Abstract
The introduction of vaccines containing the capsular polysaccharides of N. meningitidis, S. pneumonia, and H. influenzae type b has driven a significant reduction in cases of disease caused by these bacteria. The polysaccharide-specific antibody responses following vaccination are well characterized, however less is known about the B cells underlying this response. Here, we summarize the plasma cell (PC) and memory B cell (BMEM) responses following plain polysaccharide and protein-polysaccharide conjugate vaccination, drawing together studies covering a range of vaccines and age groups. These studies show that infant primary PC and BMEM responses to polysaccharide-conjugate vaccines are low in relation to older age groups but are significantly higher following booster doses. PC kinetics have generally been found to follow a similar pattern irrespective of vaccine type or age group, whereas divergent BMEM responses have been reported following plain polysaccharide and conjugate vaccination. A degree of correlation between early BMEM responses and maintenance of protective antibody levels has been identified in some studies, but the relationship between the 2 remains unclear. Identification of the B cell subsets involved and the mechanisms by which they are induced may provide a better understanding of the role of B cells in maintaining protective immunity through vaccination.
Keywords: antibody, B cells, B lymphocytes, glycoconjugate, immune response, polysaccharide, thymus-dependent, thymus-independent, vaccination
Introduction
Encapsulated organisms such as Haemophilus influenzae type b, Neisseria meningitidis, and Streptococcus pneumoniae cause a significant burden of disease worldwide mainly affecting individuals at the extremes of age.1-3 The outer surface of all 3 bacteria is covered by a polysaccharide capsule, the composition and structure of which differs between and within each bacterial species. The polysaccharide capsule represents an important virulence factor, and is a major target for polysaccharide-specific antibodies that can be successfully generated by vaccines.4 There are 2 types of vaccines against these organisms which use the polysaccharide capsule as the vaccine antigen; plain polysaccharide vaccines and glycoconjugate vaccines. Natural immunity to encapsulated bacteria is provided by a combination of physical barriers, innate immune mechanisms and parts of the adaptive immune system including T cells and antibodies. Polysaccharide-containing vaccines aim to induce antigen-specific humoral immune responses, which can be quantified in terms of the serum antibody concentration. Antibodies are produced by B cells that have been triggered and activated by antigen and have undergone differentiation into antibody-secreting plasma cells. Plain polysaccharide vaccines act by cross-linking B cell receptors on the surface of naïve B cells, directing plasma cell production, whereas the protein component of glycoconjugate vaccines recruits T cell help and the generation of both plasma and memory B cells.4 Extracellular spaces, which have been invaded by pathogens and which are usually the place where they multiply, are protected by antibodies which kill extracellular microorganisms through complement-mediated bacteriolysis, opsonophagocytosis, or antibody-dependent cellular cytotoxicity.
Although there are some data indicating how best to achieve high concentrations of (functional) antibodies, little is known about the B cells that underlie such an immune response. Circulating B cells are a dynamic population and previous studies have defined the kinetics of the B cell response and permitted B cell frequencies before and after immunization to be related to immediate and medium-term antibody responses. In this manuscript we aim to describe the kinetics (timing of responses) and magnitude of both polysaccharide-specific plasma cell (PC) and memory B cell (BMEM) responses following vaccination, summarizing data from previous studies in children, adolescents, and adults.5-13 Table 1 shows characteristics of studies describing plasma and memory B cell kinetics following glycoconjugate vaccination. Data derived from these studies were used to generate Figures 1 and 2.
Table 1. Characteristics of studies describing plasma and memory B cell kinetics following immunization with glycoconjugate vaccines.
| Study reference | Age (y) |
Number of samples per study time point* |
Vaccine | Carrier protein | Number of priming doses [time since last vaccination] |
Study time points (days) |
|---|---|---|---|---|---|---|
| Plasma cells | ||||||
| Kelly et al.7 | 13–15 | 3–34 | MenCCV | CRM197 | 1 [2–3 y] | 0, 3, 4, 5, 6, 7, 8, 9, 10, 12, 28 |
| Clutterbuck et al.6 | 28–44 | 6 | PCV-7† | CRM197 | 1 [1 y] | 0, 6, 7, 15 |
| Blanchard Rohner et al.5 | 1 | 5–26 | MenCCV | CRM197 | 3 [8 mo] | 0, 2, 4, 6, 8, 9, 30 |
| Kelly et al.8 | 0.17 | 33 | MenCCV | CRM197 | - | 4, 6, 8, 10, 12, 14, 16, 30 |
| Kelly et al.8 | 0.33 | 29 | MenCCV | CRM197 | 2 [1 mo] | 4, 6, 8, 10, 12, 14, 16 |
| Memory B cells | ||||||
| Kelly et al.7 | 13–15 | 3–40 | MenCCV | CRM197 | 1 [2–3 y] | 0, 3, 4, 5, 6, 7, 28, 365 |
| Clutterbuck et al.6 | 28–44 | 6 | PCV-7† | CRM197 | 1 [1 y] | 0, 6, 7, 15, 28 |
| Blanchard Rohner et al.5 | 1 | 4–28 | MenCCV | CRM197 | 3 [8 mo] | 0, 2, 4, 6, 8, 9, 30 |
| Kelly et al.8 | 0.17 | 31 | MenCCV | CRM197 | - | 4, 6, 8, 10, 12, 14, 16, 30 |
| Kelly et al.8 | 0.33 | 17 | MenCCV | CRM197 | 2 [1 mo] | 4, 6, 8, 12, 14, 16, 30 |
| Perrett et al.9 | 7 | 52 | MenCCV | TT | 3 [6 y] | 0, 7, 28, 365 |
| Perrett et al.9 | 8 | 25 | MenCCV | TT | 2 [6 y] | 0, 28, 365 |
| Perrett et al.9 | 8.3 | 53 | MenCCV | TT | 1 [6 y] | 0, 7, 28, 365 |
| Perrett et al.9 | 9.3 | 30 | MenCCV | TT | 1 [6 y] | 0, 28, 365 |
| Perrett et al.9 | 10.3 | 27 | MenCCV | TT | 1 [6 y] | 0, 28, 365 |
| Perrett et al.9 | 11.3 | 27 | MenCCV | TT | 1 [6 y] | 0, 28, 365 |
| Perrett et al.9 | 12.1 | 29 | MenCCV | TT | 1 [6 y] | 0, 7, 28, 365 |
Number of samples between study time points may differ markedly and the range is given if known, otherwise the total number of samples is shown; †Values from serotype 4 were used to describe plasma and memory B cell kinetics; CRM197, cross-reactive material (non- toxic recombinant form of diphtheria toxin); MenCCV, meningococcal serogroup C conjugate vaccine; PCV-7, 7-valent pneumococcal conjugate vaccine; TT, tetanus toxoid.
