Abstract
Platelet numbers are intricately regulated to avoid spontaneous bleeding or arterial occlusion and organ damage. The growth factor thrombopoietin (TPO) drives platelet biogenesis by inducing megakaryocyte production. A recent study in mice identified a feedback mechanism by which clearance of aged, desialylated platelets stimulates TPO synthesis by hepatocytes. This new finding generated renewed interest in platelet clearance mechanisms. Here, different established and emerging mechanisms of platelet senescence and clearance will be reviewed with specific emphasis on the role of posttranslational modifications.
Introduction
In addition to their essential role in hemostasis to repair vascular damage, platelets are also considered important participants in inflammatory events through secretion of cytokines and growth factors. Platelets interact with white blood cells and vascular endothelial cells both directly by contact-dependent mechanisms and indirectly through secreted chemokine-driven mechanisms. The human body produces and removes 1011 platelets daily to maintain a normal steady-state platelet count of 150 000 to 400 000 platelets per microliter of blood. Thus, regulation of platelet production and destruction to maintain steady platelet counts is critical not only for hemostasis, but also in maintaining a healthy and balanced immune response. Chronic inflammation is often associated with reactive high platelet counts, and responses to acute infections may be accompanied by sudden reduction or increase of platelets (thrombocytopenia or thrombocytosis, respectively), placing platelets as reporters of disease progression or healing. To this date the mechanisms that regulate platelet numbers at steady state and pathologic conditions remain under-characterized. This review will focus on the role of glycans and novel emerging clearance mechanisms in regulating in vivo and in vitro platelet lifespan and clearance.
Role of GPIbα in Platelet Clearance
Glycoprotein (GP) Ibα is the major subunit of the platelet receptor complex for von Willebrand factor (VWF) that also contains the GPIbβ and GPIX subunits (Figure 1) [1, 2]. In addition to VWF, GPIbα also binds to a number of ligands or counter-receptors in circulation, including the integrin αMβ2 discussed below. It is abundantly and exclusively expressed on the surface of platelets and megakaryocytes. The extracellular domain of GPIbα consists of an N-terminal ligand-binding domain (LBD), a heavily O-glycosylated mucin-like region that contains multiple unstructured repeating sequences, and a quasi-stable mechanosensory domain (MSD) [3-5]. In addition to the mucin-like region, the GPIbα N-terminal LBD and the extracellular domains of GPIbβ and GPIX are decorated with N-glycans in human platelets. By one estimate, missing the GPIb-IX complex could result in an ∼80% reduction of sialic acid content per unit surface area of the platelet [6]. While past studies of platelets from patients and model animals, as well as biochemical characterization of GPIbα with its ligands, have firmly established its importance in mediating hemostasis and platelet aggregation [7-11], recent evidence suggests that glycans on GPIbα play a critical role in mediating platelet clearance via receptors containing carbohydrate-binding domains on the macrophage αMβ2 integrin and the hepatic Ashwell-Morell Receptor (AMR) [12-18].
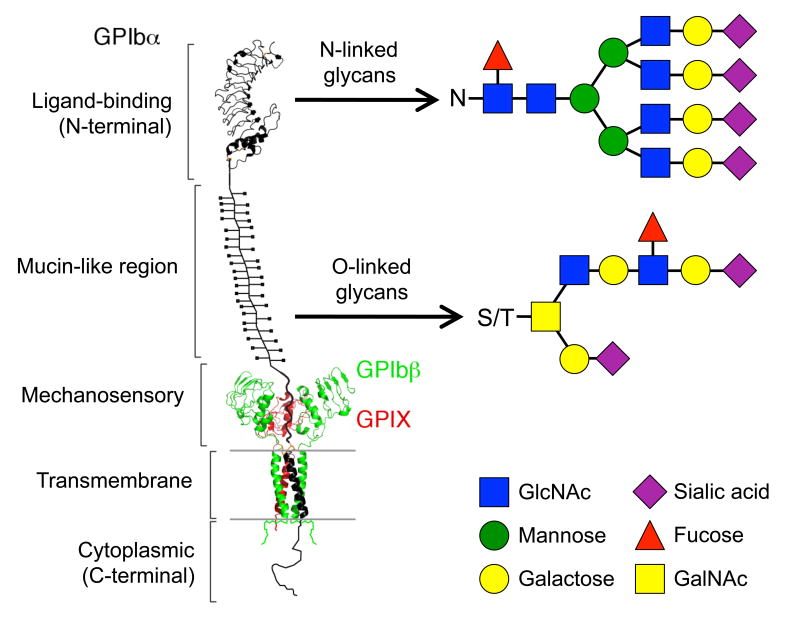
Figure 1. Ribbon diagram depicting the structure of the platelet GPIb-IX complex and examples of its associated O- and N-linked glycans.
The extracellular domain of GPIbα consists of: an N-terminal ligand-binding domain, ligands of which include VWF and the integrin αMβ2; a heavily O-glycosylated mucin-like region; and a quasi-stable mechanosensory domain. The GPIbα LBD and the extracellular domains of GPIbβ and GPIX are decorated with N-linked glycans, i.e., oligosaccharides (N-acetylglucosamine; GlcNAc) attached to a nitrogen atom of an asparagine residue (N). O-linked glycosylation is the attachment of oligosaccharides (N-acetylgalactosamine; GalNAc) to an oxygen atom of a protein serine or threonine residue (S/T).
VWF in the circulation does not spontaneously bind platelet GPIbα. When it becomes immobilized at the injured vessel wall and undergoes a conformational change, VWF binds to GPIbα, thereby recruiting and activating platelets at the site of injury [19, 20]. If circumstances allow, VWF will bind to GPIbα, and the binding often coincides with onset of platelet clearance. For instance, VWF bearing a type 2B mutation exhibits an increased affinity for GPIbα and spontaneously binds to platelets in circulation [21-23]. Type 2B von Willebrand disease (VWD) patients often present thrombocytopenia, albeit to various extents [24]. Consistently, recent characterization of transgenic mice expressing type 2B VWF showed that VWF-platelet complexes in these mice are recognized and cleared by macrophages in the liver and spleen [25].
