Abstract
The objectives of this study were to estimate incidence density rates (IDR) of herpes zoster (HZ) and to analyze the association with sociodemographic characteristics and selected chronic medical conditions. The study cohort consisted of the adult population included in the Public Health System of the Autonomous Community of Madrid, Spain on 1/10/2009 (5 244 402 persons). Data source were electronic medical records from primary care between 1/10/2009–31/12/2012. Individual socioeconomic status (SES) was inferred by geocoding. Poisson regression analyses were stratified by sex, to identify factors associated with HZ. We identified 81 541 incident cases of HZ (61.7% in women and 46.5% in the group aged 60 and over). IDR was 4.11 per 1000 person-years in men and 5.95 in women. IDR were higher with age, in autochthonous population, those with lower SES and in patients with immunodeficiencies. After adjustment, higher incidence rate ratios were found with age, autochthonous origin, lower SES, and in patients with HIV-infection/AIDS (3.20, CI95% 2.90–3.53 in men and 2.98, CI95% 2.58–3.45 in women), and other immunodeficiencies (1.57, CI95% 1.41–1.75 and 1.65, CI95% 1.50–1.80). COPD, asthma, DM, ischemic heart disease, other cardiovascular diseases, and cancer were also associated with an increased incidence of HZ. We conclude that older, autochthonous patients with lower SES and with certain underlying medical conditions had a higher probability of suffering HZ. Electronic databases are useful for estimating the incidence of HZ, and for finding associations with sociodemographic and clinical characteristics. Identifying unrecognized risk factors for HZ, such as asthma or cardiovascular diseases, is crucial to interpret the epidemiology of HZ, to target vaccination programs and to monitor their effect.
Keywords: chronic conditions, herpes zoster, incidence, primary health care, risk factor, vaccine, varicella
Introduction
Herpes zoster (HZ) results from the reactivation of latent varicella zoster virus (VZV). The triggers for reactivation have not been identified and probably involve multiple factors. However, specific components of cell-mediated immunity (CMI) have an important role in controlling the development of zoster by preventing reactivation within the neuron or the full clinical expression of reactivated VZV as zoster. These CMI components are believed to be partially or substantially maintained by periodic immunologic boosting.1
It has been speculated that a universal varicella vaccination program might alter the epidemiology of HZ due to the expected decline of the boosting of CMI because of the reduced VZV circulation. Several studies have found that rates of HZ have consistently increased since the implementation of varicella vaccination programs.2-5 In the Autonomous Community of Madrid varicella vaccination for children aged 15 mo was included in the systematic vaccination schedule in 2006, and incidence rates of HZ increased significantly from 2005 to 2012.6 Other studies reported increases in HZ incidence rates both prior to and after the implementation of varicella vaccination programs.7-11 These trends could not be entirely explained by known major risk factors for HZ, suggesting the presence of other contributing factors.
Age is the most important risk factor for the development of HZ. The lifetime risk is estimated to be 10–30% and incidence increases markedly with age, affecting up to 50% of people who live to 85 y.7,12 The risk approximately doubles for each decade after 50 y of age.13 This age-related increase in HZ is due to a VZV-specific decline in CMI with increasing age.14 Higher incidences of HZ have been described in females,6 with a female/male incidence rate ratio ranging between 1.13 and 1.56,15 and an average 28% age-standardized excess incidence in females over males.16 Female gender has been identified as an independent risk factor for HZ in the 25- to 64-y-old age group.17
Other risk factors for HZ reactivation identified in analytical studies included ethnicity, genetic susceptibility, exogenous boosting of immunity from varicella contacts, underlying cell-mediated immune disorders, mechanical trauma, psychological stress, depression, and immunotoxin exposure.12,18 Several studies have shown that people with suppressed CMI from immunosuppressive disorders or therapies are at higher risk of HZ, with incidences ranging from 25.0–91.5/1000 person-years.12 The incidence of HZ is substantially greater among persons with hematological malignancies, solid tumors, human immunodeficiency virus (HIV) infection, hematopoietic stem cell and solid organ transplantation, and systemic lupus erythematosis.1
However, population-based studies have reported that approximately 90% of HZ cases occur among immunocompetent patients, for whom risk factors for HZ are not well characterized.4
Recent studies increasingly focus on identifying chronic conditions with possible associations with HZ, looking for unrecognized risk factors. Associations between HZ and diabetes mellitus (DM) had been investigated with different results,19,20 until a large population-based study clearly showed that DM is an independent risk factor for HZ.21 More recently, other studies have found higher risk of HZ associated with pathologies like chronic obstructive pulmonary disease (COPD), childhood asthma, coronary artery disease, renal failure, hypertension, hyperlipidemia, hypothyroidism, and osteoarthritis.22-25 However, those factors did not explain, or contribute substantially to, the burden or increasing incidence of HZ.23 Unrecognized risk factors clearly exist for HZ, and it is crucial to identify them in order to interpret the epidemiology of HZ and target prevention and treatment strategies. The HZ vaccine is not yet approved in Spain. A better understanding of the population at greatest risk may be useful to guide future vaccination policies and monitor the effect of varicella and HZ vaccination programs.
The objectives of this study were to estimate the incidence rates of HZ, and to analyze the association of HZ with sociodemographic characteristics and selected chronic medical conditions on the basis of data drawn from electronic clinical records in primary care (ECRPC).
Results
At the beginning of the study (1 October 2009) 5,244,402 people of 18 y of age and over were included in the MRPHS, representing 99.0% of the residents of the Region of Madrid (5,299,273 person, census on 1 January 2010). Of these, 52.6% were female and 24.0% were over 60 y (Table 1). Of that total, 20.1% were foreign-born population, with Latin America being the most frequent region of origin (10.7%), followed by Central and Eastern Europe (4.1%). Of the men, 16.7% had at least one of the chronic conditions included in the risk factor analysis, with the most common being DM (6.0%), the group of other cardiovascular diseases (3.4%), and asthma (3.1%). Among the women, the figure was 15.1% with DM (4.9%), asthma (4.7%), and other cardiovascular diseases (3.1%). The maximum number of medical conditions identified was 8 (7 in women).