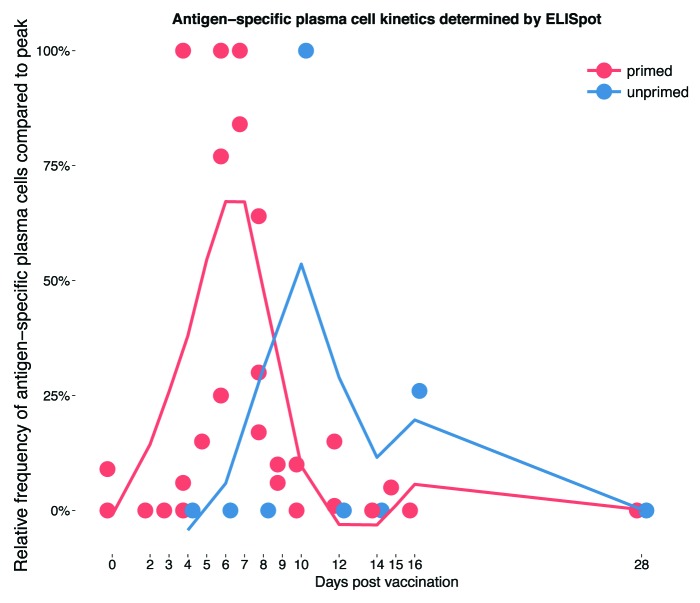
Figure 1. The kinetics of the plasma cell response to polysaccharide-containing vaccines in vaccine primed and unprimed subjects. Data taken from studies reporting on at least 2 time points between baseline and 1 mo following vaccination.5-8 Values are plotted as a percentage of the maximum response within the observed time period and then smoothed (using a locally weighted polynomial regression model [method “loess”] of the ggplot2 package20 in R21) across all 4 studies represented by the lines for vaccine primed and unprimed infants, respectively.
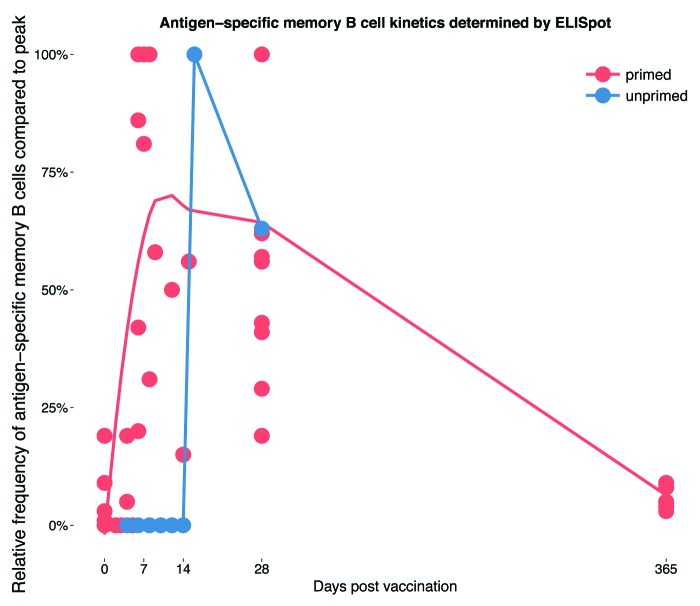
Figure 2. The kinetics of the memory B cell response to polysaccharide-containing vaccines in primed and unprimed individuals. Data taken from studies reporting on at least one additional time point other than baseline and 1 mo post-vaccination.5-8 Values are plotted as a percentage of the maximum response within the observed time period and then smoothed (see Figure legend 1; for the “unprimed” group data points were directly connected as smoothing in a similar way was not possible) across all 5 studies represented by the lines for vaccine primed and unprimed individuals, respectively.