Ristocetin induces spontaneous binding of plasma VWF to GPIbα and platelets [26] and had to be pulled from clinical treatment due to complications of thrombocytopenia and blood clotting [27]. Animal studies corroborate the human data, as botrocetin, a snake venom that induces binding of plasma VWF to GPIbα, induced thrombocytopenia when injected into animals [28, 29]. How the VWF-platelet complexes are recognized and cleared remains to be defined. Recent observations have shown that the juxtamembrane MSD in GPIbα undergoes unfolding when a mechanical pulling force is exerted on the LBD through the bound A1 domain of VWF [5]. Botrocetin-mediated VWF binding may induce similar MSD unfolding on the platelet surface, which in turn triggers signaling into the platelet, desialylation on the platelet surface and platelet clearance (R.L., unpublished data). Since the shedding cleavage site is located in the middle of the MSD, desialylation may induce additional GPIbα shedding [30], which effectively cleaves the MSD in half and likely leaves both halves unfolded, thereby propagating the signal for platelet clearance. Whether desialylation triggers structural changes in GPIbα is unclear.
Sialic acid deficiency on platelet GPIbα does not alter VWF binding in mice lacking ST3GalIV [31]. However, binding to GPIbα of desialylated plasma VWF from these mice is increased in the presence, but not in the absence of botrocetin, indicating that VWF desialylation enhances binding to GPIbα once it is activated. By contrast, human VWF desialylated by the Vibrio cholerae neuraminidase can interact with GPIbα in the absence of any stimulus such as ristocetin [32]. It is unclear whether the discrepancy between these observations is due to the different species (mouse vs. human) or methods (genetic ST3GalIV deletion vs. unspecific enzymatic desialylation) used.
Earlier reports have shown that ristocetin-induced VWF binding to GPIbα also induces platelet apoptosis, presumably by up-regulating the levels of pro-apoptotic Bax and Bak [33]. While the underlying molecular mechanisms, including the location and extent of desialylation, remain to be elucidated, these findings suggest a novel mechanism linking platelet activation to platelet desialylation, apoptosis and clearance, in which changes of glycosylation of GPIbα plays a central role. These findings also suggest that under certain circumstances VWF may be considered as a “platelet clearance factor”.
In Vivo Aging: Senescence-induced platelet clearance by the Ashwell-Morell Receptor
Loss of sialic acid has recently been identified as a determinant of senescent platelet removal [34]. Platelets lose sialic acid during circulation and are cleared via the hepatic AMR, a transmembrane heteroligomeric glycoprotein complex composed of ASGPR1 (HL-1) and ASGPR2 (HL-2) subunits. This highly conserved receptor has been largely regarded as an endocytic receptor [35], and since its discovery four decades ago its regulatory role has remained largely unclear. Specifically, mice lacking either the ASGPR1 or ASGPR2 subunit do not accumulate plasma proteins or lipids lacking sialic acid, which has been the predicted outcome of eliminating one of the AMR subunits [35]. It has therefore been a surprising discovery that platelets with reduced α2,3-linked sialic acid during sepsis, after cold storage (in-vitro aging), or in mice lacking the sialyltransferase ST3GalIV are cleared by the hepatic AMR (Figure 2) [15-17, 31, 34].
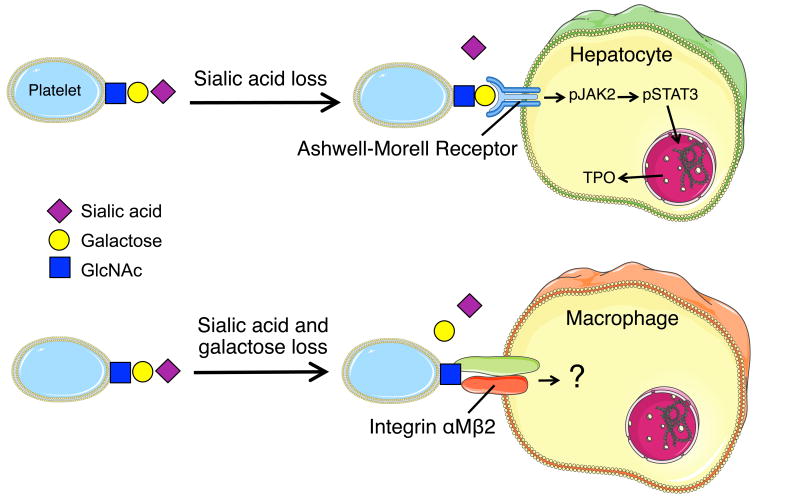
Figure 2. In vivo and in vitro platelet aging.
(A) Senescent platelets lose sialic acid while circulating leading to exposure of penultimate surface galactose. Desialylated platelets bind to the hepatic Ashwell-Morell Receptor (AMR) on hepatocytes to initiate the JAK2-STAT3 signaling cascade and thrombopoietin (TPO) production to regulate steady state platelet numbers. (B) Platelets stored at 4°C lose both sialic acid and galactose and are cleared by the AMR on hepatocytes and the αMβ2 integrin (via its αM subunit lectin domain) on hepatic macrophages (Kupffer cells). It remains to be determined if engagement of cold stored platelets with either receptor induces intracellular signaling.