Table 1. Characteristics of the population of 18 y of age and over registered in the Individualized Health Card database the 1st October 2009—Autonomous Community of Madrid.
| Total | Men | Women | ||||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| Total | 5 244 402 | 2 483 553 | 2 760 849 | |||
| Age group | ||||||
| <30 y | 994 286 | 19 | 488 427 | 19.7 | 505 859 | 18.3 |
| 30–44 y | 1 803 540 | 34.4 | 901 146 | 36.3 | 902 394 | 32.7 |
| 45–59 y | 1 187 082 | 22.6 | 566 310 | 22.8 | 620 772 | 22.5 |
| 60–74 y | 775 941 | 14.8 | 352 882 | 14.2 | 423 059 | 15.3 |
| ≥75 y | 483 553 | 9.2 | 174 788 | 7 | 308 765 | 11.2 |
| Origin | ||||||
| Spain | 4 190 526 | 79.9 | 1 981 379 | 79.8 | 2 209 147 | 80 |
| Europe-15 | 82 687 | 1.6 | 40 801 | 1.6 | 41 886 | 1.5 |
| Central and Eastern Europe | 213 097 | 4.1 | 102 458 | 4.1 | 110 639 | 4 |
| North Africa | 88 705 | 1.7 | 53 275 | 2.1 | 35 430 | 1.3 |
| Sub-Saharan Africa | 39 437 | 0.8 | 24 607 | 1 | 14 830 | 0.5 |
| Latin America | 561 267 | 10.7 | 245 590 | 9.9 | 315 677 | 11.4 |
| North America | 6 911 | 0.1 | 3 129 | 0.1 | 3 782 | 0.1 |
| Asia/Oceania | 61 544 | 1.2 | 32 182 | 1.3 | 29 362 | 1.1 |
| Socioeconomic level | ||||||
| Q1 (highest) | 1 642 663 | 31.3 | 745 691 | 30 | 896 972 | 32.5 |
| Q2 | 1 293 563 | 24.7 | 616 721 | 24.8 | 676 842 | 24.5 |
| Q3 | 1 240 744 | 23.7 | 596 754 | 24 | 643 990 | 23.3 |
| Q4 (lowest) | 1 067 432 | 20.4 | 524 387 | 21.1 | 543 045 | 19.7 |
| Medical Condition | ||||||
| Asthma | 207 092 | 3.9 | 77 047 | 3.1 | 130 045 | 4.7 |
| COPD | 88 003 | 1.7 | 63 873 | 2.6 | 24 130 | 0.9 |
| Ischaemic heart disease | 109 963 | 2.1 | 73 954 | 3 | 36 009 | 1.3 |
| Heart failure | 35 547 | 0.7 | 13 598 | 0.5 | 21 949 | 0.8 |
| Other cardiovascular diseases | 168 506 | 3.2 | 83 575 | 3.4 | 84 931 | 3.1 |
| Diabetes | 284 895 | 5.4 | 149 200 | 6 | 135 695 | 4.9 |
| HIV-infection/AIDS | 15 269 | 0.3 | 11 405 | 0.5 | 3864 | 0.1 |
| Other immunodeficiency | 20 749 | 0.4 | 10 593 | 0.4 | 10 156 | 0.4 |
| Cancer | 113 775 | 2.2 | 51 687 | 2.1 | 62 088 | 2.2 |
| Number of conditions | ||||||
| None | 4 409 617 | 84.1 | 2 067 251 | 83.2 | 2 342 366 | 84.8 |
| 1 | 669 107 | 12.8 | 323 527 | 13 | 345 580 | 12.5 |
| ≥2 | 165 678 | 3.2 | 93 775 | 3.7 | 72 903 | 2.6 |
COPD, chronic obstructive pulmonary disease.
Throughout the study period (3 y and 3 mo) 81,541 incident cases of HZ were identified in that population, 61.7% of them in women and 46.5% in the group aged 60 and over (Table 2). The IDR was 4.11 per 1000 person-years in men and 5.95 per 1000 person-years in women, with a sharp increase with age, ranging from 2.47 per 1000 person-years among young adults to 10.36 per 1000 person-years among the elderly (≥ 75 y of age). The IDR was higher in autochthonous population.
Table 2. Distribution of herpes zoster cases and incidence density rate (cases/1000/year) of cases diagnosed between 01/10/2009 and 31/12/2012 in adult population—Autonomous Community of Madrid.
| Total | Men | Women | ||||
|---|---|---|---|---|---|---|
| Incident cases | IDR per 1000 person-years | Incident cases | IDR per 1000 person-years | Incident cases | IDR per 1000 person-years | |
| Total | 81 541 | 5.08 | 31 233 | 4.11 | 50 308 | 5.95 |
| Age group | ||||||
|
<30 y |
7474 | 2.47 | 3362 | 2.26 | 4112 | 2.68 |
|
30–44 y |
14 983 | 2.69 | 6600 | 2.38 | 8383 | 3.00 |
|
45–59 y |
21 191 | 5.72 | 7489 | 4.24 | 13 702 | 7.06 |
|
60–74 y |
23 777 | 9.93 | 9254 | 8.53 | 14 523 | 11.09 |
|
≥75 y |
14 116 | 10.36 | 4528 | 9.29 | 9588 | 10.95 |
| Origin | ||||||
|
Spain |
73 105 | 5.61 | 28 053 | 4.54 | 45 052 | 6.56 |
|
Europe-15 |
751 | 2.97 | 308 | 2.48 | 443 | 3.45 |
|
Central and Eastern Europe |
1435 | 2.46 | 525 | 1.89 | 910 | 2.98 |
|
North Africa |
741 | 2.86 | 351 | 2.30 | 390 | 3.67 |
|
Sub-Saharan Africa |
170 | 1.71 | 91 | 1.48 | 79 | 2.06 |
|
Latin America |
4884 | 3.01 | 1693 | 2.40 | 3191 | 3.48 |
|
North America |
48 | 2.35 | 21 | 2.29 | 27 | 2.39 |
|
Asia/Oceania |
407 | 2.28 | 191 | 2.11 | 216 | 2.46 |
| Socioeconomic level | ||||||
|
Q1 (highest) |
24 073 | 4.77 | 8885 | 3.87 | 15 188 | 5.52 |
|
Q2 |
20 326 | 5.13 | 7921 | 4.20 | 12 405 | 5.98 |
|
Q3 |
20 102 | 5.31 | 7737 | 4.26 | 12 365 | 6.27 |
|
Q4 (lowest) |
17 040 | 5.23 | 6690 | 4.20 | 10 350 | 6.22 |
| Medical condition | ||||||
|
Asthma |
4476 | 6.89 | 1189 | 4.90 | 3 287 | 8.08 |
|
COPD |
2894 | 11.38 | 1982 | 10.80 | 912 | 12.87 |
|
Ischaemic heart disease |
3485 | 10.74 | 2134 | 9.71 | 1351 | 12.90 |
|
Heart failure |
1141 | 12.35 | 387 | 11.08 | 754 | 13.12 |
|
Other cardiovascular diseases |
5168 | 10.47 | 2158 | 8.76 | 3010 | 12.18 |
|
Diabetes |
8012 | 9.38 | 3658 | 8.14 | 4354 | 10.75 |
|
HIV-infection/AIDS |
572 | 12.51 | 397 | 11.61 | 175 | 15.18 |
|
Other immunodeficiency |
770 | 12.90 | 328 | 10.84 | 442 | 15.02 |
|
Cancer |
3329 | 10.03 | 1325 | 9.03 | 2004 | 10.82 |
| Number of conditions | ||||||
|
None |
58 772 | 4.34 | 21 355 | 3.37 | 37 417 | 5.20 |
|
1 |
17 235 | 8.41 | 7069 | 7.12 | 10 166 | 9.62 |
|
≥2 |
5534 | 11.74 | 2809 | 10.64 | 2725 | 13.13 |
IDR, incidence density rate; COPD, chronic obstructive pulmonary disease.
