Abstract
Campylobacter jejuni is a major cause of diarrheal disease and results in high levels of morbidity and economic loss in both industrialized and developing regions of the world. To date, prior vaccine approaches have failed to confer protection against this enteric pathogen. Key challenges to the development of a practical Campylobacter vaccine for human use include a lack of understanding of Campylobacter pathogenesis and well-defined immune correlates of protection. With the discovery that C. jejuni expresses a capsule polysaccharide associated with virulence, a conjugate vaccine approach is currently being evaluated. Conjugate vaccines have been successfully developed and implemented against other invasive mucosal pathogens including Streptococcus pneumoniae, Neisseria meningitidis,and Hemophilus influenzae. Furthermore, Shigella-based conjugate vaccines based on lipopolysaccharide have shown promising results in field trials. A prototype C. jejuni conjugate vaccine is currently entering human testing.
Keywords: Campylobacter, conjugate vaccines, GBS, diarrheal disease, capsular polysaccharide
Introduction
Campylobacter jejuni is among the most common causes of diarrheal disease worldwide.1-4C. jejuni is a zoonotic pathogen and humans are most often infected by consumption of contaminated poultry, water, or raw milk.5-12 The incidence of varies widely, ranging from hyperendemic levels (40 000/100 000 children less than 5 years old) in developing regions of the world, especially Southeast Asia,4 to endemic levels (20–100/100 000 population) occurring as sporadic disease in young adults and infants in developed countries.13C. jejuni represent a significant cause of traveler’s diarrhea in persons from industrialized nations visiting hyperendemic regions.14 Although infection is not usually associated with mortality in developed countries, Campylobacter infections result in a high level of morbidity and loss of productivity. In the United States alone, it is estimated that greater than one million cases of diarrhea can be attributed to Campylobacter infection each year.15 In addition to acute diarrhea, C. jejuni is associated with a number of post infectious serious sequelae, including Guillain-Barré syndrome (GBS),16 reactive arthritis17, and irritable bowel syndrome (IBS).3,18 Moreover, an association of repeated C. jejuni infections with malnutrition has been reported in the developing world.19,20 The molecular basis for the association of C. jejuni with GBS is recognized to be due to the mimicry between lipooligosaccharide (LOS) chains of most C.jejuni strains, such that antibodies directed toward LOS cross react with human peripheral nerves.21
As a result of acute diseases and post-infectious sequelae, it has been estimated that in the US it costs over 2000 USD for the treatment of the average Campylobacter case and over 1.7 billion USD annually for all cases, and the quality-adjusted life years (QALYs) associated with Campylobacter disease totals 13 300 annually.22 Despite its significant impact on human health, relatively little is known regarding what constitutes protective immunity against this pathogen, and there is currently no licensed vaccine to prevent disease caused by C. jejuni.
Campylobacter Virulence/Pathogenesis
C. jejuni is considered an invasive enteric pathogen, but the molecular details of its pathogenesis remain enigmatic, due largely to the lack of reliable, non-primate animal models of disease. Flagella are critical to virulence for the organism to swim through the mucus lining of the intestine and to serve as a secretory organelle for a variety of proteins, some of which appear to play roles in invasion.23-27C. jejuni invasion is similar to that of Shigella spp., in that both usually invade the intestinal epithelium and are not generally found systemically, in contrast to Salmonella spp. The process of invasion of epithelial cells results in an influx of immune cells and cytokine release that is manifest as inflammatory diarrhea.28
Genome sequencing of numerous C. jejuni strains has failed to identify many virulence factors that are found in other, better-characterized pathogens. This suggests that C. jejuni utilizes unique pathogenic mechanisms. Indeed, one of the biggest surprises from the first genome sequence was the presence of an ABC capsular transport system, analogous to those found in a sub-set of other encapsulated pathogens. Studies from several labs quickly confirmed that the saccharide previously thought to be a lipopolysaccharide (LPS) was, in fact, a capsule.29,30 Mutation in the C. jejuni capsule transport system demonstrated that the capsule was the major serodeterminant of the Penner typing scheme, a passive slide hemaglutination assay that recognizes 47 different serotypes.30,31 Capsule characterization was quickly followed by studies linking this structure to virulence.32 Isogenic non-encapsulated mutants were demonstrated to be attenuated in an infant ferret model of diarrheal disease,32 exhibited reduced invasion of a human epithelial cell line in vitro,32 reduced colonization of chicken ceca33, and were more sensitive to complement mediated killing.34,35 More recent studies have also indicated that the polysaccharide capsule can be immunomodulatory.34,36
Special Challenges to C. jejuni Vaccine Development
The association of C. jejuni with GBS poses special problems to vaccine development. The use of any whole cell vaccine approach, although originally evaluated (see below), is precluded due to safety concerns. Similarly, the standard phase 2B challenge that is generally done to evaluate the efficacy of vaccines cannot be performed with C. jejuni strains expressing ganglioside mimics, although some early volunteer studies were done with such strains prior to our current understanding of GBS pathogenesis. However, despite the fact that the vast majority of C. jejuni strains express one or more ganglioside mimics in their outer LOS core, there have been some strains described that lack such mimicry. More recently, a human challenge model has been developed using such a strain, as outlined below.
