Abstract
Strategies to optimize responses to seasonal influenza vaccination in older adults include the use of adjuvants, higher antigen doses, and intradermal delivery. In this study adults aged ≥65 years (n = 450) received a single dose of 1 of 2 non-adjuvanted trivalent influenza vaccine (TIV) formulations administered intradermally (ID), both containing 6 µg of A/H1N1 and B, differing in A/H3N2 content (6 µg or 12 µg), or a single dose of 1 of 8 TIV formulations administered intramuscularly (IM) all containing 15 µg of A/H1N1 and B, differing in A/H3N2 hemagglutinin (HA) content (15 µg or 30 µg) and/or in MF59® adjuvant content (0%, 25%, 50%, or 100% of the standard dose). This paper focuses on the comparisons of low-dose non-adjuvanted ID, full-dose non-adjuvanted IM and full-dose MF59-adjuvanted IM formulations (n = 270). At day 22 post-vaccination, at least one European licensure immunogenicity criterion was met by all groups against all 3 strains; however, all three criteria were met against all 3 vaccine strains by the low-dose non-adjuvanted ID and the full-dose MF59-adjuvanted IM groups only. The full-dose MF59-adjuvanted IM group elicited significantly higher immune response vs. the low-dose non-adjuvanted ID formulations for most comparisons. The full-dose MF59 adjuvanted IM groups were associated with increased pain at the site of injection (P < 0.01) compared to the ID groups, and the low-dose non-adjuvanted ID groups were associated with increased erythema, induration, and swelling at the injection site (P < 0.0001) and unsolicited AEs compared with the IM groups. There were no differences between IM and ID groups in the frequencies of subjects experiencing solicited systemic reactions. Overall, while MF59 adjuvantation increased pain at the site of injection, and intradermal delivery increased unsolicited adverse events, erythema, induration, and swelling at the injection site, both strategies of vaccination strongly enhanced the immunogenicity of seasonal influenza vaccine in older adults compared with conventional non-adjuvanted intramuscular delivery.
Trial registration: http://www.clinicaltrials.gov: NCT00848848
The authors wish to thank Dr Jamie Stirling and Dr Hari Sai Priya Baddela (Novartis Vaccines) and Dr Patricia de Groot and Dr Amanda Prowse (CHC Europe) for editorial assistance in the preparation of this manuscript.
Keywords: influenza, vaccine, seasonal, intradermal, adjuvant, MF59
Introduction
Influenza is a highly contagious disease, which is associated with substantial morbidity and mortality, especially among vulnerable populations such as the elderly.1,2 Preventative, annual vaccination continues to be the most effective strategy to control influenza, and is recommended for all individuals aged 65 y and above.3 However, the clinical efficacy of conventional influenza vaccines decreases with advancing age, and drops from 70−90% in young adults to 17–53% in adults over 65 y of age.4 This reduced immune response in older adults is mainly due to immunosenescence, i.e., an age-related decline in innate and adaptive immune function.5,6 To compensate for the effects of immunosenescence, strategies to enhance the immunogenicity and consequent clinical efficacy of influenza vaccines in older adults are needed to alleviate the burden of influenza-related disease in this rapidly increasing section of the population.7 Several approaches have been investigated, including the use of adjuvanted trivalent influenza vaccines (aTIVs),8-10 increasing the dose of antigens,11-13, intradermal (ID) vaccine delivery,14,15 and virosomal subunit vaccines.16
MF59® (Novartis Vaccines) is an oil-in-water emulsion originally developed by the Chiron Corporation as a vaccine adjuvant.17 Previous clinical studies have demonstrated that MF59-adjuvanted influenza vaccines induce higher and broader (heterologous) antibody responses to immunization in healthy and high-risk populations, including the elderly, as compared with non-adjuvanted vaccines.8,18-20 The seasonal, MF59-adjuvanted influenza vaccine, Fluad® (Novartis Vaccines) has been approved and used in older adults in Europe since 1997. Over 85 million doses of Fluad have been distributed to date worldwide, with no safety signals.21
ID delivery of influenza vaccine may be an alternative to conventional intramuscular (IM) injection because the skin is an important natural barrier and immune organ which contains large numbers of Langerhans cells and resident dermal dendritic cells which, as professional antigen-presenting cells, are able to take up antigens at the site of injection, migrate to the draining lymph nodes, and there trigger an effective immune response by activating antigen-specific T cells.22,23 Although preliminary results of ID vaccination against influenza are promising and have demonstrated good immunogenicity with lower antigen doses compared with IM administration,24-27 the traditional ID technique for vaccine delivery (the Mantoux technique) is not easy to perform correctly and requires trained personnel and fine maneuvering of a needle into a 1−2 mm deep layer of tissue.28 To overcome these limitations, new ID injection devices have been developed to deliver vaccine more reliably and conveniently, which employ short needles (1.5 mm in length),14,29-32 and microneedles (<1.0 mm in length).32-35 MicronJet® (NanoPass Technologies) is one such novel device using microneedles. A recent study in older adults by Hung et al. demonstrated that ID delivery (MicronJet) of a non-adjuvanted, trivalent influenza vaccine (TIV) containing a total of 3.0 and 9.0 µg hemagglutinin (HA) per strain surface antigen resulted in significantly higher rates of subjects achieving titers associated with seroprotection (i.e., hemagglutination inhibition (HI) ≥ 1:40) against A/H1N1 and A/H3N2 strains than IM delivery of TIV containing a total of 15 µg HA per strain.34
The present factorial design study evaluated the immunogenicity and safety of 8 IM TIV formulations, all with 15 µg of A/H1N1, and B antigens differing in the quantity of MF59 adjuvant content (0, 25%, 50%, and 100% of standard dose) and A/H3N2 HA antigen content (15 µg and 30 µg) and of 2 ID TIV formulations with comparatively lower antigen doses (6 µg of A/H1N1 and B strains and either 6 µg or 12 µg of A/H3N2 strain) (Table 1). The data describing the relationship between MF59 dose, antigen dose and resulting immunogenicity and safety profiles for the IM formulations are published elsewhere.36 This paper focuses on the comparisons between low antigen dose ID formulations, and full antigen dose IM non-adjuvanted and fully adjuvanted formulations.
Table 1. Study groups and vaccine formulations.
| Vaccine group/ formulation |
Route of administration |
A/H1N1 antigen per dose (µg) |
A/H3N2 antigen per dose (µg) |
B strain antigen per dose (µg) |
MF59 per dose (%) |
Volume per dose (mL) |
|---|---|---|---|---|---|---|
| ID1 (n = 47) | ID | 6 | 6 | 6 | 0 | 0.2 |
| ID2 (n = 46) | ID | 6 | 12 | 6 | 0 | 0.2 |
| A (n = 44) | IM | 15 | 15 | 15 | 0 | 0.5 |
| B (n = 43) | IM | 15 | 30 | 15 | 0 | 0.5 |
| C (n = 45) | IM | 15 | 15 | 15 | 25 | 0.5 |
| D (n = 46) | IM | 15 | 30 | 15 | 25 | 0.5 |
| E (n = 46) | IM | 15 | 15 | 15 | 50 | 0.5 |
| F (n = 43) | IM | 15 | 30 | 15 | 50 | 0.5 |
| G (n = 47) | IM | 15 | 15 | 15 | 100 | 0.5 |
| H (n = 43) | IM | 15 | 30 | 15 | 100 | 0.5 |
ID, intradermal; IM, intramuscular. This paper focuses on groups ID1 and ID2 (low-dose non-adjuvanted ID); groups A and B (full-dose non-adjuvanted IM); and groups G and H (full-dose MF59-adjuvanted IM).
