Abstract
The aim was to compare the safety and immunogenicity of purified chick embryo cell vaccine (PCECV) with Zagreb 2-1-1 and Essen 1-1-1-1-1 regimens in patients with WHO category II exposure in China. Side effects including systemic and local symptoms were recorded for all patients during vaccination with purified chick embryo cell vaccine (PCECV) under Zagreb 2-1-1 or Essen 1-1-1-1-1 regimens, and the rabies neutralization antibody titers in patients’ serum at days 0, 7, 14, 45, 365 post-immunization were measured to determine the immunogenicity. Fever and pain were the most common events for systemic and local symptoms respectively, and most side effects (86.78%, 105/121) occurred after the first dose of vaccination. Safety analysis showed differences in side effects in <5-year-old patients between Zagreb and Essen regimens, especially after the first dose of vaccination (P = 0.043). Immunogenicity analysis indicated that Zagreb can achieve higher neutralization antibody titers and a greater seroconversion rate in a shorter time but had less persistence than Essen. When compared with the Essen regimen, the Zagreb regimen had a different immunogenicity in all study subjects, and different safety profile in young children, and a further study with a larger population and longer surveillance is warranted.
Keywords: rabies, vaccine, PCECV, post-exposure prophylaxis, Zagreb regimen, Essen regimen, safety, immunogenicity
Introduction
Rabies is a serious public health problem throughout the world. It is caused by RNA viruses of the family Rhabdoviridae, genus Lyssavirus,1 and is capable of causing disease that almost invariably results in death following the development of clinical symptoms.2 Rabies can infect humans through animal bites, and causes more than 55 000 deaths each year worldwide,3 with an average of 2037 cases per year in China.4 In recent years, although the rabies vaccines are widely used in most countries, the reported rabies cases are still increasing dramatically.4
Based on many years of experience, immunization of animals with recombinant rabies virus vaccines and post-exposure treatment using rabies vaccine have proved to be the most important methods to prevent and control rabies infection. However, for ethical, economic, and administrative reasons, it is difficult to conduct a large-scale vaccine campaign in animals in China. Thus, post-exposure treatment with reliable vaccines and a scientific regimen is thought to be more important for controlling rabies infection in human. Nowadays, the intramuscular post-exposure prophylaxis recommended by the World Health Organization (WHO) includes the Essen five-dose regimen and the Zagreb four-dose regimen.5,6 Although the WHO has recommended several different regimens or post-exposure prophylaxis, individual countries decide on protocols for local use.7 In China, the traditional Essen regimen has been formally approved and recommended since 1980, and is commonly used in China, including Wuhan city. Previous studies have proved that the Zagreb 2-1-1 regimen is equivalently effective, yet more economical.5 The purpose of the present study was to examine the safety and immunogenicity of vaccination with purified chick embryo cell vaccine (PCECV), a newly approved commercial vaccine, under a Zagreb 2-1-1 regimen, and in particular, in an all-aged population for up to 1-y post-immunization.
In May 2010, PCECV was ratified by the Chinese State Food and Drug Administration using the 2-1-1 regimen in healthy Chinese volunteers,5 which was thought to be a suitable option with its favorable immunogenicity and safety profile.8 As an imported commercial vaccine, the immunogenicity and safety in the Chinese population as a post-exposure prophylaxis under a 2-1-1 regimen remains unknown. Therefore, it is important to study whether or not PCECV under a 2-1-1 regimen is suitable for post-exposure Chinese patients, in particular, among different age groups.
Results
Safety analysis of vaccination
Patient information
A total of 300 patients exposed to animals were enrolled in this study and were equally divided into 2 groups (Zagreb group and Essen group). The Zagreb group included 66 males and 84 females, and the Essen group included 69 males and 81 females. Because of patient preference or exclusion criteria, 42 participants were excluded, and the remaining participants for both regimens are shown in Table 1. The mean ages of these 2 groups were 38.13 ± 27.08 y and 35.83 ± 26.48 y, respectively. There were no significant age or sex differences between the 2 groups (P = 0.459 and 0.728, respectively) (Table 1). No patient was injected with immuneglobulin, and no patient developed clinical rabies during the study period.