Lessons Learned from Experimental Campylobacter Infections
Through the use of experimental Campylobacter infections in humans, much has been learned regarding pathogenesis of disease as well as immune responses to C. jejuni. In the first human challenge, conducted by Black et al.,37 healthy adults were experimentally infected with either C. jejuni strain 81–176 or strain A3249. For challenge studies using strain 81–176, doses ranged from 106-109 colony forming units (CFU), and the percentage of volunteers who developed diarrhea was 46%. Despite no apparent dose effect, this work established that experimental infection produces signs and symptoms that are comparable to those observed from natural infections in developed countries. In these studies, volunteers who fell ill following oral challenge with 108-109 CFU strain 81–176 exhibited significant increases in Campylobacter-specific IgA and IgM. Interestingly, volunteers who were infected and did not become ill did not show any increase in C. jejuni-specific antibodies over the observation period. Volunteers who had exhibited illness during primary infection with strain 81–176 (n = 7) were subjected to a homologous re-challenge one month after recovery. Although 5/7 veterans displayed stool colonization, no diarrheal disease was observed in these previously infected volunteers. In contrast, 6/12 control volunteers developed illness following oral challenge.
Tribble et al. also examined the duration of protection in humans experimentally infected with escalating doses of C. jejuni strain 81–176.38 Robust immune responses (systemic and mucosal) were observed in all naïve individuals following challenge, irrespective of dose. Volunteers initially infected with 109 CFU were selected for homologous re-challenge studies in which some individuals would be challenged 28–49 days (short-term volunteers (STV) or 1 year [long-term volunteers (LTV)] following primary infection. STV (8/8) were completely protected against illness following re-challenge whereas 4 out of 7 LTV displayed outcomes ranging from mild to severe illness. Morevoer, LTV exhibited an attenuated illness compared with naïve control volunteers.
These early human challenge studies using C. jejuni strain 81–176, which expresses GM2 and GM3 ganglioside mimics,39 were conducted prior to our understanding that such structures are closely associated with GBS. This risk led to the development of a new refined human challenge model using C. jejuni strain CG8421 that naturally lacks ganglioside mimicry in its core.40 Dose finding studies determined that a reduced inoculum (105 CFU) of strain CG8421 led to an improved diarrhea attack rate (93%) compared with what was achieved using strain 81–176 in which 109 CFU was needed to achieve an attack rate of >75%.38,41 Furthermore, Campylobacter-specific humoral and cell-mediated immune responses were comparable to what had been previously observed during experimental infection with strain 81–176. Surprisingly, following a homologous re-challenge 3 mo after initial recovery, individuals that had recovered from a primary infection with strain CG8421 (n = 8) were not protected against illness following secondary infection, with 8/8 volunteers experiencing campylobacteriosis and no reduction in symptoms.42
The studies utilizing C. jejuni strain 81–176 are important in demonstrating that immunity can be acquired following recovery from experimental Campylobacter infection, and can be long lasting.37,38 However, these human challenge studies lack adequate descriptions of protective antigens and precise mechanisms of immunity. Although Campylobacter-specific immune responses were measured in these studies, measurements were made against relatively crude mixtures of extracted cell surface proteins from C. jejuni with no true measure of the specificity of the immune response (e.g., anti-capsular IgG).37,38,41,42 Another critical point to remember is that there are key differences between the two challenge models that likely influence the acquisition of immunity against homologous re-challenge. These differences include inocula size, duration of infection, and time to re-infection, all of which can affect the generation of long-lasting memory responses.43,44 Thus, although homologous protection was not observed in humans re-challenged with CG8421, this should not diminish the value nor preclude the use of this challenge model in assessing the efficacy of candidate vaccines against disease caused by C. jejuni.
Previous C. jejuni Vaccine Approaches
There have been limited numbers of potential C. jejuni vaccines that have advanced to human testing and most have been reviewed recently.45 NMRC developed a killed, whole cell vaccine based on strain 81–176 in the 1990s which was immunogenic and safe during Phase 1 clinical evaluation, but was unsuccessful in a Phase 2b challenge with the same strain.45 A subunit protein vaccine based on the flagellin of C. jejuni also underwent phase 1 testing but was only moderately immunogenic.45 More recently, ACE Biosciences developed another protein subunit vaccine, ACE 393, that was not efficacious in phase 2B trials with GC8421 (http://www.tdvaccines.com/index.php?module=front:home&action=content&id=7&menuid=11; Clinical trials.gov; NCT00859716).
Capsule Conjugate Vaccines
Capsule conjugate vaccines have an excellent track record with regards to safety and efficacy. Conjugate vaccines have been successfully developed and implemented against other invasive mucosal pathogens, notably Streptococcus pneumoniae, Neisseria meningiditis, and Hemophilus influenzae.46,47 These vaccines, which are administered parentarally, elicit IgG antibodies that function to prevent invasion of these pathogens (see below). There are no licensed conjugate vaccines against enteric pathogens, however. In fact, most enteric pathogens do not express polysaccharide capsules, C. jejuni being the main exception to this rule. Moreover, enteric vaccines are generally designed to elicit secretory IgA antibodies in the intestine rather than IgG.48 However, conjugate vaccines based on the LPS of Shigella have shown good efficacy in field trials.49,50 Based on these results, a conjugate vaccine approach is being evaluated for its ability to prevent diarrheal disease caused by C. jejuni. It should also be mentioned that, unlike LOS, there has been no ganglioside mimicry associated with any C. jejuni capsular polysaccharide described to date, so there should be no association of this glycan with GBS.