Results
Study participants were randomly assigned to 1 of 10 different vaccination groups (Table 1 and Methods section). A total of 450 healthy volunteers ≥65 y of age were enrolled in the study. This paper focuses on the comparisons of the following formulations: low-dose non-adjuvanted ID (groups ID1 and ID2); full-dose non-adjuvanted IM (groups A and B); and full-dose MF59-adjuvanted IM (groups G and H) (n = 270). All enrolled subjects completed the study on Day 22, except for one subject in group H who withdrew consent after day 1. A total of 91−98% of subjects were included in the Per Protocol Set (PPS) analyses (groups ID1 [43/47] and ID2 [43/46]: low-dose non-adjuvanted ID; groups A [43/44] and B [41/43]: full-dose non-adjuvanted IM; groups G [46/47] and H [42/43]: full-dose MF59-adjuvanted IM formulations). Three subjects did not meet the entry criteria, one subject received the wrong vaccine, and 8 subjects had previously received an excluded concomitant medication. The baseline demographics of the study population are summarized in Table 2. Vaccination groups were similar with respect to age, sex, weight, height, and race. Across groups, 40–56% of subjects were male with a mean age of 69 y, 70−76% of participants had previously received influenza vaccine, and 98−100% of the participants were Caucasian.
Table 2. Study population demographics.
| Group ID1 (n = 47) |
Group ID2 (n = 46) |
Group A (n = 44) |
Group B (n = 43) |
Group G (n = 47) |
Group H (n = 43) |
|
|---|---|---|---|---|---|---|
| A/H3N2 antigen per dose (µg) | 6 | 12 | 15 | 30 | 15 | 30 |
| MF59 per dose (%) | 0 | 0 | 0 | 0 | 100 | 100 |
| Age (years, SD) | 68.3 ± 3.5 | 69.6 ± 5.1 | 69.2 ± 3.6 | 69.2 ± 4.0 | 68.5 ± 3.1 | 69.0 ± 3.5 |
| Male (%) | 49 | 48 | 40 | 56 | 42 | 46 |
| Weight (kg, SD) | 78.6 ± 15.5 | 77.1 ± 10.5 | 74.8 ± 13.5 | 77.1 ± 14.7 | 76.6 ± 14.3 | 73.0 ± 13.3 |
| Height (cm, SD) | 168.4 ± 9.0 | 167.7 ± 8.2 | 167.0 ± 8.0 | 167.0 ± 9.0 | 167.0 ± 8.0 | 165.0 ± 10.0 |
| Previously vaccinated (%) | 70 | 74 | 74 | 73 | 76 | 76 |
| Caucasian (%) | 100 | 100 | 100 | 98 | 100 | 100 |
| Asian (%) | 0 | 0 | 0 | 2 | 0 | 0 |
SD, standard deviation. The demographic data for IM subject groups C, D, E, and F are published elsewhere.36
Immunogenicity
No significant differences in geometric mean antibody titers (GMTs) against A/H3N2 were observed between the 6 µg A/H3N2 and 12 µg A/H3N2 formulations of ID vaccine at days 8 (P = 0.62) and 22 (P = 0.51). Also, different quantities of A/H3N2 antigen in the ID formulations had no impact on antibody responses against A/H1N1 (day 8, P = 0.36; day 22, P = 0.91) and B (day 8, P = 0.99; day 22, P = 0.44) strains. Likewise, there were no significant differences in A/H3N2-specific antibody responses between the 15 µg A/H3N2 and 30 µg A/H3N2 IM non-adjuvanted and fully-adjuvanted formulations (day 8, P = 0.14; day 22, P = 0.16). Also, different quantities of A/H3N2 antigen in the IM formulations had no impact on antibody responses against A/H1N1 (day 8, P = 0.14; day 22, P = 0.31) and B (day 8, P = 0.35; day 22, P = 0.54) strains. Therefore, for analyses of antibody responses to A/H1N1 and B antigens, data were pooled from groups A and B (full-dose non-adjuvanted IM), groups G and H (full-dose MF59-adjuvanted IM) and groups ID1 and ID2 (low-dose non-adjuvanted ID), unless otherwise indicated. Individual study vaccine groups were compared for H3N2 antigen. Antibody responses (GMTs) to vaccine antigen strains A/Brisbane/59/2007 (H1N1), A/Uruguay 2007 (H3N2), and B/Florida/4/2006 assessed by HI assay are shown in Table 3.
Table 3. Geometric mean titers (GMTs) and geometric mean ratios (GMRs) at baseline (day 1), 1 wk (day 8), and 3 wk (day 22) after vaccination (95% CI).
| GMT | GMR | ||||
|---|---|---|---|---|---|
| A/H3N2 |
Group ID1 (6 µg H3N2) (0% MF59) |
Group A (15 µg H3N2) (0% MF59) |
Group G (15 µg H3N2) (100% MF59) |
Groups A: ID1 |
Groups G: ID1 |
| Day 1 | 14 (9.9-21) n = 43 |
17 (12-25) n = 43 |
18 (13–26) n = 46 |
1.2 (0.7–2.0) | 1.3 (0.8–2.1) |
| Day 8 | 84 (56–128) n = 43 |
81 (54–123) n = 43 |
94 (63–141) n = 46 |
1.0 (0.5–1.7) | 1.1 (0.6–2.0) |
| Day 22 | 236 (157–353) n = 43 |
158 (106–237) n = 43 |
252 (169–374) n = 45 |
0.7 (0.4–1.2) | 1.1 (0.6–1.9) |
| A/H3N2 |
Group ID2 (12 µg H3N2) (0% MF59) |
Group B (30 µg H3N2) (0% MF59) |
Group H (30 µg H3N2) (100% MF59) |
Groups B: ID2 |
Groups H: ID2 |
| Day 1 | 15 (10–21) n = 43 |
17 (12–26) n = 41 |
16 (11–24) n = 42 |
1.2 (0.7–2.1) | 1.1 (0.7–1.9) |
| Day 8 | 73 (48–110) n = 43 |
48 (31–74) n = 41 |
158 (103–242) n = 41 |
0.7 (0.4–1.2) | 2.2 (1.2–3.9)* |
| Day 22 | 195 (129–293) n = 42 |
122 (80–184) n = 41 |
333 (220–503) n = 41 |
0.6 (0.4–1.1) | 1.7 (1.0–3.1) |
| A/H1N1 |
Groups ID1+ID2 (low-dose) (0% MF59) |
Groups A+B (full-dose) (0% MF59) |
Groups G+H (full-dose) (100% MF59) |
Groups A+B: ID1+ID2 |
Groups G+H: ID1+ID2 |
| Day 1 | 20 (15–25) n = 86 |
23 (18–30) n = 84 |
21 (16–27) n = 88 |
1.2 (0.8–1.7) | 1.1 (0.7–1.5) |
| Day 8 | 56 (44–72) n = 86 |
46 (36–60) n = 84 |
99 (77–126) n = 87 |
0.8 (0.6–1.2) | 1.8 (1.2–2.5)** |
| Day 22 | 96 (74–123) n = 85 |
65 (50–83) n = 84 |
152 (118–196) n = 86 |
0.7 (0.5–1.0)* | 1.6 (1.1–2.3)* |
| B Strain |
Groups ID1+ID2 (low-dose) (0% MF59) |
Groups A+B (full-dose) (0% MF59) |
Groups G+H (full-dose) (100% MF59) |
Groups A+B: ID1+ID2 |
Groups G+H: ID1+ID2 |
| Day 1 | 12 (10–14) n = 86 |
12 (10–14) n = 84 |
11 (9.4–13) n = 88 |
1.0 (0.8–1.3) | 0.9 (0.7–1.2) |
| Day 8 | 22 (18–27) n = 86 |
20 (16–24) n = 84 |
33 (27–39) n = 87 |
0.9 (0.7–1.2) | 1.5 (1.1–1.9)** |
| Day 22 | 34 (28–43) n = 85 |
29 (23–36) n = 84 |
47 (38–58) n = 86 |
0.8 (0.6–1.1) | 1.4 (1.0–1.8)* |
Antibody titers at day 8 and 22 are baseline adjusted. Asterisks indicate significant differences between groups: *P < 0.05; **P < 0.01.
