Abstract
Entamoeba histolytica is the causative agent of amebiasis, one of the top three parasitic causes of mortality worldwide. In the majority of infected individuals, E. histolytica asymptomatically colonizes the large intestine, while in others, the parasite breaches the mucosal epithelial barrier to cause amebic colitis and can disseminate to soft organs to cause abscesses. Vaccinations using native and recombinant forms of the parasite Gal-lectin have been successful in protecting animals against intestinal amebiasis and amebic liver abscess. Protection against amebic liver abscesses has also been reported by targeting other E. histolytica components including the serine-rich protein and the 29-kDa-reductase antigen. To date, vaccines against the Gal-lectin hold the most promise but clinical trials will be required to validate its efficacy in humans. Here, we review the current strategies and future perspectives involved in the development of a vaccine against E. histolytica.
Keywords: Entamoeba histolytica, amebiasis, immunopathogenesis, immune response, mucosal immune response, cell-mediated immunity, amebic colitis, dysentery, amebic liver abscess, DNA vaccine
Introduction
Entamoeba histolytica is an enteric protozoan parasite that infects humans, and is the etiologic agent of amebiasis. Amebiasis remains a worldwide health problem, accounting for up to 100 000 deaths annually.1 It is more common in developing countries with poor sanitation, lack of clean water, and higher incidences of undernutrition2 including Bangladesh,3 South Africa,4,5 and Vietnam.6E. histolytica is one of the pathogens responsible for diarrheal diseases, which is a major cause of mortality in children in developing countries.7 Compared with other parasites, the life cycle of E. histolytica is relatively simple and consists of 2 stages: the infectious cyst and the disease-inducing (motile) trophozoite stage. When amebic cysts are ingested via fecal contaminated food or water, they pass through the stomach and excyst in the terminal ileum where they mature into trophozoites and colonize the colon. About 90% of infections are asymptomatic and the remaining 10% display a spectrum of disease that include acute diarrhea, dysentery, amebic colitis, and amebic liver abscesses (ALA).8 In asymptomatic infections, E. histolytica trophozoites live as commensals feeding on colonic microflora and nutrients of the host and form cysts that pass through stool to perpetuate the life cycle.
Drug therapies such as metronidazole and other nitroimidazole-derived compounds are effective for treating invasive parasites. However, these drugs display adverse side effects and are expensive and not easily available in certain countries and areas.9 Improvement of water purification systems and hygiene practices could decrease disease incidence but this will require considerable time, changes to government policies and monetary investments. For these reasons, the development of a vaccine and introduction of vaccination programs in developing countries represents an attractive alternative. Relative to drug treatment, vaccines are cost-effective, safe, and have less undesirable side effects. Moreover, they display high protection rates and have been proven to be efficient in the control of many infectious diseases. For instance, the vaccine against poliomyelitis has been one of the most successful resulting in 99% reduction of poliomyelitis cases from 1988 to 2003 worldwide.10 Unfortunately, no amebiasis vaccine has been approved for human clinical trials to date, but many recent vaccine development studies hold promise. In this review, we will underline the key elements to be considered during vaccine design against E. histolytica. We will first discuss the pathogenesis of E. histolytica as a source for determining suitable vaccine target proteins. Second, we will highlight the major protective immune responses elicited by E. histolytica and how certain amebiasis vaccine strategies can make use of these responses. Lastly, we will discuss current challenges faced with amebiasis research and future strategies to drive vaccine development forward.
Pathogenesis of Amebiasis
Why E. histolytica becomes invasive in certain individuals is still unresolved and suggests that this host-parasite interaction is quite complex. E. histolytica has a unique set of virulence traits that enable it to adapt to changing environments within the gut and to manipulate the host immune surveillance system. The central events in the pathogenesis of infection by E. histolytica include adhesion and colonization to the mucus layer, mucus depletion, epithelial contact-dependent killing, and invasion of tissues followed by dissemination to soft organs.11 The first step in pathogenesis involves the binding of trophozoites to the mucus layer of the colon, which is composed of secreted MUC2 mucin that forms the first line of innate host defense.12,13 This is mediated by the parasite surface galactose-N-acetyl-D-galactosamine inhibitable lectin (Gal-lectin), which binds with high affinity to galactose and N-acetyl galactosamine residues of colonic mucins.14,15 The Gal-lectin adhesin is the most well-characterized protein of E. histolytica with regards to pathogenesis and its ability to stimulate pro-inflammatory immune responses. Additionally, E. histolytica secretes high levels of cysteine proteinase 5 that cleaves the C-terminus of the MUC2 polymer, thereby degrading the mucin barrier in disease pathogenesis.13 Other virulence components of E. histolytica include amebapores, arginase, alcohol dehydrogenase, peroxiredoxin, and lipopeptidophosphoglycan, all of which contribute to activation or evasion from host defenses.11 Interestingly, E. histolytica has also been shown to produce a mucin secretagogue that induces hypersecretion of mucus from goblet cells that can deplete mucin stores.16 Underlying the protective mucus barrier is a single layer of epithelial cells, to which trophozoites can bind through Gal-lectin and trigger either apoptosis and/or phagocytosis of these cells.17 The resulting cell destruction leads to an acute pro-inflammatory response and immune cell infiltration in an attempt from the host to clear the infection. In rare cases, the parasite enters the bloodstream and travels to the liver causing extensive tissue damage and ALA, which can be fatal. Based on the central role of the Gal-lectin in disease pathogenesis, this molecule has been the subject of intense investigation for its potential role in vaccine development.