B Cell Kinetics Following Vaccination
Plasma cell kinetics
Most studies that aimed to determine polysaccharide-specific B cell frequencies were performed using the enzyme-linked immunospot (ELISpot) assay. Other approaches such as flow-cytometry have only rarely proven to be helpful and are also costly, laborious, and difficult to perform on the large scale needed in clinical vaccine trials.14,15
PCs may be detected in peripheral blood using an ex-vivo ELISpot. This technique involves measurement of the frequency of antibody-secreting cells in peripheral blood which spontaneously produce antibody.6 In the steady-state, antigen-specific PCs in the peripheral blood are only present at a very low overall frequency in comparison to the total B cell population, but rise transiently following vaccination. PC kinetics following primary vaccination have been investigated in children after the first and third dose of a 3 dose schedule of meningococcal serogroup C conjugate vaccine at 2, 3, and 4 mo of age (MenCCV).8 Following the first vaccination, PCs were low frequency but peaked at day 10 and fell to undetectable levels by day 30. PC responses following the third dose were faster both in onset and decline, with the highest frequencies detected on day 4 (the first day assessed) and reaching baseline for all but one individual by day 12. At the time of the third dose, germinal centers generated by the previous vaccine doses may still be present, having not yet undergone the process of involution. Therefore, revaccination may continue to drive a response from these germinal centers, accounting for the early peak in plasma cells.
A small but consistent second rise of MenC-specific PC was seen both in unprimed individuals after the first vaccine dose and following the third dose detectable at day 16 and day 12, respectively.8 Although only a few samples were available for testing at each time point, it is possible that these later peaks represent the time of germinal center involution as suggested by animal data.16
Unprimed B cell responses directed at polysaccharides are more difficult to assess in adults. This is because the majority of vaccine-naïve adults already have pre-existing immunity to capsular polysaccharides, which also serve as vaccine antigens.10,13,14 This immunity, in the form of BMEM and serum antibody is probably acquired following repeated nasopharyngeal carriage of encapsulated bacteria. To overcome this problem, a novel T-dependent protein antigen for unvaccinated individuals in the UK, the rabies vaccine, was used to investigate B cell kinetics in a controlled setting of primary, secondary and tertiary immune responses.12 In this study, PCs peaked at day 10 following priming, with the most rapid and highest PC responses seen at day 7 following the third dose in the naïve group, as was observed with the primary polysaccharide response in infants. Studies of adult PC kinetics following influenza vaccination have also revealed consistent patterns of response. In these studies, PCs peaked at around a week following immunization.17-19
The PC response to a booster dose of a glycoconjugate vaccine is more rapid than following a primary dose, similar to the PC kinetics following repeated protein vaccination. The peak in PC responses following booster vaccination with a MenC glycoconjugate in 1-y-old children5 or 13- to 15-y-old adolescents7 appeared at day 6, which is more rapid than following the first vaccine dose in infants but slower than that following the third dose of an infant primary series.8
Given as a booster dose, both plain polysaccharide and glycoconjugate vaccines produce a similar pattern of PC responses. No difference in polysaccharide-specific PC kinetics was found between adolescents receiving a booster of dose of MenCCV or MenC polysaccharide vaccine (MenCPS).7 Furthermore, studies of glycoconjugate vaccines have demonstrated similar PC kinetics for both polysaccharide and carrier protein antigens,6,8 although one study reported a more rapid decrease in meningococcal serogroup C (MenC) than diphtheria toxoid-specific PCs.5
The kinetics of PC responses to vaccination in primed (individuals with pre-existing immunity through previous vaccination) and unprimed children and adults compiled from studies reporting on several separate time points within the first month following vaccination are summarized in Figure 1.
Memory B cell kinetics
Culturing peripheral blood mononuclear cells (PBMCs) with polyclonal stimulators such as a combination of pokeweed mitogen, S. aureus Cowan strain and CpG DNA induces the proliferation of BMEM and their differentiation into antibody-secreting cells which can be detected using the ELISpot assay.6,22 The culture step means that the output of the ELISpot assay is not a direct measure of BMEM frequency in the peripheral blood, but is useful for the comparison of responses between individuals and time points in relation to vaccination. Other methods such as flow cytometry or a limiting dilution assay may also be used to enumerate antigen-specific BMEM, however labeling of polysaccharide antigens for flow cytometric analysis is very laborious and not straight forward due to potential non-specific binding of B cells, and the limiting dilution assay is not as sensitive as ELISpot for BMEM detection.23
BMEM responses to a first infant dose of glycoconjugate vaccine are low, with MenC specific BMEM only appearing at day 14 post-vaccination with numbers close to the limit of detection.8 Reflecting the PC response, BMEM responses to a third dose of vaccine are more rapid, becoming detectable at day 4.8 As described previously, primary B cell responses to polysaccharide antigens are difficult to study in adults, however, using rabies protein as a model vaccine, BMEM responses in unprimed adults vaccinated with rabies protein vaccine showed similar kinetics; the first BMEM became detectable 10 d post-vaccination but appeared more rapidly following additional doses.12
Following booster vaccination with a glycoconjugate vaccine at one year of age, BMEM were shown to peak at around a week,5 which is similar to booster responses in adults primed with rabies vaccine.12 When booster doses of MenCPS and MenCCV were compared in adolescents, BMEM responses were more rapid and better sustained in those given the glycoconjugate vaccine as a booster.7
Compared with PC responses, BMEM responses in peripheral blood are more prolonged. Although frequencies of BMEM also diminish following the post-vaccination peak, BMEM specific to polysaccharides6,14 as well other antigens such as the smallpox virus24 have been detected in peripheral blood months to years following vaccination. BMEM generated in germinal center responses are thought to circulate briefly in peripheral blood before transiting to secondary lymphoid organs such as the lymph nodes or spleen where they persist long-term, or from where they continue to recirculate.4 It is therefore important when interpreting the studies described in this paper to appreciate that B cells are only being measured in transit. There is currently limited knowledge of B cell activity in other compartments; however the inaccessibility of these tissues makes peripheral blood the only option in most cases.