Full-dose MF59-adjuvanted IM vaccine vs. low-dose non-adjuvanted ID vaccine
Both non-adjuvanted ID vaccines contained less antigen per dose than the IM formulations (Table 1). At day 8 post-vaccination, the pooled full-dose MF59-adjuvanted IM groups (groups G + H) demonstrated significantly higher GMTs compared with the pooled low dose non-adjuvanted ID groups (groups ID1 + ID2) against A/H1N1 and B strains (P < 0.01). GMTs against the A/H3N2 strain were significantly (P < 0.05) higher in the full-dose MF59-adjuvanted IM group compared with the low dose non-adjuvanted ID group only when the dose of A/H3N2 was doubled (group H vs. group ID2). At day 22 post-vaccination, GMTs were significantly higher in the full-dose MF59-adjuvanted IM groups compared with the low-dose non-adjuvanted ID groups against A/H1N1 and B strains (P < 0.05), but not against A/H3N2 (Table 3).
Full-dose non-adjuvanted IM vaccine vs. low-dose non-adjuvanted ID vaccine:
At day 22 post-vaccination GMTs were significantly higher in the pooled low-dose non-adjuvanted ID groups (groups ID1 + ID2) compared with the pooled full-dose non-adjuvanted IM groups (groups A + B) against A/H1N1 (P < 0.05). All other comparisons across strains between low-dose non-adjuvanted ID and full-dose non-adjuvanted IM vaccine groups at days 8 and 22 were not significant (Table 3).
Evaluation of geometric mean ratios (GMRs), seroconversion rates, and rates of subjects with HI titer ≥ 1:40 (hereafter referred to as “seroprotection” for brevity) for low-dose non-adjuvanted ID, full-dose non-adjuvanted IM, and full-dose MF59-adjuvanted IM groups against the 3 vaccine antigen strains according to licensure criteria established by the European Committee for Medicinal Products for Human Use (CHMP) are shown in Table 4. At day 8 post-vaccination at least one of the three licensure criteria was met against all three strains for the full-dose MF59-adjuvanted IM groups. The full-dose non-adjuvanted IM groups and the low-dose non-adjuvanted ID groups met at least one of three licensure criteria against A/H3N2 and A/H1N1 strains, but not against B strain. At day 22 post-vaccination, at least 1 criterion out of the 3 was met by all groups against all 3 strains; however all 3 criteria were met against all 3 vaccine strains by the low-dose non-adjuvanted ID and the full-dose MF59-adjuvanted IM groups only.
Table 4. Immunogenicity analyses against A/H3N2, A/H1N1, and B strain vaccine antigens according to the European (CHMP) licensure criteria for influenza vaccines for older adults.
| A/H3N2 | Group ID1 (6 µg H3N2) (0% MF59) |
Group A (15 µg H3N2) (0% MF59) |
Group G (15 µg H3N2) (100% MF59) |
|||
|---|---|---|---|---|---|---|
| Day 8 (n = 43) |
Day 22 (n = 43) |
Day 8 (n = 43) |
Day 22 (n = 43) |
Day 8 (n = 46) |
Day 22 (n = 46) |
|
| SC (%) | 47 | 77 | 56 | 70 | 59 | 87 |
| SP (%) | 67 | 88 | 74 | 93 | 74 | 96 |
| GMR | 5.7 | 16 | 4.6 | 9.0 | 5.7 | 15 |
| A/H3N2 |
Group ID2 (12 µg H3N2) (0% MF59) |
Group B (30 µg H3N2) (0% MF59) |
Group H (30 µg H3N2) (100% MF59) |
|||
| Day 8 (n = 43) |
Day 22 (n = 43) |
Day 8 (n = 41) |
Day 22 (n = 41) |
Day 8 (n = 42) |
Day 22 (n = 42) |
|
| SC (%) | 51 | 71 | 32 | 63 | 66 | 95 |
| SP (%) | 74 | 90 | 66 | 80 | 90 | 100 |
| GMR | 4.9 | 13 | 2.9 | 7.3 | 8.9 | 19 |
| A/H1N1 |
Groups ID1+ID2 (low-dose) (0% MF59) |
Groups A+B (full-dose) (0% MF59) |
Groups G+H (full-dose) (100% MF59) |
|||
| Day 8 (n = 86) |
Day 22 (n = 86) |
Day 8 (n = 84) |
Day 22 (n = 84) |
Day 8 (n = 87) |
Day 22 (n = 87) |
|
| SC (%) | 35 | 44 | 20 | 30 | 51 | 62 |
| SP (%) | 69 | 84 | 68 | 77 | 91 | 95 |
| GMR | 3.1 | 5.1 | 2.0 | 2.8 | 4.5 | 7.1 |
| B Strain |
Groups ID1+ID2 (low-dose) (0% MF59) |
Groups A+B (full-dose) (0% MF59) |
Groups G+H (full-dose) (100% MF59) |
|||
| Day 8 (n = 86) |
Day 22 (n = 86) |
Day 8 (n = 84) |
Day 22 (n = 84) |
Day 8 (n = 87) |
Day 22 (n = 87) |
|
| SC (%) | 15 | 36 | 15 | 30 | 36 | 49 |
| SP (%) | 36 | 61 | 32 | 49 | 51 | 66 |
| GMR | 1.9 | 3.0 | 1.7 | 2.5 | 2.8 | 4.1 |
SC, seroconversion (>30% with HI titer ≥ 40); GMR, geometric mean ratio (>2.0); SP, seroprotection (>60% with HI titer ≥ 40). Day 8 GMR values describe ratio of day 8 to day 1 HI GMTs. Day 22 GMR values describe ratio of day 22 to day 1 HI GMTs. Bold text indicates that CHMP licensure criterion was met.