The Host Immune Response to E. histolytica
A central component of the human gut defense mechanism is the production of mucosal immunoglobulins (Ig), which have important roles in maintaining intestinal homeostasis.18 Secretory IgA (sIgA) is one of the most abundant Ig produced by plasma cells within the lamina propria and functions by preventing pathogens from adhering and breaching the mucosal barrier.19 There is accumulating evidence suggesting that mucosal anti-Gal-lectin IgA responses are critical for resistance to amebic colonization and invasion.4,5 This stems from observational studies in a susceptible population of children from Bangladesh where the presence of stool IgA Gal-lectin-specific antibodies correlated with reduced re-infection rates with E. histolytica.3,20,21 In South Africa, a group of recovered ALA subjects exhibited a greater average number of anti-Gal-lectin IgA peaks compared with asymptomatic subjects over a 3 y period and these peaks were of higher amplitude and longer duration.4 Increases in anti-Gal-lectin IgA antibodies were associated with clearance of subsequent amebic infections, demonstrating that ALA subjects developed a heightened immune responsiveness and have retained memory of the parasite.4 While IgA titers correlate with protection against amebiasis, studies suggested that the presence of IgG on the other hand has a detrimental role.21,22 One study reported that Bangladeshi children with serum anti-ameba IgG antibodies developed 37% more new and severe E. histolytica infections compared with children negative for anti-ameba IgG at 2 y of follow-up.21 Furthermore, cell-mediated immune responses may also be critical for host defense and protection against E. histolytica.23 Analysis of asymptomatic carriers of E. histolytica showed that carriers had higher levels of interferon-gamma (IFN-γ), reflecting a T helper (Th) 1 response, while patients with invasive amebiasis displayed higher levels of IL-4, resembling that of a Th2 response.24 In accordance with this, protection of mice vaccinated with a portion of Gal-lectin against re-infection was mediated by IFN-γ-producing CD4+ T-cells and IL-17-secreting CD8+ T-cells.23 In vitro studies have also shown that IFN-γ treatment of macrophages induced high amebicidal activity.25 Taken together, these studies demonstrate that the host can mount both humoral and cell-mediated immune responses against E. histolytica, both of which are associated with protection. Harnessing these responses is a critical component in designing a successful vaccine against amebiasis.
Vaccine Candidates
Major requirements for the development of an effective vaccine include the establishment of immunological memory, which is dependent on eliciting a strong immune response, the identification of a protective antigen, and the use of an appropriate delivery route. Although some of the candidate proteins discussed below have been shown to be immunostimulatory on their own, the use of adjuvants are required to elicit strong antibody and cell-mediated responses. The specific adjuvants used for each vaccine study are listed in Tables 1 and 2. The most frequently used adjuvant in amebiasis vaccine studies is Freund’s adjuvant, which elicits strong antibody and cell-mediated responses in animal models, however, is too toxic for use in humans. Cholera toxin B subunit (CTB) has been shown to be non-toxic and immunogenic in humans, and has been successfully used as an adjuvant in oral and intranasal vaccinations to elicit Th2-mediated mucosal immunity.26 In order to stimulate mucosal Th1 responses, adjuvants such as cytosine guanine oligodeoxynucleotides (CpG-ODNs) have been proven effective.26 CpG-ODNs are synthetic oligodeoxynucleotides containing immunostimulatory CpG motifs that have been shown to predominantly induce Th1-polarizing cytokines.27 Therefore, the adjuvant not only acts as an immunopotentiator but can also determine the type of immune response elicited.
Table 1. Efficacy of the native and recombinant Gal-lectin vaccine trials.
| Antigen | Forma | Adjuvant/ Delivery |
Routeb | Dose of antigen (μg) | % Protective efficacyc | Ref. |
|---|---|---|---|---|---|---|
| 260-kDa | Native | Freund’s | s.c. i.p. |
Wk 0, 2, 4: 10 Wk 0, 2, 4: 10 |
43-67 ALA 86 ALA |
35 |
| 260-kDa** | Native | CTB Freund’s |
i.n. i.p. |
Wk 0, 2, 6, 7, 9: 10 Wks 4, 7: 15 |
100 AC | 63 |
| 260-kDa** | Native | CpG-ODN | i.n. i.p. |
Wk 0, 1, 3, 5: 10 Wk 5: 10 |
100 ALA | 42 |
| 260-kDa | Native | EhDNA | i.m. | Wk 0, 1, 2: 10 | 100 ALA | 64 |
| aa 1-1204* | Recombinant |
Yersinia enterocolitica |
Orally | Wk 0, 1, 2, 3, 4: 109 | 3-68 ALA | 65 |
| aa 1-436 aa 436-624 aa 799-939 aa 939-1053 |
Recombinant | Freund’s | i.p. | Wk 0, 2, 4: 50 | 6.7 ALA 37.5 ALA 11.1 ALA 62.5 ALA |
41 |
| aa 482-1138 | Recombinant His tagged Recombinant |
Freund’s Salmonella dublin |
i.p. Orally |
Wk 0, 4: 30 Days 1, 5, 7: 108-1010 |
45 ALA 18-28 ALA |
66 |
| aa 578-1154** | Recombinant | CTB Freund’s |
i.n. i.p. |
Wk 0, 2, 6, 7, 9: 10 Wk 4:15 |
34–89 AC | 63 |
| aa 649-1201 | Recombinant GST fusion | Freund’s | i.p. | Wk 0, 4, 7: 150 | 81 ALA | 67 |
| aa 758-1134 | Recombinant His tagged | Titermax | i.p. | Wk 0, 2, 4: 10 | 71 ALA | 36 |
| aa 895-998 | Recombinant His tagged |
Freund’s | i.p. | Wk 0, 2: 200 Wk 4: 400 |
N/A | 68 |
| aa 1005-1029 aa 1005-1029* aa 1005-1029* |
Synthetic peptide, KLH fusion Recombinant CTB fusion Recombinant CTB fusion |
Freund’s None Freund’s |
i.p. Orally i.p. |
Wk 0, 2, 4: 50 Wk 0, 2, 4, 13: 100 Wk 0, 2, 4: 50 |
33 ALA 0-30 ALA 0-55 ALA |
69 |
aa, amino acid; CTB, cholera toxin b; ALA, amebic liver abscess; AC, amebic colitis; Wks, weeks; Cysteine-poor domain, aa 1–436; Pseudo-repeat domain, 436–624; Cysteine-rich domain, 624–1053. *Varying fragments of residues within this region. **Experiments with combined regimens. aAll recombinant proteins were produced in Escherichia coli. bRoutes: i.p., intraperitoneally; i.n., intranasally; i.m., intramuscularly; s.c, subcutaneously; orally, by oral gavage. c%Protective Efficacy: ([number of unvaccinated animals infected-number of vaccinated animals infected]/[number of unvaccinated animals infected]) × 100.