Figure 2 combines data from the studies discussed in this section to summarize the BMEM response to vaccination in primed and unprimed individuals.5-9
Frequency of Polysaccharide-Specific B Cell Responses
Although the timing of B cell responses seems to adhere to general patterns (Figs. 1 and 2), the magnitude of these responses is more variable. In the following section we address the effect of factors such as the number of vaccine doses, age at vaccination, and vaccine type on post-vaccination B cell frequencies.
Plasma cell frequency following vaccination
As well as becoming increasingly rapid, the magnitude of the PC response is also greater with additional doses of vaccine after the primary dose. A greater frequency of PCs is generated following the third dose of MenCCV than following the first,8 although in toddlers receiving their first dose of pneumococcal conjugate vaccine at 12 mo of age, revaccination at 14 mo did not increase PC frequency in peripheral blood relative to the same time point (1 wk post-vaccination) at 12 mo.10
Despite showing similar kinetics, there are differences in the number of plasma cells induced by plain polysaccharide and glycoconjugate vaccines. In adolescents receiving either a booster dose of MenCCV or MenCPS, both of which contained the same quantity of polysaccharide antigen, the PC response was of greater magnitude in the glycoconjugate group, although there were no differences in the timing of responses.7 This suggests that a single signal from the polysaccharide component of both vaccine types is sufficient to induce the generation of PCs from pre-existing BMEM, but that T cell help may facilitate greater expansion of the PC pool.
Differences in PC frequency have also been reported in adults following different combinations of the 7-valent pneumococcal conjugate vaccine (PCV-7) and the 23-valent pneumococcal polysaccharide vaccine (PPV-23). In adults primed with PCV-7, boosting with PPV-23 resulted in a significantly higher PC response on day 7 compared with boosting with PCV-7.25 This may be because the polysaccharide quantity of each serotype is more than 10 times higher in the PPV-23 compared with the PCV-7 or may result from unconjugated polysaccharide antigens inducing the terminal differentiation of BMEM into PCs, a phenomenon which will be covered in more detail in the discussion of BMEM responses below.
Memory B cell frequency following vaccination
BMEM frequencies differ depending on the number of vaccine doses, and particularly when plain polysaccharide and glycoconjugate vaccines are compared in infants and the elderly. Greater BMEM frequencies have been reported following a 3 dose8 compared with a 2 dose primary series11 of meningococcal conjugate vaccine, however in both studies less than 30% of children still had detectable polysaccharide-specific BMEM by 12 mo of age. Despite low pre-booster BMEM frequency, robust responses have been reported following booster vaccination with glycoconjugate vaccines at 12 mo of age,5,11 suggesting that the small pool of BMEM maintained post-priming is sufficient to sustain immunological memory.
The effect of age on BMEM frequencies has been investigated in a number of studies. Greater frequencies of BMEM have been found following vaccination in adults than in children. Twelve month old toddlers with little or no prior exposure to encapsulated bacteria required at least 2 doses of PCV-7 to reach BMEM frequencies equivalent to those following a single dose in adults.10 However, all of the adults in this study already had detectable polysaccharide-specific BMEM at baseline, likely induced by prior pneumococcal colonization, in effect priming the immune system. Another study reported that age at primary vaccination (ranging between 6 mo and 34 y) with a glycoconjugate did not affect the induction and persistence of polysaccharide-specific BMEM.14
There are no studies describing BMEM kinetics following plain polysaccharide vaccination in children. Plain polysaccharide vaccines are thymus-independent (T-independent) antigens, and are not thought to induce BMEM formation. Instead, they are believed to act by inducing extensive cross-linking of the B cell receptor (BCR) on marginal zone B cells and B1 cells, inducing an extrafollicular response predominantly involving PCs.26 Children under the age of 2 y are unable to make an efficient immune response to T-independent antigens such as plain polysaccharide vaccines. This phenomenon is not well understood but present models suggest (1) an immaturity of most B cells, e.g., lack of CD21/complement receptor 2 on neonatal B cells,27-29 (2) differences in activation requirements between primary and secondary B cells with young children having primary B cells that require both BCR signaling and T cell help for activation,30 (3) low frequency of marginal zone B cells,31,32 (4) lower levels of complement C3, and (5) immaturity of marginal zone dendritic cells and the marginal zone in young children.33
Although there are no data in infants, BMEM responses to plain polysaccharide and glycoconjugate vaccines have been compared when given to adolescents and adults. In adolescents, the magnitude of BMEM response was greater following a booster dose of MenCCV than a booster of MenCPS.34 In an adult study, vaccination with PCV-7 resulted in an increase in polysaccharide-specific BMEM whereas vaccination with PPV-23 had the opposite effect, causing a reduction in BMEM frequency.25 Adults over the age of 65 are the only population to routinely receive PPV-23. This vaccine appears to offer a degree of short-term protection against invasive pneumococcal disease,35 but no reduction in rates of pneumonia or mortality.36,37 Furthermore, immunization with plain polysaccharide vaccines may reduce antibody levels induced by prior receipt of a polysaccharide-containing vaccine, an effect known as hyporesponsiveness. This phenomenon has been demonstrated in children38 and more recently in adults,39 although another smaller adult study did not identify an effect.13 Hyporesponsiveness may be explained by the effect of polysaccharide vaccines on the BMEM population. Plain polysaccharide vaccines are thought to deplete the BMEM pool by inducing strong BCR cross-linking and driving the terminal differentiation of these cells into PCs,25 or by inducing apoptosis as demonstrated in neonatal mice primed with MenCCV and boosted with MenCPS.40 A recent study identified IgM-producing B cells as an important subset in the response to PPV-23 in young adults.41 When the B cell response was compared between young and elderly adults, elderly participants had fewer IgM positive B cells and an impaired IgM response to vaccination. A shift in B cell populations has also been implicated in the increased susceptibility of HIV-infected children to pneumococcal disease.42 Here, children with HIV were found to have reduced numbers of circulating mature naïve and resting BMEM, with an over-representation of mature, activated B cells.