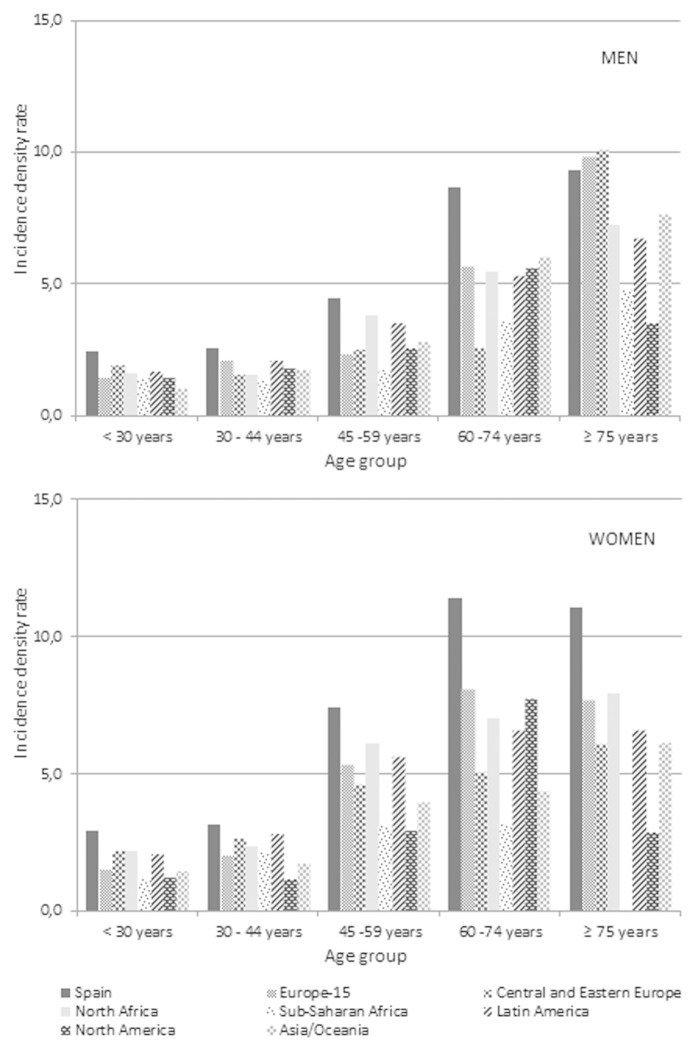
Among foreign men, Europe-15 and Latin America were the regions of origin that had higher IDR (2.42 and 2.34 per 1000 person-years, respectively). Among foreign-born women, the highest HZ IDR was observed in women from North Africa (3.61 per 1000 person-years), followed by Europe-15 and Latin America (3.39 per 1000 person-years both of them). When stratified by age and origin, the IDR were consistently higher among the autochthonous population in both sexes and all age groups except in men of 75 y and older, in which the highest incidences occurred in patients from the rest of Europe (Fig. 1).
Figure 1. Incidence density rate (cases/1000/year) of herpes zoster diagnosed between 01/10/2009 and 31/12/2012 in adult men and women, by age group and origin. Autonomous Community of Madrid.
The highest IDR appeared in the quartiles with the lowest socioeconomic status (SES), with a more pronounced gradient in women, ranging from 5.52 to 6.22 per 1000 person-years (Table 2). With regard to the medical conditions analyzed, the IDR increased markedly with the number of pathologies diagnosed, and were higher in women, exceeding 10 cases per 1000 person-years in all morbidities except asthma and other respiratory diseases. The highest IDR in both sexes was for HIV-infection/AIDS (15.18 per 1000 person-years in women and 11.61 in men) In women, this was followed by the group of other immunodeficiencies (15.02 per 1000 person-years) and heart failure (13.12 per 1000 person-years). Conversely in men, IDR in heart failure surpassed the group of other immunodeficiencies (11.08 vs. 10.84 per 1000 person-years, respectively).
In the multivariate Poisson-regression analysis increasing age was associated with an increased incidence of HZ, both in men and women, with a slight decline of the increasing gradient in the group aged 75 y and older (Table 3). Foreign origin was associated with a lower incidence of HZ compared with autochthonous population. This association was statistically significant for all the regions analyzed and in both sexes. A lower SES was associated with a higher incidence rate of HZ, with a clear gradient among women (IRR: 1.17, CI95% 1.14–1.20 in the most disadvantaged quartile). After adjustment by age, origin, SES, and other comorbidities, the medical conditions with higher IRR, both in men and women, were HIV-infection/AIDS (IRR: 3.20, CI95% 2.90–3.53 and IRR: 2.98, CI95% 2.58–3.45, respectively), followed by the group of other immunodeficiencies (IRR: 1.57, CI95% 1.41–1.75 in men and IRR: 1.65, CI95% 1.50–1.80 in women). The diagnosis of COPD, asthma, DM, ischemic heart disease and other cardiovascular diseases (except heart failure), and cancer was associated with the appearance of HZ.
Table 3. Factors associated with herpes zoster cases diagnosed between 01/10/2009 and 31/12/2012 in adult population, according to multivariate Poisson Regression analysis—Autonomous Community of Madrid.