Table 1. Safety comparison between the Zagreb and Essen regimens used in patients with WHO category II exposure to rabies.
| Zagreb | Essen | P | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–5 y old# | 6–18 y old | 19–59 y old | 60+ years old | Total | 0–5 y old# | 6–18 y old | 19–59 y old | 60+ years old | Total | ||
| Sex, M/F | 11/14 | 15/17 | 18/20 | 15/22 | 59/73 | 12/16 | 17/12 | 18/19 | 14/18 | 61/65 | 0.907 |
| Mean age (SD)‡ | 41.92 (11.66) m | 15.22 (3.12) y | 46.26 (11.12) y | 73.49 (7.37) y | 38.13 (27.08) y | 37.18 (12.53) m | 14.48 (2.84) y | 43.68 (11.76) y | 69.56 (5.81) y | 35.83 (26.48) y | 0.459 |
| Systemic symptoms (occurring during first immunization)* | 16(15) | 5(5) | 2(2) | 4(4) | 27(26) | 12(10) | 3(3) | 2(2) | 3(3) | 20(18) | 0.341 |
| Fever | 12 | 1 | 1 | 1 | 15 | 8 | 2 | 1 | 1 | 12 | |
| Malaise | 0 | 2 | 1 | 2 | 5 | 0 | 0 | 0 | 2 | 2 | |
| Allergy | 1 | 1 | 0 | 0 | 2 | 2 | 0 | 1 | 0 | 3 | |
| Restlessness | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | |
| Nausea and vomiting | 2 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 1 | |
| Diarrhea | 1 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | |
| Local symptoms (occurring during first immunization)* | 5(5) | 14(12) | 5(4) | 16(13) | 40(34) | 5(5) | 13(9) | 4(3) | 12(10) | 34 (27) | 0.556 |
| Pain | 1 | 9 | 3 | 11 | 24 | 0 | 10 | 2 | 6 | 18 | |
| Induration | 2 | 2 | 1 | 0 | 5 | 2 | 1 | 0 | 2 | 5 | |
| Edema | 1 | 1 | 0 | 1 | 3 | 1 | 1 | 1 | 1 | 4 | |
| Tenderness | 1 | 2 | 1 | 3 | 7 | 1 | 1 | 1 | 3 | 6 | |
| Erythema | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | |
*Data in brackets show the number of side effects after first immunization, which have no significant differences of total side effects incidence between Zagreb and Essen in systemic symptoms (P = 0.383, 26/27 vs 18/20) or local symptoms (P = 0.529, 34/40 vs 27/34). #Although the difference in side effects of <5-y-old patients between Zagreb and Essen (P = 0.060, 21/25 vs 17/28) was not statistically significant, it was found to be statistically significant after the first-dose immunization (P =0.043, 20/25 vs 15/28). ‡m, months; y, years.
Systemic symptoms
Both Zagreb and Essen groups presented systemic symptoms (Table 1), in which fever was the most common symptom after immunization. Most patients with fever were aged below 5 y (12/15 and 8/12 for Zagreb and Essen, respectively). All patients with fever recovered within 48 h with or without specific drugs. The severity of all side effects belonged to class I and II according to the “Preventive vaccine clinical trials, adverse events grading guidelines” issued by the China Food and Drug Administration, and no adverse events (AEs) occurred during the study. The allergies in all patients were local hives of class II, and regressed spontaneously within 72 h. For other systemic symptoms, no differences were found between the 2 groups.