Correlation of B Cell Responses to Antibody Concentrations
On a population scale a relationship between antibody concentration and clinical protection has been demonstrated, such that (functional) antibody concentrations are routinely used to predict vaccine efficacy in clinical vaccine trials. For example, the recently introduced pneumococcal conjugate vaccine, PCV-13, was licensed on the basis of its ability to elicit antibody responses above a threshold defined by the efficacy of PCV-7.43 Plasma cells have been shown to correlate with immediate increases in antibody5,7 however long-term maintenance of serum antibody cannot be accounted for by transiently induced PCs. Instead, it is thought to rely upon BMEM and long-lived PCs (LLPCs) induced by immunization. LLPCs have been identified in mice as a fraction of the PC population produced in the germinal center reaction following protein-polysaccharide vaccination.44 These cells persist in the bone marrow and can secrete antibody for prolonged periods of time. Support for the existence of LLPCs in humans comes from observations including the report that humans depleted of PC precursors (and hence circulating PCs) with rituximab are still able to maintain normal antibody levels.45 There is still debate on the exact mechanisms of survival and maintenance of LLPCs, however the “imprinting lifespan” theory appears to best fit the observed antibody kinetics in humans. This theory suggests that plasma cell life span is dependent on the strength of combined B cell signals experienced by the cell during the encounter with antigen.46 Mechanisms of long-term maintenance of BMEM are also still unclear but most likely rely on antigen-independent processes.47
While some studies have demonstrated a relationship between BMEM and antibody concentration at various intervals after immunization this has not been consistent for all antigens or between studies.9 Blanchard Rohner et al. found that BMEM levels rather than antibody levels following priming with MenCCV better discriminated those with putative protective antibody levels at 12 mo.5 Another study identified a weak positive correlation between 5 mo BMEM levels and 12 mo antibody concentrations for serotypes C and Y but not A or W in children vaccinated at 2 and 4 mo of age with the MenACWY-CRM197 conjugate vaccine.11
In the absence of pre-existing antibody, the secondary immune response initiated by memory cell populations is too slow to prevent infection by encapsulated bacteria, which can become established within hours or days. Even a large BMEM response may not guarantee protection; pneumococcal conjugate vaccines have so far failed to provide any protection against serotype 3 disease despite large BMEM responses generated toward this serotype in children vaccinated with a serotype 3 containing vaccine.48
Herd immunity induced by polysaccharide-containing vaccines is dependent on the presence of polysaccharide-specific antibody in the nasopharyngeal mucosa, which prevents bacterial colonization and carriage. The generation of antibody concentrations of sufficient magnitude to prevent colonization requires BMEM to be resident in the mucosa, however polysaccharide-specific BMEM are not found in this tissue at the steady-state, only appearing transiently following vaccination.49 This may limit the duration of herd immunity provided by glycoconjugate vaccines. Protein-specific BMEM appear to persist better in the mucosa than polysaccharide-specific BMEM, perhaps reflecting the different immunological properties of the stimulating antigen. It will be interesting to compare the extent of herd immunity and the effects on carriage provided by the recently licensed protein-based serogroup B meningococcal vaccine with that provided by glycoconjugate vaccines for other meningococcal capsular groups.
Conclusions and Perspectives
The ability to detect B cells in the circulation using techniques such as ELISpot has allowed the study of how 2 major B cell populations, plasma cells, and memory B cells, respond to vaccination. Consistent patterns have been revealed applicable to a range of ages and vaccine types. Despite efforts to correlate peripheral B cell responses with antibody levels, only weak relationships have so far been identified. This is not surprising, given the incomplete understanding of the long-term activity and maintenance of B cells following their relatively short appearance in peripheral blood. Further understanding of the role of B cells in the post-vaccination immune response requires certain challenges to be overcome. First, the ELISpot assay as it is currently performed suffers from the limitation that BMEM can only be easily measured in the peripheral blood. In contrast to antibodies, which are secreted into the serum as their site of action, BMEM only pass through the blood on their way to the secondary lymphoid tissues where they reside and meet their antigen. Further, detection of PCs and BMEM in peripheral blood may be limited by the sensitivity of the available assays, and some responses especially in young children may be below the limit of