|
|
Men | Women | ||||
|---|---|---|---|---|---|---|
| aIRR | CI 95% | aIRR | CI 95% | |||
| Age group | ||||||
|
<30 y |
1.00 | - | - | 1.00 | - | - |
|
30–44 y |
1.06 | 1.01 | 1.10 | 1.13 | 1.09 | 1.17 |
|
45–59 y |
1.78 | 1.71 | 1.86 | 2.56 | 2.48 | 2.66 |
|
60–74 y |
3.23 | 3.10 | 3.36 | 3.75 | 3.62 | 3.88 |
|
≥75 y |
2.99 | 2.85 | 3.13 | 3.26 | 3.14 | 3.39 |
| Origin | ||||||
|
Spain |
1.00 | - | - | 1.00 | - | - |
|
Europe-15 |
0.70 | 0.63 | 0.79 | 0.68 | 0.62 | 0.75 |
|
Central and Eastern Europe |
0.55 | 0.50 | 0.60 | 0.63 | 0.59 | 0.67 |
|
North Africa |
0.64 | 0.58 | 0.71 | 0.71 | 0.64 | 0.78 |
|
Sub-Saharan Africa |
0.39 | 0.32 | 0.48 | 0.38 | 0.30 | 0.47 |
|
Latin America |
0.70 | 0.67 | 0.73 | 0.72 | 0.70 | 0.75 |
|
North America |
0.60 | 0.39 | 0.92 | 0.46 | 0.32 | 0.67 |
|
Asia/Oceania |
0.58 | 0.51 | 0.67 | 0.51 | 0.44 | 0.58 |
| Socioeconomic level | ||||||
|
Q1 (highest) |
1.00 | - | - | 1.00 | - | - |
|
Q2 |
1.12 | 1.08 | 1.15 | 1.11 | 1.08 | 1.13 |
|
Q3 |
1.12 | 1.08 | 1.15 | 1.15 | 1.12 | 1.17 |
|
Q4 (lowest) |
1.14 | 1.10 | 1.18 | 1.17 | 1.14 | 1.20 |
| Medical condition* | ||||||
|
Asthma |
1.34 | 1.27 | 1.42 | 1.32 | 1.28 | 1.37 |
|
COPD |
1.28 | 1.22 | 1.34 | 1.23 | 1.15 | 1.31 |
|
Ischaemic heart disease |
1.24 | 1.18 | 1.29 | 1.17 | 1.11 | 1.24 |
|
Other cardiovascular diseases |
1.17 | 1.11 | 1.22 | 1.19 | 1.15 | 1.24 |
|
Diabetes |
1.13 | 1.09 | 1.17 | 1.04 | 1.01 | 1.08 |
|
HIV-infection/AIDS |
3.20 | 2.90 | 3.53 | 2.98 | 2.58 | 3.45 |
|
Other immunodeficiency |
1.57 | 1.41 | 1.75 | 1.65 | 1.50 | 1.80 |
|
Cancer |
1.12 | 1.06 | 1.18 | 1.24 | 1.19 | 1.30 |
aIRR, adjusted Incidence Rate Ratio (adjusted by the factors included in the multivariate model: age, origin, socioeconomic level, asthma, COPD, ischemic heart disease, other cardiovascular diseases, diabetes, HIV-infection/AIDS, other immunodeficiency, and cancer. Heart disease was not statistically significant in the multivariate model); *The comparison group were persons without the specified condition; COPD, chronic obstructive pulmonary disease.
Discussion
The results of this population-based cohort indicate an annual incidence of HZ of 5.1 per 1000 person-years, with a clear association of age, origin, SES, and some medical conditions with the occurrence of HZ. Data from ECRPC have been a useful source of information for the epidemiological analysis of HZ.
The overall IDR of HZ in this study is slightly higher than those reported for other developed countries, which range between 1.2 and 4.8 per 1000 person-years,12,15,26 although these cover all ages. Previous studies performed in primary care in other Spanish regions have estimated incidence rates of HZ of 4.25 cases per 1000 inhabitants in 2005–2006,27 4.1 per 1000 individuals in 2007 (excluding children under 15 y of age),28 and more recently 4.6 per 1000 person-year between 2007–2010 for all age groups.29 Differences in incidence among geographic areas could be influenced by the climatological factor. Although evidence of this association is limited, a recent study found that temperature is the climate variable significantly associated with higher incidence of HZ.30 Moreover, additional sunny hours may increase exposure to UV irradiation, which may act as an immunosuppressant and reduce immunity to VZV.31
Sociodemographic factors
In line with our data, higher annual incidences in the elderly and in females have been widely described.2,8,27,28,32-39 Gender differences in the incidence of herpes simplex add strong support to the hypothesis that females have a differing response to latent viral infection and that this may account for their increased risk compared with men.16
Studies conducted in numerous settings, and with various designs, have indicated an association between age and increasing zoster incidence.1 There is also an interaction between age and sex. The difference between female and male rates appears to increase gradually with age, reaching a maximum that, depending on the study, varies within the 40–69 age range and decreases at older ages.8,9,16 According to our results, the increased risk observed with age is not sustained, and appears to decrease slightly in the group aged 75 y and over. Previous studies have found that incidence increased markedly with age, with the peak occurring around 75–79 y, and decreasing above that age.26,27,29,33 The underlying cause could be a gradually reduced risk of HZ, explained by the hypothesis that exposure to VZV provides the host with progressive immunity to VZV reactivation.40 Also this age group could be more affected by the underreporting of episodes, due to their greater degree of home attendance or institutionalization. This drop in incidence has not been observed in hospitalizations.29,41 The cases in this age group may be more severe and addressed at specialized level, although it would be unusual for HZ episodes not to result in any contact with primary care.
In our study, the foreign-born population of any origin was less at risk of HZ than autochthonous population, after adjusting by age, SES, and comorbidities. Previous studies have described racial differences in the HZ risk, finding lower risk in black vs. white study participants.12,42,43 The reasons for these racial differences are unknown, however these findings may be partially explained by a delayed average age of varicella in people born in some regions (the Caribbean, Central America, Indian Subcontinent)12; by differences in immune “boosting” to VZV because of differences in household composition or community contacts; and by racial differences in the decline of cellular immunity with aging.43 Published data on incidence of HZ in underdeveloped countries are scarce, limiting the comparison with incidences found in economic immigrants. Therefore, healthy migrant bias cannot be discarded.
According to our data, the incident cases of HZ were associated with lower SES, after adjustment. An analysis of incident HZ cases between 1994 and 2002 in Canada found that for HZ, there was no evidence of disparity by SES.44 However, another study performed in Israel during 2006–2010 found that, in multivariable analyses, HZ and postherpetic neuralgia were associated with higher SES, female sex, DM, cancer history, and HIV treatment.26 The disparity of these results could be attributed to the heterogeneity of methods used to measure SES, with the consequent lack of comparability, and the added constraint of using ecological data in most studies. The different patterns in health service utilization could be affecting our data. Although access to the MRPHS for immigrant population was universal along the study period, foreign populations are more likely to visit the emergency services and less likely to have private health coverage.45 Higher-income people are more likely to have, and use, private insurance even though they have access to the Public Health System. Consequentially, this affects incidence rates. It has been hypothesized that increased psychological stress and/or lack of social support, as well as exposure to some environmental contaminants, can cause generalized cell-mediated immunosuppression leading to an increase in HZ,12 and this could partially explain social disparities in its occurrence.
Comorbidities
In our study, HIV-infection/AIDS and other immunodeficiencies (leukemias, lymphomas, cirrhosis, and others) were the medical conditions with strongest association to HZ, showing the highest IDR among the morbidities analyzed: 12.51 and 12.90 per 1000 person-years, respectively. The Israeli study mentioned above found similar IDR during 2006–2010 in immune-compromised patients (12.8 per 1000 person-years).26 In cohort studies conducted before the introduction of highly active antiretroviral therapy, incidence rates of 29.4–51.5 per 1000 person years had been reported among HIV-infected adults, reflecting 12- to 17-fold increase compared with HIV-negative persons.1,12 More recent studies have detected lower incidence rates than previously (9.0–12.2 per 1000 person-years),46,47 showing that appropriate antiretroviral therapy is an important protective factor for HZ.47
The increased incidence of HZ in patients with hematological malignancies and solid tumors has been known for years, and both the nature of the underlying cancer and the type of treatment determine the magnitude of risk.1,22 It has been demonstrated recently that, although HZ risk declined with time following a cancer diagnosis, it was still significant at least 5 y afterwards.26 The incidence of HZ in a cohort of adults diagnosed with invasive cancer from 2001 to 2005 in the US was estimated at 12 per 1000 person-years in patients with solid tumors.48 That rate is higher than ours (10.15 per 1000 person-years), although these differences could be explained at least partially by differences in the criteria for selection and grouping of tumors.