These findings led subsequently to the discovery that removal of senescent, sialic acid deprived platelets drives hepatic TPO mRNA expression in vivo and in vitro via Janus kinase 2 (JAK2) and signal transducer and activator of transcription 3 (STAT3) to increase megakaryocyte numbers and de novo platelet production (Figure 2). The notion that loss of sialic acid determines platelet lifespan is not entirely novel [36-38], however, the recent study elucidates that aged, desialylated platelets regulate hepatic TPO mRNA production in vivo via the AMR. This feedback mechanism presents the AMR-desialylated platelet pair as the critical control point for TPO homeostasis and shows that TPO expression in hepatocytes is regulated and not constitutive. Importantly, disruption of AMR-desialylated platelet signaling using JAK1/2 inhibitors AZD1480, TG101348 and BMS911543 adversely affects hepatic TPO mRNA expression and secretion in hepatocytes in vitro and in vivo [34]. Thrombocytopenia is a common adverse event of JAK1/2 inhibitor treatment, which is clinically used in myeloproliferative neoplasms [39, 40]. JAK1/2 inhibitors target hematopoietic stem and precursor-cell mutant JAK2-V617F as well as wild-type JAK2, activation of which is essential for red blood cell and platelet production [41, 42]. This new study indicates that inhibition of TPO production downstream of the hepatic AMR-JAK2 signaling cascade could additionally contribute to the thrombocytopenia associated with JAK1/2 treatment. Clinical studies are necessary to investigate this notion. In conclusion, these findings show that the regulatory mechanisms of TPO production, its effects and JAK2 regulation are complex and not completely understood. Further investigation is necessary to determine the exact relationship between the TPO receptor Mpl, TPO and JAK2.
In Vitro Aging: Senescence-induced platelet clearance by liver lectins
Platelet storage is often used as an in vitro aging system and data are compared with in vivo aging mechanisms. For decades, all platelet products have been stored at room temperature, limiting platelet storage to 5 days because of the risk of bacterial growth and loss of platelet functionality. Platelet refrigeration remains undesired because once chilled platelets are rapidly removed from the circulation, a phenomenon with profound consequences for blood banking. Investigation for almost a decade of why refrigerated platelets fail to circulate have defined two previously unsuspected, carbohydrate-dependent platelet clearance mechanisms [43]. The notion that chilled platelets are cleared because they undergo an extensive shape change when exposed to low temperatures and become trapped in the vasculature has been disproved [12, 13]. Rather, cooling of platelets induces progressive clustering of glycan-bearing receptors, causing lectins, specifically the integrin αMβ2 (Mac-1) on macrophages and the AMR on hepatocytes, to recognize chilled platelets via terminal N-acetyl glucosamine residues (GlcNAc) and β-galactose, respectively (Figure 2).
Integrins are heterodimeric transmembrane glycoproteins present on virtually all mammalian cells. The most important integrins expressed on leucocytes belong to the β2 integrin family. The heterodimeric integrin αMβ2 is expressed on the surface of many leukocytes involved in the innate immune system. The αM subunit contains a cation-dependent ligand binding I-domain, which mediates inflammation by regulating leukocyte adhesion and migration, and has been implicated in several immune processes such as phagocytosis, cell-mediated cytotoxicity, chemotaxis, and cellular activation. It is involved in the complement system, due to its capacity to bind inactivated complement component 3b (iC3b), and the integrin αMβ2 serves as a phagocytic receptor for the iC3b fragment of complement [44-46]. The αM domain also binds to platelet GPIbα, and inhibition studies using monoclonal antibodies or receptor ligands have shown that the interaction involves the macrophage-1 antigen domain (homologous to the VWF A1 domain) and the GPIbα N-terminal LBD that contains leucine-rich repeats.
In addition to binding the complement component iC3b, the αM subunit has a distinct lectin site for β-glucan [47]. The lectin domain also recognizes circulating cold-platelets and transfused cold-stored platelets via exposure of GlcNAc on platelet surfaces [14]. Additionally, mice lacking the αM subunit have slightly increased platelet counts [12], indicating that platelets are removed by this receptor under steady state conditions. Taken together, the data shows that glycan remodeling affects circulating platelet numbers. In vivo and in vitro aging leads to profound changes in glycan expression and clearance via multiple lectin-receptors localized on macrophages and hepatocytes. Cold-induced changes in GPIbα glycan composition play a role in platelet binding to macrophages [48]. Interestingly, binding of VWF to platelets increases upon prolonged cooling [16], indicating that in the case of “cold-induced platelet activation”, bound VWF could facilitate clearance via the AMR. It remains to be determined if changes in platelet surface glycosylation and VWF binding observed during in vitro aging induce TPO production by hepatocytes in human settings, and whether binding of platelets to platelet lectins, specifically the AMR, induces expression of additional factors than TPO regulating platelet homeostasis.
Role of Lysosomal Glycosidases
Sialidases (or neuraminidases) are glycoside hydrolase enzymes that cleave the glycosidic linkages of sialic (or neuraminic) acids. Sialidases are a large family found in a range of organisms. Removal of sialic acid from the cell surface glycoconjugates affects cell-cell interactions, binding to soluble molecules, viruses, bacteria and protozoa and modulates cell activity [49]. The significance of sialidase activity in the normal function of eukaryotic cells has been inferred from the heterogeneous clinical manifestations of individuals with genetic sialidase deficiencies [50-52]. At least four mammalian sialidases have been described in the human genome: sialidases 1 to 4 (Neu1-4) [53]. Neu1 is a mammalian lysosomal neuraminidase enzyme, while Neu3 is expressed on the cell surface where it may play a role in modulating the ganglioside content of the lipid bilayer.
Sialic acid loss from the platelet surface may be mediated by upregulation of platelet sialidases, i.e., Neu1 and Neu3, expressed in granular compartments and on the plasma membrane, respectively [30]. Platelet Neu1 and Neu3 have a preference for the sialic acid linkage found on glycans of the VWF receptor complex GPIbα subunit, exposing underlying β-galactose residues and priming GPIbα for metalloproteinase-mediated degradation during storage. Interestingly, platelet incubation with the sialidase inhibitor, 2-deoxy- 2,3-dehydro-N-acetylneuraminic acid (DANA), enhances the recovery and survival of platelets stored at 4°C in mice [30]. In vitro studies demonstrated that addition of DANA during platelet storage at 4°C in the presence of plasma additive solutions preserves surface sialic acid [54]. The sialidase inhibitor oseltamivir phosphate (Tamiflu®), which is clinically used to treat influenza, has also been shown to increase platelet counts in 2 patients with immune thrombocytopenia (ITP), as well as in 77 patients from the Erasmus Medical Center, Rotterdam, independently of influenza diagnosis [55]. Clinical studies are required to determine whether preventing platelet desialylation by inhibition of platelet Neu1 and Neu3 with DANA and/or oseltamivir phosphate treatment improves platelet recovery and survival in transfusion settings.
