Abstract
Background and Objectives
Cryolipolysis has previously received FDA clearance for fat reduction in the abdomen, flanks, and thighs. There is also interest in small volume fat reduction for areas such as the chin, knees, and axilla. This article reports the results of a cryolipolysis pivotal IDE study for reduction of submental fullness.
Study Design/Material and Methods
A prototype small volume vacuum applicator (CoolMini applicator, CoolSculpting System, ZELTIQ Aesthetics) was used to treat 60 subjects in the submental area. At each treatment visit, a single treatment cycle was delivered at −10°C for 60 minutes, the same temperature and duration used in current commercially‐available cryolipolysis vacuum applicators. At the investigator's discretion, an optional second treatment was delivered 6 weeks after the initial treatment. The primary efficacy endpoint was 80% correct identification of baseline photographs by independent physician review. The primary safety endpoint was monitoring incidence of device‐ and/or procedure‐related serious adverse events. Secondary endpoints included assessment of fat layer thickness by ultrasound and subject satisfaction surveys administered 12 weeks after final cryolipolysis treatment.
Results
Independent photo review from 3 blinded physicians found 91% correct identification of baseline clinical photographs. Ultrasound data indicated mean fat layer reduction of 2.0 mm. Patient questionnaires revealed 83% of subjects were satisfied, 80% would recommend submental cryolipolysis to a friend, 77% reported visible fat reduction, 77% felt that their appearance improved following the treatment, and 76% found the procedure to be comfortable. No device‐ or procedure‐related serious adverse events were reported.
Conclusion
The results of this clinical evaluation of 60 patients treated in a pivotal IDE study demonstrate that submental fat can be reduced safely and effectively with a small volume cryolipolysis applicator. Patient surveys revealed that submental cryolipolysis was well‐tolerated, produced visible improvement in the neck contour, and generated high patient satisfaction. These study results led to FDA clearance of cryolipolysis for submental fat treatment. Lasers Surg. Med. 48:3–13, 2016. © 2015 The Authors. Lasers in Surgery and Medicine Published by Wiley Periodicals, Inc.
Keywords: cryolipolysis, submentum, submental fat, non‐invasive body contouring, non‐surgical fat reduction, subcutaneous fat, ATX‐101
INTRODUCTION
The American Society for Dermatologic Survey 2015 Survey on Cosmetic Dermatologic Procedures revealed that half of the patients surveyed were considering a cosmetic procedure. Top reasons specified were to look as young as they felt, to appear more attractive, and to feel more confident. The most common area of concern was excess weight, as reported by 88%, followed by skin texture and/or discoloration, reported by 72%, and lines and wrinkles around and under the eyes, reported by 69%. The fourth most common area of interest was excess fat under the chin and neck, as reported by 67% of respondents 1. Another survey showed similar results; 77% of the patients surveyed reported noticeable excess fat under their chins and 61% expressed a desire to have submental fat reduced 2.
As patients are becoming more aware of aesthetic procedures, they are also continuing to express interest in non‐surgical treatments. The American Society for Aesthetic Plastic Surgery Cosmetic Surgery National Data Bank Statistics showed that in 2014, there were 10 million cosmetic procedures performed in the US with over 12 billion dollars spent 3. Of these cosmetic procedures, 40% were non‐surgical procedures. Non‐surgical treatments have increased 508% since 1997 3.
The increased non‐surgical cosmetic procedure volume is driven by the baby boomer generation, seeking to minimize or delay the signs of aging, particularly in their faces and necks. The soft tissue composition of the face and neck changes as it ages, with alterations in skin texture, fat volume, and position of fat. Fullness is lost due to downward movement of fat, particularly in the periorbital, forehead, glabellar, malar buccal cheek, and perioral areas; meanwhile, fat accumulation occurs in or on the infraorbital pouches, paranasolabial and labiomental folds, the jaws, and the submental areas 4. While age‐related volume loss can be addressed with fat grafting or injectable filler techniques to rejuvenate the face, volume reduction has mainly relied upon surgical intervention.
Increases in fat in the submental compartment play a crucial role in the perception of aging in the face 5. The subcutaneous fat accumulated in the preplastysmal submental compartment leads to loss of mandibular line definition and an aged or overweight appearance 5, 6. Treatment of submental fat has been primarily limited to surgical liposuction, including laser‐assisted liposuction and ultrasound‐assisted liposuction. Some non‐surgical energy‐based methods have been employed but liposuction has remained the gold standard of treatment. A survey of plastic surgeons revealed that liposuction was used in 81% of cases for submental and/or jowl fat reduction, followed in popularity by radiofrequency treatment in 7% and laser liposuction in 4% 2. While liposuction may provide the most dramatic and reliable treatment result, many patients are reluctant to undergo liposuction because of the associated ecchymosis, skin laxity, surgical recovery time, and post‐surgical elevations in blood pressure 2. Also, there are liposuction risks for vascular or neurological complications, greater financial burden, psychological disturbances, and potential for unnatural result in both form and function 4.