Table 2.Entamoeba histolytica antigens tested in animal models.
| Antigen | Animal model | Adjuvant/ Delivery |
Routea | % Protectionb | Immune response | Ref. |
|---|---|---|---|---|---|---|
| SREHP fused to maltose binding protein | Gerbil | Attenuated Salmonella typhimurium | Orally | 78% are protected against ALA | Humoral immunity: Anti-SREHP serum IgG, serum IgA, and mucosal IgA |
46 |
| DNA encoding SREHP | Mouse Gerbil |
Plasmid | i.m. | Mouse: 80% are protected against ALA Gerbils: 60% are protected against ALA |
Humoral immunity:Anti-SREHP IgG Strong lymphocyte proliferation |
70 |
| 29-kDa alkyl hydroperoxide reductase | Mice | CTB | Orally | 80% are protected against AC | Humoral immunity: Anti-Eh29 intestinal IgA and serum IgG |
52 |
| MLIF tetramer around lysine core (MLIF-MAPS) | Gerbil | None | i.m. | 100% protected against ALA (8 wk) | No humoral immunity Weak lymphocyte proliferation |
71 |
| DNA encoding Ehcp112 and Ehadh112 | Hamster | Plasmid | i.d. i.m. |
i.d.: 60% survived i.m.: 30% survived |
Poor humoral immunity for both delivery routes (only IgG measured) i.d.: Strong lymphocyte proliferation i.m.:Weak lymphocyte proliferation |
72 |
| HSBP | Guinea Pig | Freund’s | s.c. | N/A | Humoral immunity: Anti-HSBP IgG and IgM, IgA |
58 |
| EhCBP30 | Hamster | None | s.c. | 70% are protected against ALA | Humoral immunity | 59 |
a Route: i.d., intradermally; i.m., intramusculary; s.c., subcutaneously; orally, by oral gavage. b%Protection: ([number of uninfected vaccinated animals]/[total number of vaccinated animals]) × 100.
Despite the lack of an animal model that precisely mimics human amebiasis, various in vivo models have been established and contributed immensely to advance the development of a potential vaccine against amebiasis. The current readout for protection against E. histolytica is ALA development in the gerbil model (Meriones unguiculatus),28 although the use of it is restricted by the availability of immunological reagents. Monitoring of immune responses in hamsters and mice have also been used to measure protective efficacy against E. histolytica infection, however the pathological features seen in these models are not representative of the human disease.29 Whichever animal model is used, the standard procedure for vaccine trials involves vaccine administration, after which animals are challenged by the direct injection of live trophozoites into the target organ (commonly the liver), and then protective efficacy is assessed. Some of the major vaccine candidates that have been investigated include:
Gal-lectin
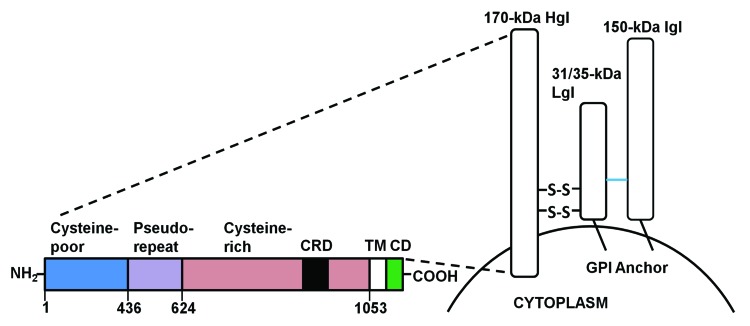
The most widely studied antigen in vaccine development is the Gal-lectin. This is a 260-kDa heterodimer protein localized on the surface of ameba, acting as an adhesin to attach to cell surfaces.15 The Gal-lectin consists of a heavy subunit (170-kDa, HgL) that is disulfide-linked to a light subunit (31/35-kDa, LgL) that is non-covalently associated with a 150-kDa intermediate subunit (Fig. 1).30 Gal-lectin is an attractive candidate for a vaccine because of its immunogenicity and its importance in disease pathogenesis, playing major roles in parasite adherence to MUC2 mucin and to target cells resulting in contact-dependent killing and phagocytosis.31 Additionally, Gal-lectin was shown to induce dendritic cell maturation and promote the production of the Th1 cytokines IL-12 and IFN-γ in vitro and in vivo.32 Moreover, the 170-kDa subunit is antigenically conserved among different strains of E. histolytica and is immunoreactive, whereas the 31/35-kDa subunit does not elicit a particular immune response.33,34 Several vaccine trials using either native or recombinant forms of the Gal-lectin have been demonstrated to be efficacious as a vaccine (reviewed in Table 1). The first experiment to use purified native Gal-lectin elicited 86% protection against ALA in the gerbil model.35 Despite these positive data, a significant limitation of using native antigens directly isolated from E. histolytica is the complexity involved with generating mass quantities of antigen and regulatory approval needed to use native protein for clinical studies. Recombinant proteins, on the other hand, can easily be produced in large quantities. Based on this rationale, a major objective was to generate a Gal-lectin subunit vaccine by mapping the carbohydrate-rich regions of the heavy subunit that elicited the greatest protective efficacy. Toward this goal, a recombinant form of the cysteine-rich region of the Gal-lectin 170-kDa subunit, which includes aa 758-1134 termed LC3, was found to be efficacious as a subunit vaccine against E. histolytica.36 Moreover, several studies that looked at the serum IgA antibodies of ALA patients found that these antibodies bound with high affinity to the LC3 regions aa 868-944 and aa 1114-1134.37 The anti-LC3 IgA antibody inhibited parasite adherence to Chinese Hamster Ovary (CHO) cells by 25 to 87%, suggesting that blocking antibodies may function by preventing E. histolytica adherence to gut epithelial cells.37
Figure 1. A schematic structure of the E. histolytica Gal-lectin. The Gal-lectin adhesin is composed of 3 subunits: the heavy subunit (Hgl) is linked by disulfide bonds to the light subunit (Lgl) and is non-covalently associated with the intermediate subunit (Igl). The heavy subunit has a short cytoplasmic tail and both the light and intermediate subunits are GPI anchored. TM, transmembrane; CD, cytoplasmic domain; CRD, carbohydrate rich domain; GPI, glycosylphosphatidylinisotol.
Furthermore, the generation of monoclonal antibodies directed against the cysteine-rich region of the Gal-lectin has allowed for the mapping of an additional 6 distinct epitopes that either enhance or inhibit amebic adherence to host cells.38,39 Monoclonal antibodies 8C12 (aa 895-998) and 1G7 (aa 596-818) were the most potent at inhibiting E. histolytica adherence to CHO cells and human colonic mucins.38,39 On the other hand, E. histolytica binding was enhanced with monoclonal antibodies 3F4 (aa 895-998) and 8A3 (aa 895-998), which interestingly recognize the same region as 8C12.38,39 These results were surprising and emphasize that precautions need to be taken when designing vaccines against this portion of the Gal-lectin. The use of the monoclonal antibody 1G7 (aa 596-1082) to the Gal-lectin heavy subunit revealed that this region was critical for stimulating cell-mediated immunity, as measured by TNF-α production.40 Consistent with this data, intraperitoneal administration of a peptide consisting of the cysteine-rich region (aa 939-1053) of the Gal-lectin heavy subunit together with Freund’s adjuvant was found to exhibit the greatest protective efficacy, in comparison to the pseudo-repeat or the cysteine-poor regions.41 These critical studies, which include fine mapping the protective epitopes coupled with studies on the biology of the parasite and host responses, emphasize the importance of choosing appropriate regions to the Gal-lectin needed to confer immune protection against E. histolytica.