Safety and tolerability
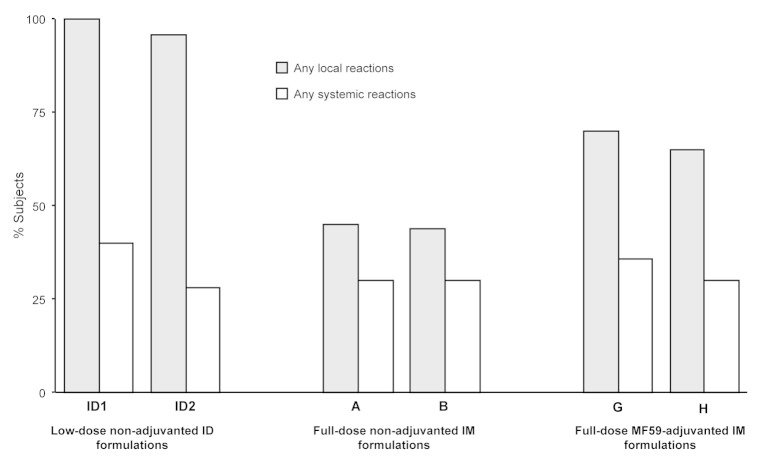
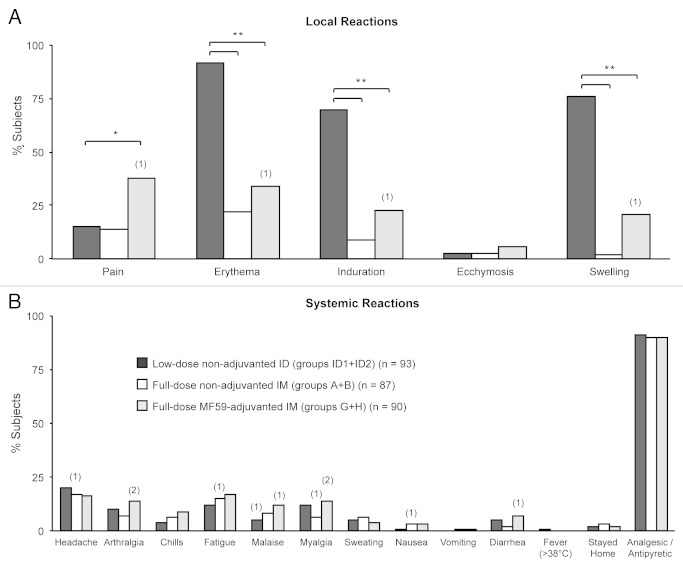
All 270 subjects from the low-dose non-adjuvanted ID, full-dose non-adjuvanted IM, and full-dose MF59-adjuvanted IM groups were included in the safety analyses. There was no detectable trend in altered frequencies of either local or systemic reactions with increasing dose of A/H3N2 antigen in either IM or ID vaccine groups. At least one local reaction was reported by 96−100% of subjects in the low-dose non-adjuvanted ID groups, 44−45% of subjects in the full-dose non-adjuvanted IM groups, and 65−70% of subjects in the full-dose MF59-adjuvanted IM groups. At least one systemic reaction was reported by 28−40% of subjects in the low-dose non-adjuvanted ID groups, 30% of subjects in the full-dose non-adjuvanted IM groups, and 30−36% of subjects in the full-dose MF59-adjuvanted IM groups (Fig. 1). For the analyses of the safety results, pooled data combining both A/H3N2 doses are shown for all groups. Erythema, induration, swelling, and pain at the site of injection were the most commonly reported solicited local reactions across the vaccine groups. Relative risk (RR) assessment by Poisson regression analyses showed an increased frequency of erythema, induration, and swelling for the low-dose non-adjuvanted ID groups compared with the full-dose non-adjuvanted and full-dose MF59-adjuvanted IM groups (RR erythema = 0.24 and 0.37; RR induration = 0.13 and 0.33; RR swelling = 0.03 and 0.28 for comparisons of low-dose non-adjuvanted ID groups vs the full-dose non-adjuvanted; and vs full-dose MF59-adjuvanted IM groups, respectively; all RRs = P < 0.01). Pain at the injection site was more frequently observed in the full-dose MF59-adjuvanted IM groups compared with the low-dose non-adjuvanted ID groups (RR, 2.58; P = 0.003; Fig. 2A). Visual analog scale (VAS) pain scores in all vaccine groups were low on the 100-point scale. The mean maximal VAS pain scores were 4.45, 3.44, and 11.00 in the low-dose non-adjuvanted ID, full-dose non-adjuvanted IM, and full-dose MF59-adjuvanted IM groups, respectively. The mean and maximum pain scores were comparable in the low-dose non-adjuvanted ID groups and the full-dose non-adjuvanted IM groups (P = 0.47), but were significantly higher in the full-dose MF59-adjuvanted IM group subjects compared with those in the low-dose non-adjuvanted ID groups (P < 0.001).
Figure 1. Percentages of subjects experiencing any solicited local and systemic adverse reactions within 1 wk (days 1−7) of vaccination.
Figure 2. Percentages of subjects experiencing solicited local (A) and systemic (B) adverse reactions following vaccination. Bars show percentages of subjects experiencing reactions after vaccination in the low-dose non-adjuvanted ID groups (groups ID1+ID2) (black bars); in the full-dose non-adjuvanted IM groups (groups A+B) (open bars); and in the full-dose MF59-adjuvanted IM groups (groups G+H) (gray bars). Numbers in parentheses above bars indicate numbers of subjects experiencing severe reactions. Asterisks indicate significant differences between groups: ∗ 2 sided P < 0.01; ∗∗ 2 sided P < 0.0001.
Few severe solicited local reactions occurred (Fig. 2A); one subject in the full-dose MF59-adjuvanted IM group experienced severe erythema, induration, and swelling, and one subject, also in the full-dose MF59-adjuvanted IM group reported severe pain. The frequencies of subjects experiencing solicited systemic reactions were similar for ID and IM vaccination groups, and Poisson regression analyses revealed no differences between the groups (Fig. 2B). The most commonly reported systemic reactions were headache and fatigue. Few severe systemic reactions were reported (Fig. 2B); these included myalgia (n = 3), arthralgia (n = 2), malaise (n = 2), headache (n = 1), nausea (n = 1), fatigue (n = 1), and diarrhea (n = 1). Only one subject, in the low-dose non-adjuvanted ID groups, reported fever (≥38 °C) following vaccination; there were no reports of severe fever (≥40 °C). Only 2−3% of subjects stayed at home, and analgesic / antipyretic medication was required by 90−91% of subjects across all vaccine groups (Fig. 2B).
Unsolicited adverse events (AEs) occurred more frequently in the low-dose non-adjuvanted ID groups (59−64%, of which 52−53% were at least possibly vaccine-related) than in the full-dose non-adjuvanted IM groups (16−25%, of which 7% were possibly vaccine-related) and full-dose MF59-adjuvanted IM groups (19−26%, of which 5−11% were possibly vaccine-related; Table 5). The most commonly reported AEs in the low-dose non-adjuvanted ID groups by preferred term (PT) were injection site erythema (47−52%, of which 45−52% were possibly vaccine-related), injection site pruritus (2−6%, all possibly vaccine related), and myalgia (6%, all possibly vaccine-related). The most commonly reported AEs by PT in the full-dose non-adjuvanted and full-dose MF59-adjuvanted IM groups were fatigue (2−7% across groups, ≤5% possibly vaccine-related), and upper respiratory tract infections (4−7% across groups, with no cases considered to be vaccine-related). Only one serious adverse event (SAE) occurred during the study, which was not considered to be vaccine-related (venous thrombosis in one subject from group A, onset 6 d after vaccination and duration of 14 d). No study withdrawals or deaths due to AEs occurred in any low-dose non-adjuvanted ID, full-dose non-adjuvanted IM, and full-dose MF59-adjuvanted IM vaccination groups.