Non‐surgical fat reduction methods have been developed and are gaining popularity. Injectable fat loss methods, such as mesotherapy and Lipodissolve, have been researched for over a decade. After initial enthusiasm, injectable fat reduction therapies lost popularity due to lack of therapeutic efficacy and unwanted side effects 7. Recently, however, the injectable deoxycholic acid formulation, ATX‐101, has undergone large‐scale randomized, controlled clinical studies, and subsequently received FDA approval for reduction of submental fat 8, 9, 10.
While the studies showed ATX‐101 to be an effective and well‐tolerated pharmacologic treatment for submental fat reduction, the study involved up to four treatment visits with up to 50 injections per visit in the European clinical trials 8, 9, 10 and up to six treatment visits with up to 50 injections per visit in the US clinical trials 11, 12. Thus, cryolipolysis was investigated as a non‐surgical fat reduction procedure which may provide greater subject comfort and convenience. Cryolipolysis, the application of controlled cooling to non‐invasively damage subcutaneous adipocytes, is based upon the greater susceptibility of lipid rich adipocytes to cold injury compared to surrounding water rich cells 13, 14, 15. Cryolipolysis has been shown to safely and effectively reduce subcutaneous fat on the body and previously had US Food and Drug Administration (FDA) clearance for treatment of the flanks, abdomen, and thighs.
To reduce submental fat, a prototype small volume vacuum cup applicator was developed and clinically investigated. This article reports the results of a multi‐center pivotal Investigational Device Exemption (IDE) study which led to FDA clearance of cryolipolysis for submental fat reduction.
MATERIALS AND METHODS
This was a multicenter, prospective, open label, non‐randomized interventional cohort study. Per protocol, each subject had one or two treatments on the submental area. If two treatments were determined appropriate by the Investigator, the treatments were performed 6 weeks apart. Each treatment consisted of a −10°C, 60 minute cooling cycle delivered to the submental area using a prototype small volume vacuum cup cryolipolysis applicator, Figure 1.
Figure 1.
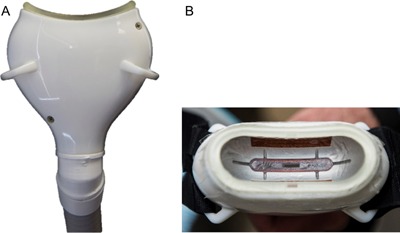
Prototype small volume applicator (A) side view and (B) top view showing contoured cooling surface.
The primary endpoints of the study were defined as a safety endpoint of monitoring the incidence of device‐ and/or procedure‐related adverse events and an efficacy endpoint of at least 80% correct identification of the pre‐treatment images by three blinded independent reviewers. The secondary endpoints in the study were the reduction in fat layer thickness, as measured by ultrasound, at 12 weeks post final‐treatment and subject satisfaction as assessed by questionnaires administered at 12 weeks post final‐treatment.
Eligible subjects were male or female, between 22 and 65 years of age, and with clearly visible submental fat greater than 1 cm, as measured by skin fold caliper. For the study population, the subject ages ranged from 25–61, with mean 49.3 years. There was not a weight or Body Mass Index (BMI) specification in the eligibility criteria. Weight ranged from 139.2 to 285.6 lbs., with mean 196.1 lbs. Body Mass Index (BMI) ranged from 23.2–46.2, with mean BMI 31.8.
For the duration of the study, subjects were instructed to avoid implementing major diet or lifestyle changes in order to maintain their weight within 5% of baseline measurement. Prior to treatment and at the 6‐ and 12‐week post‐final follow‐up visits, clinical assessments and photographs were obtained. Ultrasound images were collected at baseline and the 12‐week post‐final treatment visits. Patient surveys were also conducted at the final follow‐up visits.
Treatment efficacy was assessed by clinical photographs and ultrasound imaging. For the photographs, subjects were photographed in a seated position with five cameras simultaneously capturing front, side profile, and 45° oblique images. Photos were taken at pre‐treatment, 6‐weeks post‐treatment, and 12‐weeks post‐final treatment follow‐up visits. At all baseline and follow‐up visits, photographs were acquired using a standardized photography set‐up (Nikon D300, Nikon 60 mm lens, DynaLite strobes) to ensure consistency. For follow‐up visits, the photographer referred to baseline photographs while capturing the follow‐up photographs to ensure consistency in subject positioning and exposure. Subsequently, photos taken at the 12‐week post‐final treatment visit were compared with those taken at baseline by a blinded independent panel of three physicians board‐certified in either dermatology or plastic surgery. Independent photo review data was generated by randomizing pre‐treatment and post‐treatment photograph pairs of each subject, then asking the reviewers to determine which image was the pre‐treatment image.
Ultrasound images were acquired at baseline and 12‐weeks post‐final treatment visits with the subject lying in a supine position. A 7.5 MHz high‐resolution linear transducer was used to acquire ultrasound images of the submental treatment site (SonoSite TITAN, Bothell, Washington). The ultrasound transducer was positioned medially in the sagittal plane. To avoid affecting the images, care was taken to lightly stabilize the transducer without compressing the tissue. For follow‐up visits, the ultrasonographer carefully positioned the subjects and referred to baseline images while capturing the follow‐up images to match anatomical features in both images and ensure consistency of the image planes. Ultrasound images were post‐processed to measure anatomical features in the pre‐treatment and post‐treatment images and the fat layer reduction in the submental treatment area was calculated.
