Abstract
Abstract: Background: The rhizome of Oni-dokoro (a wild yam, Dioscorea tokoro) has extremely bitter taste and is not generally regarded edible;, however, in northern part of Japan, such as Iwate and a part of Aomori, it is used as health promoting food. To clarify the reason, we examined the biologically active compounds in the rhizome collected at Iwate and compared them from the other area in literature.
Methods: The acetonitrile extract from northern part of Japan was purified by bioassay-guided separation using antiproliferative activity to human leukemia HL-60 cell, and protodioscin (PD) was isolated and identified by instrumental analyses as the major active compound.
Results: PD known as a saponin with four sugar moieties, an inhibitor for platelet aggregation, and a low density lipoprotein (LPL) lowering agent, displayed strong growth inhibitory effect to HL-60. The literature search suggested that the rhizome from other area contained dioscin and other saponins with three sugar moieties as their major component. We assume that the edible and health promoting effect of the rhizome in the particular area is partially derived from these different components.
Conclusion: We were interested in the differences of utilization in the rhizome of wild yam Dioscorea tokoro, and examined the chemical composition in the rhizome to find protodioscin as antiproliferative compound to HL-60. In the report from other area, the rhizome exhibited dioscin as the major compound. Our study indicated that the protodioscin/dioscin composition varied regionally, although the reason is still needs to be investigated.
Keywords: Protodioscin, dioscin, Dioscorea tokoro, HL-60, antiproliferation
Introduction
In Dioscorea species, one such as Dioscorea japonica has been well utilized as the cultivated edible yam, but many of wild varieties have the rhizome with bitter and pungent taste and are not generally edible. D. tokoro is one of those wild yams and the rhizome has not been generally used for daily food. However, we found that in certain areas, especially northern part of Japan, Iwate and a part of Aomori prefectures, the rhizome has been used for health promoting food traditionally.
D. tokoro has been reported for its steroidal saponins, such as dioscin and gracillin, but not much has been described for their health promoting effects. We examined the acetonitrile extract of D. tokoro rhizome for antiproliferative activity to HL-60, human leukemia cell, isolated and identified protodioscin (PD) as the major active compound.
Materials and Methods
Cells. HL-60 cells were obtained from the RIKEN BioResource Center (Tsukuba, Japan), and were maintained in RPMI1640 medium supplemented with 10% fetal bovine serum (FBS).
Cell proliferation assay. The method was the same as described before from our research group [1]. Briefly, the cells were grown in 96-well microtiter plates, and triplicate plates were prepared. To each well HL-60 cell suspension was added, and then mixed with the medium containing serial dilution of samples to be assayed. The level of cell proliferation was measured by using alamar BlueTM (Biosource International, Lewisville, TX, USA), an oxidation-reduction indicator. After 3 days of incubation, alamar Blue was aseptically added to each well, and incubated for 24 hr. Cellular proliferation (% of untreated positive control) was calculated with the following equation (1). In the equation, A570 and A595 are the absorbance at 570 nm and 595 nm, respectively. The blank contains a sample and buffer solution to eliminate the absorbance derived from colored sample.
 (1)
(1)
Extraction and Separation. The rhizome of Dioscorea tokoro MAKINO was collected in the suburb of Tohno city, Iwate, Japan, washed and dried at the room temperature under shade for two days. The air dried rhizome (203.8 g) was cut into 2 cm cubes and homogenized in 500 mL of acetonitrile (CH3CN). The homogenate was filtered and the filtrate was condensed in vacuo to leave a brown residue. The residue was dissolved in water and the aqueous extract was re-extracted three times with ethyl acetate (EtOAc). The EtOAc was evaporated in vacuo to give a brown solid (0.29 g) with the IC50 of 19.9 ppm to HL-60. The aqueous layer was freeze-dried to give the crude brown powder (3.28 g) with the IC50 of 17.9 ppm. The antiproliferative compounds from aqueous layer were purified by repeated chromatography according to the results from the bioassay of each fractions on HL-60.