Additionally, even though the Gal-lectin is a potent immunogen on its own, appropriate adjuvants are needed to elicit a strong immune response. As IFN-γ and TNF-α-stimulated macrophages play a key role in host defense against E. histolytica, we designed a vaccine strategy that would elicit a Th1 response in order to favor this mechanism. We thus used the Th1 adjuvant CpG-ODN in conjunction with Gal-lectin in place of conventional adjuvants.27 We showed that the combination of native 260-kDa Gal-lectin and CpG-ODN induced higher levels of IFN-γ, IL-2, and IL-12 compared with controls in addition to the production of antibodies that inhibited amebic adherence to target cells by 92% and elicited 100% protection against ALA formation.27 This places the Gal-lectin and CpG-ODN as one of the promising vaccines to date (Table 1). As E. histolytica is primarily a mucosal pathogen, we modified the vaccine for intranasal delivery to elicit mucosal immune responses, followed by a boost with native Gal-lectin intraperitoneally to trigger systemic immune responses. Using the strategy of native Gal-lectin plus CpG-ODN administered intranasally, animals produced Gal-lectin-neutralizing sIgA that inhibited parasite adherence to target cells and, importantly, protected animals against ALA development.42 This was the first Gal-lectin based vaccine design that demonstrated high efficacy at both mucosal and systemic sites.
Serine-rich E. histolytica protein
Other potential protein targets and their respective percentage of protection against infections are reviewed in Table 2. Previous studies have reported much success with the use of serine-rich E. histolytica protein (SREHP) as an antigen to protect gerbils against ALA.43 SREHP consists of a high number of serine residues and multiple conserved sequences of octapeptides and dodecapeptides.44,45 Although the function of SREHP is largely unexplored, it has been shown to act as an adhesin and as a chemoattractant for E. histolytica trophozoites in vitro.44,45 Intradermal immunization with SREHP as a maltose-binding fusion protein (MBP) induced 100% protection against ALA in gerbils.43 Using attenuated bacteria as a vehicle for the delivery of antigens has also become an attractive method for immunizations. Attenuated vaccine strains of Salmonella typhimurium46 and Vibrio cholera47 have been used to deliver SREHP to stimulate systemic and mucosal immune responses and protection against amebic infections were observed. Another strategy to induce IgA and IgG mucosal immune responses is by administering an antigen conjugated with CTB.48 CTB was found to enhance the immunogenicity of SREHP when mice were orally immunized with the dodecapeptide repeat of SREHP conjugated to CTB, however, administration of a supplemental dose of CTB was required to achieve maximal protection.48 In another study, a construct consisting of the SREHP-cholera toxin A2 fusion and its co-expression with CTB in Escherichia coli was as effective in inducing mucosal immune responses without supplemental CTB and its associated side-effects.49 The aforementioned studies indicate that improvements are continually being made to attain a safe and effective vaccine.
E. histolytica 29-kDa antigen
Alongside Gal-lectin and SREHP, E. histolytica 29-kDa antigen (Eh29) is also considered a prime target protein for an amebiasis vaccine. Eh29 is an alkyl hydroperoxide reductase involved in the detoxification of reactive oxygen species secreted by the microflora or immune cells.50 One study has shown that 54% of hamsters vaccinated against Eh29 were protected against ALA.51 A subsequent study using Eh29 conjugated to CTB conferred protection against intracecal amebiasis, which was associated with anti-Eh29 IgA antibodies in the intestine and Eh29-specific IgG antibodies in serum.52 In summary, both SREHP and Eh29 appear to hold promise for vaccine development against amebic infections. There is no consensus as to which adjuvant or mode of delivery works the best because a successful vaccine also depends on the type of antigen and administration route chosen. For this reason, more immunization trials are required to find the optimal vaccine regimen appropriate for the animal models.
DNA Vaccines
The development of DNA vaccines is fairly recent and involves the introduction of DNA sequences of a specific antigen of interest into a bacterial plasmid.53 Upon delivery of the plasmid to host cells, the gene is expressed and leads to the production of the corresponding protein.53 This peptide is then recognized by the host cell as foreign, thereby stimulating the production of antibodies against it.53 DNA vaccines have been shown to induce strong humoral and cell-mediated responses and were successful in conferring protection against pathogens in many animal models.54 Several optimization strategies are also available to improve DNA vaccines in hopes of stimulating strong immune responses and protection against parasites.54 In our laboratory, we generated a codon-optimized DNA vaccine encoding a portion of the E. histolytica Gal-lectin.55 A gerbil codon usage was used to re-write the Gal-lectin Hgl and when mice were vaccinated intradermally with the DNA plasmid, they developed a Th1-specific cellular immune response in addition to serum antibodies against the Gal-lectin recombinant region.55 These studies have not been explored further as high vaccine efficacy was not achieved. Multivalence can also be achieved with DNA vaccines by inserting more than one antigen into a single plasmid or the administration of several plasmids. In one such study, the EhCPADH complex, formed by 2 surface molecules, cysteine proteinase 112 (EhCP112) and an adhesin (EhADH112), was used as a source of antigens for a multivalent DNA vaccine.56 In comparison to immunization with each plasmid alone (EhCP112 or EhADH112), co-immunization of hamsters with the 2 DNA plasmids induced significantly greater levels of anti-E. histolytica IgG.56 These studies are in their infancy and, unless other single or multivalent DNA vaccines are developed and tested, the utility of DNA vaccine against E. histolytica will most likely remain unexplored.
Recent Vaccine Candidates
Recent newly identified protein targets for vaccine design include the heparin sulfate binding protein (HSBP) and the 30-kDa collagen binding protein (CBP30) of E. histolytica. It is widely recognized that adherence is the first step in the pathogenesis of amebiasis. In addition to adherence to MUC2 and glycolipids, several surface proteoglycans, including heparin sulfate proteins, act as adhesion receptors for E. histolytica.57,58 HSBPs, which bind to host heparin sulfate proteins, were isolated from E. histolytica and used to vaccinate guinea pigs.58 This regime stimulated the production of IgG and IgA against HSBPs.58 Antibody levels, especially IgA, peaked much higher after subsequent challenge infection with E. histolytica, indicative of the establishment of immunological memory.58 Another study used recombinant CBP30 fused to portions of the heat shock protein 70-kDa of Trypanosoma cruzi and found a 70% reduction in ALA formation in hamsters vaccinated with recombinant CBP30 alone or the CBP30 fusion protein compared with the non-vaccinated group.59 Although these studies propose interesting target proteins, further studies are required to elucidate the role of these proteins in disease pathogenesis and/or parasite metabolism. Collectively, based on the many studies conducted, the readout for E. histolytica infection, such as the presence or absence of amebic colitis or ALA formation and quantitative analyses including antibody titers and protection efficacy, are valuable measurements to include to allow comparison across studies. Some of these measurements were lacking in the 2 aforementioned studies.