Subject satisfaction data was collected by a written questionnaire at the 12‐week post‐final treatment follow up visits. This questionnaire was composed of 5‐point Likert scale questions, as well as free‐text responses. Safety was monitored by documentation of adverse events and clinical assessment of the treatment site. Subjects were assessed throughout the study for adverse events.
The cryolipolysis submental treatment is illustrated in Figure 2. A protective gel was applied to the skin, the contoured cup small volume applicator was positioned in the center of the treatment area, and vacuum suction was initiated. The vacuum adhered the applicator to the treatment area and velcro straps provided additional support throughout the 60 minute treatment. At the conclusion of the treatment cycle, the applicator was removed, revealing firm, frozen tissue. At the conclusion of the cycle, the treatment area was manually massaged for 2 minutes allowing the tissue to rewarm and regain its original shape.
Figure 2.
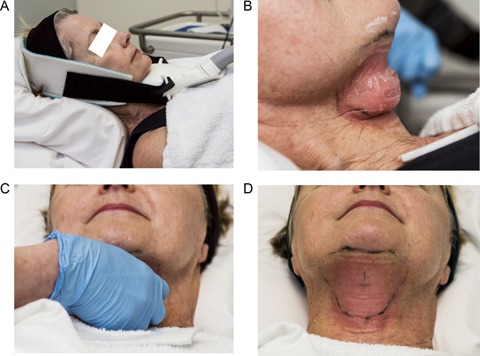
Cryolipolysis treatment steps for the submental area. (A) The small volume applicator was secured by vacuum suction and velcro straps. (B) Frozen tissue immediately following applicator removal. (C) Manual massage of the treatment area. D) Normalized tissue contour and lingering erythema following massage.
Per protocol, the treatment plan allowed subjects to receive either one or two cooling cycles (temperature −10°C, duration 60 minutes) performed 6 weeks apart, based on Investigator determination. Fifty‐nine (59) subjects received two cooling cycles and one (1) subject (BUR‐007) received one cooling cycle. Of the 59 subjects who received two cooling cycles, two subjects had one cycle that was incomplete due to device interference error.
One subject (KIL‐017) had the second treatment cycle interrupted due to interference at 47 minutes and the other subject (ZEL‐001) had the second treatment cycle interrupted at 40 minutes elapsed time. There were two additional subjects whose treatment cycles ended due to device interference error after 50 minutes of treatment; treatment of 50 minutes or longer was considered a full treatment, consistent with current ZELTIQ commercial treatment guidelines. Subject KIL‐012 had the first treatment cycle terminated at 52 minutes and subject BUR‐006 had the first treatment cycle terminated at 59 minutes.
RESULTS
From three clinical sites, 60 patients were enrolled and completed treatment. Fifty‐nine subjects received two cooling cycles, and one subject received one cooling cycle on the submental area. All subjects remained within the allowed 5% weight change limit; therefore, no subjects were excluded from treatment efficacy analysis due to weight change.
Forty‐eight of the subjects enrolled in the study were female and 12 were male. The Fitzpatrick Skin Type of the subjects ranged from I–V, with most subjects falling into categories II (47%) and III (42%). Ethnicity of subjects was primarily Caucasian (87%), Hispanic (8%), African American (2%), and Other (3%).
Figures 3, 4, 5, 6, 7, 8, 9 show representative subjects at baseline and at 12‐weeks post‐final treatment. Visible reduction in submental fullness is demonstrated from the pre‐ and post‐treatment photographs. From the independent photo review, three blinded, independent physicians reviewed the photographs in randomized pairs. Of the 60 subjects, two subjects (ZEL‐001 and KIL‐017) had a partial cooling cycle due to device interference error and were excluded from the efficacy analysis. Therefore, 58 subjects were considered for analysis. The overall correct identification rate was 91.4% (159 /174) for this population, with the three reviewers correctly identifying 95% (55/58), 91% (53/58), and 88% (51/58) of photo pairs. The results from the independent panel review of the photographs are statistically significant (P < 0.0001). The primary efficacy endpoint of at least 80% correct identification of the pre‐treatment images was met.
Figure 3.
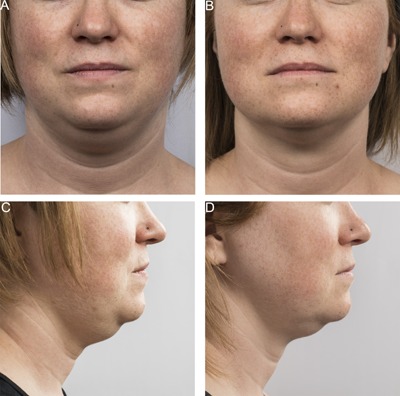
Baseline (A, C) and 12 week post‐treatment (B, D) photos for a 36 year‐old female. Weight change −3.5 lbs. (−2%) from baseline. Procedure by Dr. Brian Zelickson.