Isolation. The aqueous extract (3.28 g) was chromatographed over HP-20 (Nihon Rensui, Tokyo, Japan) with the eluent as CH3CN in H2O (0, 20, 40, 60, 80, 100%) to give 6 fractions, fr.1 (1787.2 mg), fr.2 (384.3 mg), fr.3 (1027.0 mg), fr.4 (51.9 mg), fr.5 (18.9 mg), and fr.6 (215.8 mg). The antiproliferation assay showed only fraction 3 was active. A part of fr.3 (703.4 mg) was separated over octadecylsilicate (ODS) (Nakarai-tesk Co., Kyoto, Japan) 50 g with CH3CN in H2O as the eluent (20, 25, 30, 35, 40, 60, 80, and 100%) to give 8 fractions, fr.3-1 (39.4 mg), fr.3-2(500.0 mg), fr.3-3 (299.3 mg), fr.3-4 (203.7 mg), fr.3-5 (12.2 mg), fr.3-6 (4.5 mg), fr.3-7 (0.7 mg), and fr.3-8 (3.3 mg). Since the fr.3-3 was active to HL-60, it was re-chromatographed over ODS (Nakarai-tesk Co., Kyoto, Japan) 30 g with CH3CN in H2O as the eluent (20, 25, 30, 40, 60, 80,and 100%) to give 12 fractions, fr.3-3-1 (3.4 mg), fr.3-3-2 (28.9 mg), fr.3-3-3-1 (19.7 mg), fr.3-3-3-2 (215.3 mg), fr.3-3-3-3 (1.3 mg), fr.3-3-3-4 (0 mg), fr.3-3-4 (5.5 mg), fr.3-3-5 (2.8 mg), fr.3-3-6 (0.4 mg), fr3-3-7 (0.5 mg), fr3-3-8 (2.5 mg). The fr3-3-3-2 (215.3 mg)contained only one compound and the yield was 1.54 mg/g fresh weight. The IC50 value to HL-60 antiproliferation was 5.1 μM (Fig. 1), and the chemical structure was analyzed by instrumental analyses.
Fig. (1).
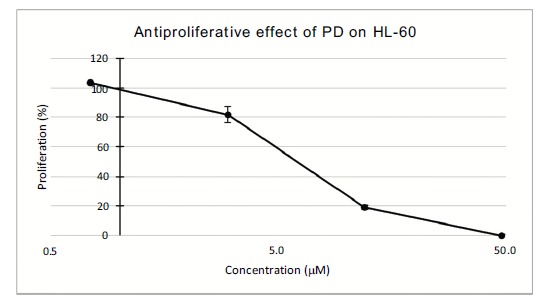
Antiprolirative effect of PD on HL-60.
Structure elucidation. The spectral data are as follows. Colorless oil. HR-ESI-MS: m/z 1071.5367 observed for [M + Na]+, 1071.5346 calcd. for [C51H84O22Na]+. 1H-NMR and 13C-NMR (pyridine-d5, δH at 600 MHz and δC at 150 MHz) data in ppm are shown in Table 1.
Table 1.
1H-NMR and 13C-NMR (pyridine-d5) data of protodioscin.