Local symptoms
Several patients developed local symptoms after PCECV immunization, in which pain was the most common symptom in both the Zagreb and Essen groups. However, in 42 patients of both regimens, only one patient aged <5 y reported the local symptom of pain, which might have been the result of difficulty in expression experienced by infants. There were no differences in other local symptoms (induration, edema, tenderness, erythema) between the patients aged <5 y and >5 y (Table 1). Although no significant differences between the 2 groups were found for all side effects, Zagreb showed more side effects in <5-y-old patients, especially after first-dose immunization (P = 0.043, Table 1).
Immunogenicity analysis
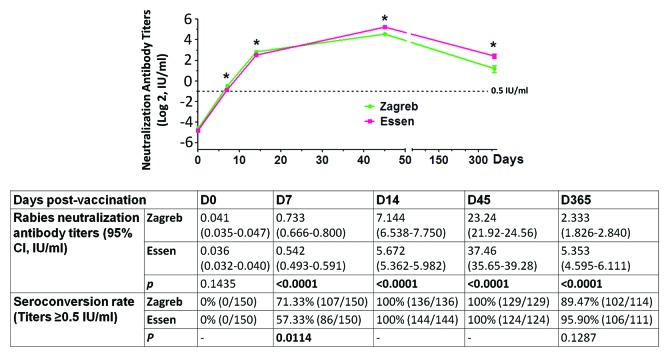
The immunogenicity analysis was performed at 0 (D0), 7 (D7), 14 (D14), 45 (D45), and 365 d (D365) post-immunization. Figure 1 shows the trend of rabies neutralization antibody titers in patients for up to 1 y post-immunization. All patients in both the Zagreb and Essen groups converted to positive (rabies neutralization antibody titer ≥0.5 IU/mL) for rabies antibody at day 14, which was proof of adequate immune response after vaccination. Interestingly, when comparing the 2 groups at day 7, Zagreb had a higher rabies neutralization antibody titer mean value (0.733 ± 0.034 vs 0.542 ± 0.025, P < 0.001) and more seroconverted patients (71.33% vs 57.33%, P = 0.0114), but the rabies neutralization antibody titers of the 2 groups significantly decreased to only about 10% and 14% at day 365, respectively, when compared with that at day 45 (mean value of 2.33 vs 23.24 IU/mL for Zagreb and 5.35 vs 37.46 IU/mL for Essen) (Fig. 1), and the neutralization antibody titers in patients of Zagreb group were significantly lower than that of Essen patients after the day 45 (P < 0.001, Fig. 1).
Figure 1. The difference in immunogenicity between Zagreb and Essen groups before (day 0) and after rabies vaccination. Zagreb showed significantly higher rabies neutralization antibody titers (P < 0.001) and seroconversion rate (P = 0.0114) than Essen at day 7 post-vaccination, but had lower rabies neutralization antibody titers at days 45 and 365 post-vaccination. The data shown are the mean ± 95% confidence interval (*P < 0.001).
Discussion
In order to achieve prompt and adequate immune response post-exposure to rabies, several administration methods and vaccination programs have been explored in recent years.5,9,10 The present study conducted in Wuhan, China, describes the immunogenicity and safety of vaccination under a 2-1-1 regimen or 1-1-1-1-1 regimen based on 1 y of surveillance. For the vaccination of rabies, several immunizations with protein antigen are aimed to increase the affinity of antibody and the number of memory B cells, not just to get higher antibody titers. Thus, serum rabies neutralization antibody titers were measured to evaluate the immunogenicity of Zagreb 2-1-1 regimen with PCECV after 1 y of immunization, and the systemic and local symptoms were also observed, especially in different age groups with WHO category II rabies exposure.
Side effects were thought to be the biggest challenge for rabies vaccination using the Zagreb 2-1-1 regimen, especially for young children and elderly subjects. Although most systemic symptoms and local symptoms occurred after the first injection, a possible result of physiological responses to primary immunization, there was no difference in side effects between the Zagreb and Essen regimens (P = 0.383 and 0.529, respectively) during the first-dose immunization (Table 1). In this study, pain was found to be the most common local reaction after immunization, which is the same as the finding reported by Liu et al.5 However, fever was the most common systemic symptom in our study, differing to that of Liu et al., which maybe because most patients with fever in our study were aged <5 y while Liu et al. performed the research in healthy adults.5 When comparing the side effects in <5-y-old patients, differences can be found between the Zagreb and Essen regimens both in complete doses and during the first immunization (Table 1). As the number of patients aged <5 y was only about 50, a larger population study in this age group is needed, and particular care needs to be exercised when clinical immunization using the Zagreb regimen is used in young children.