detection. The reliability of the assays used to measure B cell frequency is also an important consideration. Data indicate that the cultured ELISpot assay for the detection of BMEM is reliable across operators and laboratories (Trück et al., unpublished observations), however protocols may need to be standardized before data from multiple centers can be compared. A further challenge is the identification of the B cell subsets involved in the response to vaccination. T-dependent and T-independent antigens induce different subsets of B cells,15,49 but the mechanisms by which each antigen drives its signature B cell phenotype is unclear, as is the contribution of each subset to the maintenance of serum antibody. Identification of the subsets involved may allow prediction of the duration of protection and clinical outcomes such as efficacy and carriage using early B cell responses as a surrogate marker of immunity. An understanding of the signals influencing the induction of each subset may lead to the development of new vaccine strategies, which are more effective at preventing carriage and maintaining herd immunity. In conclusion, B cells are key players in the induction and maintenance of vaccine-induced immunity. A better understanding of the phenotype and role of the B cell subsets involved has the potential to provide a useful new tool for predicting the effectiveness and duration of vaccine-induced immunity.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Glossary
Abbreviations:
- BMEM
memory B cells
- PC
plasma cells
- BCR
B cell receptor
- LLPC
long-lived plasma cell
- MenCCV
meningococcal serogroup C conjugate vaccine
- MenCPS
meningococcal serogroup C polysaccharide vaccine
- PCV-7
7-valent pneumococcal conjugate vaccine
- PPV-23
23-valent pneumococcal polysaccharide vaccine
References
- 1.Watt JP, Wolfson LJ, O’Brien KL, Henkle E, Deloria-Knoll M, McCall N, Lee E, Levine OS, Hajjeh R, Mulholland K, et al. Hib and Pneumococcal Global Burden of Disease Study Team Burden of disease caused by Haemophilus influenzae type b in children younger than 5 years: global estimates. Lancet. 2009;374:903–11. doi: 10.1016/S0140-6736(09)61203-4. [DOI] [PubMed] [Google Scholar]
- 2.O’Brien KL, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M, McCall N, Lee E, Mulholland K, Levine OS, Cherian T, Hib and Pneumococcal Global Burden of Disease Study Team Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. 2009;374:893–902. doi: 10.1016/S0140-6736(09)61204-6. [DOI] [PubMed] [Google Scholar]
- 3.Khatami A, Pollard AJ. The epidemiology of meningococcal disease and the impact of vaccines. Expert Rev Vaccines. 2010;9:285–98. doi: 10.1586/erv.10.3. [DOI] [PubMed] [Google Scholar]
- 4.Blanchard-Rohner G, Pollard AJ. Long-term protection after immunization with protein-polysaccharide conjugate vaccines in infancy. Expert Rev Vaccines. 2011;10:673–84. doi: 10.1586/erv.11.14. [DOI] [PubMed] [Google Scholar]
- 5.Blanchard Rohner G, Snape MD, Kelly DF, John T, Morant A, Yu LM, Borkowski A, Ceddia F, Borrow R, Siegrist CA, et al. The magnitude of the antibody and memory B cell responses during priming with a protein-polysaccharide conjugate vaccine in human infants is associated with the persistence of antibody and the intensity of booster response. J Immunol. 2008;180:2165–73. doi: 10.4049/jimmunol.180.4.2165. [DOI] [PubMed] [Google Scholar]
- 6.Clutterbuck EA, Salt P, Oh S, Marchant A, Beverley P, Pollard AJ. The kinetics and phenotype of the human B-cell response following immunization with a heptavalent pneumococcal-CRM conjugate vaccine. Immunology. 2006;119:328–37. doi: 10.1111/j.1365-2567.2006.02436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelly DF, Snape MD, Clutterbuck EA, Green S, Snowden C, Diggle L, Yu LM, Borkowski A, Moxon ER, Pollard AJ. CRM197-conjugated serogroup C meningococcal capsular polysaccharide, but not the native polysaccharide, induces persistent antigen-specific memory B cells. Blood. 2006;108:2642–7. doi: 10.1182/blood-2006-01-009282. [DOI] [PubMed] [Google Scholar]
- 8.Kelly DF, Snape MD, Perrett KP, Clutterbuck EA, Lewis S, Blanchard Rohner G, Jones M, Yu LM, Pollard AJ. Plasma and memory B-cell kinetics in infants following a primary schedule of CRM 197-conjugated serogroup C meningococcal polysaccharide vaccine. Immunology. 2009;127:134–43. doi: 10.1111/j.1365-2567.2008.02934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perrett KP, Jin C, Clutterbuck E, John TM, Winter AP, Kibwana E, Yu LM, Curtis N, Pollard AJ. B cell memory to a serogroup C meningococcal conjugate vaccine in childhood and response to booster: little association with serum IgG antibody. J Immunol. 2012;189:2673–81. doi: 10.4049/jimmunol.1200451. [DOI] [PubMed] [Google Scholar]
- 10.Clutterbuck EA, Oh S, Hamaluba M, Westcar S, Beverley PC, Pollard AJ. Serotype-specific and age-dependent generation of pneumococcal polysaccharide-specific memory B-cell and antibody responses to immunization with a pneumococcal conjugate vaccine. Clin Vaccine Immunol. 2008;15:182–93. doi: 10.1128/CVI.00336-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blanchard-Rohner G, Snape MD, Kelly DF, O’Connor D, John T, Clutterbuck EA, Ohene-Kena B, Klinger CL, Odrljin T, Pollard AJ. The B-cell response to a primary and booster course of MenACWY-CRM₁₉₇ vaccine administered at 2, 4 and 12 months of age. Vaccine. 2013;31:2441–8. doi: 10.1016/j.vaccine.2013.03.036. [DOI] [PubMed] [Google Scholar]