Challenges in Vaccine Development and Future Strategies
Apart from the identification of E. histolytica immunogenic proteins, the right combination of doses, boosts, and adjuvants need to be optimized in order to develop a successful vaccine against amebiasis. A hallmark of successful vaccines is the induction of long-term memory which, unfortunately, has yet to be demonstrated in animal models. The majority of the Gal-lectin based subunit studies follow a vaccine regimen that consisted of administering the first dose and boosters between wk 0–9, and evaluating protection (following challenge with live E. histolytica) against ALA within 1–11 wk following boosters (Table 1). Immune responses and protection should be re-evaluated at later time points relevant for the measure of immunological memory.
Beyond rodent models, only one study has explored vaccines in a non-human primate model of amebiasis.60 When the Gal-lectin was administered with CTB as an adjuvant in baboons, the natural hosts for E. histolytica, it afforded a moderate level of protection against E. histolytica re-infection.60,61 Baboons were challenged with E. histolytica using colonoscopy into the lumen of the small intestine and cecum, which is a less invasive method without causing early inflammatory responses due to injections. Vaccinated baboons displayed high titers of intestinal anti-peptide IgA, intestinal anti-lectin IgA, and serum anti-peptide IgG antibodies and did not show signs of inflammatory colitis or parasite invasion.60 In comparison to the gerbil model of ALA, this study was a good representation of the early stages of amebiasis, since the majority of people who are infected usually present symptoms of intestinal inflammation. Another set of rigorous testing of these vaccine regimens will still be required in humans before it is licensed for use as only a small number of baboons were used in the study. Nonetheless, the non-human primate study represents a significant step toward further advancing anti-E. histolytica vaccines beyond the pre-clinical stage. A major impediment to proceeding to human clinical trials, however, is the willingness to invest in neglected tropical diseases such as amebiasis,62 which affects low and middle income countries. Furthermore, it is essential to keep in mind that socioeconomic barriers need to be overcome before conducting clinical trials, as this requires more than just delivering a vaccine and will consist of educating and informing populations about clinical trials. As there are currently no vaccines available for enteric parasites, E. histolytica vaccine research can provide the basis for developing strategies against other parasites. Ongoing research efforts in the mechanisms of pathogenesis will continually shed light on vaccine design against E. histolytica and hopefully one day, amebiasis can be eradicated.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
K.C. is a Canada Research Chair (Tier 1) in Gastrointestinal Inflammation and his research is supported by grants from the Natural Sciences and Engineering Research Council of Canada (NSERC), the Canadian Institutes of Health Research (CIHR) and the Crohn’s and Colitis Foundation of Canada. J.S.P. is supported in part, by a fellowship from the Alberta Inflammatory Bowel Disease Consortium awarded by Alberta Innovates Health Solutions. J.Q. is supported by a scholarship from the HPI NSERC CREATE program.
Glossary
Abbreviations:
- ALA
amebic liver abscess
- AC
amebic colitis
- Ig
immunoglobulin
- SREHP
serine-rich E. histolytica protein
- CTB
cholera toxin B
References
- 1.WHO Amoebiasis. Wkly Epidemiol Rec. 1997;72:97–9. [PubMed] [Google Scholar]
- 2.Mondal D, Haque R, Sack RB, Kirkpatrick BD, Petri WA., Jr. Attribution of malnutrition to cause-specific diarrheal illness: evidence from a prospective study of preschool children in Mirpur, Dhaka, Bangladesh. Am J Trop Med Hyg. 2009;80:824–6. [PMC free article] [PubMed] [Google Scholar]
- 3.Haque R, Ali IM, Sack RB, Farr BM, Ramakrishnan G, Petri WA., Jr. Amebiasis and mucosal IgA antibody against the Entamoeba histolytica adherence lectin in Bangladeshi children. J Infect Dis. 2001;183:1787–93. doi: 10.1086/320740. [DOI] [PubMed] [Google Scholar]
- 4.Abd-Alla MD, Jackson TFGH, Rogers T, Reddy S, Ravdin JI. Mucosal immunity to asymptomatic Entamoeba histolytica and Entamoeba dispar infection is associated with a peak intestinal anti-lectin immunoglobulin A antibody response. Infect Immun. 2006;74:3897–903. doi: 10.1128/IAI.02018-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ravdin JI, Abd-Alla MD, Welles SL, Reddy S, Jackson TFHG. Intestinal antilectin immunoglobulin A antibody response and immunity to Entamoeba dispar infection following cure of amebic liver abscess. Infect Immun. 2003;71:6899–905. doi: 10.1128/IAI.71.12.6899-6905.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blessmann J, Van Linh P, Nu PAT, Thi HD, Muller-Myhsok B, Buss H, Tannich E. Epidemiology of amebiasis in a region of high incidence of amebic liver abscess in central Vietnam. Am J Trop Med Hyg. 2002;66:578–83. doi: 10.4269/ajtmh.2002.66.578. [DOI] [PubMed] [Google Scholar]
- 7.Hien BTT, Trang T, Scheutz F, Cam PD, Mølbak K, Dalsgaard A. Diarrhoeagenic Escherichia coli and other causes of childhood diarrhoea: a case-control study in children living in a wastewater-use area in Hanoi, Vietnam. J Med Microbiol. 2007;56:1086–96. doi: 10.1099/jmm.0.47093-0. [DOI] [PubMed] [Google Scholar]
- 8.Haque R, Huston CD, Hughes M, Houpt E, Petri WA., Jr. Amebiasis. N Engl J Med. 2003;348:1565–73. doi: 10.1056/NEJMra022710. [DOI] [PubMed] [Google Scholar]
- 9.Bansal D, Malla N, Mahajan RC. Drug resistance in amoebiasis. Indian J Med Res. 2006;123:115–8. [PubMed] [Google Scholar]