In this study, persistence data were collected by analyzing the rabies neutralization antibody titers in patients’ serum at day 365 post-immunization. Figure 1 shows that all patients received adequate rabies neutralization antibody (titers ≥0.5 IU/mL) at day 14, and the highest rabies neutralization antibody titers in patients presented at day 45. Compared with the Essen group, rabies neutralization antibody titers in the Zagreb group were significantly higher at days 7 and 14 post-immunization, but were significantly lower from day 45. However, there was no significant difference in the seroconversion rates between these 2 groups from day 14, and about 90% of patients had rabies neutralization antibody titers of ≥0.5 IU/mL at day 365 post-immunization. The seroconversion rates are in agreement with our previous study,11 in which we performed a clinical study with Essen with 5 y of surveillance, and the rates decreased to only 34% at year 5.
Several limitations need to be acknowledged. First, this study was not performed randomly. However, as mentioned above, the patients were divided single-blind, the demographic variables and the neutralization antibody titers when enrolled showed no difference between 2 groups (Fig. 1; Table 1). Thus, the results reported in this study should be reliable. Second, the demographic and clinical information during the period were collected by telephone, which cannot avoid potential underreporting. However, underreporting to a certain degree is unavoidable for the clinical study, and no sensitive information was inquired, the underreporting in this study should be low.
Although a few subjects quit the study because of the long and cumbersome surveillance, the Zagreb regimen has proved to have the same safety profile as Essen in most patients, and showed a quick response to immunization. A further study is necessary to determine the safety of Zagreb in young children and the immunogenicity over a longer period, which will provide the necessary information to strengthen immunization.
Materials and Methods
Study design and participants
Sample size estimation
The sample size estimation of this study was conducted according to the “Practical Manual of Sample Size Determination in Health Studies” (WHO, 1996). The inspection level α (one side) was set to 2.5% and the power of test 1-β was 90%. The real difference δ was 0 (i.e., the 2 methods are actually equivalent from the aspect of the titer of day 14) and the non-inferiority margin value δ0 was –1.5. The standard deviation of log2 (antibody titer) was 2.59 and the sample amount ratio was 1:1. When the sample amount of each group is 64, there is a 90% power of test for the 2 groups of 0.025 one-sided t test to reject the null hypothesis of inferiority, and support another hypothesis, that is, when assuming there is no difference between the 2 schemes and the equivalent limit of titer (log2) is –1.5, Zagreb is not inferior to Essen according to the antibody titer of day 14. Considering a 15% expulsion rate, each group should at least include 75 cases. Considering the possible lack of follow-up during 1 y’s follow-up visit, the final sample amount was determined to be 300 and each group contained 150 cases.
Participants
From August 2010 to December 2011, 300 patients who visited the clinic of Wuhan Centers for Disease Prevention and Control with WHO category II exposure to rabies were enrolled, and divided single-blind and equally into 2 groups: Zagreb (2-1-1 regimen) and Essen (1-1-1-1-1 regimen) (Fig. 2). All patients had lived in Wuhan city for more than 6 mo, and visited the clinic of Wuhan Centers for Disease Prevention and Control within 24 h post-exposure. The patients were excluded if they had been previously vaccinated with rabies vaccine, or presented with other clinical diseases.