More evidence has been generated showing that platelet numbers are regulated by sialidase activity in clinical setting. For example, desialylation is associated with apoptosis and phagocytosis of platelets in patients with prolonged isolated thrombocytopenia after allo- hematopoietic stem cell transplantation [56]. The sialidase inhibitor oseltamivir phosphate reduced platelet clearance in these patients, indicating that sialidases remove sialic acid from circulating platelets. Investigators also reported successful treatment with oseltamivir phosphate in a patient with chronic ITP positive for anti-GPIb-IX autoantibody [57]. Hence, platelet sialic acid content is an important factor that dictates platelet interaction with other cellular systems (hepatocytes, macrophages) to induce clearance. However, other roles of sialic acid loss have to be identified.
Resting platelets also express β-galactosidase in granular compartments and on their surface [30]. β-galactosidases hydrolize β-galactosides into monosaccharides, and the breakdown of lactose to galactose and glucose is used as energy source, contributing therefore to cell survival. Substrates of β-galactosidase include lactose, ganglioside GM1, lactosylceramides, and various glycoproteins [58]. Since β-galactosidase is highly expressed and accumulates in lysosomes in senescent cells, it is used as a senescence biomarker both in vivo and in vitro in qualitative and quantitative assays, despite its limitations [59, 60]. β-galactosidase expression and activity increases on platelet surfaces upon storage (K.M.H., unpublished data), presumably mediating surface terminal galactose cleavage, which may explain the exposure of βGlcNAc on platelet surface glycoconjugtes and clearance via macrophage αMβ2 integrin. It is unclear if β-galactosidase accumulation occurs in platelets upon aging in vitro and in vivo as observed in other cells, a finding which would reinforce the role of this enzyme in platelet senescence.
The term “lysosome” was coined to convey the idea of a membrane-bound lytic organelle that contains hydrolases active at acid pH within cells. Lysosomes are thought to be the endpoint of the endocytic pathway to which proteins and extracellular particles are delivered for degradation by a number of proteases and lipases [61]. However, several studies show that lysosomes also function as secretory organelles. Degradative and secretory functions of lysosomes can be finely controlled, providing important regulatory mechanisms in the immune system and a number of other cell types, specifically megakaryocytes and platelets. Genetic defects causing secretory lysosome defects are associated with albinism, immunodeficiency and platelet disorders [62]. The fact that platelet sialidase and β-galactosidase activities increase upon cold storage [30] and upon activation (K.M.H., unpublished data) suggests the presence of these enzymes in secretory lysosomes. Our unpublished results together with previous reported studies support the notion that platelets and likely megakaryocytes contain secretory lysosomes that can release neuraminidase and β-galactosidase activities (Figure 3). However this notion and the cellular mechanisms triggering their secretion during in vivo aging remain to be investigated.
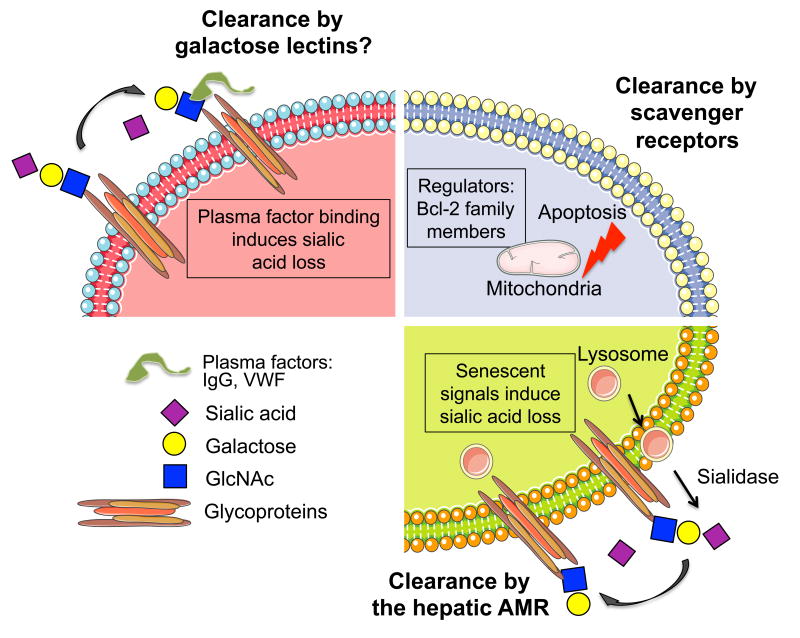
Figure 3. Platelet clearance mechanisms.
Platelets circulate with a lifespan of 7-10 days in humans. Platelet clearance is mediated by autoreactive antibodies towards the integrin αIIbβ3 and the VWF receptor complex GPIb-IX subunit GPIbα. Some anti-GPIbα, but not anti-αIIbβ3 antibodies induce platelet desialylation, thereby diverging platelet clearance to hepatic AMRs. Binding of VWF may also induce platelet desialylation and clearance under certain circumstances. Following plasma factor (autoantibodies or VWF) binding, platelets may be also cleared by galactose-binding lectins. Platelet survival also depends on the interplay between pro-survival and pro-apoptotic members of the Bcl-2 family, which are critical regulators of the intrinsic apoptotic pathway. Platelet clearance via scavenger receptors is accelerated in mice lacking the pro-survival proteins Bcl-2, Bcl-xL and Mcl-1. In vivo aging (senescence signal) induces platelet desialylation, possibly by intrinsic sialidases normally contained in intracellular lysosome compartments.