- 10.WHO Progress towards global poliomyelitis eradication: preparation for the oral poliovirus vaccine cessation era. Wkly Epidemiol Rec. 2004;79:349–55. [PubMed] [Google Scholar]
- 11.Mortimer L, Chadee K. The immunopathogenesis of Entamoeba histolytica. Exp Parasitol. 2010;126:366–80. doi: 10.1016/j.exppara.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 12.Dharmani P, Srivastava V, Kissoon-Singh V, Chadee K. Role of intestinal mucins in innate host defense mechanisms against pathogens. J Innate Immun. 2009;1:123–35. doi: 10.1159/000163037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lidell ME, Moncada DM, Chadee K, Hansson GC. Entamoeba histolytica cysteine proteases cleave the MUC2 mucin in its C-terminal domain and dissolve the protective colonic mucus gel. Proc Natl Acad Sci U S A. 2006;103:9298–303. doi: 10.1073/pnas.0600623103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chadee K, Petri WA, Jr., Innes DJ, Ravdin JI. Rat and human colonic mucins bind to and inhibit adherence lectin of Entamoeba histolytica. J Clin Invest. 1987;80:1245–54. doi: 10.1172/JCI113199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chadee K, Johnson ML, Orozco E, Petri WA, Jr., Ravdin JI. Binding and internalization of rat colonic mucins by the galactose/N-acetyl-D-galactosamine adherence lectin of Entamoeba histolytica. J Infect Dis. 1988;158:398–406. doi: 10.1093/infdis/158.2.398. [DOI] [PubMed] [Google Scholar]
- 16.Chadee K, Meerovitch E. Entamoeba histolytica: early progressive pathology in the cecum of the gerbil (Meriones unguiculatus) Am J Trop Med Hyg. 1985;34:283–91. doi: 10.4269/ajtmh.1985.34.283. [DOI] [PubMed] [Google Scholar]
- 17.Huston CD, Houpt ER, Mann BJ, Hahn CS, Petri WA., Jr. Caspase 3-dependent killing of host cells by the parasite Entamoeba histolytica. Cell Microbiol. 2000;2:617–25. doi: 10.1046/j.1462-5822.2000.00085.x. [DOI] [PubMed] [Google Scholar]
- 18.Lamm ME. Interaction of antigens and antibodies at mucosal surfaces. Annu Rev Microbiol. 1997;51:311–40. doi: 10.1146/annurev.micro.51.1.311. [DOI] [PubMed] [Google Scholar]
- 19.Lamm ME. Current concepts in mucosal immunity. IV. How epithelial transport of IgA antibodies relates to host defense. Am J Physiol. 1998;274:G614–7. doi: 10.1152/ajpgi.1998.274.4.g614. [DOI] [PubMed] [Google Scholar]
- 20.Haque R, Mondal D, Duggal P, Kabir M, Roy S, Farr BM, Sack RB, Petri WA., Jr. Entamoeba histolytica infection in children and protection from subsequent amebiasis. Infect Immun. 2006;74:904–9. doi: 10.1128/IAI.74.2.904-909.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haque R, Duggal P, Ali IM, Hossain MB, Mondal D, Sack RB, Farr BM, Beaty TH, Petri WA., Jr. Innate and acquired resistance to amebiasis in bangladeshi children. J Infect Dis. 2002;186:547–52. doi: 10.1086/341566. [DOI] [PubMed] [Google Scholar]
- 22.Kaur U, Sharma AK, Sharma M, Vohra H. Distribution of Entamoeba histolytica Gal/GalNAc lectin-specific antibody response in an endemic area. Scand J Immunol. 2004;60:524–8. doi: 10.1111/j.0300-9475.2004.01512.x. [DOI] [PubMed] [Google Scholar]
- 23.Guo X, Barroso L, Lyerly DM, Petri WA, Jr., Houpt ER. CD4+ and CD8+ T cell- and IL-17-mediated protection against Entamoeba histolytica induced by a recombinant vaccine. Vaccine. 2011;29:772–7. doi: 10.1016/j.vaccine.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sánchez-Guillén MdelC, Pérez-Fuentes R, Salgado-Rosas H, Ruiz-Argüelles A, Ackers J, Shire A, Talamás-Rohana P. Differentiation of entamoeba histolytica/entamoeba dispar by PCR and their correlation with humoral and cellular immunity in individuals with clinical variants of amoebiasis. Am J Trop Med Hyg. 2002;66:731–7. doi: 10.4269/ajtmh.2002.66.731. [DOI] [PubMed] [Google Scholar]
- 25.Denis M, Chadee K. Cytokine activation of murine macrophages for in vitro killing of Entamoeba histolytica trophozoites. Infect Immun. 1989;57:1750–6. doi: 10.1128/iai.57.6.1750-1756.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holmgren J, Adamsson J, Anjuère F, Clemens J, Czerkinsky C, Eriksson K, Flach C-F, George-Chandy A, Harandi AM, Lebens M, et al. Mucosal adjuvants and anti-infection and anti-immunopathology vaccines based on cholera toxin, cholera toxin B subunit and CpG DNA. Immunol Lett. 2005;97:181–8. doi: 10.1016/j.imlet.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 27.Ivory CPA, Keller K, Chadee K. CpG-oligodeoxynucleotide is a potent adjuvant with an Entamoeba histolytica Gal-inhibitable lectin vaccine against amoebic liver abscess in gerbils. Infect Immun. 2006;74:528–36. doi: 10.1128/IAI.74.1.528-536.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chadee K, Meerovitch E. The pathogenesis of experimentally induced amebic liver abscess in the gerbil (Meriones unguiculatus) Am J Pathol. 1984;117:71–80. [PMC free article] [PubMed] [Google Scholar]
- 29.Tsutsumi V, Shibayama M. Experimental amebiasis: a selected review of some in vivo models. Arch Med Res. 2006;37:210–20. doi: 10.1016/j.arcmed.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 30.Petri WA, Jr., Chapman MD, Snodgrass T, Mann BJ, Broman J, Ravdin JI. Subunit structure of the galactose and N-acetyl-D-galactosamine-inhibitable adherence lectin of Entamoeba histolytica. J Biol Chem. 1989;264:3007–12. [PubMed] [Google Scholar]
- 31.Huston CD, Boettner DR, Miller-Sims V, Petri WA., Jr. Apoptotic killing and phagocytosis of host cells by the parasite Entamoeba histolytica. Infect Immun. 2003;71:964–72. doi: 10.1128/IAI.71.2.964-972.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ivory CPA, Chadee K. Activation of dendritic cells by the Gal-lectin of Entamoeba histolytica drives Th1 responses in vitro and in vivo. Eur J Immunol. 2007;37:385–94. doi: 10.1002/eji.200636476. [DOI] [PubMed] [Google Scholar]
- 33.Beck DL, Tanyuksel M, Mackey AJ, Haque R, Trapaidze N, Pearson WR, Loftus B, Petri WA. Entamoeba histolytica: sequence conservation of the Gal/GalNAc lectin from clinical isolates. Exp Parasitol. 2002;101:157–63. doi: 10.1016/S0014-4894(02)00113-3. [DOI] [PubMed] [Google Scholar]
