Abstract
Neisseria meningitidis is differentiated into 12 distinct serogroups, of which A, B, C, W, X, and Y are medically most important and represent an important health problem in different parts of the world. The epidemiology of N. meningitidis is unpredictable over time and across geographic regions. Recent epidemiological surveillance has indicated an increase of serogroup Y invasive meningococcal disease in some parts of Europe as shown in the epidemiological data for 2010 and 2011 from various European countries previously published in this journal.1,2 Here, data from 33 European countries is reported indicating that the emergence of serogroup Y continued in 2012 in various regions of Europe, especially in Scandinavia, while in Eastern and South-Eastern Europe the importance of serogroup Y remained low.
Keywords: epidemiology, Europe, invasive meningococcal disease, serogroup Y, meningococcal vaccines, surveillance
Introduction
N. meningitidis continues to cause substantial rates of illness, risk of long-term sequelae and death worldwide, and is associated with significant costs.3,4 The incidence of invasive meningococcal disease (IMD) is highest in infants and young children, with a second, although lower peak of disease among adolescents and young adults. Moreover, incidence of IMD increases in the elderly. Six immunologically distinct serogroups of N. meningitidis (A, B, C, W, X, and Y) have been associated with significant pathogenic potential. Worldwide, over 90% of IMD is caused by serogroups A, B, C, Y, and W.
Country specific incidence rates and serogroup and age distributions provide important information for the public health authorities to determine optimal national immunization policies against IMD. Various meningococcal conjugate vaccines based on capsular polysaccharides have been developed, including monovalent serogroup A and C vaccines, and three quadrivalent ACWY vaccines and recently, a protein based serogroup B vaccine has been licensed in Europe. These vaccines are used according to the regional epidemiological situation and serogroup distribution.
Until recently, serogroup Y has been of minor importance in Europe, accounting for approximately 2% or less of reported IMD cases and mainly observed among elderly.5 Recently, an increase in both absolute numbers and relative proportion of serogroup Y cases has been reported in various European countries for 2010 and 2011 and a shift to younger groups of age in some countries.1,2 Here, we want to continue reporting on epidemiological data of serogroup Y in Europe and present data which has been collected for 2012 by various national reference laboratories by their locally established surveillance methods.
Results
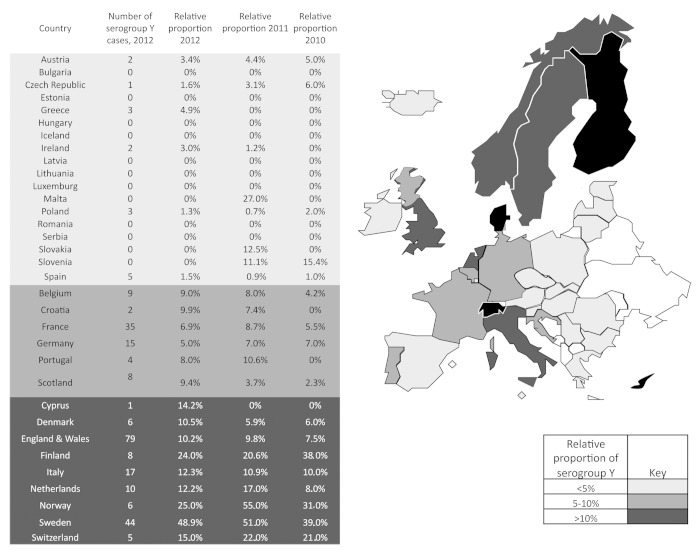
Over the last ten years, the relative importance of serogroup Y has increased in various regions in Europe. In recently published data for 2010 and 2011, the highest relative proportion for serogroup Y IMD has been reported from Scandinavian countries (up to 55%), followed by the most central/Western European countries (5–10%), while the relative proportion has been lowest (<5%) in most of the Eastern/South-Eastern European countries.1,2 For 2012, this picture has not significantly changed. In Figure 1, the absolute number and relative proportion of serogroup Y cases in 2012 are presented for 33 European countries from data that was available. Similar to 2010 and 2011, the relative proportion of serogroup Y cases remained high in Scandinavia—Sweden 49%, Norway 25%, and Finland 24%, and in Denmark, an increase has been reported from 5.9% in 2011 to 10.5% in 2012. In Central, Southern, and Western Europe, the number of reported cases did not significantly change compared with the previous year, and with a few exceptions was in the range between 5 and 10%. Notably, in all countries from Eastern and South-Eastern Europe from where data was available, the relative importance of serogroup Y remained low (<5%). This relatively low proportion of serogroup Y has also been reported from the most Western European region, Iceland and Ireland, while a slight, but continuous increase over the last three years has been observed in Scotland and England and Wales reaching 9.4% and 10.2% respectively in 2012.