Table 5. Percentages of subjects experiencing unsolicited adverse events within 3 wk (day 1 to day 22) of vaccination.
| Group ID1 (n = 47) |
Group ID2 (n = 46) |
Group A (n = 44) |
Group B (n = 41) |
Group G (n = 47) |
Group H (n = 43) |
|
|---|---|---|---|---|---|---|
| A/H3N2 antigen per dose (µg) | 6 | 12 | 15 | 30 | 15 | 30 |
| MF59 per dose (%) | 0 | 0 | 0 | 0 | 100 | 100 |
| Any AEs (%) | 64 | 59 | 25 | 16 | 26 | 19 |
| Vaccine-related AEs (%) | 53 | 52 | 7 | 7 | 11 | 5 |
| SAEs (%) | 0 | 0 | 2 | 0 | 0 | 0 |
| AEs leading to withdrawal (%) | 0 | 0 | 0 | 0 | 0 | 0 |
AEs, adverse events; SAEs, serious adverse events.
Discussion
In the elderly population, seasonal influenza vaccines with enhanced immunogenicity are needed to address the challenge of immunosenescence. Strategies to optimize responses to vaccination in older adults include the use of adjuvants,8,36 higher antigen doses,11,12 ID delivery,14,25,33,34,37 and addition of a second B strain (quadrivalent influenza vaccine, QIV). Here we report the findings of a study which compared the immunogenicity and safety profiles of low-dose non-adjuvanted ID TIV (containing either 6 µg or 12 µg of A/H3N2 antigen), full-dose non-adjuvanted IM TIV, and full-dose MF59-adjuvanted IM TIV (both IM groups containing either 15 µg or 30 µg of A/H3N2 antigen).
Doubling the A/H3N2 antigen content of vaccine (IM, 15 µg to 30 µg; ID, 6 µg to 12 µg) did not result in increased A/H3N2-specific antibody responses whether formulations were non-adjuvanted or fully MF59-adjuvanted. ID vaccine delivery resulted in significantly higher A/H1N1-specific antibody responses 3 wk after vaccination compared with conventional, non-adjuvanted IM groups. IM administration of full-dose MF59-adjuvanted vaccine resulted in significantly increased A/H1N1- and B-strain-specific antibody responses compared with ID delivery of low-dose non-adjuvanted formulations 1 and 3 wk after vaccination; full-dose MF59-adjuvanted IM responses against A/H3N2 were significantly higher than low-dose non-adjuvanted ID group responses only 1 wk after vaccination. Secondary/memory immune responses primed by previous exposure to antigens via either natural infection or vaccination are heightened and accelerated by MF59 adjuvant, as demonstrated by previous human data from trials of pandemic A/H5N138 and A/H1N1 (2009) vaccines.39 Both low-dose non-adjuvanted ID and full-dose MF59-adjuvanted IM formulations met the CHMP licensure criteria for influenza vaccines against all three strains as early as 1 wk after vaccination. These data emphasize the potential advantages of MF59 adjuvant and ID administration over conventional non-adjuvanted vaccines in rapidly inducing seroprotective antibody responses, which could be particularly important in cases of late vaccination during the influenza season.
As shown in Table 3, in a few cases the P values, which demonstrate significant differences between vaccination group GMRs appear to contradict the overlapping CIs. This is due to a well-known statistical phenomenon.40 Rejection of the null hypothesis by the method of examining overlap implies rejection by the standard method (i.e ANCOVA in this study), whereas failure to reject by the method of examining overlap does not imply failure to reject by the standard method. As a consequence, the method of examining overlap is more conservative (i.e., rejects the null hypothesis less often) than the standard method when the null hypothesis is true, and it mistakenly fails to reject the null hypothesis more frequently than does the standard method when the null hypothesis is false.
In agreement with the data presented here, previous results in older adult populations have demonstrated that ID vaccination with 6 µg or 15 µg of HA antigen per strain elicited equivalent25 or superior14,34,41 antibody responses, respectively, compared with IM administration of conventional, non-adjuvanted vaccines, despite the use of 2.5−5.0-fold less antigen per dose. Microneedles for ID injections offer the advantage of standardizing injection depth, and, with their minute length, are less intimidating for the vaccine recipient. A recent study evaluated the immunogenicity of ID administered, non-adjuvanted vaccine (Intanza®, Sanofi Pasteur MSD SNC) compared with IM administered, MF59-adjuvanted vaccine (Fluad) in older subjects, however, the overall non-inferiority objective could not be demonstrated because the ID vaccine failed the non-inferiority comparison for one strain (A/H3N2);42 non-inferiority was reported only in a post-hoc analysis for all three strains after correction for baseline antibody titers.43 In the aforementioned study by Van Damme et al.,42 15 µg HA per strain was used for ID vaccine formulations, an amount higher than the 6 µg or 12 µg of A/H3N2 antigen used in the present study; it is clear that the results obtained in these 2 studies cannot be compared due to the differences in antigen quantity and strain used for the formulation of the ID vaccines, and because of the different administration devices used. Therefore, further studies comparing the immunogenicity and safety profiles resulting from ID and IM administration of similar doses of seasonal influenza vaccines are warranted.
Importantly, both the use of adjuvanted vaccines and ID delivery allow for dose/antigen sparing. Minimal use of antigenic material is essential to ensure the widest possible availability of both seasonal and pandemic influenza vaccines when supplies are limited by high-demand and restraints in global manufacturing capacity. Consistent with previous studies,24,26,29,32,37,42 ID vaccination was associated with an increased frequency of mild to moderate local reactions which accompanied the inflammatory process taking place in the skin. For example, injection site erythema was experienced by 92% of subjects in the ID groups compared with 22% and 34% in the IM non-adjuvanted and IM MF59-adjuvanted groups, respectively. The percentages of subjects reporting pain at the injection site, and VAS pain scores, were higher among full-dose MF59-adjuvanted IM groups compared with low-dose non-adjuvanted ID groups; although the VAS pain scores in all groups were very low on the 100-scale. Frequencies of systemic reactions were similar in both ID and IM groups, and fever (≥38 °C) occurred in only one subject. One SAE was reported, which was not vaccine-related. No premature study withdrawals or deaths due to AEs occurred in any vaccination group.
In conclusion, IM administration of full-dose MF59-adjuvanted vaccine and ID delivery of low-dose non-adjuvanted vaccine rapidly induced antibody titers sufficient to meet the European licensure criteria. An acceptable safety profile was observed in all vaccination groups, although MF59-adjuvanted formulations increased pain at the site of injection, and intradermal formulations increased unsolicited adverse events, erythema, induration, and swelling at the injection site. Overall, these data demonstrate that ID vaccine delivery and addition of MF59 adjuvant to IM vaccine delivery offer promising alternatives to IM non-adjuvanted influenza vaccine in older adults.
Methods
Study design and participants
This multicenter, randomized, observer-blind study was conducted across 4 sites in Germany, one site in Poland, and one site in Belgium between October 2008 and February 2009. The study was undertaken according to Good Clinical Practice guidelines and the Declaration of Helsinki. Ethics review committees of participating centers approved the protocols, and written informed consent was obtained from all participants prior to enrolment. Healthy volunteers ≥65 y of age who were mentally competent and in general good health as determined by medical history, a physical examination, and the clinical judgment of the investigators were enrolled in the study. The main exclusion criteria were: immunization with any influenza vaccine within 6 mo before study enrolment; immunization with any experimental influenza vaccine containing adjuvant within 2 y before study enrolment; any serious disease; hypersensitivity to vaccine components; an impaired or altered immune system; known or suspected history of drug or alcohol abuse; history of bleeding diathesis; conditions associated with prolonged bleeding time, or current use of anticoagulation therapy; laboratory-confirmed influenza disease within 12 mo before study enrolment; receipt of another vaccine or investigational agent within 30 d before study enrolment; infection requiring systemic antibiotic or antiviral therapy within 14 d before study enrolment; and fever (oral temperature ≥ 38 °C) within 7 d before study enrolment.