Role of Apoptosis In Platelet Clearance
Platelet survival also depends on the interplay between pro-survival and pro-apoptotic members of the Bcl-2 family, which are critical regulators of the intrinsic apoptotic pathway (Figure 3). Platelet survival is extended in mice lacking the pro-apoptotic proteins Bak and Bax, whereas platelet clearance is accelerated in mice lacking the pro-survival proteins Bcl-2, Bcl-xL, and Mcl-1, and in mice treated with the BH3 mimetic ABT-737 (inhibitor of pro-survival Bcl-2, Bcl-xL, and Bcl-w). Whether members of the Bcl-2 family alter platelet surface sialic acid content or whether these mechanisms converge at certain points during platelet lifetime is unclear. Interestingly, the primary platelet clearance site following administration of ABT-737 is the liver in dogs, presumably via scavenger receptors, whereas the spleen does not appear to regulate the platelet lifespan in mice [63]. Evidence shows that in other cells Neu3 expression and activity is associated with resistance to apoptosis, specifically in the leukemic cell line K562 [64]. Interestingly, silencing of Neu3 in K562 triggers megakaryocytic differentiation of K562 cells. High expression of Neu3 in cancer cells has also been associated with protection against apoptosis in tumor cells [49, 65, 66]. More data are needed to establish if glycan degradation in vivo (i.e., sialic acid loss) triggers the intrinsic apoptotic machinery in platelets, linking glycan degradation and intrinsic apoptotic machinery in the clearance mechanisms regulating platelet survival.
Data show that newborn and adult mice have similar platelet production rates, but neonatal platelets survived 1 day longer in circulation. A study of pro-apoptotic and anti-apoptotic Bcl-2 family proteins shows that neonatal platelets have higher levels of the anti-apoptotic protein Bcl-2 and are more resistant to apoptosis induced by the Bcl-2/Bcl-xL inhibitor ABT-737 than adult platelets. However, genetic ablation or pharmacologic inhibition of Bcl-2 alone does not shorten neonatal platelet survival or reduce platelet counts in newborn mice, indicating the existence of redundant or alternative mechanisms mediating the prolonged lifespan of neonatal platelets [67]. Whether glycans (i.e., increase in platelet surface sialic acid) play a role in the prolonged survival of neonatal platelets remains to be established.
Immune Platelet Clearance
ITP is a common bleeding disorder caused primarily by platelet autoantibodies that accelerate platelet destruction, alter platelet function, and/or inhibit platelet production [68]. These autoantibodies are mainly directed against the two most abundant platelet GP complexes, GPIIb-IIIa (integrin αIIbβ3) and GPIb-IX (Figure 3). The prevailing model posits that antibody-mediated platelet destruction occurs in the spleen [69-71], where the interaction between the Fc portion of platelet-associated immunoglobulin G antibodies and Fcγ receptors (FcγRs) on macrophages initiates phagocytosis. However, in contrast to anti-αIIbβ3-mediated ITP, anti-GPIbα-mediated ITP is often refractory to therapies targeting FcγR pathways or splenectomy. Recent findings show that certain anti-GPIbα antibodies trigger platelet desialylation, a process that deviates platelet clearance from splenic macrophage Fc receptors to the hepatic AMR, showing that FcγR-independent platelet clearance mechanisms in ITP exist [71-73]. The mechanism of how anti-GPIbα antibody binding leads to desialylation remains to be established. It is noteworthy that many antibodies targeting GPIbβ and GPIX subunits in the GPIb-IX complex do not cause platelet clearance [74-76]. It has also been recently reported that not all anti-GPIbα antibodies induce platelet clearance [77, 78]. It is likely that platelets secrete active Neu1 and Neu3 upon antibody binding and/or platelet activation [30]. The notion that the AMR plays a significant role in the clearance of anti-GPIbα-opsonized and desialylated platelets provides a potential explanation for refractoriness to splenectomy, as well as to steroid and intravenous immunoglobulin therapies.
Conclusion
In summary, the regulatory mechanisms of platelet numbers are intricately designed to maintain a balance of platelet production and removal. Under steady state conditions, sialic acid loss is sensed by hepatic AMR receptors to induce TPO secretion and de novo platelet production. Novel mechanisms are emerging to define how platelets specifically lose sialic acid, specifically involving GPIbα. Apoptotic events also dictate the platelet lifespan. However, how the system senses platelet loss due to apoptosis to induce new platelet production remains to be determined.
Acknowledgments
This work was supported by National Institutes of Health (NIH) grants HL107146, HL089224. (to K.M.H.) and HL082808, HL123984 (to R.L.); and bridge grants from the Brigham Research Institute and the American Society of Hematology (to H.F.).
Footnotes
DECLARATION OF INTEREST: The authors report no conflicts of interest.