Figure 1. Relative proportion of N. meningitidis serogroup Y in various European countries in 2012. The figure is based on data communicated by the scientists listed in the Acknowledgment and/or published in web pages of national public institutes.10 The data for 2012 are compared with data from 2010 and 2011, which have been communicated earlier.1,2 Color coding refers 2012 data. Data were not available to the authors for countries shown in white.
Discussion
The observed increase of MenY disease in Europe has important public health implications and will require further close monitoring. It is worth noting that not all data presented here have been collected based on the same types of case definitions and surveillance methods and local methodologies differences may affect overall results. However, after the third year (2010, 2011, and 2012) of detailed collecting and reporting of data we conclude that the trend of increase of relative importance of serogroup Y in various regions of Europe is evident and was confirmed. While we have reported data from 23 countries for 2011, we were able to increase the number to 33 for 2012, which results in a more complete and more valid overall picture. One should carefully analyze this epidemiological data set. Especially, in countries with small populations and/or low IMD incidence, a few cases can have a large impact in findings, e.g., the sudden high numbers for Malta and Slovakia in 2011 were not repeated in 2012 and therefore, the trend over several years should be interpreted. This is also true for Slovenia and Cyprus; in the latter country just one case has been reported in 2012, which has a great impact on the relative proportion in this country due to its low number of inhabitants. In Denmark, the relative increase of serogroup Y from 5.9% to 10.5% may be more the result of a decrease of the incidence of IMD in total, because the absolute number of serogroup Y cases increased only by one case from five to six. In Bulgaria, only a small number of isolates have been serogrouped. Thus, one cannot exclude underestimation of the absolute numbers of serogroup Y cases and the relative proportion of this serogroup.
The emergence of serogroup C in the 1990s–2000s has successfully been arrested in various European countries by the implementation of monovalent serogroup C conjugate vaccine. Catch-up campaigns and vaccination of adolescents who are the main reservoir for transmission of the meningococci resulted in a herd effect indirectly protecting those individuals not belonging to the age cohort who were vaccinated.
Currently, booster vaccinations of adolescents are considered to maintain the herd protection by keeping the carriage rate low with serogroup C meningococci of this age group.6 Consequently, UK has modified the vaccination schedule for serogroup C vaccine by introducing a booster injection for the 12 to 16 y olds because a booster dose is assessed essential to maintain protection into adolescence.7,8
In order to broaden the serogroup protection beyond serogroup C, some European countries (e.g., Austria, Czech Republic, Poland, Greece, and some regions in Italy) recommend the quadrivalent meningococcal serogroup ACWY conjugate vaccine for vaccination of adolescents. Reed and coworkers9 have performed a phase 3 carriage study in English university students with MenACWY-CRM vaccine. 2969 students in 10 universities across England were enrolled from September to December 2010 to receive one dose of MenACWY-CRM vaccine (n = 956) or other vaccines. Across the cumulative time points over one year, MenACWY-CRM vaccination was associated with a carriage reduction efficacy 32.7% against serogroup ACWY strains. The authors concluded that these results raise the possibility of an impact on individual carriage, which may translate into greater herd protection in settings where the vaccines are implemented broadly.
If serogroup Y continues to increase in certain regions of Europe, country-specific recommendations to use MenACWY vaccine instead of MenC vaccine may be considered targeting children or adolescents or some combinations of these vaccines and age groups. An efficient prevention of meningococcal disease however requires vaccination of large proportion of the community especially in order to induce herd effects.