- 12.Blanchard-Rohner G, Pulickal AS, Jol-van der Zijde CM, Snape MD, Pollard AJ. Appearance of peripheral blood plasma cells and memory B cells in a primary and secondary immune response in humans. Blood. 2009;114:4998–5002. doi: 10.1182/blood-2009-03-211052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baxendale HE, Keating SM, Johnson M, Southern J, Miller E, Goldblatt D. The early kinetics of circulating pneumococcal-specific memory B cells following pneumococcal conjugate and plain polysaccharide vaccines in the elderly. Vaccine. 2010;28:4763–70. doi: 10.1016/j.vaccine.2010.04.103. [DOI] [PubMed] [Google Scholar]
- 14.Henneken M, Burdin N, Thoroddsen E, Sigurdardottir ST, Trannoy E, Jonsdottir I. Meningococcal serogroup C polysaccharide specific memory B cells, directly enumerated by labeled polysaccharide, are not affected by age at vaccination. Vaccine. 2010;28:2097–103. doi: 10.1016/j.vaccine.2009.12.039. [DOI] [PubMed] [Google Scholar]
- 15.Khaskhely N, Mosakowski J, Thompson RS, Khuder S, Smithson SL, Westerink MA. Phenotypic analysis of pneumococcal polysaccharide-specific B cells. J Immunol. 2012;188:2455–63. doi: 10.4049/jimmunol.1102809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu YJ, Zhang J, Lane PJ, Chan EY, MacLennan IC. Sites of specific B cell activation in primary and secondary responses to T cell-dependent and T cell-independent antigens. Eur J Immunol. 1991;21:2951–62. doi: 10.1002/eji.1830211209. [DOI] [PubMed] [Google Scholar]
- 17.Halliley JL, Kyu S, Kobie JJ, Walsh EE, Falsey AR, Randall TD, Treanor J, Feng C, Sanz I, Lee FE. Peak frequencies of circulating human influenza-specific antibody secreting cells correlate with serum antibody response after immunization. Vaccine. 2010;28:3582–7. doi: 10.1016/j.vaccine.2010.02.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sasaki S, He XS, Holmes TH, Dekker CL, Kemble GW, Arvin AM, Greenberg HB. Influence of prior influenza vaccination on antibody and B-cell responses. PLoS One. 2008;3:e2975. doi: 10.1371/journal.pone.0002975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cox RJ, Brokstad KA, Zuckerman MA, Wood JM, Haaheim LR, Oxford JS. An early humoral immune response in peripheral blood following parenteral inactivated influenza vaccination. Vaccine. 1994;12:993–9. doi: 10.1016/0264-410X(94)90334-4. [DOI] [PubMed] [Google Scholar]
- 20.Wickham HW. [Endless loop including component title]. Springer New York; 2009. Available from: http://had.co.nz/ggplot2/book
- 21.R Core Team. R: A Language and Environment for Statistical Computing [Internet]. R Foundation for Statistical Computing 2013. Available from: http://www.R-project.org/
- 22.Crotty S, Aubert RD, Glidewell J, Ahmed R. Tracking human antigen-specific memory B cells: a sensitive and generalized ELISPOT system. J Immunol Methods. 2004;286:111–22. doi: 10.1016/j.jim.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 23.Blanchard-Rohner G, Galli G, Clutterbuck EA, Pollard AJ. Comparison of a limiting dilution assay and ELISpot for detection of memory B-cells before and after immunisation with a protein-polysaccharide conjugate vaccine in children. J Immunol Methods. 2010;358:46–55. doi: 10.1016/j.jim.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 24.Crotty S, Felgner P, Davies H, Glidewell J, Villarreal L, Ahmed R. Cutting edge: long-term B cell memory in humans after smallpox vaccination. J Immunol. 2003;171:4969–73. doi: 10.4049/jimmunol.171.10.4969. [DOI] [PubMed] [Google Scholar]
- 25.Clutterbuck EA, Lazarus R, Yu LM, Bowman J, Bateman EA, Diggle L, Angus B, Peto TE, Beverley PC, Mant D, et al. Pneumococcal conjugate and plain polysaccharide vaccines have divergent effects on antigen-specific B cells. J Infect Dis. 2012;205:1408–16. doi: 10.1093/infdis/jis212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Defrance T, Taillardet M, Genestier L. T cell-independent B cell memory. Curr Opin Immunol. 2011;23:330–6. doi: 10.1016/j.coi.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 27.Dempsey PW, Allison ME, Akkaraju S, Goodnow CC, Fearon DT. C3d of complement as a molecular adjuvant: bridging innate and acquired immunity. Science. 1996;271:348–50. doi: 10.1126/science.271.5247.348. [DOI] [PubMed] [Google Scholar]
- 28.Hostetter MK. Serotypic variations among virulent pneumococci in deposition and degradation of covalently bound C3b: implications for phagocytosis and antibody production. J Infect Dis. 1986;153:682–93. doi: 10.1093/infdis/153.4.682. [DOI] [PubMed] [Google Scholar]
- 29.Rijkers GT, Sanders EA, Breukels MA, Zegers BJ. Infant B cell responses to polysaccharide determinants. Vaccine. 1998;16:1396–400. doi: 10.1016/S0264-410X(98)00098-X. [DOI] [PubMed] [Google Scholar]