| Position | δH (J in Hz) | δC | Position | δH (J in Hz) | δC |
|---|---|---|---|---|---|
| 1 | 37.1 | 3-O-Glc-1' | 4.96 (d, 6.60) | 100.2 | |
| 2 | 30.1 | 2' | 78.0 | ||
| 3 | 78.6 | 3' | 76.9 | ||
| 4 | 38.9 | 4' | 78.5 | ||
| 5 | 140.7 | 5' | 77.8 | ||
| 6 | 5.34 (br d, appox. 4.2) | 121.7 | 6' | 61.2 | |
| 7 | 32.1 | 2'-O-Rha-1” | 6.41 (br s) | 102.0 | |
| 8 | 31.6 | 2” | 72.5 | ||
| 9 | 50.3 | 3” | 72.7 | ||
| 10 | 37.5 | 4” | 73.9 | ||
| 11 | 21.0 | 5” | 69.5 | ||
| 12 | 39.7 | 6” | 1.77 (d, 6.60) | 18.6 | |
| 13 | 40.7 | 4'-O-Rha-1”' | 5.87 (br s) | 102.8 | |
| 14 | 56.5 | 2”' | 72.5 | ||
| 15 | 32.3 | 3”' | 72.8 | ||
| 16 | 81.3 | 4”' | 74.1 | ||
| 17 | 64.1 | 5”' | 70.4 | ||
| 18 | 0.82 (s) | 16.3 | 6”' | 1.64 (d, 6.00) | 18.5 |
| 19 | 1.05 (s) | 19.4 | 26-O-Glc-1”” | 4.87 (br s) | 104.9 |
| 20 | 40.4 | 2”” | 75.1 | ||
| 21 | 1.20 (d, 6.60) | 16.2 | 3”” | 77.9 | |
| 22 | 112.6 | 4”” | 71.7 | ||
| 23 | 30.8 | 5”” | 78.5 | ||
| 24 | 28.1 | 6”” | 62.8 | ||
| 25 | 34.2 | ||||
| 26 | 79.0 | ||||
| 27 | 1.01 (d, 6.00) | 17.1 |
Results and Discussion
The wild yam Dioscorea tokoro is distributed among wide area in China and Japan, but because of its astringent and bitter taste, the rhizome is not edible for the most of those areas. However, in Iwate and Aomori area in Japan, it is used as the health supporting food. The rhizome grown wild is usually 1~3 cm in diameter and 10~20 cm in length, so that it is not for staple food, but believed in the effect of recovering from tiredness. We were interested in the compounds of the rhizome which might be different from growth area to area. We collected the rhizome at Tohno, Iwate, and the extract was separated by the repeated chromatography, resulted in the isolation and identification of protodioscin (Fig. 2) [2, 3], with a potent antiproliferative activity to HL-60 (IC50 = 5.1 μM, Fig. 1).
Fig. (2).
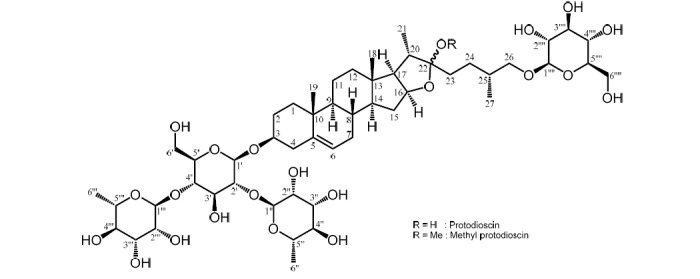
The structure of Protodioscin.
During the extraction, the use of MeOH as the extracting solvent resulted in the isolation of PD methyl ether (Fig. 2), with the yield of 1.06 mg/g fresh weight. Additionally, when ethanol was used as the extracting solvent, PD ethyl ether (m/z 1099.5694 ([M+Na]+), calculated for C53H88O22Na+ = 1099.5659) was detected by ESI-HR-MS (positive). We concluded that the methyl and ethyl ethers should be the artifact during the extraction, and thus CH3CN was used for extraction. We finally isolated an active compound and its physico-chemical data were identical as PD in the literature [3], with the yield of 1.54 mg/g fresh weight. From these results and the fact that the analysis of our CH3CN extract did not show PD methyl ether molecular ion peak on LC-MS, the original material should only contain free hydroxyl at 22-carbon.
PD is one of the steroidal saponins originally found in Dioscorea nipponica [4], and later from several Dioscorea species and other plant material, but this is the first report from D. tokoro. In Dioscorea species, the major saponin being reported as dioscin possessing 3 sugars at carbon-1 (Fig. 3), while PD has 4 sugars attached, and the three at carbon-1 but the fourth sugar at carbon-26. According to the previous report, PD displayed strong growth inhibitory effect against HL-60 cells by the induction of apoptosis but weak effect on KATO III cells [5]. PD is also known to have platelet-aggregation inhibitory activity [6] and antihyperlipidemic effect with the LDL increment [7].