We would like to add here some recommendations regarding surveillance and typing of serogroup Y IMD:
(1) Typing and fine typing of serogroup Y isolates, because countries with persistence of high proportion and high numbers of serogroup Y IMD (like Sweden) showed dominant new combination of MLST: porA VR1 and VR2:FetA; (2) Complete genome sequencing may also help understanding the emergence of MenY in Europe; (3) Surveillance of shift to younger age groups that may be associated to the introduction of new clones; (4) Systematic exploring the complement deficiencies in serogroup Y IMD that may not be linked to the emergence of new clones.
Disclosure of Potential Conflicts of Interest
M.B. is a full-time employee of Novartis Vaccines and Diagnostics, a manufacturer of various meningococcal vaccines. A.S. has received assistance to attend scientific meetings and honoraria for lecturing (Baxter, GlaxoSmithKline, Novartis, and Pfizer) and her laboratory has received research funding from GlaxoSmithKline, Novartis, and Pfizer. The other authors have no conflict of interest to declare concerning this work. The authors alone are responsible for the view expressed in this publication and do not necessarily represent the decisions, policy, or views of the institutes or company.
Acknowledgments
The authors are grateful to many colleagues for support, especially for sharing data, which has yet not been published or has been published in national languages and for the procurement of country-specific disease data.
Data and information were kindly provided (in alphabetical order) by Despo Pieridou-Bagatzouni, Nicosia General Hospital, Nicosia, Cyprus (23 August, 2013); Hans Blystad of the Norwegian Institute of the Public Health, Oslo, Norway (29 May, 2013); Suzana Bukovski of the University Hospital for Infectious Diseases “Dr. Fran Mihaljević,” Zagreb, Croatia (10 July, 2013); Rosa Cano of the Centro National de Epidemiología, Madrid, Spain (7 June, 2013); Davor Culic of the Meningococcus and Hemophilus Reference Laboratory, Srbija, Serbia (5 June, 2013); Stephane Paul Emonet of the Hôpitaux Universitaires de Genève, Switzerland (7 June, 2013); Thorolfur Gudnason of the Centre for Health Security and Communicable Disease Control, Reykjavík, Iceland (21 May, 2013); Sigrid Heuberger of the Österreichische Agentur für Gesundheit und Ernährungssicherheit GmbH, Nationales Referenzzentrum für Meningokokken, Graz, Austria (31 May, 2013); Steen Hoffmann of the Statens Serum Institut, Copenhagen, Denmark (22 May, 2013); Waleria Hryniewicz of the National Medicines Institute, Warsaw, Poland (30 April, 2013); Susanne Jacobsson of the Örebro University Hospital, Örebro, Sweden (13 June, 2013); Kadri Kermes of the United Laboratory of Tartu University Clinicum, Tartu, Estonia (16 May, 2013); Maria Koliou, Unit for Surveillance and Control of Communicable Diseases, Ministry of Health, Nicosia, Cyprus (29 August, 2013); Alenka Kraigher of the National Institute of Public Health, Ljubljana, Slovenia (7 June, 2013); Paula Kriz of the National Reference Laboratory for Meningococcal Infections, National Institute for Public Health, Prague, Czech Republic (15 May, 2013); Katalin Kristalovics and Judit Krisztina Horváth of the National Centre for Epidemiology, Budapest, Hungary (14 January, 2013); Irina Lucenko of the Centre for Disease Prevention and Control of Latvia, Riga, Latvia (2 August, 2013); Dimitar Nashev, National Center for infectious and Parasitic Diseases, Sofia, Bulgaria (9 September, 2013); David Pace of the Mater Dei Hospital, Msida, Malta (28 July, 2013); Maria Joao Simões of the Instituto Nacional de Saúde Dr. Ricardo Jorge, Lisboa, Portugal (16 January, 2014); Anna Skoczynska of the National Medicines Institute, Warsaw, Poland (15 May, 2013); Paola Stefanelli of the Istituto Superiore di Sanitá, Rome, Italy (22 May 2013); Muhamed-Kheir Taha of the Pasteur Institute, Paris, France (6 June, 2013); Greta Gargasiené of the Centre for Communicable Diseases and AIDS, Vilnius, Lithuania (23 May, 2013); Gérard Scheiden of the General Directorate of Health, Luxemburg, Luxemburg (7 June, 2013); Renata Šmit, University of Ljubljana, Faculty of Pharmacy, Ljubljana, Slovenia (17 January, 2014); Alison Smith-Palmer of the Health Protection Scotland, Glasgow, Scotland (14 May, 2013); Majia Toropainen of the National Institute for Health and Welfare, Helsinki, Finland (16 May, 2013); Georgina Tzanakaki of the National School of Public Health, Athens, Greece (14 May, 2013); Alena Vaculíková of the Public Health Authority, NRC for Meningococci, Bratislava, Slovak Republic (14 May, 2013); Arie van der Ende of the Reference Laboratory for Bacterial Meningitis, Amsterdam, The Netherlands (7 June, 2013).