References
- 1.Huizinga EG, et al. Structures of glycoprotein Iba and its complex with von Willebrand factor A1 domain. Science. 2002;297(5584):1176–9. doi: 10.1126/science.107355. [DOI] [PubMed] [Google Scholar]
- 2.Li R, Emsley J. The organizing principle of the platelet glycoprotein Ib-IX-V complex. Journal of thrombosis and haemostasis. 2013;11(4):605–14. doi: 10.1111/jth.12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berndt MC, et al. Purification and preliminary characterization of the glycoprotein Ib complex in the human platelet membrane. Eur J Biochem. 1985;151(3):637–49. doi: 10.1111/j.1432-1033.1985.tb09152.x. [DOI] [PubMed] [Google Scholar]
- 4.Lopez JA, et al. Cloning of the α chain of human platelet glycoprotein Ib: a transmembrane protein with homology to leucine-rich α2-glycoprotein. Proc Natl Acad Sci U S A. 1987;84(16):5615–9. doi: 10.1073/pnas.84.16.5615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang W, et al. Identification of a juxtamembrane mechanosensitive domain in the platelet mechanosensor glycoprotein Ib-IX complex. Blood. 2015;125(3):562–9. doi: 10.1182/blood-2014-07-589507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gröttum KA, Solum NO. Congenital thrombocytopenia with giant platelets: a defect in the platelet membrane. Br J Haematol. 1969;16(3):277–90. doi: 10.1111/j.1365-2141.1969.tb00402.x. [DOI] [PubMed] [Google Scholar]
- 7.Ware J, Russell S, Ruggeri ZM. Generation and rescue of a murine model of platelet dysfunction: the Bernard-Soulier syndrome. Proc Natl Acad Sci U S A. 2000;97(6):2803–8. doi: 10.1073/pnas.050582097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanaji T, Russell S, Ware J. Amelioration of the macrothrombocytopenia associated with the murine Bernard-Soulier syndrome. Blood. 2002;100(6):2102–7. doi: 10.1182/blood-2002-03-0997. [DOI] [PubMed] [Google Scholar]
- 9.Kleinschnitz C, et al. Targeting platelets in acute experimental stroke: impact of glycoprotein Ib, VI, and IIb/IIIa blockade on infarct size, functional outcome, and intracranial bleeding. Circulation. 2007;115(17):2323–30. doi: 10.1161/CIRCULATIONAHA.107.691279. [DOI] [PubMed] [Google Scholar]
- 10.Savage B, Almus-Jacobs F, Ruggeri ZM. Specific synergy of multiple substrate-receptor interactions in platelet thrombus formation under flow. Cell. 1998;94(5):657–66. doi: 10.1016/s0092-8674(00)81607-4. [DOI] [PubMed] [Google Scholar]
- 11.Gu M, et al. Analysis of the roles of 14-3-3 in the platelet glycoprotein Ib-IX-mediated activation of integrin αIIbβ3 using a reconstituted mammalian cell expression model. J Cell Biol. 1999;147(5):1085–96. doi: 10.1083/jcb.147.5.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoffmeister K, et al. The clearance mechanism of chilled blood platelets. Cell. 2003;10(112):87–97. doi: 10.1016/s0092-8674(02)01253-9. [DOI] [PubMed] [Google Scholar]
- 13.Hoffmeister K, et al. Glycosylation restores survival of chilled blood platelets. Science. 2003;301(5639):1531–1534. doi: 10.1126/science.1085322. [DOI] [PubMed] [Google Scholar]
- 14.Josefsson E, et al. The macrophage alphaMbeta2 integrin alphaM lectin domain mediates the phagocytosis of chilled platelets. J Biol Chem. 2005;280(18):18025–18032. doi: 10.1074/jbc.M501178200. [DOI] [PubMed] [Google Scholar]
- 15.Grewal PK, et al. The Ashwell receptor mitigates the lethal coagulopathy of sepsis. Nat Med. 2008;14(6):648–55. doi: 10.1038/nm1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rumjantseva V, et al. Dual roles for hepatic lectin receptors in the clearance of chilled platelets. Nat Med. 2009;15(11):1273–80. doi: 10.1038/nm.2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grewal PK, et al. Inducing host protection in pneumococcal sepsis by preactivation of the Ashwell-Morell receptor. Proc Natl Acad Sci U S A. 2013 doi: 10.1073/pnas.1313905110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rumjantseva V, Hoffmeister KM. Novel and unexpected clearance mechanisms for cold platelets. Transfus Apher Sci. 2010;42(1):63–70. doi: 10.1016/j.transci.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Savage B, Saldivar E, Ruggeri ZM. Initiation of platelet adhesion by arrest onto fibrinogen or translocation on von Willebrand factor. Cell. 1996;84(2):289–97. doi: 10.1016/s0092-8674(00)80983-6. [DOI] [PubMed] [Google Scholar]
- 20.Denis C, et al. A mouse model of severe von Willebrand disease: defects in hemostasis and thrombosis. Proc Natl Acad Sci U S A. 1998;95(16):9524–9. doi: 10.1073/pnas.95.16.9524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruggeri ZM, et al. Heightened interaction between platelets and factor VIII/von Willebrand factor in a new subtype of von Willebrand's disease. N Engl J Med. 1980;302(19):1047–51. doi: 10.1056/NEJM198005083021902. [DOI] [PubMed] [Google Scholar]
- 22.Randi AM, et al. Recombinant von Willebrand factor Arg578-->Gln. A type IIB von Willebrand disease mutation affects binding to glycoprotein Ib but not to collagen or heparin. J Biol Chem. 1992;267(29):21187–92. [PubMed] [Google Scholar]
- 23.Miyata S, et al. Conformational changes in the A1 domain of von Willebrand factor modulating the interaction with platelet glycoprotein Ibalpha. J Biol Chem. 1996;271(15):9046–53. doi: 10.1074/jbc.271.15.9046. [DOI] [PubMed] [Google Scholar]
- 24.Lillicrap D. von Willebrand disease: advances in pathogenetic understanding, diagnosis, and therapy. Blood. 2013;122(23):3735–40. doi: 10.1182/blood-2013-06-498303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Casari C, et al. Accelerated uptake of VWF/platelet complexes in macrophages contributes to VWD type 2B-associated thrombocytopenia. Blood. 2013;122(16):2893–902. doi: 10.1182/blood-2013-03-493312. [DOI] [PubMed] [Google Scholar]