Capsular polysaccharides are thymus-independent (TI-2) antigens that generally do not elicit potent immune responses.51-53 Although adults can generate antibody responses to purified capsules administered as a vaccine,54 children less than 2 years of age do not develop strong responses to TI-2 antigens.55 Furthermore, it has been determined that to elicit B cell responses against TI-2 antigens, the polysaccharide must have a mass in excess of 100 kDa with a minimum of 20 epitopes per polymer.56 Based on limited data, the capsule polysaccharide of C. jejuni is < 10 kDa, and it seems unlikely that free C. jejuni CPS would be capable of inducing effective antibody responses.57 B cell responses to TI-2 antigens are characterized by the production of short-lived, low-affinity IgM.51,52 The generation of long-lived, high affinity IgG (i.e., class switched) is dependent upon B cells receiving cognate T cell help.58 In a conjugate vaccine, TI-2 antigens are covalently linked to a carrier protein to optimize immune responses. The protein component allows responding B cells to receive costimulatory signals from T cells that allow IgG antibody class switching, and the generation of memory cells possessing antibody with a higher avidity for CPS.58,59
To evaluate a capsule conjugate approach against C. jejuni, CPS was purified from C. jejuni strain 81–176 (HS23/36) and conjugated to a mutant diphtheria toxin subunit, CRM197, using reductive amination.57 To determine if this research grade vaccine was immunogenic, BALB/c mice were immunized subcutaneously with escalating amounts (1, 5, or 25 μg vaccine by weight) of CPS-CRM197 co-administered with Alhydrogel (alum). These doses correspond to approximately 0.1, 0.5, and 2.5 μg of conjugated polysaccharide respectively. Three doses were administered at 2-, 4-, or 6-wk intervals. Regardless of regimen, vaccination with all dose levels induced significant anti-CPS IgG responses in immunized mice compared with baseline levels with maximal titers appearing after the third dose. Additionally, immunized mice displayed elevated levels of serum CPS-specific IgG for up to 26 wk following the final immunization. This was the first evidence that a C. jejuni CPS-CRM197 conjugate vaccine was capable of inducing robust, long-lived antibody responses.
Although a C. jejuni conjugate vaccine was demonstrated to be immunogenic in mice and protective in an intranasal mouse infection model, it remained unclear if immunization could prevent diarrhea caused by C. jejuni. Previously, a model of Campylobacter-induced diarrheal disease that mimics aspects of human illness was developed in Aotus nancymaae monkeys.60 First, before determining if CPS-CRM197 could confer protection against illness, A. nancymaae monkeys were immunized with escalating doses of research grade CPS-CRM197 to assess the immunogenicity of the conjugate.57 Subcutaneous immunization of A. nancymaae with escalating amounts (1, 5, and 25 μg by weight corresponding to approximately 0.1, 0.5, and 2.5 μg of polysaccharide, respectively) of a research-grade CPS-CRM197 vaccine demonstrated a clear dose-dependent effect on CPS-specific IgG production. Vaccinates receiving either 5 or 25 μg of conjugate vaccine per dose demonstrated significantly increased levels of plasma anti-capsular IgG compared with baseline titers following a three dose regimen. Interestingly, no increases in specific IgA production was observed in serum samples of any group.
To assess the protective efficacy of a research-grade CPS-CRM197 conjugate vaccine, A. nancymaae non-human primates were immunized with three doses (25 μg/dose by weight, corresponding to 2.5 μg/dose conjugated CPS) at 6-wk intervals.57 A group of control animals (n = 10) received saline as a sham treatment. A total of 9 wk following the immunization series, monkeys were orally challenged with ~1011 CFU of C. jejuni strain 81–176. Immunized A. nancymaae demonstrated statistically significant protection against diarrhea caused by C. jejuni challenge, with 100% (14/14) exhibiting no diarrhea compared with 70% (7/10) of the animals in the control group that developed diarrhea. Therefore, these initial experiments in mice and non-human primates established that a conjugate vaccine comprised of capsule polysaccharide from C. jejuni covalently linked to CRM197 was immunogenic and conferred protection against diarrheal disease.
Valency Questions
Any effective conjugate vaccine against C. jejuni will have to be multivalent, but the number of serotypes that would be included remains an open question. As mentioned above, capsule is the sero-determinant of the Penner serotyping scheme, of which there are 47 C. jejuni serotypes.31 Some of these serotypes fall into cross-reacting complexes (Fig. 1). Moreover, recent sequence analyses of the capsule loci of Penner type strains indicate that variation in the capsule loci associates with serotype differences, but the 47 described serotypes can be collapsed into 35 complexes based on DNA content (Poly, unpublished). While this number appears large, it is important to remember that S. pneumoniae has 97 serotypes. The first pneumococcal vaccine licensed by Wyeth in 2000 was based on the 7 most common S. pneumoniae serotypes identified in North America and Europe and showed a high efficacy. This vaccine was recently optimized to a total of 13 serotypes including the emerging 19A serotype. Like the licensed vaccine for pneumococcal disease, an effective C. jejuni CPS conjugate vaccine needs to be formulated to protect against the most common/virulent C. jejuni strains identified in developing countries where the disease is hyperendemic.