Authors’ Contributions
M.B. drafted the outline of the manuscript. All authors were actively involved in reviewing the content and editing the text of the manuscript. All authors read and approved the final version of the manuscript.
Appeal
Readers of this article who can contribute with data regarding European countries which are not listed in Figure 1 are kindly requested to contact M.B.
Glossary
Abbreviations:
- CRM
Cross-Reactive Material a non-toxic mutant of diphtheria toxin
- IMD
invasive meningococcal disease
- MenACWY
meningococcal serogroups ACWY
References
- 1.Bröker M, Jacobsson S, DeTora L, Pace D, Taha M-K. Increase of meningococcal serogroup Y cases in Europe: a reason for concern? Hum Vaccin Immunother. 2012;8:685–8. doi: 10.4161/hv.20098. [DOI] [PubMed] [Google Scholar]
- 2.Bröker M, Jacobsson S, Kuusi M, Pace D, Simões MJ, Skoczynska A, Taha MK, Toropainen M, Tzanakaki G. Meningococcal serogroup Y emergence in Europe: update 2011. Hum Vaccin Immunother. 2012;8:1907–11. doi: 10.4161/hv.21794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vyse A, Anonychuk A, Jäkel A, Wieffer H, Nadel S. The burden and impact of severe and long-term sequelae of meningococcal disease. Expert Rev Anti Infect Ther. 2013;11:597–604. doi: 10.1586/eri.13.42. [DOI] [PubMed] [Google Scholar]
- 4.Anonychuk A, Woo G, Vyse A, Demarteau N, Tricco AC. The cost and public health burden of invasive meningococcal disease outbreaks: a systematic review. Pharmacoeconomics. 2013;31:563–76. doi: 10.1007/s40273-013-0057-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.European Union Invasive Bacterial Infections Surveillance (EU-IBIS) Network. Invasive Neisseria meningitidis in Europe 2006. Health Protection Agency, London 2006. Available from http://www.cuibis.org
- 6.Pollard AJ, Green C, Sadarangani M, Snape MD. Adolescents need a booster of serogroup C meningococcal vaccine to protect them and maintain population control of the disease. Arch Dis Child. 2013;98:248–51. doi: 10.1136/archdischild-2012-303103. [DOI] [PubMed] [Google Scholar]
- 7.Borrow R, Abad R, Trotter C, van der Klis FRM, Vazquez JA. Effectiveness of meningococcal serogroup C vaccine programmes. Vaccine. 2013;31:4477–86. doi: 10.1016/j.vaccine.2013.07.083. [DOI] [PubMed] [Google Scholar]
- 8.England NHS. Important changes to the national immunization programme in 2013-14, and introduction of rotavirus vaccination for babies at two and three months. Gateway Reference Number: 00047. April 30, 2013. Available from https://www.gov.uk/./130429_Rotavirus_tripartite_letter_FINAL.pdf
- 9.Reed RC, Baxter D, Chadwick DR, Faust SN, Finn A, Gordon S, et al. Impact of a quadrivalent conjugate (MenACWY-CRM) or a serogroup B (4CMenB) meningococcal vaccine on meningococcal carriage in English university students. 31. Annual Meeting of the European Society for Paediatric Infectious Diseases, Milan, May 28- June 1, 2013, Abstract A-534-0044-01472. [Google Scholar]
- 10.Web pages of national public institutes are not listed here, because many of them are in national languages, but not in English. Readers interested in the appropriate documents or links are requested to approach the corresponding author.