Fig. (3).
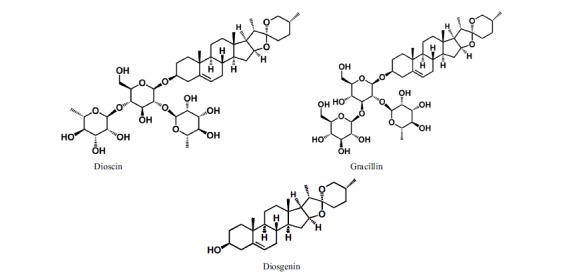
The structure of dioscin, gracillin, and diosgenin.
There were some reports about the saponins such as dioscin and gracilln, and sapogenin such as diosgenin (Fig. 3) from D. tokoro, whose structures were closely related to PD. Dioscin is reported to have platelet-aggregation inhibitory [6], antifungal [8], and hypoglycemic [9] activities, and gracillin was reported as an antiparacitic agent [10]. Interestingly, D. tokoro rhizome extracts exhibited different compound contents depending on the locations of the plant collected. The rhizome in Fukuoka, Kyushu Japan [2] contained dioscin and gracillin, and dioscin as the major saponin, but no report on protodioscin. However, in our Iwate material, we found protodioscin as major saponin, and dioscin was only detected from the analyses of thin layer chromatography and ESI-HR-MS.
In the local area where D. tokoro is used as food, the rhizome is steamed and served. We prepared the steamed (20 minutes) rhizome and examined the amount of PD undecomposed. A piece of the rhizome was cut into halves and one was steamed. Both the steamed and the raw material were extracted by CH3CN, and the extracts were measured by LC-MS using the isolated PD as standard. The amount in the steamed sample was 0.87 mg/g fresh weight while in the raw material 0.65 mg/g fresh weight, which was 1.34 times larger. This suggested that PD is resistant to the steam treatment. The reason that the isolated amounts from the steamed was higher than the raw probably because the cell wall was destroyed by heat and the components were easier to be extracted into the solvent.
The wild yam Dioscorea tokoro is grown among wide area of China and Japan, but to the best of our knowledge, the rhizome is utilized in a very limited area. We examined the rhizome from Iwate area for the biologically active compounds by the guidance of HL-60 antiproliferating activity, and identify protodioscin as major component. Since the literature search suggested that the rhizome obtained in Kyoto seemed rich in dioscin [2], while Iwate sample appeared protodioscin as major saponin.
The reason of differences in their utilization is still unknown, the sample examined in this research showed different saponin contents. The structural difference of dioscin, and protodioscin, should result from different biosynthetic enzymes. We are currently investigating the glucosidases biosynthetically related to saponins in Dioscorea species.
Conclusion
We were interested in the differences of utilization in the rhizome of wild yam Dioscorea tokoro, and examined the chemical composition in the rhizome to find protodioscin as antiproliferative compound to HL-60. In the report from other area, rhizome exhibited dioscin as the major compound. Our study indicated that the protodioscin/dioscin composition varied regionally, and the reason still needs to be investigated.
ACKNOWLEDGEMENTS
The HR-ESI-MS (Exactive, Thermo Fisher Scientific, Inc.) and 400 MHz NMR (JNM ECP400, JEOL) spectra were measured at Akita prefectural university, and the 600 MHz NMR (JNM-ECA600, JEOL) spectra were measured at the Venture incubation center, Akita University (AU-VIC).