Recent studies have identified DM as an independent risk factor for HZ, as that risk increased by 5–6% among diabetics in adjusted analysis.21,23 A study performed in Spain in 2006 found higher incidence rates than ours, totalling 12.4 cases per 1000 person-years in men and 18.7 in women. However, that study covered the population aged 30 y and older.49 It has been reported that CMI to VZV in DM patients was significantly lower than in healthy individuals.50
The incidence of HZ in COPD patients in our study was twice that of the general population. The detection of COPD as an independent risk factor for HZ has scarcely, and only recently, been described. In line with our results, a population-based cohort study performed in Taiwan to examine the HZ risk in patients with COPD showed that, after controlling for other risk factors, it was higher compared with the general population, and greater among patients who used inhaled or oral corticosteroids therapy.25 Another case-control study with data from 2007 found a risk of HZ 1.35-fold higher in COPD patients, after adjustment for other risk factors.23 It has been hypothesized that the immune dysregulation found in COPD may put patients at higher risk of developing HZ.25
Regarding the increased incidence detected in asthmatic patients, a strong association between asthma and HZ, independently of the use of corticosteroids, has been described in children. This could be related to a possible impaired innate and adaptive immunity in these patients.24
Our data also reveal an association of HZ with ischemic heart disease and other cardiovascular diseases (arrhythmias and valvular pathology) but not with heart failure in the multivariate analysis. Coronary artery disease has been already described as an independent risk factor,23 although the pathophysiological explanation remains unclear.
Some potential limitations of this study need to be addressed. Studies that use electronic clinical records as their data source are subject to the limitation of a possible lack of registry completeness and quality in diagnostic coding,although validation studies showed that the positive predictive value of the ICD codes for HZ was good (84–94%).4,13,35,51,52 Virological confirmation of the diagnosis of HZ was not available in our data, so some cases might have been misdiagnosed as zoster. Family physicians have good clinical judgment with regard to diagnosing HZ, and clinical diagnosis is confirmed serologically in 91% of patients older than 50 who have HZ.53 Another potential limitation is that any patients with HZ who went to a private practitioner might not have been detected in our study, leading to an underestimate of the disease. However, this proportion of patients would be small given the public health care provision in Spain, as well as the availability of subsidized drugs for the treatment through primary care.
The attribution of SES by geocoding can produce errors of classification. The analysis of the effect of origin was partially limited because the information on the residence time in Spain among patients born outside the country was not available. The possible effect of dissimilar patterns of use of health services could not be corrected for in this analysis. The higher consultation rates among patients with comorbidities could increase the chance of diagnosing HZ, leading to a slight overestimation of the incidence on them. On the other hand, the medical conditions were considered only at the beginning of the period, and this may have produced the opposite effect. Data did not permit categorization of patients suffering from a medical condition according to duration, severity and/or treatment, which would have made it possible to identify the risk more accurately. The influence of using immunosuppressive medication could not be analyzed, due to lack of this data. Information about severity and complications of HZ was not available, therefore their effect could not be either analyzed.
Our study showed that older patients, autochthonous, with lower SES and with certain underlying medical conditions had a higher probability of suffering HZ. Electronic databases have proved useful in estimating the incidence of HZ, and tin finding associations with sociodemographic and clinical characteristics of the population.
The identification of previously unknown risk factors, such as asthma or cardiovascular diseases, can lead to more specific investigations and help to understand the pathophysiology of HZ.
Understanding the epidemiology and risk factors for HZ might also lead to changes in policy regarding use of varicella and HZ vaccines.1,54 Vaccination against HZ is likely to be cost-effective when the vaccine is given at approximately 65 y of age, assuming average vaccine protection against moderate/severe pain and post-herpetic neuralgia is longer than 10 y.55 The identification of increased incidences of HZ associated with medical conditions other than immunodeficiency, and therefore with no contraindication to vaccination, can help to prioritize interventions and to optimize vaccination strategies, reducing the high burden of illness due to HZ and the incidence of post-herpetic neuralgia.
Materials and Methods
We performed a cohort study using data from the ECRPC recorded between 1 October 2009 and 31 December 2012.
The study cohort was the entire adult population (18 y of age and over) with access to the Madrid Regional Public Health System (MRPHS) on 1 October 2009. Information about patients in MRPHS is recorded in the Individualized Health Card (IHC) database, that gathers personal data of the patient, as well as the assignment to a primary care health center and to health professionals. When someone loses the right to receive medical assistance (due to exitus, transfer to another region, etc.) his data are withdrawn from the IHC database. Each patient in the IHC database has an electronic clinical record in primary care, linked by nominal data and by a personal ID. In our region ECRPC has full implementation in all consultations since 2006.For each person, details of sex, age at the beginning of the period, and country of birth were obtained, as well as health center and information regarding the end of follow up (death date or date of exit of the IHC database). The country of birth is required information for the registration of a person on the IHC database. It is available in more than 99.9% of the population included. Countries of birth were grouped into 7 regions: Europe-15 (Austria, Belgium, Denmark, France, Finland, Germany, Greece, Ireland, Italy, Luxembourg, Netherlands, Portugal, Sweden, and the UK), Central and Eastern Europe, North Africa, Sub-Saharan Africa, Latin America, North America (USA and Canada), and Asia/Oceania.
Clinical information was obtained from data recorded in the ECRPC between 1 October 2009 and 31 December 2012. The ECRPC is universally used by primary care professionals. Health care episodes are routinely recorded in ECRPC by health professionals, coded as per the International Classification for Primary Care (ICPC), issued by the World Organization of Family Doctors (WONCA). Medical conditions of interest were selected according to their clinical relevance, prevalence and/or proven association with HZ. The list of pathologies with their corresponding ICPC codes can be seen in Table 4. The pathologies were grouped according to their effect on the immune system, to ease comparisons and to independently assess their association with herpes zoster. For each person, information was beginning of the period. All episodes coded as S70, which corresponds to herpes zoster, registered during the study period were obtained too, with their onset date and accompanying descriptive text. The descriptive clinical text accompanying the ICPC code S70, available in 100% of the episodes, was used to discard episodes that clearly did not correspond to HZ, or when an HZ code was given for a diagnosis of history of HZ or family history of HZ in a person with no evidence of an acute HZ episode (1.4% of the total). When a patient had more than one episode registered in the ECRPC with onset date occurring during the study period, only the first one was considered.