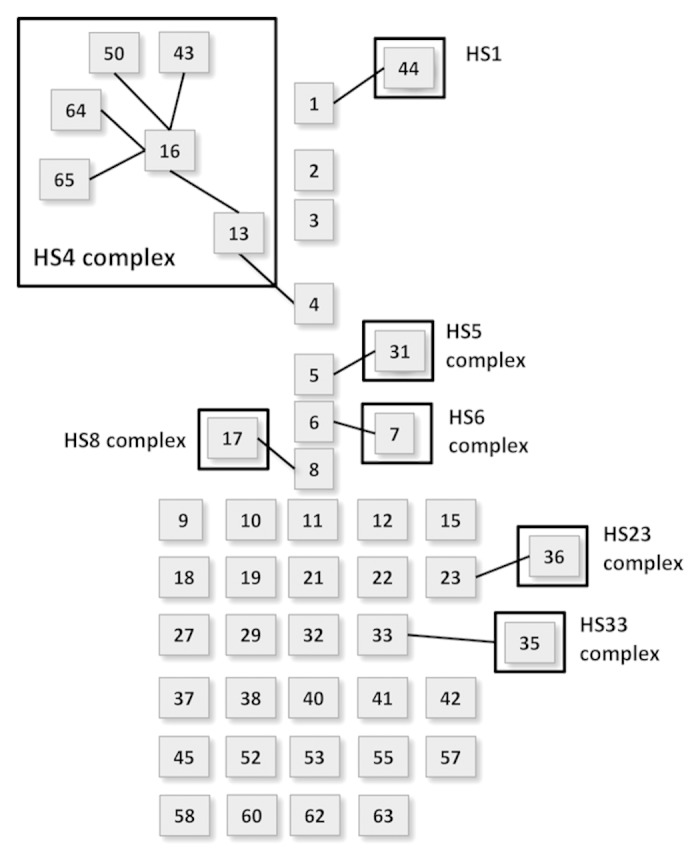
Figure 1. Illustration of the 47 described C. jejuni Penner serotypes and its 35 complexes.
A systematic review of Penner serotyping published in the past 30 y was recently performed. A total of 59 studies, compiling almost 22 000 sporadic human cases from 5 continents were reviewed,61 and three serotypes, HS1, HS2, and HS4, were the most common in all sites comprising as high as 50% of the strains. However, the majority of the strains examined were from Europe (87%) and very few from hyper endemic areas where the burden of disease is higher, like South America where no data were reported. Due to its complexity and cost, Penner serotyping has been gradually replaced in the last decade by more efficient molecular typing methods including Pulse field gel electrophoresis (PFGE) and multi locus sequence typing (MLST).62 To gather additional information on CPS distribution in developing countries, Poly et al. developed a multiplex PCR method to rapidly determine capsule types. The initial description covered 14 major capsule types,62 but the method has now been expanded to cover all 47 Penner types (Poly, manuscript in preparation) and has been deployed to numerous labs worldwide. Preliminary results indicate that in addition to HS1, HS2, and HS4, an additional 4 to 6 CPS types would cover >70% of C. jejuni CPS types worldwide.
Correlates of Protection
Currently there are no well-defined correlates of protection against diarrheal disease caused by Campylobacter. Tribble et al. demonstrated that protection against homologous re-challenge with C. jejuni strain 81–176 correlated with in vitro IFN-γ production by peripheral blood mononuclear cells (PBMCs), and that protective responses were observed above a threshold value of 400 pg/ml.38 In addition, a protective role for IgA has been suggested given the biology of Campylobacter.38 Currently, there are no studies detailing the human immune response to the capsule polysaccharide of C. jejuni following either experimental or natural infection. However, multiple studies examining natural infection have provided evidence that age-related increases in serum antibodies against Campylobacter are associated with resistance to infection and illness,63-66 and in initial studies performed in A. nancymaae where monkeys were immunized with a C. jejuni capsule conjugate vaccine, significant levels of CPS-specific plasma IgG were observed with no concomitant increase in IgA levels. As mentioned previously, following challenge these immunized monkeys were completely protected against diarrheal disease, demonstrating that protection in this model correlates with anti-capsular antibody responses.57 Although these studies demonstrate this link clearly, the precise mechanism of immunity remains to be determined.
Correlates of protection are established for pathogens in which licensed conjugate vaccines have been developed. Specific assays have been developed to measure vaccine-induced responses against pneumococcal and meningococcal disease. What these assays have in common is that they are measures of functional antibody activity. Protection against meningitis correlates with the serum bactericidal titer that is achieved following immunization,67-69 whereas the correlate for protection against pneumococcal disease is the opsonophagocytic activity of immune serum.70,71 Bactericidal activity is conferred by the ability of an antibody, generally IgM or IgG, to activate the classical complement pathway leading to formation of the membrane attack complex, which can directly kill bacteria. In contrast antibodies with opsonophagocytic functions bind pathogens and allow for uptake into phagocytes via Fc receptors. Common between the aforementioned pathogens and Campylobacter is that all are invasive, mucosal pathogens, and their polysaccharide capsules are critical for serum resistance. Thus, the evaluation of functional antibodies elicited by immunization with a C. jejuni conjugate vaccine is merited.
Future Work
Recently, a prototype cGMP-produced C. jejuni CPS-CRM197 monovalent conjugate vaccine based on the HS23/36 capsule type (CJCV1) was produced under contract with a contract research organization. This vaccine was immunogenic in mice and is currently being evaluated in a toxicology study. Following pre-clinical testing, future plans are to evaluate CJCV1 in a first-in-human Phase 1 clinical trial for safety and immunogenicity. As our candidate vaccine moves into clinical evaluations, the human challenge model utilizing C. jejuni strain CG8421 (HS23/36) will be of great value in assessing the efficacy of this prototype cGMP-produced conjugate vaccine in a Phase 2b clinical trial. This will establish a proof-of-principle that a conjugate vaccine can protect against diarrhea caused by C. jejuni in humans. However, additional work is needed to advance the goal of creating a multivalent C. jejuni conjugate vaccine for human use. Key to the development of a practical vaccine is identifying the valency needed to provide adequate vaccine coverage and identifying correlates of protection against diarrheal disease.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by the Military Infectious Diseases Research Program, Work Unit no. 6000.RAD1.DA3.A0308. The opinions and assertions contained herein are the private ones of the authors and do not reflect the official policy of the Department of Navy, Department of Defense or the US government.