- 30.Lucas AH, Reason DC. Polysaccharide vaccines as probes of antibody repertoires in man. Immunol Rev. 1999;171:89–104. doi: 10.1111/j.1600-065X.1999.tb01343.x. [DOI] [PubMed] [Google Scholar]
- 31.Weill JC, Weller S, Reynaud CA. Human marginal zone B cells. Annu Rev Immunol. 2009;27:267–85. doi: 10.1146/annurev.immunol.021908.132607. [DOI] [PubMed] [Google Scholar]
- 32.Weller S, Braun MC, Tan BK, Rosenwald A, Cordier C, Conley ME, Plebani A, Kumararatne DS, Bonnet D, Tournilhac O, et al. Human blood IgM “memory” B cells are circulating splenic marginal zone B cells harboring a prediversified immunoglobulin repertoire. Blood. 2004;104:3647–54. doi: 10.1182/blood-2004-01-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martin F, Kearney JF. Marginal-zone B cells. Nat Rev Immunol. 2002;2:323–35. doi: 10.1038/nri799. [DOI] [PubMed] [Google Scholar]
- 34.Kelly DF, Snape MD, Clutterbuck EA, Green S, Snowden C, Diggle L, Yu LM, Borkowski A, Moxon ER, Pollard AJ. CRM197-conjugated serogroup C meningococcal capsular polysaccharide, but not the native polysaccharide, induces persistent antigen-specific memory B cells. Blood. 2006;108:2642–7. doi: 10.1182/blood-2006-01-009282. [DOI] [PubMed] [Google Scholar]
- 35.Andrews NJ, Waight PA, George RC, Slack MP, Miller E. Impact and effectiveness of 23-valent pneumococcal polysaccharide vaccine against invasive pneumococcal disease in the elderly in England and Wales. Vaccine. 2012;30:6802–8. doi: 10.1016/j.vaccine.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 36.Huss A, Scott P, Stuck AE, Trotter C, Egger M. Efficacy of pneumococcal vaccination in adults: a meta-analysis. CMAJ. 2009;180:48–58. doi: 10.1503/cmaj.080734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moberley SA, Holden J, Tatham DP, Andrews RM. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev. 2008:CD000422. doi: 10.1002/14651858.CD000422.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Brien KL, Hochman M, Goldblatt D. Combined schedules of pneumococcal conjugate and polysaccharide vaccines: is hyporesponsiveness an issue? Lancet Infect Dis. 2007;7:597–606. doi: 10.1016/S1473-3099(07)70210-4. [DOI] [PubMed] [Google Scholar]
- 39.Lazarus R, Clutterbuck E, Yu LM, Bowman J, Bateman EA, Diggle L, Angus B, Peto TE, Beverley PC, Mant D, et al. A randomized study comparing combined pneumococcal conjugate and polysaccharide vaccination schedules in adults. Clin Infect Dis. 2011;52:736–42. doi: 10.1093/cid/cir003. [DOI] [PubMed] [Google Scholar]
- 40.Brynjolfsson SF, Henneken M, Bjarnarson SP, Mori E, Del Giudice G, Jonsdottir I. Hyporesponsiveness following booster immunization with bacterial polysaccharides is caused by apoptosis of memory B cells. J Infect Dis. 2012;205:422–30. doi: 10.1093/infdis/jir750. [DOI] [PubMed] [Google Scholar]
- 41.Leggat DJ, Thompson RS, Khaskhely NM, Iyer AS, Westerink MA. The immune response to pneumococcal polysaccharides 14 and 23F among elderly individuals consists predominantly of switched memory B cells. J Infect Dis. 2013;208:101–8. doi: 10.1093/infdis/jit139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iwajomo OH, Finn A, Moons P, Nkhata R, Sepako E, Ogunniyi AD, Williams NA, Heyderman RS. Deteriorating pneumococcal-specific B-cell memory in minimally symptomatic African children with HIV infection. J Infect Dis. 2011;204:534–43. doi: 10.1093/infdis/jir316. [DOI] [PubMed] [Google Scholar]
- 43.Mitchell R, Trück J, Pollard AJ. Use of the 13-valent pneumococcal conjugate vaccine in children and adolescents aged 6 - 17 years. Expert Opin Biol Ther. 2013;13:1451–65. doi: 10.1517/14712598.2013.824419. [DOI] [PubMed] [Google Scholar]
- 44.Manz RA, Hauser AE, Hiepe F, Radbruch A. Maintenance of serum antibody levels. Annu Rev Immunol. 2005;23:367–86. doi: 10.1146/annurev.immunol.23.021704.115723. [DOI] [PubMed] [Google Scholar]
- 45.Edwards JC, Szczepanski L, Szechinski J, Filipowicz-Sosnowska A, Emery P, Close DR, Stevens RM, Shaw T. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med. 2004;350:2572–81. doi: 10.1056/NEJMoa032534. [DOI] [PubMed] [Google Scholar]
- 46.Amanna IJ, Slifka MK. Mechanisms that determine plasma cell lifespan and the duration of humoral immunity. Immunol Rev. 2010;236:125–38. doi: 10.1111/j.1600-065X.2010.00912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mackay F, Figgett WA, Saulep D, Lepage M, Hibbs ML. B-cell stage and context-dependent requirements for survival signals from BAFF and the B-cell receptor. Immunol Rev. 2010;237:205–25. doi: 10.1111/j.1600-065X.2010.00944.x. [DOI] [PubMed] [Google Scholar]
- 48.Trück J, Mitchell R, Bowman J, Thompson A, Clutterbuck EA, Snape MD, et al. The memory B cell response to a booster dose of a 13-valent (PCV-13) or 10-valent (PHiD-CV) pneumococcal conjugate vaccine. 31st Annual Meeting of the European Society for Paediatric Infectious Diseases; 2013 May 28-June 1; Milan, Italy. [Google Scholar]
- 49.Clarke ET, Williams NA, Dull PM, Findlow J, Borrow R, Finn A, Heyderman RS. Polysaccharide-protein conjugate vaccination induces antibody production but not sustained B-cell memory in the human nasopharyngeal mucosa. Mucosal Immunol. 2013;6:288–96. doi: 10.1038/mi.2012.70. [DOI] [PubMed] [Google Scholar]