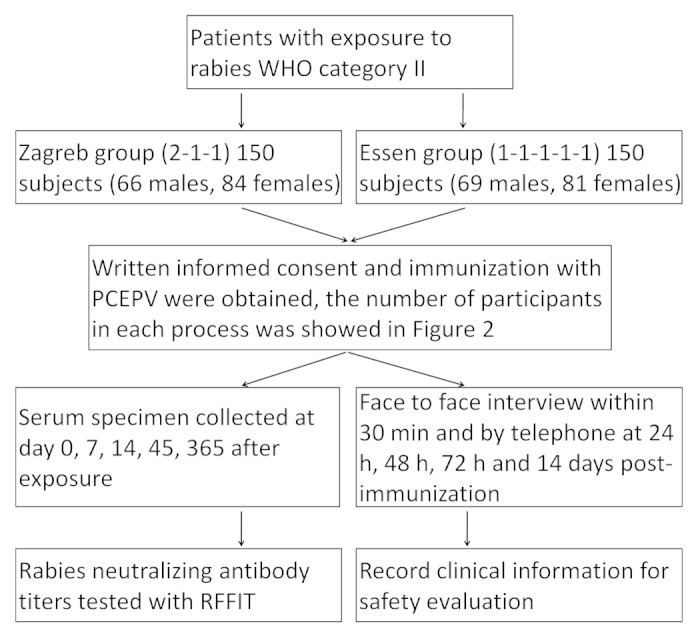
Figure 2. Flowchart of the safety and immunogenicity study.
Vaccine
The PCECV studied was commercially available, and had been approved and tested by the National Institute for the Control of Pharmaceutical and Biological products. The imported PCECV (Rabipur, 201103011-2, Novartis Vaccines and Diagnostics) had an antigen content of 6.4 IU/dose (registered potency tested by the China Food and Drug Administration).
Immunization and blood collection
Immunization was performed by injection in the deltoid muscles of the left and/or right arms. For the Zagreb group (2-1-1 regimen), 2 doses of PCECV were injected in the deltoid muscles both of the left and right arms on day 0 (D0), and then one dose administered on day 7 (D7) and day 21 (D21) respectively, which gave a total of 4 doses over 3 visits. For the Essen group (1-1-1-1-1 regimen), one dose of PCECV was injected in the deltoid muscles of the left or right arm at D0, day 3 (D3), day 7 (D7), day 14 (D14), and day 28 (D28) respectively, which gave a total of 5 doses over 5 visits. In order to study the immunogenicity in the patients, post-vaccination blood samples were collected at D0, D7, D14, D45, and D365, sera was separated and frozen at –70 °C for analysis.
Safety monitoring
Side effects were observed for 30 min after each vaccination in both regimens. At initial follow-up, a telephone visit was conducted at 24 h, 48 h, 72 h, and 14 d post-immunization, to record any adverse reactions. Before the injection of PCECV, a face-to-face survey was also performed to collect the demographic and clinical information during the period between the 2 visits. Clinical data were defined according to the “Preventive vaccine clinical trials, adverse events grading guidelines” issued by the China Food and Drug Administration.
Immunogenicity analysis
In order to compare the immunogenicity of patients with PCECV, rabies neutralizing antibody titers in the serum were measured under masked conditions using a rapid fluorescent focus inhibition test (RFFIT). The rabies neutralization antibody titer was measured with RFFIT in patients’ serum by the virology laboratory of our institute. The reference standard (30 IU/dose) was purchased from the National Institute for Biological Standards and Control (NIBSC). The rabies neutralizing antibody titers were analyzed in all patients’ serum before and after immunization. Successful protection is achieved when the rabies neutralization antibody titer is <0.5 IU/mL at D0 and ≥0.5 IU/mL after immunization according to WHO recommendations (WHO, 1992).
Statistical analysis
Where appropriate, data were expressed as mean ± standard deviation (SD) if not defined. Categorical variables were tested with chi-square of the Fisher exact test, and comparison between Zagreb and Essen was tested with the Student t test. Statistical analysis was performed with GraphPad Instat statistical software (GraphPad Software). Statistical significance was defined as a P value < 0.05.