- 26.Scott JP, Montgomery RR, Retzinger GS. Dimeric ristocetin flocculates proteins, binds to platelets, and mediates von Willebrand factor-dependent agglutination of platelets. J Biol Chem. 1991;266(13):8149–55. [PubMed] [Google Scholar]
- 27.Gangarosa EJ, et al. Hematologic complications arising during ristocetin therapy; relation between dose and toxicity. N Engl J Med. 1958;259(4):156–61. doi: 10.1056/NEJM195807242590402. [DOI] [PubMed] [Google Scholar]
- 28.Sanders WE, et al. Thrombotic thrombocytopenia with von Willebrand factor deficiency induced by botrocetin. An animal model. Lab Invest. 1988;59(4):443–52. [PubMed] [Google Scholar]
- 29.Sanders WE, Jr, et al. Thrombotic thrombocytopenia induced in dogs and pigs. The role of plasma and platelet vWF in animal models of thrombotic thrombocytopenic purpura. Arterioscler Thromb Vasc Biol. 1995;15(6):793–800. doi: 10.1161/01.atv.15.6.793. [DOI] [PubMed] [Google Scholar]
- 30.Jansen G, et al. Desialylation accelerates platelet clearance following refrigeration and initiates GPIbα metalloproteinase-mediated cleavage in mice. Blood. 2012;119:1263–73. doi: 10.1182/blood-2011-05-355628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sorensen AL, et al. Role of sialic acid for platelet life span: exposure of beta-galactose results in the rapid clearance of platelets from the circulation by asialoglycoprotein receptor-expressing liver macrophages and hepatocytes. Blood. 2009;114(8):1645–54. doi: 10.1182/blood-2009-01-199414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Marco L, et al. Interaction of asialo von Willebrand factor with glycoprotein Ib induces fibrinogen binding to the glycoprotein IIb/IIIa complex and mediates platelet aggregation. J Clin Invest. 1985;75(4):1198–203. doi: 10.1172/JCI111816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li S, et al. The glycoprotein Ibα-von Willebrand factor interaction induces platelet apoptosis. Journal of thrombosis and haemostasis. 2010;8(2):341–50. doi: 10.1111/j.1538-7836.2009.03653.x. [DOI] [PubMed] [Google Scholar]
- 34.Grozovsky R, et al. The Ashwell-Morell receptor regulates hepatic thrombopoietin production via JAK2-STAT3 signaling. Nat Med. 2015;21(1):47–54. doi: 10.1038/nm.3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grewal PK. The Ashwell-Morell receptor. Methods Enzymol. 2010;479:223–41. doi: 10.1016/S0076-6879(10)79013-3. [DOI] [PubMed] [Google Scholar]
- 36.Crook M. Sialic Acid: its importance to platelet function in health and disease. Platelets. 1991;2(1):1–10. doi: 10.3109/09537109109005496. [DOI] [PubMed] [Google Scholar]
- 37.Crook M. Platelet sialic Acid and its significance to platelet life-spans. Platelets. 1990;1(3):167. doi: 10.3109/09537109009005484. [DOI] [PubMed] [Google Scholar]
- 38.Reimers HJ, et al. Experimental modification of platelet survival. Adv Exp Med Biol. 1977;82:231–3. doi: 10.1007/978-1-4613-4220-5_48. [DOI] [PubMed] [Google Scholar]
- 39.Levine RL, Gilliland DG. Myeloproliferative disorders. Blood. 2008;112(6):2190–8. doi: 10.1182/blood-2008-03-077966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.LaFave LM, Levine RL. JAK2 the future: therapeutic strategies for JAK-dependent malignancies. Trends Pharmacol Sci. 2012;33(11):574–82. doi: 10.1016/j.tips.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 41.Park SO, et al. Conditional deletion of Jak2 reveals an essential role in hematopoiesis throughout mouse ontogeny: implications for Jak2 inhibition in humans. PLoS One. 2013;8(3):e59675. doi: 10.1371/journal.pone.0059675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grisouard J, et al. Selective deletion of Jak2 in adult mouse hematopoietic cells leads to lethal anemia and thrombocytopenia. Haematologica. 2014;99(4):e52–4. doi: 10.3324/haematol.2013.100016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hoffmeister KM. The role of lectins and glycans in platelet clearance. J Thromb Haemost. 2011;9(Suppl 1):35–43. doi: 10.1111/j.1538-7836.2011.04276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Springer T. Traffic signals for lymphocyte recirculation and leucocyte emigration: The multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 45.Xia Y, et al. Function of the lectin domain of Mac-1/complement receptor type 3 (CD11b/CD18) in regulating neutrophil adhesion. J Immunol. 2002;169:6417–6426. doi: 10.4049/jimmunol.169.11.6417. [DOI] [PubMed] [Google Scholar]
- 46.Petty HR, Todd RF., III Receptor-receptor interactions of complement receptor type 3 in neutrophil membranes. J Leukoc Biol. 1993;54:492–494. doi: 10.1002/jlb.54.5.492. [DOI] [PubMed] [Google Scholar]
- 47.Thornton B, et al. Analysis of the sugar specifty and molecular location of the b-glucan-binding lectin site of compement receptor type 3 (CD11b/D18) J Immonol. 1996;156:1235–1246. [PubMed] [Google Scholar]
- 48.Badlou B, et al. Role of glycoprotein Ibalpha in phagocytosis of platelets by macrophages. Transfusion. 2006;46(12):2090–2099. doi: 10.1111/j.1537-2995.2006.01034.x. [DOI] [PubMed] [Google Scholar]
- 49.Miyagi T, Yamaguchi K. Mammalian sialidases: physiological and pathological roles in cellular functions. Glycobiology. 2012;22(7):880–96. doi: 10.1093/glycob/cws057. [DOI] [PubMed] [Google Scholar]
- 50.Stamatos NM, et al. Differential expression of endogenous sialidases of human monocytes during cellular differentiation into macrophages. FEBS J. 2005;272(10):2545–56. doi: 10.1111/j.1742-4658.2005.04679.x. [DOI] [PubMed] [Google Scholar]
- 51.Pshezhetsky AV, Ashmarina M. Lysosomal multienzyme complex: biochemistry, genetics, and molecular pathophysiology. Prog Nucleic Acid Res Mol Biol. 2001;69:81–114. doi: 10.1016/s0079-6603(01)69045-7. [DOI] [PubMed] [Google Scholar]
- 52.Pshezhetsky AV, Ashmarina LI. Desialylation of surface receptors as a new dimension in cell signaling. Biochemistry (Mosc) 2013;78(7):736–45. doi: 10.1134/S0006297913070067. [DOI] [PubMed] [Google Scholar]