- 34.Petri WA, Jr., Broman J, Healy G, Quinn T, Ravdin JI. Antigenic stability and immunodominance of the Gal/GalNAc adherence lectin of Entamoeba histolytica. Am J Med Sci. 1989;297:163–5. doi: 10.1097/00000441-198903000-00006. [DOI] [PubMed] [Google Scholar]
- 35.Petri WA, Jr., Ravdin JI. Protection of gerbils from amebic liver abscess by immunization with the galactose-specific adherence lectin of Entamoeba histolytica. Infect Immun. 1991;59:97–101. doi: 10.1128/iai.59.1.97-101.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soong C-J, Kain KC, Abd-Alla M, Jackson TFHG, Ravdin JI. A recombinant cysteine-rich section of the Entamoeba histolytica galactose-inhibitable lectin is efficacious as a subunit vaccine in the gerbil model of amebic liver abscess. J Infect Dis. 1995;171:645–51. doi: 10.1093/infdis/171.3.645. [DOI] [PubMed] [Google Scholar]
- 37.Abd-Alla MD, Jackson TFGH, Soong GC, Mazanec M, Ravdin JI. Identification of the Entamoeba histolytica galactose-inhibitable lectin epitopes recognized by human immunoglobulin A antibodies following cure of amebic liver abscess. Infect Immun. 2004;72:3974–80. doi: 10.1128/IAI.72.7.3974-3980.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petri WA, Jr., Snodgrass TL, Jackson TFHG, Gathiram V, Simjee AE, Chadee K, Chapman MD. Monoclonal antibodies directed against the galactose-binding lectin of Entamoeba histolytica enhance adherence. J Immunol. 1990;144:4803–9. [PubMed] [Google Scholar]
- 39.Mann BJ, Chung CY, Dodson JM, Ashley LS, Braga LL, Snodgrass TL. Neutralizing monoclonal antibody epitopes of the Entamoeba histolytica galactose adhesin map to the cysteine-rich extracellular domain of the 170-kilodalton subunit. Infect Immun. 1993;61:1772–8. doi: 10.1128/iai.61.5.1772-1778.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Séguin R, Mann BJ, Keller K, Chadee K. Identification of the galactose-adherence lectin epitopes of Entamoeba histolytica that stimulate tumor necrosis factor-alpha production by macrophages. Proc Natl Acad Sci U S A. 1995;92:12175–9. doi: 10.1073/pnas.92.26.12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lotter H, Zhang T, Seydel KB, Stanley SL, Jr., Tannich E. Identification of an epitope on the Entamoeba histolytica 170-kD lectin conferring antibody-mediated protection against invasive amebiasis. J Exp Med. 1997;185:1793–801. doi: 10.1084/jem.185.10.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ivory CPA, Chadee K. Intranasal immunization with Gal-inhibitable lectin plus an adjuvant of CpG oligodeoxynucleotides protects against Entamoeba histolytica challenge. Infect Immun. 2007;75:4917–22. doi: 10.1128/IAI.00725-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang T, Cieslak PR, Stanley SL., Jr. Protection of gerbils from amebic liver abscess by immunization with a recombinant Entamoeba histolytica antigen. Infect Immun. 1994;62:1166–70. doi: 10.1128/iai.62.4.1166-1170.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stanley SL, Jr., Tian K, Koester JP, Li E. The serine-rich Entamoeba histolytica protein is a phosphorylated membrane protein containing O-linked terminal N-acetylglucosamine residues. J Biol Chem. 1995;270:4121–6. doi: 10.1074/jbc.270.8.4121. [DOI] [PubMed] [Google Scholar]
- 45.Stanley SL, Jr., Becker A, Kunz-Jenkins C, Foster L, Li E. Cloning and expression of a membrane antigen of Entamoeba histolytica possessing multiple tandem repeats. Proc Natl Acad Sci U S A. 1990;87:4976–80. doi: 10.1073/pnas.87.13.4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang T, Stanley SL., Jr. Oral immunization with an attenuated vaccine strain of Salmonella typhimurium expressing the serine-rich Entamoeba histolytica protein induces an antiamebic immune response and protects gerbils from amebic liver abscess. Infect Immun. 1996;64:1526–31. doi: 10.1128/iai.64.5.1526-1531.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ryan ET, Butterton JR, Zhang T, Baker MA, Stanley SL, Jr., Calderwood SB. Oral immunization with attenuated vaccine strains of Vibrio cholerae expressing a dodecapeptide repeat of the serine-rich Entamoeba histolytica protein fused to the cholera toxin B subunit induces systemic and mucosal antiamebic and anti-V. cholerae antibody responses in mice. Infect Immun. 1997;65:3118–25. doi: 10.1128/iai.65.8.3118-3125.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang T, Li E, Stanley SL., Jr. Oral immunization with the dodecapeptide repeat of the serine-rich Entamoeba histolytica protein (SREHP) fused to the cholera toxin B subunit induces a mucosal and systemic anti-SREHP antibody response. Infect Immun. 1995;63:1349–55. doi: 10.1128/iai.63.4.1349-1355.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sultan F, Jin L-L, Jobling MG, Holmes RK, Stanley SL., Jr. Mucosal immunogenicity of a holotoxin-like molecule containing the serine-rich Entamoeba histolytica protein (SREHP) fused to the A2 domain of cholera toxin. Infect Immun. 1998;66:462–8. doi: 10.1128/iai.66.2.462-468.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bruchhaus I, Richter S, Tannich E. Removal of hydrogen peroxide by the 29 kDa protein of Entamoeba histolytica. Biochem J. 1997;326:785–9. doi: 10.1042/bj3260785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Soong C-JG, Torian BE, Abd-Alla MD, Jackson TFHG, Gatharim V, Ravdin JI. Protection of gerbils from amebic liver abscess by immunization with recombinant Entamoeba histolytica 29-kilodalton antigen. Infect Immun. 1995;63:472–7. doi: 10.1128/iai.63.2.472-477.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carrero JC, Contreras-Rojas A, Sánchez-Hernández B, Petrosyan P, Bobes RJ, Ortiz-Ortiz L, Laclette JP. Protection against murine intestinal amoebiasis induced by oral immunization with the 29 kDa antigen of Entamoeba histolytica and cholera toxin. Exp Parasitol. 2010;126:359–65. doi: 10.1016/j.exppara.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 53.Donnelly JJ, Wahren B, Liu MA. DNA vaccines: progress and challenges. J Immunol. 2005;175:633–9. doi: 10.4049/jimmunol.175.2.633. [DOI] [PubMed] [Google Scholar]