Figure 4.
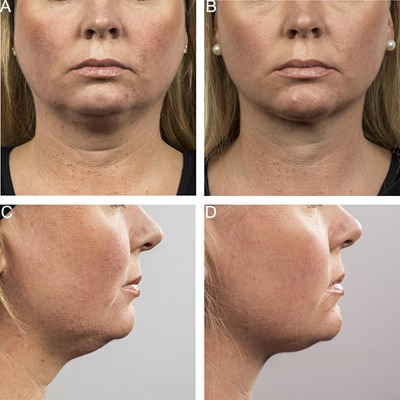
Baseline (A, C) and 12 week post‐treatment (B, D) photos for a 39 year‐old female. Weight change −4.4 lbs. (−3%) from baseline. Procedure by Dr. Suzanne Kilmer.
Figure 5.
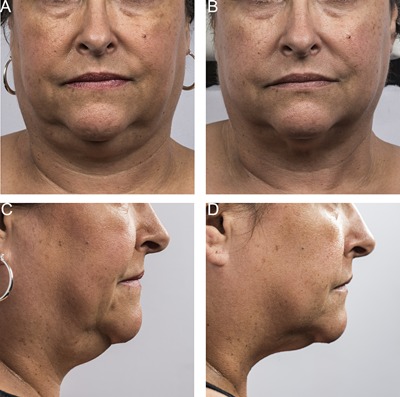
Baseline (A, C) and 12 week post‐treatment (B, D) photos for a 50 year‐old female. Weight change +1.7 lbs. (+1%) from baseline. Procedure by Dr. Jay Burns.
Figure 6.
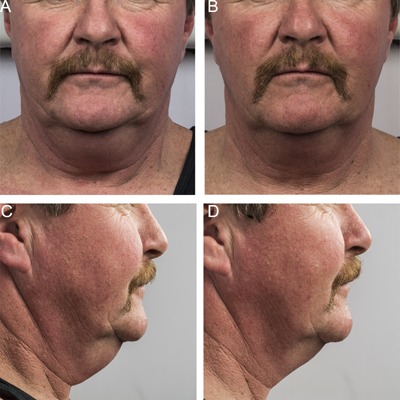
Baseline (A, C) and 12 week post‐treatment (B, D) photos for a 58 year‐old male. Weight change +2.4 lbs. (+1%) from baseline. Procedure by Dr. Brian Zelickson.
Figure 7.
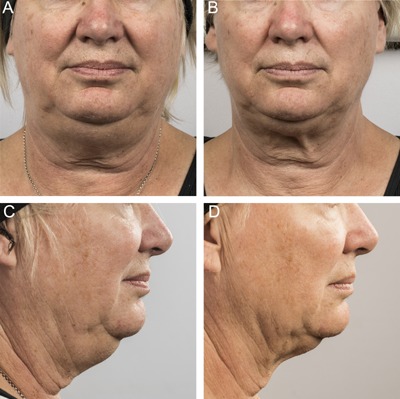
Baseline (A, C) and 12 week post‐treatment (B, D) photos for a 58 year‐old female. Weight change −8.2 lbs. (−5%) from baseline. Procedure by Dr. Suzanne Kilmer.
Figure 8.
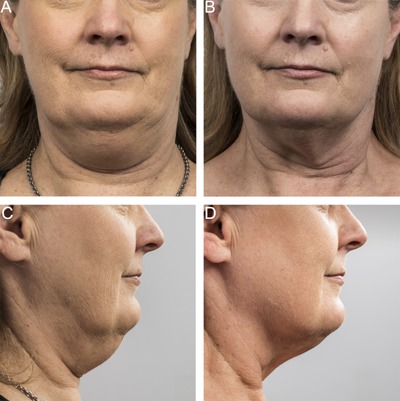
Baseline (A, C) and 12 week post‐treatment (B, D) photos for a 55 year‐old female. Weight change −1.4 lbs. (−1%) from baseline. Procedure by Dr. Jay Burns.
Figure 9.
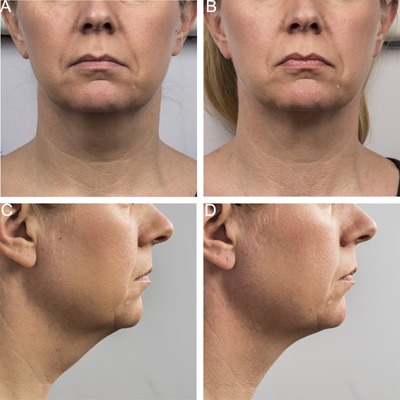
Baseline (A, C) and 12 week post‐treatment (B, D) photos for a 44 year‐old female. Weight change −4.5 lbs. (‐3%) from baseline. Procedure by Dr. Brian Zelickson.