Ethics
The protocol of this study was approved by the Institutional Review Board of Wuhan Centers for Disease Prevention and Control, and written informed consent was obtained from all participants, or their parents or legal guardians in the case of children up to 18 y of age. All the materials and methods used in this study were approved by the Chinese State Food and Drug Administration (Approval No: 2008B0914, 2010B00516, 2008S00001), and have been used in many countries in the world, including China,5,6 thus no clinical trial registration is needed for this study. In addition, we performed a clinical observation, not a clinical trial, to collect the clinical data, which won’t affect the vaccination policy for PCECV and 2 regimens in China, but provide a reference for the clinical doctors and patients when choosing a regimen.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Glossary
Abbreviations:
- PCECV
purified chick embryo cell vaccine
- RFFIT
rapid fluorescent focus inhibition test
- WHO
World Health Organization
References
- 1.Kopel E, Oren G, Sidi Y, David D. Inadequate antibody response to rabies vaccine in immunocompromised patient. Emerg Infect Dis. 2012;18:1493–5. doi: 10.3201/eid1809.111833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evans JS, Horton DL, Easton AJ, Fooks AR, Banyard AC. Rabies virus vaccines: is there a need for a pan-lyssavirus vaccine? Vaccine. 2012;30:7447–54. doi: 10.1016/j.vaccine.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 3.Song M, Tang Q, Wang DM, Mo ZJ, Guo SH, Li H, Tao XY, Rupprecht CE, Feng ZJ, Liang GD. Epidemiological investigations of human rabies in China. BMC Infect Dis. 2009;9:210. doi: 10.1186/1471-2334-9-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu WY, Liang GD. Current status of canine rabies in China. Biomed Environ Sci. 2012;25:602–5. doi: 10.3967/0895-3988.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 5.Liu H, Huang G, Tang Q, Li J, Cao S, Fu C, Cao Q, Liu B, Pan H, Wang M. The immunogenicity and safety of vaccination with purified Vero cell rabies vaccine (PVRV) in China under a 2-1-1 regimen. Hum Vaccin. 2011;7:220–4. doi: 10.4161/hv.7.2.14003. [DOI] [PubMed] [Google Scholar]
- 6.Rabies WECo. Guide for post-exposure treatment. Eighth report. WHO technical report 824. Geneva Switzerland: World Health Organization 1992. [Google Scholar]
- 7.Warrell MJ. Current rabies vaccines and prophylaxis schedules: preventing rabies before and after exposure. Travel Med Infect Dis. 2012;10:1–15. doi: 10.1016/j.tmaid.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 8.Malerczyk C, Vakil HB, Bender W. Rabies pre-exposure vaccination of children with purified chick embryo cell vaccine (PCECV) Hum Vaccin Immunother. 2013;9:1454–9. doi: 10.4161/hv.24502. [DOI] [PubMed] [Google Scholar]
- 9.Chhabra M, Ichhpujani RL, Bhardwaj M, Tiwari KN, Panda RC, Lal S. Safety and immunogenicity of the intradermal Thai red cross (2-2-2-0-1-1) post exposure vaccination regimen in the Indian population using purified chick embryo cell rabies vaccine. Indian J Med Microbiol. 2005;23:24–8. doi: 10.4103/0255-0857.13868. [DOI] [PubMed] [Google Scholar]
- 10.Sudarshan MK, Gangaboraiah B, Ravish HS, Narayana DH. Assessing the relationship between antigenicity and immunogenicity of human rabies vaccines when administered by intradermal route: results of a metaanalysis. Hum Vaccin. 2010;6:562–5. doi: 10.4161/hv.6.7.11934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang X, Zhu Z, Wang C. Persistence of rabies antibody 5 years after postexposure prophylaxis with vero cell antirabies vaccine and antibody response to a single booster dose. Clin Vaccine Immunol. 2011;18:1477–9. doi: 10.1128/CVI.05090-11. [DOI] [PMC free article] [PubMed] [Google Scholar]