Treatments and procedures
Participants were randomly assigned to 1 of 10 vaccination groups (see below and Table 1). Subjects in IM groups A to H received one 0.5 mL dose administered into the deltoid muscle, preferably of the non-dominant arm. Subjects in groups ID1 and ID2 received one 0.2 mL dose administered intradermally using the MicronJet delivery system from NanoPass Technologies. Immunogenicity assessments were performed on day 1 (baseline/pre-vaccination), day 8 (1 wk post-vaccination), and day 22 (3 wk post-vaccination). Blood samples of 15 mL were obtained by venipuncture at each time point. Subsets of participants in groups A to H were randomly selected for additional blood draws to assess cell-mediated immune responses. Serum was isolated by centrifugation and stored at −18 °C or below until shipped to the Novartis Vaccines Clinical Serology for immunogenicity analysis. Data on comparisons across IM formulations and on cell-mediated immune responses have been published elsewhere.36
Vaccines
The trivalent influenza vaccines included in this study were formulated for either ID (groups ID1 and ID2) or IM (groups A through H) administration (Table 1). ID vaccines were not adjuvanted and contained 6 µg of A/H1N1 and B strains and either 6 µg or 12 µg of A/H3N2 strain HA antigens. IM vaccines contained 15 µg of A/H1N1 and B strains, and either 15 µg or 30 µg A/H3N2 HA antigens. IM groups were further differentiated from each other based on the quantity of MF59 (0%, 25%, 50%, or 100% of the standard dose contained in the licensed seasonal TIV, Fluad®). The IM group A (non-adjuvanted, 15 µg antigen per strain) had the same antigen content as the licensed seasonal TIV, Agrippal/Agriflu® (Novartis Vaccines). The IM group G (100% MF59-adjuvanted, 15 µg antigen per strain) had the same antigen content as the licensed seasonal TIV, Fluad® (Novartis Vaccines). HA antigens were derived from influenza strains A/Brisbane/59/2007 (H1N1), A/Uruguay/716/2007 (H3N2), and B/Florida/4/2006, as recommended by the World Health Organization for the 2008–09 influenza season in the northern hemisphere. A standard / full dose (100%) of MF59 adjuvant consists of 9.75 mg of squalene, 1.18 mg polysorbate 80, 1.18 mg sorbitan trioleate, 0.66 mg sodium citrate dehydrate, and 0.04 mg citric acid monohydrate. Vaccine formulations containing less than the standard / full dose of MF59 were prepared by diluting full MF59 dose vaccine with non-adjuvanted vaccine. The MicronJet microneedle ID administration device consists of an array of 4 microneedles made of silicon crystal, each needle 0.45 mm in length.32 The needles are bonded to the tip of a plastic adaptor, which can be mounted on any standard syringe; therefore, the device is used like any other needle, except that the microneedles can only be used for intradermal delivery due to their limited length.
Immunogenicity assessment
Serum samples collected at baseline (day 1) and at days 8 and 22 post-vaccination were assessed for antibody content by HI assay, according to standard methods.44 HI antibody responses on days 1, 8, and 22 were expressed as GMTs and GMRs of post-vaccination to pre-vaccination titers (day 8: day 1 titers, and day 22: day 1 titers). The European CHMP licensure criteria defined seroconversion in an individual vaccinee as a pre-vaccination HI titer < 10 (seronegative at baseline) to a post-vaccination HI titer ≥ 40, or a ≥4-fold increase in HI titer for subjects with a pre-vaccination titer ≥ 10 (seropositive at baseline).
Safety and tolerability assessment
Safety assessments included reports of solicited local and systemic adverse reactions and unsolicited AEs. The frequency and severity of solicited local and systemic reactions were recorded on diary cards for 7 consecutive days following vaccination (day 1 to day 8). Reactions continuing beyond day 8 were recorded as unsolicited AEs. All unsolicited reports of AEs and use of concomitant medications were collected throughout the entire study period (day 1 to 22). Any SAEs were reported to the study sponsor within 24 h of onset. All subjects were observed for at least 30 min after vaccination to monitor for immediate adverse events. Solicited local reactions were pain at the site of injection, erythema, induration, swelling, and ecchymosis. Pain at the injection site was also assessed by a 100 mm visual analog scale, ranging from 0 (worst imaginable state of health) to 100 (best imaginable state of health). Pain scores included the average mean score over 7 consecutive days and the maximum score. Solicited systemic reactions were headache, chills, fatigue, arthralgia, malaise, myalgia, nausea, sweating, vomiting, diarrhea, and fever. The use of analgesic or antipyretic medication, and events causing subjects to remain at home were also monitored as indicators of reactogenicity. The severity of local and systemic reactions was graded according to US. Center for Biologics Evaluation and Research (CBER) guidelines.44 The severity of unsolicited AEs was categorized as mild, moderate, or severe, if they resulted in no limitation of, some limitation of, or inability to perform normal daily activities, respectively. AEs were defined as serious AEs (SAEs) if they were fatal or life-threatening; required or prolonged hospitalization; resulted in permanent disability; led to congenital abnormality; required intervention to prevent permanent impairment or damage; or were a significant medical event that may have jeopardized the subject. Assessments of the causal relationship of AEs to vaccination were classified by the investigator as either not related, possibly related, or probably related.
Statistical analyses
Immunogenicity analyses were run on the PPS, which included all enrolled subjects who received vaccine, provided evaluable serum samples at relevant time points, and experienced no major protocol deviations. Immunogenicity endpoints were analyzed according to the CHMP licensure criteria for elderly subjects.44 The following criteria applied: the proportion of subjects achieving seroconversion (HI titer ≥ 40) or significantly (≥4-fold) increased antibody titers should be >30% (seroconversion criterion); the proportion of subjects achieving an HI titer ≥ 1:40 should be >60% (seroprotection criterion); GMRs should be >2.0 (GMR criterion). Adequate immunogenicity was confirmed when at least one of the three licensure criteria was met. Assuming: log-normal distributed antibody titers; a common standard deviation of 0.7 for log10 titers; and a two-sided, type I error of 5%—the study was 80% powered to demonstrate significance with a sample size of 42 subjects per group, if the difference between groups was ≥ factor 3.0. Taking advantage of group pooling, a sample size of 88 subjects per group led to a minimum relevant difference of factor 2.0. The results of this study showed that increasing the A/H3N2 antigen content of vaccines did not affect antibody responses against A/H1N1 and B strains; therefore, the following group data was pooled for analyses of A/H1N1 and B strain responses: intradermal groups ID1 + ID2; IM non-adjuvanted groups A (15 µg A/H3N2) + B (30 µg A/H3N2); and IM full (100%) MF59 dose groups G (15 µg A/H3N2) + H (30 µg A/H3N2). This report focuses on data from groups ID1 and ID2, A and B, and G and H for the assessment of differences in immunogenicity and safety following low-dose non-adjuvanted ID, full-dose non-adjuvanted IM, and full-dose fully-adjuvanted IM vaccinations, respectively. Data for comparisons across the IM groups (A to H) are published in a separate paper.36 Group differences in terms of ratios of GMTs were estimated along with two-tailed 95% confidence intervals fitting an analysis of covariance model with vaccine group as qualitative factor and baseline titer as quantitative covariate; significance was declared if the two-sided P value was <0.05. Safety analyses included data from all vaccinated subjects where at least one safety observation was recorded. Safety and tolerability data are summarized by vaccine group and percentages of subjects experiencing a specific event. Poisson regression analyses were extended to local and systemic reactions, and low-dose non-adjuvanted ID groups were compared with full-dose non-adjuvanted IM and full-dose MF59-adjuvanted IM groups in terms of risk ratios including two-sided 95% confidence intervals and two-sided P values. Statistical evaluation was performed using SAS® version 9.1 software (SAS Institute).
