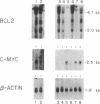
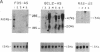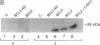Abstract
A gene-transfer approach was used to explore the function of the BCL2 (B-cell lymphoma/leukemia 2) gene in a human T-cell line, Jurkat. Though stable introduction of a BCL2 expression plasmid into Jurkat T cells was by itself insufficient, the combined transfer of BCL2 and MYC genes markedly enhanced the tumorigenicity of these cells in athymic mice. Moreover, a BCL2 antisense expression plasmid ablated tumor formation by Jurkat cells, providing further evidence that this oncogene contributes to the regulation of the in vivo growth of these human T lymphocytes. In addition to their influence on tumor formation, BCL2 sense and antisense expression plasmids increased and decreased, respectively, the in vitro survival of Jurkat T cells in serum-free medium. These observations extend to T cells the finding of synergy of BCL2 with MYC previously reported for B cells and provide evidence that BCL2 can regulate the growth of human T cells.
Full text
PDF
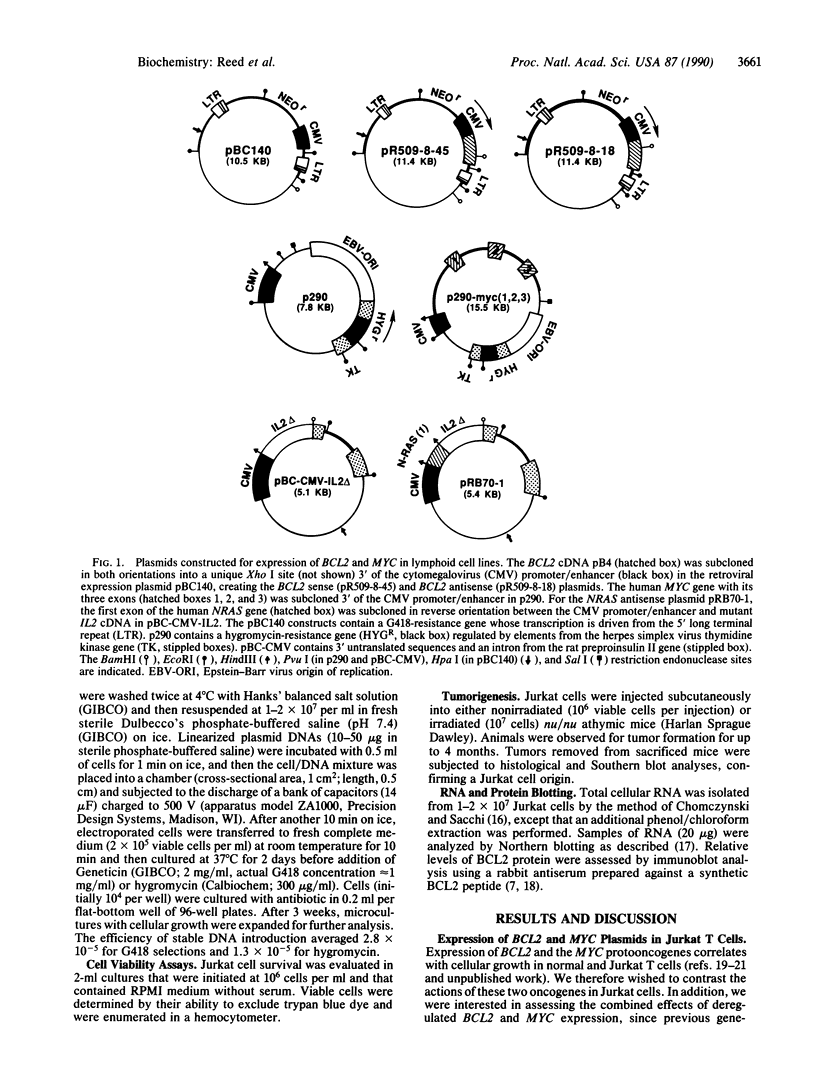

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Coppola J. A., Parker J. M., Schuler G. D., Cole M. D. Continued withdrawal from the cell cycle and regulation of cellular genes in mouse erythroleukemia cells blocked in differentiation by the c-myc oncogene. Mol Cell Biol. 1989 Apr;9(4):1714–1720. doi: 10.1128/mcb.9.4.1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen B. R. Trans-activation of human immunodeficiency virus occurs via a bimodal mechanism. Cell. 1986 Sep 26;46(7):973–982. doi: 10.1016/0092-8674(86)90696-3. [DOI] [PubMed] [Google Scholar]
- Curran T., MacConnell W. P., van Straaten F., Verma I. M. Structure of the FBJ murine osteosarcoma virus genome: molecular cloning of its associated helper virus and the cellular homolog of the v-fos gene from mouse and human cells. Mol Cell Biol. 1983 May;3(5):914–921. doi: 10.1128/mcb.3.5.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla-Favera R., Wong-Staal F., Gallo R. C. Onc gene amplification in promyelocytic leukaemia cell line HL-60 and primary leukaemic cells of the same patient. Nature. 1982 Sep 2;299(5878):61–63. doi: 10.1038/299061a0. [DOI] [PubMed] [Google Scholar]
- De Jong D., Voetdijk B. M., Beverstock G. C., van Ommen G. J., Willemze R., Kluin P. M. Activation of the c-myc oncogene in a precursor-B-cell blast crisis of follicular lymphoma, presenting as composite lymphoma. N Engl J Med. 1988 May 26;318(21):1373–1378. doi: 10.1056/NEJM198805263182106. [DOI] [PubMed] [Google Scholar]
- Freytag S. O. Enforced expression of the c-myc oncogene inhibits cell differentiation by precluding entry into a distinct predifferentiation state in G0/G1. Mol Cell Biol. 1988 Apr;8(4):1614–1624. doi: 10.1128/mcb.8.4.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauwerky C. E., Hoxie J., Nowell P. C., Croce C. M. Pre-B-cell leukemia with a t(8; 14) and a t(14; 18) translocation is preceded by follicular lymphoma. Oncogene. 1988 May;2(5):431–435. [PubMed] [Google Scholar]
- Greene W. C., Robb R. J., Depper J. M., Leonard W. J., Drogula C., Svetlik P. B., Wong-Staal F., Gallo R. C., Waldmann T. A. Phorbol diester induces expression of Tac antigen on human acute T lymphocytic leukemic cells. J Immunol. 1984 Aug;133(2):1042–1047. [PubMed] [Google Scholar]