Copyright Statement
PG.. is an employee of the US Government. This work was prepared as part of official duties. Title 17 U.S.C. §105 provides that “Copyright protection under this title is not available for any work of the United States Government.” Title 17 U.S.C. §101 defines a US Government work as a work prepared by a military service member or employee of the US Government as part of that person’s official duties.
Glossary
Abbreviations:
- GBS
Guillain-Barré syndrome
- LOS
lipooligosaccharide
- LPS
lipopolysaccharide
References
- 1.Blaser MJ. Epidemiologic and clinical features of Campylobacter jejuni infections. J Infect Dis. 1997;176(Suppl 2):S103–5. doi: 10.1086/513780. [DOI] [PubMed] [Google Scholar]
- 2.WHO. The global view of campylobacteriosis: report of an expert consultation, Utrecht, Netherlands, 9-11 July 2012. Available from: http://wwwwhoint/foodsafety/publications/foodborne_disease/global_view_campylobacterosis/en/ accessed 13 January 2014 Geneva: World Health Organization 2013.
- 3.Riddle MS, Gutierrez RL, Verdu EF, Porter CK. The chronic gastrointestinal consequences associated with campylobacter. Curr Gastroenterol Rep. 2012;14:395–405. doi: 10.1007/s11894-012-0278-0. [DOI] [PubMed] [Google Scholar]
- 4.Coker AO, Isokpehi RD, Thomas BN, Amisu KO, Obi CL. Human campylobacteriosis in developing countries. Emerg Infect Dis. 2002;8:237–44. doi: 10.3201/eid0803.010233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Griffiths PL, Park RW. Campylobacters associated with human diarrhoeal disease. J Appl Bacteriol. 1990;69:281–301. doi: 10.1111/j.1365-2672.1990.tb01519.x. [DOI] [PubMed] [Google Scholar]
- 6.Friedman CR, Hoekstra RM, Samuel M, Marcus R, Bender J, Shiferaw B, Reddy S, Ahuja SD, Helfrick DL, Hardnett F, et al. Emerging Infections Program FoodNet Working Group Risk factors for sporadic Campylobacter infection in the United States: A case-control study in FoodNet sites. Clin Infect Dis. 2004;38(Suppl 3):S285–96. doi: 10.1086/381598. [DOI] [PubMed] [Google Scholar]
- 7.Heuvelink AE, van Heerwaarden C, Zwartkruis-Nahuis A, Tilburg JJ, Bos MH, Heilmann FG, Hofhuis A, Hoekstra T, de Boer E. Two outbreaks of campylobacteriosis associated with the consumption of raw cows’ milk. Int J Food Microbiol. 2009;134:70–4. doi: 10.1016/j.ijfoodmicro.2008.12.026. [DOI] [PubMed] [Google Scholar]
- 8.Jakopanec I, Borgen K, Vold L, Lund H, Forseth T, Hannula R, Nygård K. A large waterborne outbreak of campylobacteriosis in Norway: the need to focus on distribution system safety. BMC Infect Dis. 2008;8:128. doi: 10.1186/1471-2334-8-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beaudeau P, de Valk H, Vaillant V, Mannschott C, Tillier C, Mouly D, Ledrans M. Lessons learned from ten investigations of waterborne gastroenteritis outbreaks, France, 1998-2006. J Water Health. 2008;6:491–503. doi: 10.2166/wh.2008.051. [DOI] [PubMed] [Google Scholar]
- 10.Neimann J, Engberg J, Mølbak K, Wegener HC. A case-control study of risk factors for sporadic campylobacter infections in Denmark. Epidemiol Infect. 2003;130:353–66. doi: 10.1017/s0950268803008355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Danis K, Di Renzi M, O’Neill W, Smyth B, McKeown P, Foley B, Tohani V, Devine M. Risk factors for sporadic Campylobacter infection: an all-Ireland case-control study. Euro Surveill. 2009;14:14. [PubMed] [Google Scholar]
- 12.Gallay A, Bousquet V, Siret V, Prouzet-Mauléon V, Valk Hd, Vaillant V, Simon F, Le Strat Y, Mégraud F, Desenclos JC. Risk factors for acquiring sporadic Campylobacter infection in France: results from a national case-control study. J Infect Dis. 2008;197:1477–84. doi: 10.1086/587644. [DOI] [PubMed] [Google Scholar]
- 13.Jones TF, Scallan E, Angulo FJ. FoodNet: overview of a decade of achievement. Foodborne Pathog Dis. 2007;4:60–6. doi: 10.1089/fpd.2006.63. [DOI] [PubMed] [Google Scholar]
- 14.Riddle MS, Sanders JW, Putnam SD, Tribble DR. Incidence, etiology, and impact of diarrhea among long-term travelers (US military and similar populations): a systematic review. Am J Trop Med Hyg. 2006;74:891–900. [PubMed] [Google Scholar]
- 15.Janssen R, Krogfelt KA, Cawthraw SA, van Pelt W, Wagenaar JA, Owen RJ. Host-pathogen interactions in Campylobacter infections: the host perspective. Clin Microbiol Rev. 2008;21:505–18. doi: 10.1128/CMR.00055-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ang CW, Jacobs BC, Laman JD. The Guillain-Barré syndrome: a true case of molecular mimicry. Trends Immunol. 2004;25:61–6. doi: 10.1016/j.it.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 17.Pope JE, Krizova A, Garg AX, Thiessen-Philbrook H, Ouimet JM. Campylobacter reactive arthritis: a systematic review. Semin Arthritis Rheum. 2007;37:48–55. doi: 10.1016/j.semarthrit.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pimentel M, Chatterjee S, Chang C, Low K, Song Y, Liu C, Morales W, Ali L, Lezcano S, Conklin J, et al. A new rat model links two contemporary theories in irritable bowel syndrome. Dig Dis Sci. 2008;53:982–9. doi: 10.1007/s10620-007-9977-z. [DOI] [PubMed] [Google Scholar]