Disclosure of Potential Conflicts of Interest
U.N., K.L., F.C., G.G., N.G., and G.D.G. are permanent employees of Novartis Vaccines, Inc. G.L.R. and F.C. are permanent employees of the Centre for Vaccinology, CEVAC. Y.L. is a permanent employee of NanoPass Technologies, Ltd.
Funding
This study was supported by funds provided by Novartis Vaccines, Inc.
Author Contributions
All authors participated in the conception, design, and implementation of the trial. All authors were involved in the interpretation of analyzed data and the decision to submit for publication.
Acknowledgments
Glossary
Abbreviations:
- AE
adverse event
- TIV
trivalent influenza vaccine
- aTIV
adjuvanted trivalent influenza vaccine
- GMR
geometric mean ratio
- GMT
geometric mean titer
- HA
hemagglutinin
- HI
hemagglutination inhibition
- ID
intradermal
- IM
intramuscular
- PPS
per protocol set
- SAE
serious adverse event
- VAS
visual analogue scale
- CHMP
Committee for Medicinal Products for Human Use
- CBER
Center for Biologics Evaluation and Research
References
- 1.Gasparini R, Amicizia D, Lai PL, Panatto D. Clinical and socioeconomic impact of seasonal and pandemic influenza in adults and the elderly. Hum Vaccin Immunother. 2012;8:21–8. doi: 10.4161/hv.8.1.17622. [DOI] [PubMed] [Google Scholar]
- 2.Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, Fukuda K. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289:179–86. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Media center. Seasonal influenza fact sheet number 211. 2009: http://www.who.int/mediacentre/factsheets/fs211/en/.
- 4.Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine. 2006;24:1159–69. doi: 10.1016/j.vaccine.2005.08.105. [DOI] [PubMed] [Google Scholar]
- 5.Cao W, Kim JH, Chirkova T, Reber AJ, Biber R, Shay DK, Sambhara S. Improving immunogenicity and effectiveness of influenza vaccine in older adults. Expert Rev Vaccines. 2011;10:1529–37. doi: 10.1586/erv.11.137. [DOI] [PubMed] [Google Scholar]
- 6.Lambert ND, Ovsyannikova IG, Pankratz VS, Jacobson RM, Poland GA. Understanding the immune response to seasonal influenza vaccination in older adults: a systems biology approach. Expert Rev Vaccines. 2012;11:985–94. doi: 10.1586/erv.12.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McElhaney JE. The unmet need in the elderly: designing new influenza vaccines for older adults. Vaccine. 2005;23(Suppl 1):S10–25. doi: 10.1016/j.vaccine.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 8.Banzhoff A, Nacci P, Podda A. A new MF59-adjuvanted influenza vaccine enhances the immune response in the elderly with chronic diseases: results from an immunogenicity meta-analysis. Gerontology. 2003;49:177–84. doi: 10.1159/000069172. [DOI] [PubMed] [Google Scholar]
- 9.Parodi V, de Florentiis D, Martini M, Ansaldi F. Inactivated influenza vaccines: recent progress and implications for the elderly. Drugs Aging. 2011;28:93–106. doi: 10.2165/11586770-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 10.Sindoni D, La Fauci V, Squeri R, Cannavò G, Bacilieri S, Panatto D, Gasparini R, Amicizia D. Comparison between a conventional subunit vaccine and the MF59-adjuvanted subunit influenza vaccine in the elderly: an evaluation of the safety, tolerability and immunogenicity. J Prev Med Hyg. 2009;50:121–6. [PubMed] [Google Scholar]
- 11.Couch RB, Winokur P, Brady R, Belshe R, Chen WH, Cate TR, Sigurdardottir B, Hoeper A, Graham IL, Edelman R, et al. Safety and immunogenicity of a high dosage trivalent influenza vaccine among elderly subjects. Vaccine. 2007;25:7656–63. doi: 10.1016/j.vaccine.2007.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Falsey AR, Treanor JJ, Tornieporth N, Capellan J, Gorse GJ. Randomized, double-blind controlled phase 3 trial comparing the immunogenicity of high-dose and standard-dose influenza vaccine in adults 65 years of age and older. J Infect Dis. 2009;200:172–80. doi: 10.1086/599790. [DOI] [PubMed] [Google Scholar]
- 13.Chen WH, Cross AS, Edelman R, Sztein MB, Blackwelder WC, Pasetti MF. Antibody and Th1-type cell-mediated immune responses in elderly and young adults immunized with the standard or a high dose influenza vaccine. Vaccine. 2011;29:2865–73. doi: 10.1016/j.vaccine.2011.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holland D, Booy R, De Looze F, Eizenberg P, McDonald J, Karrasch J, McKeirnan M, Salem H, Mills G, Reid J, et al. Intradermal influenza vaccine administered using a new microinjection system produces superior immunogenicity in elderly adults: a randomized controlled trial. J Infect Dis. 2008;198:650–8. doi: 10.1086/590434. [DOI] [PubMed] [Google Scholar]
- 15.Falsey AR. New emerging technologies and the intradermal route: the novel way to immunize against influenza. Vaccine. 2010;28(Suppl 4):D24–32. doi: 10.1016/j.vaccine.2010.08.026. [DOI] [PubMed] [Google Scholar]
- 16.de Bruijn I, Meyer I, Gerez L, Nauta J, Giezeman K, Palache B. Antibody induction by virosomal, MF59-adjuvanted, or conventional influenza vaccines in the elderly. Vaccine. 2007;26:119–27. doi: 10.1016/j.vaccine.2007.10.051. [DOI] [PubMed] [Google Scholar]
- 17.O’Hagan DT, Rappuoli R, De Gregorio E, Tsai T, Del Giudice G. MF59 adjuvant: the best insurance against influenza strain diversity. Expert Rev Vaccines. 2011;10:447–62. doi: 10.1586/erv.11.23. [DOI] [PubMed] [Google Scholar]
- 18.Esposito S, D’Angelo E, Daleno C, Peia F, Scala A, Serra D, Mirra N, Galeone C, Principi N. Immunogenicity, safety and tolerability of monovalent 2009 pandemic influenza A/H1N1 MF59-adjuvanted vaccine in patients with β-thalassemia major. Vaccine. 2010;28:7825–8. doi: 10.1016/j.vaccine.2010.09.058. [DOI] [PubMed] [Google Scholar]
- 19.Frey S, Poland G, Percell S, Podda A. Comparison of the safety, tolerability, and immunogenicity of a MF59-adjuvanted influenza vaccine and a non-adjuvanted influenza vaccine in non-elderly adults. Vaccine. 2003;21:4234–7. doi: 10.1016/S0264-410X(03)00456-0. [DOI] [PubMed] [Google Scholar]
- 20.Vesikari T, Pellegrini M, Karvonen A, Groth N, Borkowski A, O’Hagan DT, Podda A. Enhanced immunogenicity of seasonal influenza vaccines in young children using MF59 adjuvant. Pediatr Infect Dis J. 2009;28:563–71. doi: 10.1097/INF.0b013e31819d6394. [DOI] [PubMed] [Google Scholar]
- 21.O’Hagan DT, Ott GS, De Gregorio E, Seubert A. The mechanism of action of MF59 - an innately attractive adjuvant formulation. Vaccine. 2012;30:4341–8. doi: 10.1016/j.vaccine.2011.09.061. [DOI] [PubMed] [Google Scholar]