Table 4. International Classification for Primary Care (ICPC) codes for selected chronic conditions analyzed.
| Medical Condition | ICPC CODE | |
|---|---|---|
| Asthma | Asthma | R96 |
| COPD | Chronic obstructive pulmonary disease | R95 |
| Chronic bronchitis | R79 | |
| Chronic bronchitis (icpc-1) | R91 | |
| Ischaemic heart disease | Ischaemic heart disease with angina | K74 |
| Acute myocardial infarction | K75 | |
| Ischaemic heart disease without angina | K76 | |
| Heart failure | Heart failure | K77 |
| Other cardiovascular diseases | Atrial fibrillation/flutter | K78 |
| Pulmonary heart disease | K82 | |
| Heart valve disease not otherwise specified | K83 | |
| Heart disease other | K84 | |
| Diabetes | Diabetes insulin dependent | T89 |
| Diabetes non-insulin dependent | T90 | |
| HIV infection/AIDS | HIV-infection/AIDS | B90 |
| Other immunodeficiency | Ruptured spleen traumatic | B76 |
| Hodgkin disease/lymphoma | B72 | |
| Leukemia | B73 | |
| Malignant neoplasm blood other | B74 | |
| Congenital anomaly blood/lymph other | B79 | |
| Liver disease not otherwise specified: restricted to “Cirrhosis “ | D97 | |
| Cancer | Malignancy not otherwise specified | A79 |
| Malignant neoplasm stomach | D74 | |
| Malignant neoplasm colon/rectum | D75 | |
| Malignant neoplasm pancreas | D76 | |
| Malignant neoplasm digest other/not otherwise specified | D77 | |
| Neoplasm of eye/adnexa | F74 | |
| Neoplasm of ear | H75 | |
| Neoplasm cardiovascular | K72 | |
| Malignant neoplasm musculoskeletal | L71 | |
| Malignant neoplasm nervous system | N74 | |
| Malignant neoplasm bronchus/lung | R84 | |
| Malinant neoplasm respiratory, other | R85 | |
| Malignant neoplasm of skin: restricted to “Melanoma “ | S77 | |
| Malignant neoplasm of kidney | U75 | |
| Malignant neoplasm of bladder | U76 | |
| Malignant neoplasm cervix | X75 | |
| Malignant neoplasm breast female | X76 | |
| Malignant neoplasm prostate | Y77 | |
Information on the SES of the individual was not included in the ECRPC. The SES may be inferred from the general sociodemographic composition of the area of residence of a patient (geocoding) using census-derived information. The smaller the chosen area of residence, the more homogeneous it is in terms of its sociodemographic composition.56 Each health center of the MRPHS provides assistance to people living in its area of influence, ranging in size from 5000 to 25 000 patients. A deprivation index based on census data of 2001 was used to assign SES to each patient. This deprivation index includes 5 indicators, related to work (unemployment, manual, and casual workers) and education (insufficient education, considered overall and in young people).57 The corresponding quartile of the deprivation index was assigned to each health center and, by extension, to the patients assisted in it.
Statistical Analysis
Descriptive analysis was performed on the population characteristics, stratified by gender.
Incidence density rates (IDR) were calculated considering the follow up time for each person until the onset date of HZ, when it occurred, until the end of follow up, i.e: death date or date of exit of the IHC database, or until the end of the study in the remaining cases. IDR were expressed as cases of HZ per 1000 person-years.
Bivariate and multivariate robust Poisson regressions were performed to identify the factors associated with incident HZ cases. The factors analyzed were age, origin, SES, and presence of the following medical conditions: asthma, COPD, ischemic heart disease, heart failure, other cardiovascular diseases, diabetes, HIV-infection/AIDS, other immunodeficiency, and cancer. Models for men and women were analyzed separately. Results from Poisson regression analyses are expressed as incidence rate ratios (IRR) with 95% confidence intervals (95% CI). Statistical significance was set at P < 0.05. Analyses were performed with SPSS 21.0 software.
The prior consent of patients and approval by an ethics committee were not required, given the characteristics of the study and the current legislation. Patient information is available for scientific purposes, with the right to privacy fully guaranteed. Access to medical records for research purposes requires patients' personal identification data to be kept separate from clinical data, so as to ensure anonymity.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Harpaz R, Ortega-Sanchez IR, Seward JF, Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC) Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2008;57(RR-5):1–30, quiz CE2-4. [PubMed] [Google Scholar]
- 2.Leung J, Harpaz R, Molinari NA, Jumaan A, Zhou F. Herpes zoster incidence among insured persons in the United States, 1993-2006: evaluation of impact of varicella vaccination. Clin Infect Dis. 2011;52:332–40. doi: 10.1093/cid/ciq077. [DOI] [PubMed] [Google Scholar]
- 3.Rimland D, Moanna A. Increasing incidence of herpes zoster among Veterans. Clin Infect Dis. 2010;50:1000–5. doi: 10.1086/651078. [DOI] [PubMed] [Google Scholar]
- 4.Yawn BP, Saddier P, Wollan PC, St Sauver JL, Kurland MJ, Sy LS. A population-based study of the incidence and complication rates of herpes zoster before zoster vaccine introduction. Mayo Clin Proc. 2007;82:1341–9. doi: 10.4065/82.11.1341. [DOI] [PubMed] [Google Scholar]
- 5.Yih WK, Brooks DR, Lett SM, Jumaan AO, Zhang Z, Clements KM, Seward JF. The incidence of varicella and herpes zoster in Massachusetts as measured by the Behavioral Risk Factor Surveillance System (BRFSS) during a period of increasing varicella vaccine coverage, 1998-2003. BMC Public Health. 2005;5:68. doi: 10.1186/1471-2458-5-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Esteban-Vasallo MD, Gil-Prieto R, Domínguez-Berjón MF, Astray-Mochales J, Gil de Miguel A. Temporal trends in incidence rates of herpes zoster among patients treated in primary care centers in Madrid (Spain), 2005-2012. J Infect. 2014;68:378–86. doi: 10.1016/j.jinf.2013.09.035. [DOI] [PubMed] [Google Scholar]
- 7.Brisson M, Edmunds WJ, Law B, Gay NJ, Walld R, Brownell M, Roos L, De Serres G. Epidemiology of varicella zoster virus infection in Canada and the United Kingdom. Epidemiol Infect. 2001;127:305–14. doi: 10.1017/S0950268801005921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Russell ML, Schopflocher DP, Svenson L, Virani SN. Secular trends in the epidemiology of shingles in Alberta. Epidemiol Infect. 2007;135:908–13. doi: 10.1017/S0950268807007893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toyama N, Shiraki K, Society of the Miyazaki Prefecture Dermatologists Epidemiology of herpes zoster and its relationship to varicella in Japan: A 10-year survey of 48,388 herpes zoster cases in Miyazaki prefecture. J Med Virol. 2009;81:2053–8. doi: 10.1002/jmv.21599. [DOI] [PubMed] [Google Scholar]