- Haldar S., Beatty C., Tsujimoto Y., Croce C. M. The bcl-2 gene encodes a novel G protein. Nature. 1989 Nov 9;342(6246):195–198. doi: 10.1038/342195a0. [DOI] [PubMed] [Google Scholar]
- McDonnell T. J., Deane N., Platt F. M., Nunez G., Jaeger U., McKearn J. P., Korsmeyer S. J. bcl-2-immunoglobulin transgenic mice demonstrate extended B cell survival and follicular lymphoproliferation. Cell. 1989 Apr 7;57(1):79–88. doi: 10.1016/0092-8674(89)90174-8. [DOI] [PubMed] [Google Scholar]
- Nunez G., Seto M., Seremetis S., Ferrero D., Grignani F., Korsmeyer S. J., Dalla-Favera R. Growth- and tumor-promoting effects of deregulated BCL2 in human B-lymphoblastoid cells. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4589–4593. doi: 10.1073/pnas.86.12.4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed J. C., Haldar S., Cuddy M. P., Croce C., Makover D. Deregulated BCL2 expression enhances growth of a human B cell line. Oncogene. 1989 Sep;4(9):1123–1127. [PubMed] [Google Scholar]
- Reed J. C., Sabath D. E., Hoover R. G., Prystowsky M. B. Recombinant interleukin 2 regulates levels of c-myc mRNA in a cloned murine T lymphocyte. Mol Cell Biol. 1985 Dec;5(12):3361–3368. doi: 10.1128/mcb.5.12.3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed J. C., Tsujimoto Y., Alpers J. D., Croce C. M., Nowell P. C. Regulation of bcl-2 proto-oncogene expression during normal human lymphocyte proliferation. Science. 1987 Jun 5;236(4806):1295–1299. doi: 10.1126/science.3495884. [DOI] [PubMed] [Google Scholar]
- Reed J. C., Tsujimoto Y., Epstein S. F., Cuddy M., Slabiak T., Nowell P. C., Croce C. M. Regulation of bcl-2 gene expression in lymphoid cell lines containing normal #18 or t(14;18) chromosomes. Oncogene Res. 1989;4(4):271–282. [PubMed] [Google Scholar]
- Schneider M. D., Perryman M. B., Payne P. A., Spizz G., Roberts R., Olson E. N. Autonomous expression of c-myc in BC3H1 cells partially inhibits but does not prevent myogenic differentiation. Mol Cell Biol. 1987 May;7(5):1973–1977. doi: 10.1128/mcb.7.5.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto M., Jaeger U., Hockett R. D., Graninger W., Bennett S., Goldman P., Korsmeyer S. J. Alternative promoters and exons, somatic mutation and deregulation of the Bcl-2-Ig fusion gene in lymphoma. EMBO J. 1988 Jan;7(1):123–131. doi: 10.1002/j.1460-2075.1988.tb02791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimoto Y., Croce C. M. Analysis of the structure, transcripts, and protein products of bcl-2, the gene involved in human follicular lymphoma. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5214–5218. doi: 10.1073/pnas.83.14.5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimoto Y. Overexpression of the human BCL-2 gene product results in growth enhancement of Epstein-Barr virus-immortalized B cells. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1958–1962. doi: 10.1073/pnas.86.6.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaux D. L., Cory S., Adams J. M. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988 Sep 29;335(6189):440–442. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- Weiss L. M., Warnke R. A., Sklar J., Cleary M. L. Molecular analysis of the t(14;18) chromosomal translocation in malignant lymphomas. N Engl J Med. 1987 Nov 5;317(19):1185–1189. doi: 10.1056/NEJM198711053171904. [DOI] [PubMed] [Google Scholar]