- 22.Bos JD. Skin Immune System-Cutaneous immunology and clinical immunodermatology 3th editon ed: CRC Press; 2005. [Google Scholar]
- 23.Glenn GM, Kenney RT. Mass vaccination: solutions in the skin. Curr Top Microbiol Immunol. 2006;304:247–68. doi: 10.1007/3-540-36583-4_14. [DOI] [PubMed] [Google Scholar]
- 24.Auewarakul P, Kositanont U, Sornsathapornkul P, Tothong P, Kanyok R, Thongcharoen P. Antibody responses after dose-sparing intradermal influenza vaccination. Vaccine. 2007;25:659–63. doi: 10.1016/j.vaccine.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 25.Belshe RB, Newman FK, Cannon J, Duane C, Treanor J, Van Hoecke C, Howe BJ, Dubin G. Serum antibody responses after intradermal vaccination against influenza. N Engl J Med. 2004;351:2286–94. doi: 10.1056/NEJMoa043555. [DOI] [PubMed] [Google Scholar]
- 26.Belshe RB, Newman FK, Wilkins K, Graham IL, Babusis E, Ewell M, Frey SE. Comparative immunogenicity of trivalent influenza vaccine administered by intradermal or intramuscular route in healthy adults. Vaccine. 2007;25:6755–63. doi: 10.1016/j.vaccine.2007.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kenney RT, Frech SA, Muenz LR, Villar CP, Glenn GM. Dose sparing with intradermal injection of influenza vaccine. N Engl J Med. 2004;351:2295–301. doi: 10.1056/NEJMoa043540. [DOI] [PubMed] [Google Scholar]
- 28.Laurent A, Mistretta F, Bottigioli D, Dahel K, Goujon C, Nicolas JF, Hennino A, Laurent PE. Echographic measurement of skin thickness in adults by high frequency ultrasound to assess the appropriate microneedle length for intradermal delivery of vaccines. Vaccine. 2007;25:6423–30. doi: 10.1016/j.vaccine.2007.05.046. [DOI] [PubMed] [Google Scholar]
- 29.Icardi G, Orsi A, Ceravolo A, Ansaldi F. Current evidence on intradermal influenza vaccines administered by Soluvia™ licensed micro injection system. Hum Vaccin Immunother. 2012;8:67–75. doi: 10.4161/hv.8.1.18419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kis EE, Winter G, Myschik J. Devices for intradermal vaccination. Vaccine. 2012;30:523–38. doi: 10.1016/j.vaccine.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 31.Laurent PE, Bonnet S, Alchas P, Regolini P, Mikszta JA, Pettis R, Harvey NG. Evaluation of the clinical performance of a new intradermal vaccine administration technique and associated delivery system. Vaccine. 2007;25:8833–42. doi: 10.1016/j.vaccine.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 32.Van Damme P, Oosterhuis-Kafeja F, Van der Wielen M, Almagor Y, Sharon O, Levin Y. Safety and efficacy of a novel microneedle device for dose sparing intradermal influenza vaccination in healthy adults. Vaccine. 2009;27:454–9. doi: 10.1016/j.vaccine.2008.10.077. [DOI] [PubMed] [Google Scholar]
- 33.Hung IF, Levin Y, To KK. Quantitative and qualitative analysis of antibody response after dose sparing intradermal 2009 H1N1 vaccination. Vaccine. 2012;30:2707–8. doi: 10.1016/j.vaccine.2011.12.069. [DOI] [PubMed] [Google Scholar]
- 34.Hung IF, Levin Y, To KK, Chan KH, Zhang AJ, Li P, Li C, Xu T, Wong TY, Yuen KY. Dose sparing intradermal trivalent influenza (2010/2011) vaccination overcomes reduced immunogenicity of the 2009 H1N1 strain. Vaccine. 2012;30:6427–35. doi: 10.1016/j.vaccine.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 35.Leroux-Roels I, Vets E, Freese R, Seiberling M, Weber F, Salamand C, Leroux-Roels G. Seasonal influenza vaccine delivered by intradermal microinjection: A randomised controlled safety and immunogenicity trial in adults. Vaccine. 2008;26:6614–9. doi: 10.1016/j.vaccine.2008.09.078. [DOI] [PubMed] [Google Scholar]
- 36.Della Cioppa G, Nicolay U, Lindert K, Leroux-Roels G, Clement F, Castellino F, Galli G, Groth N, Del Giudice G. Superior immunogenicity of seasonal influenza vaccines containing full dose of MF59 (®) adjuvant: results from a dose-finding clinical trial in older adults. Hum Vaccin Immunother. 2012;8:216–27. doi: 10.4161/hv.18445. [DOI] [PubMed] [Google Scholar]
- 37.Chi RC, Rock MT, Neuzil KM. Immunogenicity and safety of intradermal influenza vaccination in healthy older adults. Clin Infect Dis. 2010;50:1331–8. doi: 10.1086/652144. [DOI] [PubMed] [Google Scholar]
- 38.Galli G, Hancock K, Hoschler K, DeVos J, Praus M, Bardelli M, Malzone C, Castellino F, Gentile C, McNally T, et al. Fast rise of broadly cross-reactive antibodies after boosting long-lived human memory B cells primed by an MF59 adjuvanted prepandemic vaccine. Proc Natl Acad Sci U S A. 2009;106:7962–7. doi: 10.1073/pnas.0903181106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clark TW, Pareek M, Hoschler K, Dillon H, Nicholson KG, Groth N, Stephenson I. Trial of 2009 influenza A (H1N1) monovalent MF59-adjuvanted vaccine. N Engl J Med. 2009;361:2424–35. doi: 10.1056/NEJMoa0907650. [DOI] [PubMed] [Google Scholar]
- 40.Schenker N, Gentleman NF. On judging the significance of differences by examining the overlap between confidence intervals. Am Stat. 2001;55:182–6. doi: 10.1198/000313001317097960. [DOI] [Google Scholar]
- 41.Arnou R, Icardi G, De Decker M, Ambrozaitis A, Kazek MP, Weber F, Van Damme P. Intradermal influenza vaccine for older adults: a randomized controlled multicenter phase III study. Vaccine. 2009;27:7304–12. doi: 10.1016/j.vaccine.2009.10.033. [DOI] [PubMed] [Google Scholar]
- 42.Van Damme P, Arnou R, Kafeja F, Fiquet A, Richard P, Thomas S, Meghlaoui G, Samson SI, Ledesma E. Evaluation of non-inferiority of intradermal versus adjuvanted seasonal influenza vaccine using two serological techniques: a randomised comparative study. BMC Infect Dis. 2010;10:134. doi: 10.1186/1471-2334-10-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Damme P. Evaluation of non-inferiority of intradermal versus adjuvanted seasonal influenza vaccine using two serological techniques: a randomised comparative study. . BMC Infect Dis. 2010;10:134. doi: 10.1186/1471-2334-10-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.U.S. Food and Drug Administration. Center for Biological Evaluation and Research. Guidance for industry: clinical data needed to support the licensure for pandemic influenza vaccines. 2007: http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Vaccines/ucm074786.htm.
- 45.Committee for Proprietary Medicinal Products. Note for guidance on harmonisation of requirements for influenza vaccines (CPMP/BWP/214/96). 1997: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003945.pdf.