- 10.Chao DY, Chien YZ, Yeh YP, Hsu PS, Lian IB. The incidence of varicella and herpes zoster in Taiwan during a period of increasing varicella vaccine coverage, 2000-2008. Epidemiol Infect. 2012;140:1131–40. doi: 10.1017/S0950268811001786. [DOI] [PubMed] [Google Scholar]
- 11.Nelson MR, Britt HC, Harrison CM. Evidence of increasing frequency of herpes zoster management in Australian general practice since the introduction of a varicella vaccine. Med J Aust. 2010;193:110–3. [PubMed] [Google Scholar]
- 12.Thomas SL, Hall AJ. What does epidemiology tell us about risk factors for herpes zoster? Lancet Infect Dis. 2004;4:26–33. doi: 10.1016/S1473-3099(03)00857-0. [DOI] [PubMed] [Google Scholar]
- 13.Donahue JG, Choo PW, Manson JE, Platt R. The incidence of herpes zoster. Arch Intern Med. 1995;155:1605–9. doi: 10.1001/archinte.1995.00430150071008. [DOI] [PubMed] [Google Scholar]
- 14.Levin MJ, Smith JG, Kaufhold RM, Barber D, Hayward AR, Chan CY, Chan IS, Li DJ, Wang W, Keller PM, et al. Decline in varicella-zoster virus (VZV)-specific cell-mediated immunity with increasing age and boosting with a high-dose VZV vaccine. J Infect Dis. 2003;188:1336–44. doi: 10.1086/379048. [DOI] [PubMed] [Google Scholar]
- 15.Pinchinat S, Cebrián-Cuenca AM, Bricout H, Johnson RW. Similar herpes zoster incidence across Europe: results from a systematic literature review. BMC Infect Dis. 2013;13:170. doi: 10.1186/1471-2334-13-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fleming DM, Cross KW, Cobb WA, Chapman RS. Gender difference in the incidence of shingles. Epidemiol Infect. 2004;132:1–5. doi: 10.1017/S0950268803001523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Opstelten W, Van Essen GA, Schellevis F, Verheij TJ, Moons KG. Gender as an independent risk factor for herpes zoster: a population-based prospective study. Ann Epidemiol. 2006;16:692–5. doi: 10.1016/j.annepidem.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 18.Irwin MR, Levin MJ, Carrillo C, Olmstead R, Lucko A, Lang N, Caulfield MJ, Weinberg A, Chan IS, Clair J, et al. Major depressive disorder and immunity to varicella-zoster virus in the elderly. Brain Behav Immun. 2011;25:759–66. doi: 10.1016/j.bbi.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown GR. Herpes zoster: correlation of age, sex, distribution, neuralgia, and associated disorders. South Med J. 1976;69:576–8. doi: 10.1097/00007611-197605000-00028. [DOI] [PubMed] [Google Scholar]
- 20.Ragozzino MW, Melton LJ, 3rd, Kurland LT, Chu CP, Perry HO. Population-based study of herpes zoster and its sequelae. Medicine (Baltimore) 1982;61:310–6. doi: 10.1097/00005792-198209000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Heymann AD, Chodick G, Karpati T, Kamer L, Kremer E, Green MS, Kokia E, Shalev V. Diabetes as a risk factor for herpes zoster infection: results of a population-based study in Israel. Infection. 2008;36:226–30. doi: 10.1007/s15010-007-6347-x. [DOI] [PubMed] [Google Scholar]
- 22.Hata A, Kuniyoshi M, Ohkusa Y. Risk of Herpes zoster in patients with underlying diseases: a retrospective hospital-based cohort study. Infection. 2011;39:537–44. doi: 10.1007/s15010-011-0162-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joesoef RM, Harpaz R, Leung J, Bialek SR. Chronic medical conditions as risk factors for herpes zoster. Mayo Clin Proc. 2012;87:961–7. doi: 10.1016/j.mayocp.2012.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim BS, Mehra S, Yawn B, Grose C, Tarrell R, Lahr B, Juhn YJ. Increased risk of herpes zoster in children with asthma: a population-based case-control study. J Pediatr. 2013;163:816–21. doi: 10.1016/j.jpeds.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang YW, Chen YH, Wang KH, Wang CY, Lin HW. Risk of herpes zoster among patients with chronic obstructive pulmonary disease: a population-based study. CMAJ. 2011;183:E275–80. doi: 10.1503/cmaj.101137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weitzman D, Shavit O, Stein M, Cohen R, Chodick G, Shalev V. A population based study of the epidemiology of Herpes Zoster and its complications. J Infect. 2013;67:463–9. doi: 10.1016/j.jinf.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 27.García Cenoz M, Castilla J, Montes Y, Morán J, Salaberri A, Elía F, Floristán Y, Rodrígo I, Irisarri F, Arriazu M, et al. [Varicella and herpes zoster incidence prior to the introduction of systematic child vaccination in Navarre, 2005-2006] An Sist Sanit Navar. 2008;31:71–80. doi: 10.4321/s1137-66272008000100006. [DOI] [PubMed] [Google Scholar]
- 28.Cebrián-Cuenca AM, Díez-Domingo J, Rodríguez MS, Puig-Barberá J, Navarro-Pérez J, Herpes Zoster Research Group of the Valencian Community Epidemiology of herpes zoster infection among patients treated in primary care centres in the Valencian community (Spain) BMC Fam Pract. 2010;11:33. doi: 10.1186/1471-2296-11-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morant-Talamante N, Díez-Domingo J, Martínez-Úbeda S, Puig-Barberá J, Alemán-Sánchez S, Pérez-Breva L. Herpes zoster surveillance using electronic databases in the Valencian Community (Spain) BMC Infect Dis. 2013;13:463. doi: 10.1186/1471-2334-13-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu PY, Wu HD, Chou TC, Sung FC. Varicella vaccination alters the chronological trends of herpes zoster and varicella. PLoS One. 2013;8:e77709. doi: 10.1371/journal.pone.0077709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zak-Prelich M, Borkowski JL, Alexander F, Norval M. The role of solar ultraviolet irradiation in zoster. Epidemiol Infect. 2002;129:593–7. doi: 10.1017/S0950268802007793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pierik JG, Gumbs PD, Fortanier SA, Van Steenwijk PC, Postma MJ. Epidemiological characteristics and societal burden of varicella zoster virus in the Netherlands. BMC Infect Dis. 2012;12:110. doi: 10.1186/1471-2334-12-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gialloreti LE, Merito M, Pezzotti P, Naldi L, Gatti A, Beillat M, Serradell L, di Marzo R, Volpi A. Epidemiology and economic burden of herpes zoster and post-herpetic neuralgia in Italy: a retrospective, population-based study. BMC Infect Dis. 2010;10:230. doi: 10.1186/1471-2334-10-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Melker H, Berbers G, Hahné S, Rümke H, van den Hof S, de Wit A, Boot H. The epidemiology of varicella and herpes zoster in The Netherlands: implications for varicella zoster virus vaccination. Vaccine. 