- 19.Lee G, Pan W, Peñataro Yori P, Paredes Olortegui M, Tilley D, Gregory M, Oberhelman R, Burga R, Chavez CB, Kosek M. Symptomatic and asymptomatic Campylobacter infections associated with reduced growth in Peruvian children. PLoS Negl Trop Dis. 2013;7:e2036. doi: 10.1371/journal.pntd.0002036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.da Silva Quetz J, Lima IF, Havt A, de Carvalho EB, Lima NL, Soares AM, Mota RM, Guerrant RL, Lima AA. Campylobacter jejuni and Campylobacter coli in children from communities in Northeastern Brazil: molecular detection and relation to nutritional status. Diagn Microbiol Infect Dis. 2010;67:220–7. doi: 10.1016/j.diagmicrobio.2010.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ang CW, De Klerk MA, Endtz HP, Jacobs BC, Laman JD, van der Meché FG, van Doorn PA. Guillain-Barré syndrome- and Miller Fisher syndrome-associated Campylobacter jejuni lipopolysaccharides induce anti-GM1 and anti-GQ1b Antibodies in rabbits. Infect Immun. 2001;69:2462–9. doi: 10.1128/IAI.69.4.2462-2469.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoffmann S, Batz MB, Morris JG., Jr. Annual cost of illness and quality-adjusted life year losses in the United States due to 14 foodborne pathogens. J Food Prot. 2012;75:1292–302. doi: 10.4315/0362-028X.JFP-11-417. [DOI] [PubMed] [Google Scholar]
- 23.Konkel ME, Babakhani F, Joens LA. Invasion-related antigens of Campylobacter jejuni. J Infect Dis. 1990;162:888–95. doi: 10.1093/infdis/162.4.888. [DOI] [PubMed] [Google Scholar]
- 24.Poly F, Ewing C, Goon S, Hickey TE, Rockabrand D, Majam G, Lee L, Phan J, Savarino NJ, Guerry P. Heterogeneity of a Campylobacter jejuni protein that is secreted through the flagella filament. Infect Immun. 2007 doi: 10.1128/IAI.00159-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barrero-Tobon AM, Hendrixson DR. Identification and analysis of flagellar coexpressed determinants (Feds) of Campylobacter jejuni involved in colonization. Mol Microbiol. 2012;84:352–69. doi: 10.1111/j.1365-2958.2012.08027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Christensen JE, Pacheco SA, Konkel ME. Identification of a Campylobacter jejuni-secreted protein required for maximal invasion of host cells. Mol Microbiol. 2009;73:650–62. doi: 10.1111/j.1365-2958.2009.06797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neal-McKinney JM, Konkel ME. The Campylobacter jejuni CiaC virulence protein is secreted from the flagellum and delivered to the cytosol of host cells. Front Cell Infect Microbiol. 2012;2:31. doi: 10.3389/fcimb.2012.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kopecko DJ, Hu L, Zaal KJ. Campylobacter jejuni--microtubule-dependent invasion. Trends Microbiol. 2001;9:389–96. doi: 10.1016/S0966-842X(01)02107-2. [DOI] [PubMed] [Google Scholar]
- 29.Parkhill J, Wren BW, Mungall K, Ketley JM, Churcher C, Basham D, Chillingworth T, Davies RM, Feltwell T, Holroyd S, et al. The genome sequence of the food-borne pathogen Campylobacter jejuni reveals hypervariable sequences. Nature. 2000;403:665–8. doi: 10.1038/35001088. [DOI] [PubMed] [Google Scholar]
- 30.Karlyshev AV, McCrossan MV, Wren BW. Demonstration of polysaccharide capsule in Campylobacter jejuni using electron microscopy. Infect Immun. 2001;69:5921–4. doi: 10.1128/IAI.69.9.5921-5924.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Penner JL, Hennessy JN. Passive hemagglutination technique for serotyping Campylobacter fetus subsp. jejuni on the basis of soluble heat-stable antigens. J Clin Microbiol. 1980;12:732–7. doi: 10.1128/jcm.12.6.732-737.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bacon DJ, Szymanski CM, Burr DH, Silver RP, Alm RA, Guerry P. A phase-variable capsule is involved in virulence of Campylobacter jejuni 81-176. Mol Microbiol. 2001;40:769–77. doi: 10.1046/j.1365-2958.2001.02431.x. [DOI] [PubMed] [Google Scholar]
- 33.Grant AJ, Coward C, Jones MA, Woodall CA, Barrow PA, Maskell DJ. Signature-tagged transposon mutagenesis studies demonstrate the dynamic nature of cecal colonization of 2-week-old chickens by Campylobacter jejuni. Appl Environ Microbiol. 2005;71:8031–41. doi: 10.1128/AEM.71.12.8031-8041.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maue AC, Mohawk KL, Giles DK, Poly F, Ewing CP, Jiao Y, Lee G, Ma Z, Monteiro MA, Hill CL, et al. The polysaccharide capsule of Campylobacter jejuni modulates the host immune response. Infect Immun. 2013;81:665–72. doi: 10.1128/IAI.01008-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bacon DJ, Szymanski CM, Burr DH, Silver RP, Alm RA, Guerry P. A phase-variable capsule is involved in virulence of Campylobacter jejuni 81-176. Mol Microbiol. 2001;40:769–77. doi: 10.1046/j.1365-2958.2001.02431.x. [DOI] [PubMed] [Google Scholar]