Ultrasound images were analyzed to calculate fat layer reduction. Figure 10 shows representative ultrasound images captured at baseline and 12 weeks post‐final treatment. One subject was unavailable for ultrasound images and two subjects (ZEL‐001 and KIL‐017) had incomplete treatment, therefore ultrasound fat layer reduction was calculated for 57 subjects. The ultrasound measurement of the treatment areas showed a mean fat layer reduction of 2.0 mm, with a standard deviation of 2.0 mm and a range from an increase of 2.0 mm to a reduction of 5.9 mm. Reduction in fat layer was statistically significant (P < 0.0001). The mean 2.0 mm reduction correlated to a mean 20% submental fat layer reduction.
Figure 10.
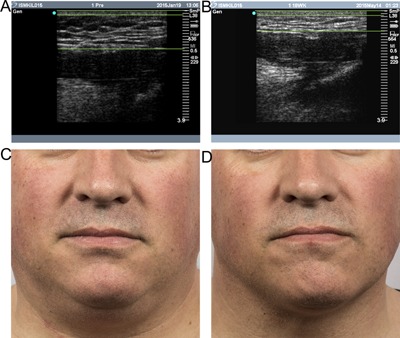
Baseline (A, C) and 12 week post‐treatment (B, D) ultrasound images and photos demonstrate fat layer reduction for a 47 year‐old male. Lines indicate the skin layer at the top of the ultrasound images and the fat thickness. Weight change −4.2 lbs. (−2%) from baseline. Procedure by Dr. Suzanne Kilmer.
Patient survey data from the follow‐up questionnaire were tabulated for all subjects. From the surveys, 83% of subjects were satisfied, 80% would recommend submental cryolipolysis to a friend, 77% reported visible fat reduction and felt that their appearance improved following the treatment, and 76% found the procedure to be comfortable.
Clinical assessment of the treatment sites was performed immediately post‐treatment and at the 1‐week, 6‐week and 12‐week follow‐up visits. All subjects were evaluated for side effects at the treatment sites and assessed for any adverse events. Study staff contacted subjects weekly for follow‐up regarding any side effects or adverse events noted. At each time point, subjects were assessed for common side effects including erythema, edema, bruising, numbness, and tingling at the treatment site. In addition, any other side effects were also assessed and recorded.
Tables 1 and 2 show clinical assessment data following the first and second treatments using a numerical scale in which 0 = none, 1 = minor, 2 = moderate, and 3 = severe. Immediately post‐treatment, the most common effects within the treatment area were erythema, edema, and numbness. At the 1‐week follow‐up visit, most incidences of erythema and edema had resolved and numbness was the most prevalent side effect. At the 1‐week visit after the first treatment, three incidences of mild swelling and one incidence of mild bruising were reported. After the second treatment, there were no reports of swelling or bruising at the 1‐week follow‐up visit. By the 6‐week follow‐up visit after the first treatment, all reports of swelling and bruising had resolved, and four incidences of mild numbness were reported. At the 6‐week follow‐up visit after the second treatment, two incidences of mild numbness were reported. Reports of sensitivity, itching, and tenderness were reported in the category Other. By the 12‐week post‐final treatment visit, all side effects had resolved.
Table 1.
Clinical Assessment of the Submental Treatment Area Following First Treatment
| Clinical assessment post‐treatment #1 | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Immediate post‐treatment #1 | 1‐Week follow‐up | 6‐Week follow‐up | 12‐Week follow‐up | |||||||||||||
| 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | |
| Blanching | 45 | 14 | 1 | 0 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Bruising | 58 | 2 | 0 | 0 | 59 | 1 | 0 | 0 | 60 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Erythema/purpura | 0 | 16 | 44 | 0 | 59 | 0 | 1 | 0 | 60 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Edema/swelling | 23 | 7 | 30 | 0 | 57 | 3 | 0 | 0 | 60 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Numbness | 6 | 17 | 23 | 14 | 17 | 30 | 12 | 1 | 56 | 4 | 0 | 0 | 1 | 0 | 0 | 0 |
| Tingling | 42 | 15 | 3 | 0 | 50 | 10 | 0 | 0 | 60 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Other | 0 | 0 | 0 | 1 | 0 | 11 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Table 2.
Clinical Assessment of the Submental Treatment Area Following Second Treatment
| Clinical assessment post‐treatment #2 | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Immediate post‐treatment #2 | 1‐Week follow‐up | 6‐Week follow‐up | 12‐Week follow‐up | |||||||||||||
| 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | |
| Blanching | 40 | 19 | 0 | 0 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Bruising | 59 | 0 | 0 | 0 | 59 | 0 | 0 | 0 | 57 | 0 | 0 | 0 | 59 | 0 | 0 | 0 |
| Erythema/purpura | 1 | 42 | 16 | 0 | 58 | 1 | 0 | 0 | 57 | 0 | 0 | 0 | 59 | 0 | 0 | 0 |
| Edema/swelling | 24 | 31 | 4 | 0 | 59 | 0 | 0 | 0 | 57 | 0 | 0 | 0 | 59 | 0 | 0 | 0 |
| Numbness | 1 | 30 | 10 | 18 | 31 | 25 | 3 | 0 | 55 | 2 | 0 | 0 | 59 | 0 | 0 | 0 |
| Tingling | 42 | 13 | 2 | 2 | 57 | 1 | 1 | 0 | 57 | 0 | 0 | 0 | 59 | 0 | 0 | 0 |
| Other | 0 | 0 | 0 | 0 | 0 | 4 | 1 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
There were four device‐ and/or procedure‐related adverse events consisting of two incidents of prolonged erythema (resolved at 17 and 20 days), one incident of hyperpigmentation (resolved at 27 days), and one subject report of fullness sensation in the back of the throat due to swelling (resolved at 40 days). All four of the device‐ and/or procedure‐related adverse events resolved without treatment. The primary safety endpoint for the study was satisfied and there were no device‐ or procedure‐related serious adverse events and no unanticipated adverse device effects occurred during the study.