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
References
- 1.Kawaii S., Ikuina T., Hikima T., Tokiwano T., Yoshizawa Y. Relationship between structure and antiproliferative activity of polymethoxyflavones towards HL60 cells. Anticancer Res. 2012;32(12):5239–5244. [PubMed] [Google Scholar]
- 2.Tsukamoto T., Kawasaki T., Yamauchi T. Japanese Dioscoreaceae. VIII. Saponins from the rhizomes of Dioscorea tokoro Makino. Yakugaku Zasshi. 1957;77(8):1225–1229. [Google Scholar]; Kawasaki T., Yamauchi T. Structures of prosapogenin-B and -A of dioscin and cooccurrence of B with dioscin in the rhizoma of Dioscorea tokoro Makino. Chem. Pharm. Bull. (Tokyo) 1968;16(6):1070–1075. doi: 10.1248/cpb.16.1070. [DOI] [PubMed] [Google Scholar]
- 3.Zhao Y., Feng B., Kang L-P., Lin B., Huang H-Z., Cong Y-W., Ma B-P. Biotransformatin of dichotomin by pectinex BE XXL. Chin. J. Nat. Med. 2009;7(5):381–389. [Google Scholar]
- 4.Tsukamoto T., Ueno Y. Research on the glycoside from Dioscorea tokoro Makino (report I), about dioscin, Dioscorea-sapotoxin and diosgenin. Yakugaku Zasshi. 1936;56(10):802–807. [Google Scholar]; Tsukamoto T., Ueno Y., Ohta Z. About the structure of diosgenins (part I), research on the glycosides from Dioscorea tokoro Makino (report II). Yakugaku Zasshi. 1936;56(11):931–940. [Google Scholar]; Tsukamoto T., Ueno Y., Ohta Z. About the structure of diosgenins (part II), research on the glycoside from Dioscorea tokoro Makino (report III). Yakugaku Zasshi. 1937;57(11):985–991. [Google Scholar]
- 5.Hibasami H., Moteki H., Ishikawa K., Katsuzaki H., Imai K., Yoshioka K., Ishii Y., Komiya T. Protodioscin isolated from fenugreek (Trigonella foenumgraecum L.) induces cell death and morphological change indicative of apoptosis in leukemic cell line H-60, but not in gastric cancer cell line KATO III. Int. J. Mol. Med. 2003;1(1):23–26. [PubMed] [Google Scholar]
- 6.Li H., Huang W., Wen Y., Gong G., Zhao Q., Yu G. Anti-thrombotic activity and chemical characterization of steroidal saponins from Dioscorea zingiberensis C.H. Wright. Fitoterapia. 2010;81(8):1147–1156. doi: 10.1016/j.fitote.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 7.Wang T., Choi R.C., Li J., Li J., Bi C.W., Zang L., Liu Z., Dong T.T., Bi K., Tsim K.W. Antihyperlipidemic effect of protodioscin, an active ingredient isolated from the rhizomes of Dioscorea nipponica. Planta Med. 2010;76(15):1642–1646. doi: 10.1055/s-0030-1249960. [DOI] [PubMed] [Google Scholar]
- 8.Choa J., Choia H., Leea J., Kimb M-S., Sohnb H-Y., Leea D.G. The antifungal activity and membrane-disruptive action of dioscin extracted from Dioscorea nipponica. Biochim. Biophys. Acta. 2013;1828(3):1153–1158. doi: 10.1016/j.bbamem.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 9.Yoshikawa M., Xu F., Morikawa T., Pongpiriyadacha Y., Nakamura S., Asao Y., Kumahara Y., Matsuda H. Medicinal flowers. XII. (1) New spirostane-type steroid saponins with antidiabetogenic activity from Borassus flabellifer. Chem. Pharm. Bull. (Tokyo) 2007;55(2):308–316. doi: 10.1248/cpb.55.308. [DOI] [PubMed] [Google Scholar]
- 10.Zheng W., Yan C.M., Zhang Y.B., Li Z.H., Li Z., Li X.Y., Wang Z.W., Wang X., Chen W.Q., Yu X.H. Antiparasitic efficacy of Gracillin and Zingibernsis newsaponin from Costus speciosus (Koen ex. Retz) Sm. against Ichthyophthirius multifiliis. Parasitology. 2015;142(3):473–479. doi: 10.1017/S0031182014001358. [DOI] [PubMed] [Google Scholar]