- 36.Rose A, Kay E, Wren BW, Dallman MJ. The Campylobacter jejuni NCTC11168 capsule prevents excessive cytokine production by dendritic cells. Med Microbiol Immunol. 2012;201:137–44. doi: 10.1007/s00430-011-0214-1. [DOI] [PubMed] [Google Scholar]
- 37.Black RE, Levine MM, Clements ML, Hughes TP, Blaser MJ. Experimental Campylobacter jejuni infection in humans. J Infect Dis. 1988;157:472–9. doi: 10.1093/infdis/157.3.472. [DOI] [PubMed] [Google Scholar]
- 38.Tribble DR, Baqar S, Scott DA, Oplinger ML, Trespalacios F, Rollins D, Walker RI, Clements JD, Walz S, Gibbs P, et al. Assessment of the duration of protection in Campylobacter jejuni experimental infection in humans. Infect Immun. 2010;78:1750–9. doi: 10.1128/IAI.01021-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guerry P, Szymanski CM, Prendergast MM, Hickey TE, Ewing CP, Pattarini DL, Moran AP. Phase variation of Campylobacter jejuni 81-176 lipooligosaccharide affects ganglioside mimicry and invasiveness in vitro. Infect Immun. 2002;70:787–93. doi: 10.1128/IAI.70.2.787-793.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Poly F, Read TD, Chen YH, Monteiro MA, Serichantalergs O, Pootong P, Bodhidatta L, Mason CJ, Rockabrand D, Baqar S, et al. Characterization of two Campylobacter jejuni strains for use in volunteer experimental-infection studies. Infect Immun. 2008;76:5655–67. doi: 10.1128/IAI.00780-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tribble DR, Baqar S, Carmolli MP, Porter C, Pierce KK, Sadigh K, Guerry P, Larsson CJ, Rockabrand D, Ventone CH, et al. Campylobacter jejuni strain CG8421: a refined model for the study of Campylobacteriosis and evaluation of Campylobacter vaccines in human subjects. Clin Infect Dis. 2009;49:1512–9. doi: 10.1086/644622. [DOI] [PubMed] [Google Scholar]
- 42.Kirkpatrick BD, Lyon CE, Porter CK, Maue AC, Guerry P, Pierce KK, Carmolli MP, Riddle MS, Larsson CJ, Hawk D, et al. Lack of homologous protection against Campylobacter jejuni CG8421 in a human challenge model. Clin Infect Dis. 2013;57:1106–13. doi: 10.1093/cid/cit454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002;2:251–62. doi: 10.1038/nri778. [DOI] [PubMed] [Google Scholar]
- 44.Griffin A, Baraho-Hassan D, McSorley SJ. Successful treatment of bacterial infection hinders development of acquired immunity. J Immunol. 2009;183:1263–70. doi: 10.4049/jimmunol.0900772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tribble DR, Baqar S, Thompson SA. Development of a Human Vaccine. In: Nachamkin I, Szymanski CM, Blaser MJ, eds. Campylobacter. Washington D.C.: ASM Press, 2008:429-44. [Google Scholar]
- 46.Lesinski GB, Westerink MA. Vaccines against polysaccharide antigens. Curr Drug Targets Infect Disord. 2001;1:325–34. doi: 10.2174/1568005014605964. [DOI] [PubMed] [Google Scholar]
- 47.Knuf M, Kowalzik F, Kieninger D. Comparative effects of carrier proteins on vaccine-induced immune response. Vaccine. 2011;29:4881–90. doi: 10.1016/j.vaccine.2011.04.053. [DOI] [PubMed] [Google Scholar]
- 48.Takahashi I, Nochi T, Yuki Y, Kiyono H. New horizon of mucosal immunity and vaccines. Curr Opin Immunol. 2009;21:352–8. doi: 10.1016/j.coi.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 49.Passwell JH, Harlev E, Ashkenazi S, Chu C, Miron D, Ramon R, Farzan N, Shiloach J, Bryla DA, Majadly F, et al. Safety and immunogenicity of improved Shigella O-specific polysaccharide-protein conjugate vaccines in adults in Israel. Infect Immun. 2001;69:1351–7. doi: 10.1128/IAI.69.3.1351-1357.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cohen D, Ashkenazi S, Green MS, Gdalevich M, Robin G, Slepon R, Yavzori M, Orr N, Block C, Ashkenazi I, et al. Double-blind vaccine-controlled randomised efficacy trial of an investigational Shigella sonnei conjugate vaccine in young adults. Lancet. 1997;349:155–9. doi: 10.1016/S0140-6736(96)06255-1. [DOI] [PubMed] [Google Scholar]
- 51.Mond JJ, Vos Q, Lees A, Snapper CM. T cell independent antigens. Curr Opin Immunol. 1995;7:349–54. doi: 10.1016/0952-7915(95)80109-X. [DOI] [PubMed] [Google Scholar]
- 52.Mond JJ, Lees A, Snapper CM. T cell-independent antigens type 2. Annu Rev Immunol. 1995;13:655–92. doi: 10.1146/annurev.iy.13.040195.003255. [DOI] [PubMed] [Google Scholar]