- 53.Monti E, et al. Sialidases in vertebrates: a family of enzymes tailored for several cell functions. Adv Carbohydr Chem Biochem. 2010;64:403–79. doi: 10.1016/S0065-2318(10)64007-3. [DOI] [PubMed] [Google Scholar]
- 54.Skripchenko A, et al. Addition of sialidase or p38 MAPK inhibitors does not ameliorate decrements in platelet in vitro storage properties caused by 4 degrees C storage. Vox Sang. 2014;107(4):360–7. doi: 10.1111/vox.12174. [DOI] [PubMed] [Google Scholar]
- 55.Jansen AJ, et al. Sialidase inhibition to increase platelet counts: A new treatment option for thrombocytopenia. Am J Hematol. 2015;90(5):E94–5. doi: 10.1002/ajh.23953. [DOI] [PubMed] [Google Scholar]
- 56.Zhang XH, et al. Desialylation is associated with apoptosis and phagocytosis of platelets in patients with prolonged isolated thrombocytopenia after allo-HSCT. J Hematol Oncol. 2015;8(1):116. doi: 10.1186/s13045-015-0216-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shao L, et al. Successful treatment with oseltamivir phosphate in a patient with chronic immune thrombocytopenia positive for anti-GPIb/IX autoantibody. Platelets. 2015;26(5):495–7. doi: 10.3109/09537104.2014.948838. [DOI] [PubMed] [Google Scholar]
- 58.Sandhoff K, Harzer K. Gangliosides and gangliosidoses: principles of molecular and metabolic pathogenesis. J Neurosci. 2013;33(25):10195–208. doi: 10.1523/JNEUROSCI.0822-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sikora E, Mosieniak G, Sliwinska MA. Morphological and functional characteristic of senescent cancer cells. Curr Drug Targets. 2015 doi: 10.2174/1389450116666151019094724. [DOI] [PubMed] [Google Scholar]
- 60.Klement K, Goodarzi AA. DNA double strand break responses and chromatin alterations within the aging cell. Exp Cell Res. 2014;329(1):42–52. doi: 10.1016/j.yexcr.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 61.Kornfeld S, Mellman I. The biogenesis of lysosomes. Annu Rev Cell Biol. 1989;5:483–525. doi: 10.1146/annurev.cb.05.110189.002411. [DOI] [PubMed] [Google Scholar]
- 62.Stinchcombe J, Bossi G, Griffiths G. Linking albinism and immunity: the secrets of secretory lysosomes. Science. 2004;305(5680):55–59. doi: 10.1126/science.1095291. [DOI] [PubMed] [Google Scholar]
- 63.Kile BT. The role of apoptosis in megakaryocytes and platelets. Br J Haematol. 2014;165(2):217–26. doi: 10.1111/bjh.12757. [DOI] [PubMed] [Google Scholar]
- 64.Tringali C, et al. Silencing of membrane-associated sialidase Neu3 diminishes apoptosis resistance and triggers megakaryocytic differentiation of chronic myeloid leukemic cells K562 through the increase of ganglioside GM3. Cell Death Differ. 2009;16(1):164–74. doi: 10.1038/cdd.2008.141. [DOI] [PubMed] [Google Scholar]
- 65.Miyagi T, et al. Sialidase significance for cancer progression. Glycoconj J. 2012;29(8-9):567–77. doi: 10.1007/s10719-012-9394-1. [DOI] [PubMed] [Google Scholar]
- 66.Yamaguchi K, et al. Reduced susceptibility to colitis-associated colon carcinogenesis in mice lacking plasma membrane-associated sialidase. PLoS One. 2012;7(7):e41132. doi: 10.1371/journal.pone.0041132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu ZJ, et al. Expansion of the neonatal platelet mass is achieved via an extension of platelet lifespan. Blood. 2014;123(22):3381–9. doi: 10.1182/blood-2013-06-508200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cines DB, et al. The ITP syndrome: pathogenic and clinical diversity. Blood. 2009;113(26):6511–21. doi: 10.1182/blood-2009-01-129155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nugent D, et al. Pathogenesis of chronic immune thrombocytopenia: increased platelet destruction and/or decreased platelet production. Br J Haematol. 2009;146(6):585–96. doi: 10.1111/j.1365-2141.2009.07717.x. [DOI] [PubMed] [Google Scholar]
- 70.Cines DB, McMillan R. Pathogenesis of chronic immune thrombocytopenic purpura. Curr Opin Hematol. 2007;14(5):511–4. doi: 10.1097/MOH.0b013e3282ba5552. [DOI] [PubMed] [Google Scholar]
- 71.McMillan R. The pathogenesis of chronic immune thrombocytopenic purpura. Semin Hematol. 2007;44(4 Suppl 5):S3–S11. doi: 10.1053/j.seminhematol.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 72.Li J, et al. Desialylation is a mechanism of Fc-independent platelet clearance and a therapeutic target in immune thrombocytopenia. Nat Commun. 2015;6:7737. doi: 10.1038/ncomms8737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li J, et al. Severe platelet desialylation in a patient with glycoprotein Ib/IX antibody-mediated immune thrombocytopenia and fatal pulmonary hemorrhage. Haematologica. 2014;99(4):e61–3. doi: 10.3324/haematol.2013.102897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bergmeier W, et al. Structural and functional characterization of the mouse von Willebrand factor receptor GPIb-IX with novel monoclonal antibodies. Blood. 2000;95(3):886–93. [PubMed] [Google Scholar]
- 75.Maurer E, et al. Targeting platelet GPIbβ reduces platelet adhesion, GPIb signaling and thrombin generation and prevents arterial thrombosis. Arteriosclerosis, thrombosis, and vascular biology. 2013;33(6):1221–9. doi: 10.1161/ATVBAHA.112.301013. [DOI] [PubMed] [Google Scholar]
- 76.Stolla M, et al. CalDAG-GEFI deficiency protects mice in a novel model of Fcgamma RIIA-mediated thrombosis and thrombocytopenia. Blood. 2011;118(4):1113–20. doi: 10.1182/blood-2011-03-342352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liang X, et al. Dimerization of glycoprotein Ibα is not sufficient to induce platelet clearance. J Thromb Haemost. 2015 doi: 10.1111/jth.13221. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yan R, et al. Glycoprotein Ibalpha clustering induces macrophage-mediated platelet clearance in the liver. Thromb Haemost. 2015;113(1):107–17. doi: 10.1160/TH14-03-0217. [DOI] [PubMed] [Google Scholar]