholar]
- 51. Brower KS, Del Vecchio MT, Aronoff SC. The etiologies of non‐CF bronchiectasis in childhood: a systematic review of 989 subjects. BMC Pediatr. 2014;14:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rogers GB, Zain NM, Bruce KD, et al. A novel microbiota stratification system predicts future exacerbations in bronchiectasis. Ann Am Thorac Soc. 2014;11:496–503. [DOI] [PubMed] [Google Scholar]
- 53. Finklea JD, Khan G, Thomas S, et al. Predictors of mortality in hospitalized patients with acute exacerbation of bronchiectasis. Respir Med. 2010;104:816–821. [DOI] [PubMed] [Google Scholar]
- 54. Naidich DP, McCauley DI, Khouri NF, et al. Computed tomography of bronchiectasis. J Comput Assist Tomogr. 1982;6:437–444. [DOI] [PubMed] [Google Scholar]
- 55. Chalmers JD, Goeminne P, Aliberti S, et al. The bronchiectasis severity index. An international derivation and validation study. Am J Respir Crit Care Med. 2014;189:576–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Martínez‐García MÁ, de Gracia J, Vendrell Relat M, et al. Multidimensional approach to non‐cystic fibrosis bronchiectasis: the FACED score. Eur Respir J. 2014;43:1357–1367. [DOI] [PubMed] [Google Scholar]
- 57. Guan WJ, Gao YH, Xu G, et al. Bronchodilator response in adults with bronchiectasis: correlation with clinical parameters and prognostic implications. J Thorac Dis. 2016;8:14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Guan WJ, Gao YH, Xu G, et al. Characterization of lung function impairment in adults with bronchiectasis. PLoS ONE. 2014;9:e113373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Guan WJ, Gao YH, Xu G, et al. Inflammatory responses, spirometry, and quality of life in subjects with bronchiectasis exacerbations. Respir Care. 2015;60:1180–1189. [DOI] [PubMed] [Google Scholar]
- 60. King PT, Holdsworth SR, Freezer NJ, et al. Microbiologic follow‐up study in adult bronchiectasis. Respir Med. 2007;101:1633–1638. [DOI] [PubMed] [Google Scholar]
- 61. Wilson CB, Jones PW, O'Leary CJ, et al. Effect of sputum bacteriology on the quality of life of patients with bronchiectasis. Eur Respir J. 1997;10:1754–1760. [DOI] [PubMed] [Google Scholar]
- 62. McDonnell MJ, Jary HR, Perry A, et al. Non cystic fibrosis bronchiectasis: a longitudinal retrospective observational cohort study of Pseudomonas persistence and resistance. Respir Med. 2015;109:716–726. [DOI] [PubMed] [Google Scholar]
- 63. Guan WJ, Gao YH, Xu G, et al. Effect of airway Pseudomonas aeruginosa isolation and infection on steady‐state bronchiectasis in Guangzhou, China. J Thorac Dis. 2015;7:625–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wickremasinghe M, Ozerovitch LJ, Davies G, et al. Non‐tuberculous mycobacteria in patients with bronchiectasis. Thorax. 2005;60:1045–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Mirsaeidi M, Hadid W, Ericsoussi B, Rodgers D, Sadikot RT. Non‐tuberculous mycobacterial disease is common in patients with non‐cystic fibrosis bronchiectasis. Int J Infect Dis. 2013;17:e1000–e1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Maiz L, Giron R, Olveira C, Vendrell M, Nieto R, Martinez‐Garcia MA. Prevalence and factors associated with nontuberculous mycobacteria in non‐cystic fibrosis bronchiectasis: a multicenter observational study. BMC Infect Dis. 2016;16:437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Chu H, Zhao L, Xiao H, et al. Prevalence of nontuberculous mycobacteria in patients with bronchiectasis: a meta‐analysis. Arch Med Sci. 2014;10:661–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Marras TK, Chedore P, Ying AM, Jamieson F. Isolation prevalence of pulmonary non‐tuberculous mycobacteria in Ontario, 1997 2003. Thorax. 2007;62:661–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Damaraju D, Jamieson F, Chedore P, Marras TK. Isolation of non‐tuberculous mycobacteria among patients with pulmonary tuberculosis in Ontario, Canada. Int J Tuberc Lung Dis. 2013;17:676–681. [DOI] [PubMed] [Google Scholar]
- 70. Kendall BA, Winthrop KL. Update on the epidemiology of pulmonary nontuberculous mycobacterial infections. Semin Respir Crit Care Med. 2013;34:87–94. [DOI] [PubMed] [Google Scholar]
- 71. Griffith DE, Aksamit T, Brown‐Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367–416. [DOI] [PubMed] [Google Scholar]
- 72. Borekci S, Halis AN, Aygun G, Musellim B. Bacterial colonization and associated factors in patients with bronchiectasis. Ann Thorac Med. 2016;11:55–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Finch S, McDonnell MJ, Abo‐Leyah H, Aliberti S, Chalmers JD. A comprehensive analysis of the impact of pseudomonas aeruginosa colonization on prognosis in adult bronchiectasis. Ann Am Thorac Soc. 2015;12:1602–1611. [DOI] [PubMed] [Google Scholar]
- 74. Tunney MM, Einarsson GG, Wei L, et al. Lung microbiota and bacterial abundance in patients with bronchiectasis when clinically stable and during exacerbation. Am J Respir Crit Care Med. 2013;187:1118–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lee AL, Burge AT, Holland AE. Airway clearance techniques for bronchiectasis. Cochrane Database Syst Rev. 2015;11:CD008351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Brodt AM, Stovold E, Zhang L. Inhaled antibiotics for stable non‐cystic fibrosis bronchiectasis: a systematic review. Eur Respir J. 2014;44:382–393. [DOI] [PubMed] [Google Scholar]
- 77. Gao YH, Guan WJ, Xu G, et al. Macrolide therapy in adults and children with non‐cystic fibrosis bronchiectasis: a systematic review and meta‐analysis. PLoS ONE. 2014;9:e90047. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