DISCUSSION
This multi‐center pivotal IDE study evaluated cryolipolysis for submental fat reduction using a small volume vacuum applicator. There were four cases of device interference errors occurring during treatment and these were likely due to patient motion. Device interference errors are triggered when any of the thermistors embedded in the cryolipolysis vacuum cup detect an aberrant change in temperature, such as those caused by patient motion, electrical noise, or condensation, and the system software discontinues the treatment cycle. It is important to instruct patients to remain relaxed and still throughout the treatment, to refrain from speaking or looking around the treatment room. In addition to patient instructions to minimize applicator device errors during treatment, it is believed that the commercial version of the small volume applicator will provide more secure patient adhesion than the prototype version did. A more comfortable and secure strap has been developed to support the applicator and will attach to a special treatment pillow which will support the patient's head and neck throughout the treatment. The improved applicator and strap design is intended to more securely attach the applicator to the patient during the treatment procedure.
Aside from the inconvenience of the four device interference errors, all treatments for the 60 subjects proceeded smoothly. Procedural comfort was compared to published data from cryolipolysis studies for outer thighs and pseudogynecomastia. The submental cryolipolysis treatments were reported to be comfortable by 76% of subjects, which is similar to survey results for outer thigh (76%) and pseudogynecomastia treatment (79%) 16, 17. While it is unknown what percentage of ATX‐101 subjects felt the procedure was comfortable, the cryolipolysis and ATX‐101 procedures can be compared in terms of typical side effects. Submental cryolipolysis produced typical side effects such as numbness and erythema, generally described as mild, whereas the ATX‐101 procedure produced side effects such as bruising and pain, generally described as mild to moderate, with significant swelling lasting for 1 week. At one week following submental cryolipolysis treatment, swelling was reported in 3% (3 of 119 treatments) and bruising in 1% (1 of 119 treatments), all of mild degree and resolving without intervention. Based upon the side effect profile, submental cryolipolysis probably has higher patient tolerability than the ATX‐101 procedure.
Efficacy was demonstrated by independent review of clinical photographs, finding 91% correct identification of baseline clinical photographs. Mean fat layer reduction was measured by ultrasound to be 2.0 mm. This mean result is lower compared to other recent cryolipolysis studies, such as 2.8 mm for inner thighs and 2.6 mm for lateral thighs 16, 18. For a small volume treatment, such as the submental area, however, the fat layer reduction was appreciable.
Eight subjects showed increase in the fat layer thickness. The increase was typically less than 1 mm and attributed to variability in the ultrasound measurement technique. For the two subjects that had greater than 1 mm increase, they also showed weight increase of 2.0 and 7.5 lbs., which may have contributed to the increased thickness. The increased fat thickness is not believed to be paradoxical adipose hyperplasia since the treated tissue was not firmer than the surrounding untreated fat tissue 19. The study investigators are familiar with potential adverse events associated with cryolipolysis and did not identify any study subjects with paradoxical adipose hyperplasia following submental treatment.
Patient surveys showed 83% of submental cryolipolysis subjects were satisfied and 77% reported visible fat reduction. From the inner and outer thigh studies, the subject surveys revealed 93% and 86% of subjects were satisfied and 84% and 86% noticed visible fat reduction, respectively 16, 18. It should be noted that study subjects received treatments free of charge. For submental fat patients in a commercial practice, satisfaction rate may differ because of patient expectations and cost of treatment.
While the submental study subject survey results showed lower rates of satisfaction and visible fat reduction, the independent photo review results were comparable 16, 18. The blinded review of clinical photographs by a panel of independent physicians found a similar rate of correct identification of baseline photographs for various cryolipolysis studies; 91% of submental subjects were correctly identified, compared to 91% of inner thigh and 87% of outer thigh baseline clinical photographs 16, 18. In contrast, from the pseudogynecomastia study, surveys revealed that 95% of subjects noticed visual improvement in the treatment area, but the independent photo review found 82% correct identification of baseline, thus a lower objective assessment of efficacy but a higher patient reported rate of efficacy 17. It's interesting to note that although 83% subject satisfaction for the submental study is lower than other recent cryolipolysis study results, the rate of satisfaction with submental treatment is higher than the 65% reported in the pooled study for European subjects receiving 2 mg/cm2 of ATX‐101 8.