- 53.Mosier DE, Mond JJ, Goldings EA. The ontogeny of thymic independent antibody responses in vitro in normal mice and mice with an X-linked B cell defect. J Immunol. 1977;119:1874–8. [PubMed] [Google Scholar]
- 54.Lesinski GB, Westerink MA. Novel vaccine strategies to T-independent antigens. J Microbiol Methods. 2001;47:135–49. doi: 10.1016/S0167-7012(01)00290-1. [DOI] [PubMed] [Google Scholar]
- 55.Rijkers GT, Sanders EA, Breukels MA, Zegers BJ. Infant B cell responses to polysaccharide determinants. Vaccine. 1998;16:1396–400. doi: 10.1016/S0264-410X(98)00098-X. [DOI] [PubMed] [Google Scholar]
- 56.Dintzis RZ, Okajima M, Middleton MH, Greene G, Dintzis HM. The immunogenicity of soluble haptenated polymers is determined by molecular mass and hapten valence. J Immunol. 1989;143:1239–44. [PubMed] [Google Scholar]
- 57.Monteiro MA, Baqar S, Hall ER, Chen YH, Porter CK, Bentzel DE, Applebee L, Guerry P. Capsule polysaccharide conjugate vaccine against diarrheal disease caused by Campylobacter jejuni. Infect Immun. 2009;77:1128–36. doi: 10.1128/IAI.01056-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McHeyzer-Williams LJ, McHeyzer-Williams MG. Antigen-specific memory B cell development. Annu Rev Immunol. 2005;23:487–513. doi: 10.1146/annurev.immunol.23.021704.115732. [DOI] [PubMed] [Google Scholar]
- 59.Crotty S, Ahmed R. Immunological memory in humans. Semin Immunol. 2004;16:197–203. doi: 10.1016/j.smim.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 60.Jones FR, Baqar S, Gozalo A, Nunez G, Espinoza N, Reyes SM, Salazar M, Meza R, Porter CK, Walz SE. New World monkey Aotus nancymae as a model for Campylobacter jejuni infection and immunity. Infect Immun. 2006;74:790–3. doi: 10.1128/IAI.74.1.790-793.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pike BL, Guerry P, Poly F, Pike BL, Guerry P, Poly F. Global Distribution of Campylobacter jejuni Penner Serotypes: A Systematic Review. PLoS One. 2013;8:e67375. doi: 10.1371/journal.pone.0067375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Poly F, Serichatalergs O, Schulman M, Ju J, Cates CN, Kanipes M, Mason C, Guerry P. Discrimination of major capsular types of Campylobacter jejuni by multiplex PCR. J Clin Microbiol. 2011;49:1750–7. doi: 10.1128/JCM.02348-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Taylor DN, Echeverria P, Pitarangsi C, Seriwatana J, Bodhidatta L, Blaser MJ. Influence of strain characteristics and immunity on the epidemiology of Campylobacter infections in Thailand. J Clin Microbiol. 1988;26:863–8. doi: 10.1128/jcm.26.5.863-868.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Taylor DN, Perlman DM, Echeverria PD, Lexomboon U, Blaser MJ. Campylobacter immunity and quantitative excretion rates in Thai children. J Infect Dis. 1993;168:754–8. doi: 10.1093/infdis/168.3.754. [DOI] [PubMed] [Google Scholar]
- 65.Blaser MJ, Black RE, Duncan DJ, Amer J. Campylobacter jejuni-specific serum antibodies are elevated in healthy Bangladeshi children. J Clin Microbiol. 1985;21:164–7. doi: 10.1128/jcm.21.2.164-167.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Blaser MJ, Taylor DN, Echeverria P. Immune response to Campylobacter jejuni in a rural community in Thailand. J Infect Dis. 1986;153:249–54. doi: 10.1093/infdis/153.2.249. [DOI] [PubMed] [Google Scholar]
- 67.Romero-Steiner S, Fernandez J, Biltoft C, Wohl ME, Sanchez J, Feris J, Balter S, Levine OS, Carlone GM. Functional antibody activity elicited by fractional doses of Haemophilus influenzae type b conjugate vaccine (polyribosylribitol phosphate-tetanus toxoid conjugate) Clin Diagn Lab Immunol. 2001;8:1115–9. doi: 10.1128/CDLI.8.6.1115-1119.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Romero-Steiner S, Spear W, Brown N, Holder P, Hennessy T, Gomez De Leon P, Carlone GM. Measurement of serum bactericidal activity specific for Haemophilus influenzae type b by using a chromogenic and fluorescent metabolic indicator. Clin Diagn Lab Immunol. 2004;11:89–93. doi: 10.1128/CDLI.11.1.89-93.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Frasch CE, Borrow R, Donnelly J. Bactericidal antibody is the immunologic surrogate of protection against meningococcal disease. Vaccine. 2009;27(Suppl 2):B112–6. doi: 10.1016/j.vaccine.2009.04.065. [DOI] [PubMed] [Google Scholar]
- 70.Romero-Steiner S, Frasch CE, Carlone G, Fleck RA, Goldblatt D, Nahm MH. Use of opsonophagocytosis for serological evaluation of pneumococcal vaccines. Clin Vaccine Immunol. 2006;13:165–9. doi: 10.1128/CVI.13.2.165-169.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fleck RA, Romero-Steiner S, Nahm MH. Use of HL-60 cell line to measure opsonic capacity of pneumococcal antibodies. Clin Diagn Lab Immunol. 2005;12:19–27. doi: 10.1128/CDLI.12.1.19-27.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]