2006;24:3946–52. doi: 10.1016/j.vaccine.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 35.Mullooly JP, Riedlinger K, Chun C, Weinmann S, Houston H. Incidence of herpes zoster, 1997-2002. Epidemiol Infect. 2005;133:245–53. doi: 10.1017/S095026880400281X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lionis CD, Vardavas CI, Symvoulakis EK, Papadakaki MG, Anastasiou FS, Antonopoulou MD, Apostolakis CM, Dimitrakopoulos SA, Fountakis GI, Grammatikopoulos IA, et al. Measuring the burden of herpes zoster and post herpetic neuralgia within primary care in rural Crete, Greece. BMC Fam Pract. 2011;12:136. doi: 10.1186/1471-2296-12-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pérez-Farinós N, Ordobás M, García-Fernández C, García-Comas L, Cañellas S, Rodero I, Gutiérrez-Rodríguez A, García-Gutiérrez J, Ramírez R. Varicella and herpes zoster in Madrid, based on the Sentinel General Practitioner Network: 1997-2004. BMC Infect Dis. 2007;7:59. doi: 10.1186/1471-2334-7-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Insinga RP, Itzler RF, Pellissier JM, Saddier P, Nikas AA. The incidence of herpes zoster in a United States administrative database. J Gen Intern Med. 2005;20:748–53. doi: 10.1111/j.1525-1497.2005.0150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Socan M, Blasko M. Surveillance of varicella and herpes zoster in Slovenia, 1996 - 2005. Euro Surveill. 2007;12:12. [Google Scholar]
- 40.Oxman MN. Herpes zoster pathogenesis and cell-mediated immunity and immunosenescence. J Am Osteopath Assoc. 2009;109(Suppl 2):S13–7. [PubMed] [Google Scholar]
- 41.Gil-Prieto R, Walter S, González-Escalada A, García-Garcia L, Marín-García P, Gil-de-Miguel A. Different vaccination strategies in Spain and its impact on severe varicella and zoster. Vaccine. 2014;32:277–83. doi: 10.1016/j.vaccine.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 42.Schmader K, George LK, Burchett BM, Pieper CF, Hamilton JD. Racial differences in the occurrence of herpes zoster. J Infect Dis. 1995;171:701–4. doi: 10.1093/infdis/171.3.701. [DOI] [PubMed] [Google Scholar]
- 43.Schmader K, George LK, Burchett BM, Pieper CF. Racial and psychosocial risk factors for herpes zoster in the elderly. J Infect Dis. 1998;178(Suppl 1):S67–70. doi: 10.1086/514254. [DOI] [PubMed] [Google Scholar]
- 44.Russell ML, Schopflocher DP, Svenson LW. Health disparities in chickenpox or shingles in Alberta? Can J Public Health. 2008;99:41–5. doi: 10.1007/BF03403739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aerny Perreten N, Ramasco Gutiérrez M, Cruz Maceín JL, Rodríguez Rieiro C, Garabato González S, Rodríguez Laso A. [Health and its determinants in the immigrant population of the region of Madrid] Gac Sanit. 2010;24:136–44. doi: 10.1016/j.gaceta.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 46.Blank LJ, Polydefkis MJ, Moore RD, Gebo KA. Herpes zoster among persons living with HIV in the current antiretroviral therapy era. J Acquir Immune Defic Syndr. 2012;61:203–7. doi: 10.1097/QAI.0b013e318266cd3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jansen K, Haastert B, Michalik C, Guignard A, Esser S, Dupke S, Plettenberg A, Skaletz-Rorowski A, Brockmeyer NH. Incidence and risk factors of herpes zoster among hiv-positive patients in the german competence network for HIV/AIDS (KompNet): a cohort study analysis. BMC Infect Dis. 2013;13:372. doi: 10.1186/1471-2334-13-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Habel LA, Ray GT, Silverberg MJ, Horberg MA, Yawn BP, Castillo AL, Quesenberry CP, Jr., Li Y, Sadier P, Tran TN. The epidemiology of herpes zoster in patients with newly diagnosed cancer. Cancer Epidemiol Biomarkers Prev. 2013;22:82–90. doi: 10.1158/1055-9965.EPI-12-0815. [DOI] [PubMed] [Google Scholar]
- 49.Aldaz P, Díaz JA, Loayssa JR, Dronda MJ, Oscáriz M, Castilla J. [Herpes zoster incidence in diabetic patients] An Sist Sanit Navar. 2013;36:57–62. doi: 10.4321/S1137-66272013000100006. [DOI] [PubMed] [Google Scholar]
- 50.Okamoto S, Hata A, Sadaoka K, Yamanishi K, Mori Y. Comparison of varicella-zoster virus-specific immunity of patients with diabetes mellitus and healthy individuals. J Infect Dis. 2009;200:1606–10. doi: 10.1086/644646. [DOI] [PubMed] [Google Scholar]
- 51.Jumaan AO, Yu O, Jackson LA, Bohlke K, Galil K, Seward JF. Incidence of herpes zoster, before and after varicella-vaccination-associated decreases in the incidence of varicella, 1992-2002. J Infect Dis. 2005;191:2002–7. doi: 10.1086/430325. [DOI] [PubMed] [Google Scholar]
- 52.Klompas M, Kulldorff M, Vilk Y, Bialek SR, Harpaz R. Herpes zoster and postherpetic neuralgia surveillance using structured electronic data. Mayo Clin Proc. 2011;86:1146–53. doi: 10.4065/mcp.2011.0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Opstelten W, van Loon AM, Schuller M, van Wijck AJ, van Essen GA, Moons KG, Verheij TJ. Clinical diagnosis of herpes zoster in family practice. Ann Fam Med. 2007;5:305–9. doi: 10.1370/afm.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reynolds MA, Chaves SS, Harpaz R, Lopez AS, Seward JF. The impact of the varicella vaccination program on herpes zoster epidemiology in the United States: a review. J Infect Dis. 2008;197(Suppl 2):S224–7. doi: 10.1086/522162. [DOI] [PubMed] [Google Scholar]
- 55.Drolet M, Oxman MN, Levin MJ, Schmader KE, Johnson RW, Patrick D, Mansi JA, Brisson M. Vaccination against herpes zoster in developed countries: state of the evidence. Hum Vaccin Immunother. 2013;9:1177–84. doi: 10.4161/hv.23491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gavrielov-Yusim N, Friger M. Use of administrative medical databases in population-based research. J Epidemiol Community Health. 2014;;68:283–7. doi: 10.1136/jech-2013-202744. [DOI] [PubMed] [Google Scholar]
- 57.Domínguez-Berjón MF, Borrell C, Cano-Serral G, Esnaola S, Nolasco A, Pasarín MI, Ramis R, Saurina C, Escolar-Pujolar A. [Constructing a deprivation index based on census data in large Spanish cities(the MEDEA project)] Gac Sanit. 2008;22:179–87. doi: 10.1157/13123961. [DOI] [PubMed] [Google Scholar]