A limitation of the study is that the submental fat reduction wasn't quantified using a standardized scale, thus providing an assessment of the relative clinical improvement. This study was designed similarly to previous cryolipolysis studies which evaluated efficacy by blinded independent review of clinical photographs, ultrasound measurement of fat layer reduction, and patient surveys 16, 17, 18. These study methods found that a mean 2.0 mm reduction in submental fat was coincident with 91% correct identification of baseline clinical photographs and 83% subject satisfaction. For future cryolipolysis submental studies, it may be beneficial to utilize a standardized scale to facilitate efficacy comparison with other submental fat reduction procedures.
The study subjects were treated with one cryolipolysis cycle per treatment visit and most subjects underwent a second cycle 6 weeks after the initial treatment. These treatments adhered to the study protocol, but the authors believe that patients with more significant submental fullness would benefit from additional treatment cycles, up to two and three cycles per visit, in a left and right configuration or center, left, and right for larger submental fat pockets.
While the study subjects were satisfied with the non‐surgical fat reduction procedure, in a commercial aesthetic practice, some patients may have additional concerns about residual skin laxity or platysmal band prominence following submental fat reduction. For those patients, it may be desirable to employ a secondary procedure, such as a radiofrequency (RF) or microfocused ultrasound (MFUS) procedure for skin tightening or neuromodulator injection to reduce platysmal band prominence. We feel that it is unlikely that cryolipolysis would cause skin laxity in the submental area. The study used a vacuum setting of 50 for submental cryolipolysis, sufficient to gently draw submental fat into the vacuum cup without causing skin stretching and laxity. Some investigators have even suggested that skin tightening occurs as an unexpected benefit of cryolipolysis 20, 21.
As with the Treatment to Transformation commercial protocol, treatment plans should be developed to meet the individual patient's needs and some patients may achieve more dramatic fat reduction and greater satisfaction from additional treatment cycles and repeat treatments, as needed. While the small volume vacuum applicator was well‐suited for submental treatment, the authors see promise for utilizing the device for small volume treatments elsewhere, such as reducing undesirable fat bulges around the knees and axilla.
There is great interest in submental fat reduction. Along with the recently FDA approved ATX‐101 injectable pharmacologic treatment, cryolipolysis appears to provide a safe and effective non‐surgical option for reduction of submental fat. While these non‐surgical options are promising, however, they are not appropriate for all patients seeking neck and facial rejuvenation by reducing submental fat. For patients with excessive skin laxity or platysmal banding, surgical procedures will still be necessary to improve the neckline contour 2. But for many patients wishing to reduce submental fat, cryolipolysis with a small volume applicator provides a safe and effective treatment option.
A small volume vacuum applicator was evaluated for cryolipolysis treatment of submental fat. There was no incidence of device‐ or procedure‐related serious adverse events and common cryolipolysis side effects, such as erythema and numbness, were typically mild, transient, and self‐resolving. Cryolipolysis was demonstrated to be safe, effective, well‐tolerated, and producing high patient satisfaction for submental fat reduction.
CONCLUSION
As demonstrated by 60 patients treated in a pivotal IDE study, submental fat can be reduced safely and effectively with a small volume cryolipolysis applicator. As shown by patient surveys, cryolipolysis was well‐tolerated, produced visible improvement in the neck contour, and generated high patient satisfaction. Results of this clinical study led to FDA clearance of cryolipolysis for submental fat reduction.
DISCLOSURES
Dr. Kilmer is on the medical advisory boards for Candela/Syneron, Living Proof/Strateris, Lumenis, Lutronics, and Zeltiq and receives research support from Candela/Syneron, Living Proof/ Strateris, Lumenis, Lutronics, Zeltiq, Allergan, Cutera, Cynosure/Palomar, and Valeant. Dr. Burns is on the medical advisory boards for Zeltiq, Ulthera, Cynosure, Myoscience, and Thermi‐Aesthetics; receives honoraria from Valeant, Sciton, Zeltiq, and Ulthera; receives funding through research grants from Valeant, Zeltiq, and Ulthera; owns stock and/or stock options in Zeltiq; and receives equipment discounts or loans from Palomar, Cynosure, Sciton, Zimmer, Zeltiq, and Cutera. Dr. Zelickson is on the medical advisory boards for Zeltiq, Lumenis, Candela, Ulthera, and Eleme; received research grants from Sciton, Candela, Palomar, Syneron, Silk'n, Valeant, Lumenis, Ulthera, Zeltiq, Canfield, and Wave Rx.
ACKNOWLEDGMENTS
This clinical study was sponsored by ZELTIQ Aesthetics, manufacturers of the CoolSculpting System.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and have disclosed the following: Dr. Kilmer is the founding director of the Laser and Skin Surgery Center of Northern California and is a Clinical Professor of Dermatology at the University of California, Davis. Dr. Burns has a private practice at Dallas Plastic Surgery Institute. Dr. Zelickson is the founding director Zel Skin and Laser Specialists and is an Adjunct Professor of Dermatology at the University of Minnesota.