- 54.Ivory C, Chadee K. DNA vaccines: designing strategies against parasitic infections. Genet Vaccines Ther. 2004;2:17. doi: 10.1186/1479-0556-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gaucher D, Chadee K. Construction and immunogenicity of a codon-optimized Entamoeba histolytica Gal-lectin-based DNA vaccine. Vaccine. 2002;20:3244–53. doi: 10.1016/S0264-410X(02)00303-1. [DOI] [PubMed] [Google Scholar]
- 56.Madriz X, Martínez MB, Rodríguez MA, Sierra G, Martínez-López C, Riverón AM, Flores L, Orozco E. Expression in fibroblasts and in live animals of Entamoeba histolytica polypeptides EhCP112 and EhADH112. Microbiology. 2004;150:1251–60. doi: 10.1099/mic.0.26938-0. [DOI] [PubMed] [Google Scholar]
- 57.Rostand KS, Esko JD. Microbial adherence to and invasion through proteoglycans. Infect Immun. 1997;65:1–8. doi: 10.1128/iai.65.1.1-8.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kaur U, Khurana S, Saikia UN, Dubey ML. Immunogenicity and protective efficacy of heparan sulphate binding proteins of Entamoeba histolytica in a guinea pig model of intestinal amoebiasis. Exp Parasitol. 2013;135:486–96. doi: 10.1016/j.exppara.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 59.González-Vázquez MC, Carabarin-Lima A, Baylón-Pacheco L, Talamás-Rohana P, Rosales-Encina JL. Obtaining of three recombinant antigens of Entamoeba histolytica and evaluation of their immunogenic ability without adjuvant in a hamster model of immunoprotection. Acta Trop. 2012;122:169–76. doi: 10.1016/j.actatropica.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 60.Abd Alla MD, Wolf R, White GL, Kosanke SD, Cary D, Verweij JJ, Zhang M-J, Ravdin JI. Efficacy of a Gal-lectin subunit vaccine against experimental Entamoeba histolytica infection and colitis in baboons (Papio sp.) Vaccine. 2012;30:3068–75. doi: 10.1016/j.vaccine.2012.02.066. [DOI] [PubMed] [Google Scholar]
- 61.Jackson TF, Sargeaunt PG, Visser PS, Gathiram V, Suparsad S, Anderson CB. Entamoeba histolytica: naturally occurring infections in baboons. Arch Invest Med (Mex) 1990;21(Suppl 1):153–6. [PubMed] [Google Scholar]
- 62.Bethony JM, Cole RN, Guo X, Kamhawi S, Lightowlers MW, Loukas A, Petri W, Reed S, Valenzuela JG, Hotez PJ. Vaccines to combat the neglected tropical diseases. Immunol Rev. 2011;239:237–70. doi: 10.1111/j.1600-065X.2010.00976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Houpt E, Barroso L, Lockhart L, Wright R, Cramer C, Lyerly D, Petri WA. Prevention of intestinal amebiasis by vaccination with the Entamoeba histolytica Gal/GalNac lectin. Vaccine. 2004;22:611–7. doi: 10.1016/j.vaccine.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 64.Ivory CPA, Prystajecky M, Jobin C, Chadee K. Toll-like receptor 9-dependent macrophage activation by Entamoeba histolytica DNA. Infect Immun. 2008;76:289–97. doi: 10.1128/IAI.01217-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lotter H, Rüssmann H, Heesemann J, Tannich E. Oral vaccination with recombinant Yersinia enterocolitica expressing hybrid type III proteins protects gerbils from amebic liver abscess. Infect Immun. 2004;72:7318–21. doi: 10.1128/IAI.72.12.7318-7321.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mann BJ, Burkholder BV, Lockhart LA. Protection in a gerbil model of amebiasis by oral immunization with Salmonella expressing the galactose/N-acetyl D-galactosamine inhibitable lectin of Entamoeba histolytica. Vaccine. 1997;15:659–63. doi: 10.1016/S0264-410X(96)00236-8. [DOI] [PubMed] [Google Scholar]
- 67.Zhang T, Stanley SL., Jr. Protection of gerbils from amebic liver abscess by immunization with a recombinant protein derived from the 170-kilodalton surface adhesin of Entamoeba histolytica. Infect Immun. 1994;62:2605–8. doi: 10.1128/iai.62.6.2605-2608.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dodson JM, Lenkowski PW, Jr., Eubanks AC, Jackson TFGH, Napodano J, Lyerly DM, Lockhart LA, Mann BJ, Petri WA., Jr. Infection and immunity mediated by the carbohydrate recognition domain of the Entamoeba histolytica Gal/GalNAc lectin. J Infect Dis. 1999;179:460–6. doi: 10.1086/314610. [DOI] [PubMed] [Google Scholar]
- 69.Lotter H, Khajawa F, Stanley SL, Jr., Tannich E. Protection of gerbils from amebic liver abscess by vaccination with a 25-mer peptide derived from the cysteine-rich region of Entamoeba histolytica galactose-specific adherence lectin. Infect Immun. 2000;68:4416–21. doi: 10.1128/IAI.68.8.4416-4421.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang T, Stanley SL., Jr. DNA vaccination with the serine rich Entamoeba histolytica protein (SREHP) prevents amebic liver abscess in rodent models of disease. Vaccine. 1999;18:868–74. doi: 10.1016/S0264-410X(99)00343-6. [DOI] [PubMed] [Google Scholar]
- 71.Giménez-Scherer JA, Cárdenas G, López-Osuna M, Velázquez JR, Rico G, Isibasi A, Maldonado MdelC, Morales ME, Fernández-Diez J, Kretschmer RR. Immunization with a tetramer derivative of an anti-inflammatory pentapeptide produced by Entamoeba histolytica protects gerbils (Meriones unguiculatus) against experimental amoebic abscess of the liver. Parasite Immunol. 2004;26:343–9. doi: 10.1111/j.0141-9838.2004.00718.x. [DOI] [PubMed] [Google Scholar]
- 72.Martínez MB, Rodríguez MA, García-Rivera G, Sánchez T, Hernández-Pando R, Aguilar D, Orozco E. A pcDNA-Ehcpadh vaccine against Entamoeba histolytica elicits a protective Th1-like response in hamster liver. Vaccine. 2009;27:4176–86. doi: 10.1016/j.vaccine.2009.04.051. [DOI] [PubMed] [Google Scholar]