REFERENCES
- 1.American Society for Dermatologic Surgery (ASDS) 2015. Consumer Survey on Cosmetic Dermatologic Procedures.
- 2. Schlessinger J, Weiss SR, Jewell M, Narurkar V, Weinkle S, Gold MH, Bazerkanian E. Perceptions and practices in submental fat treatment: A survey of physicians and patients. Skinmed 2013; 11(1):27–31. [PubMed] [Google Scholar]
- 3.American Society for Aesthetic Plastic Surgery (ASAPS) 2014. Cosmetic Surgery National Data Bank Statistics. [DOI] [PubMed]
- 4. Jianu DM, Filipescu M, Jianu SA, Nita AC, Chirita DA. The synergy between lasers and adipose surgery in face and neck rejuvenation: A new approach from personal experience. Laser Ther 2012; 21(3):215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hatef DA, Koshy JC, Sandoval SE, Echo AP, Izaddoost SA, Hollier LH. The submental fat compartment of the neck. Semin Plast Surg 2009; 23(4):288–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Patel BC. Aesthetic surgery of the aging neck: Options and techniques. Orbit 2006; 25(4):327–356. Review. [DOI] [PubMed] [Google Scholar]
- 7. Duncan D, Rotunda AM. Injectable therapies for localized fat loss: State of the art. Clin Plast Surg 2011; 38(3):489–501. [DOI] [PubMed] [Google Scholar]
- 8. McDiarmid J, Ruiz JB, Lee D, Lippert S, Hartisch C, Havlickova B. Results from a pooled analysis of two European, randomized, placebo‐controlled, phase 3 studies of ATX‐101 for the pharmacologic reduction of excess submental fat. Aesthetic Plast Surg 2014; 38(5):849–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ascher B, Hoffmann K, Walker P, Lippert S, Wollina U, Havlickova B. Efficacy, patient‐reported outcomes and safety profile of ATX‐101 (deoxycholic acid), an injectable drug for the reduction of unwanted submental fat: Results from a phase III, randomized, placebo‐controlled study. J Eur Acad Dermatol Venereol 2014; 28(12):1707–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rzany B, Griffiths T, Walker P, Lippert S, McDiarmid J, Havlickova B. Reduction of unwanted submental fat with ATX‐101 (deoxycholic acid), an adipocytolytic injectable treatment: Results from a phase III, randomized, placebo‐controlled study. Br J Dermatol 2014; 170(2):445–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Phase 3 Study of Deoxycholic Acid Injection (ATX‐101) Versus Placebo for the Reduction of Localized Subcutaneous Fat in the Submental Area. Data accessed from U.S. National Institutes of Health website ClinicalTrials.gov identifier NCT 0154 6142.
- 12.Phase 3 Study of Deoxycholic Acid Injection (ATX‐101) Versus Placebo for the Reduction of Localized Subcutaneous Fat in the Submental Area (REFINE‐1). Data accessed from U.S. National Institutes of Health website ClinicalTrials.gov identifier NCT01542034.
- 13. Manstein D, Laubach H, Watanabe K, Farinelli W, Zurakowski D, Anderson RR. Selective cryolysis: A novel method of non invasive fat removal. Lasers Surg Med 2009; 40:595–604. [DOI] [PubMed] [Google Scholar]
- 14. Zelickson B, Egbert BM, Preciado J, Allison J, Springer K, Rhoades RW, Manstein D. Cryolipolysis for non invasive fat cell destruction: Initial results from a pig model. Dermatol Surg 2009; 35:1462–1470. [DOI] [PubMed] [Google Scholar]
- 15. Avram MM, Harry RS. Cryolipolysis for subcutaneous fat layer reduction. Lasers Surg Med 2009; 41(10):703–708. [DOI] [PubMed] [Google Scholar]
- 16. Stevens WG, Bachelor EP. Cryolipolysis conformable surface applicator for non‐surgical fat reduction in lateral thighs. Aesthet Surg J 2015; 35(1):66–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Munavalli GS, Panchaprateep R. Cryolipolysis for targeted fat reduction and improved appearance of the enlarged male breast. Dermatol Surg 2015; 41(9):1043–1051. [DOI] [PubMed] [Google Scholar]
- 18. Zelickson BD, Burns AJ, Kilmer SL. Cryolipolysis for safe and effective inner thigh fat reduction. Lasers Surg Med 2015; 47(2):120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jalian HR, Avram MM, Garibyan L, Mihm MC, Anderson RR. Paradoxical adipose hyperplasia after cryolipolysis. JAMA Dermatol 2014; 150(3):317–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Carruthers J, Stevens WG, Carruthers A, Humphrey S. Cryolipolysis and skin tightening. Dermatol Surg 2014; 40(Suppl 12):S184–S189. [DOI] [PubMed] [Google Scholar]
- 21. Stevens WG. Does cryolipolysis lead to skin tightening? A first report of cryodermadstringo. Aesthet Surg J 2014; 34(6):NP32–NP34. [DOI] [PubMed] [Google Scholar]


