ABSTRACT
Objective
To assess the efficacy of vaginal progesterone for the prevention of preterm birth and neonatal morbidity and mortality in asymptomatic women with a twin gestation and a sonographic short cervix (cervical length ≤ 25 mm) in the mid‐trimester.
Methods
This was an updated systematic review and meta‐analysis of individual patient data (IPD) from randomized controlled trials comparing vaginal progesterone with placebo/no treatment in women with a twin gestation and a mid‐trimester sonographic cervical length ≤ 25 mm. MEDLINE, EMBASE, POPLINE, CINAHL and LILACS (all from inception to 31 December 2016), the Cochrane Central Register of Controlled Trials, Research Registers of ongoing trials, Google Scholar, conference proceedings and reference lists of identified studies were searched. The primary outcome measure was preterm birth < 33 weeks' gestation. Two reviewers independently selected studies, assessed the risk of bias and extracted the data. Pooled relative risks (RRs) with 95% confidence intervals (CI) were calculated.
Results
IPD were available for 303 women (159 assigned to vaginal progesterone and 144 assigned to placebo/no treatment) and their 606 fetuses/infants from six randomized controlled trials. One study, which included women with a cervical length between 20 and 25 mm, provided 74% of the total sample size of the IPD meta‐analysis. Vaginal progesterone, compared with placebo/no treatment, was associated with a statistically significant reduction in the risk of preterm birth < 33 weeks' gestation (31.4% vs 43.1%; RR, 0.69 (95% CI, 0.51–0.93); moderate‐quality evidence). Moreover, vaginal progesterone administration was associated with a significant decrease in the risk of preterm birth < 35, < 34, < 32 and < 30 weeks' gestation (RRs ranging from 0.47 to 0.83), neonatal death (RR, 0.53 (95% CI, 0.35–0.81)), respiratory distress syndrome (RR, 0.70 (95% CI, 0.56–0.89)), composite neonatal morbidity and mortality (RR, 0.61 (95% CI, 0.34–0.98)), use of mechanical ventilation (RR, 0.54 (95% CI, 0.36–0.81)) and birth weight < 1500 g (RR, 0.53 (95% CI, 0.35–0.80)) (all moderate‐quality evidence). There were no significant differences in neurodevelopmental outcomes at 4–5 years of age between the vaginal progesterone and placebo groups.
Conclusion
Administration of vaginal progesterone to asymptomatic women with a twin gestation and a sonographic short cervix in the mid‐trimester reduces the risk of preterm birth occurring at < 30 to < 35 gestational weeks, neonatal mortality and some measures of neonatal morbidity, without any demonstrable deleterious effects on childhood neurodevelopment. Published 2017. This article is a U.S. Government work and is in the public domain in the USA. Ultrasound in Obstetrics & Gynecology published by John Wiley & Sons Ltd on behalf of the International Society of Ultrasound in Obstetrics and Gynecology.
Keywords: cervical length, prematurity, preterm delivery, progestins, progestogens, transvaginal ultrasound
INTRODUCTION
Twin births have become more prevalent in developed countries over the last decades1, 2, 3. In 2014, the twin birth rate in the USA was 33.9 per 1000 live births, the highest rate ever recorded4. Twin gestations are at increased risk of maternal, perinatal and infant morbidity and mortality, as well as long‐term neurodevelopmental disability5, 6, 7, 8, 9, 10, 11, 12, 13. Moreover, twin gestations also have a significant impact on healthcare costs and quality of life for both the parents and the children7, 14, 15.
Preterm birth is the most important factor determining neonatal morbidity and mortality among twins. The risk of preterm birth < 37 and < 32 weeks' gestation is eight‐ to ninefold higher in twin than in singleton gestations4. Several interventions have been proposed to reduce the rate of preterm birth in twin gestations, such as bed rest16, prophylactic tocolysis17, nutritional advice18, administration of 17α‐hydroxyprogesterone caproate19, vaginal progesterone19, cerclage20 and cervical pessary21, 22. Unfortunately, these interventions have not been shown to reduce the risk of preterm birth in unselected twin gestations.
A short cervix, traditionally defined as a transvaginal sonographic cervical length (CL) ≤ 25 mm in the mid‐trimester of pregnancy, is an important risk factor for spontaneous preterm birth and has emerged as one of the strongest and most consistent predictors of preterm birth in asymptomatic women with singleton23, 24, 25, 26, 27, 28, 29 or twin gestations30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43. Currently, there is compelling evidence that administration of vaginal progesterone to asymptomatic women with a singleton gestation and a sonographic short cervix decreases the risk of preterm birth and neonatal morbidity and mortality44, 45, 46. The efficacy of vaginal progesterone in women with a twin gestation and a short cervix has been less studied.
A meta‐analysis of individual patient data (IPD) published in 2012 reported on the efficacy of vaginal progesterone in preventing preterm birth and neonatal morbidity and mortality in asymptomatic women with a twin gestation and a CL ≤ 25 mm in the mid‐trimester47. A total of 52 women (104 fetuses/infants) from three randomized controlled trials (RCTs) were included in the study. The use of vaginal progesterone was associated with a significant 44% reduction in the risk of composite neonatal morbidity and mortality (relative risk (RR), 0.56 (95% CI, 0.30–0.97)) and a 30% non‐significant reduction in the risk of preterm birth < 33 weeks' gestation (RR, 0.70 (95% CI, 0.34–1.44)). Since that time, additional RCTs evaluating the use of vaginal progesterone in twin gestations have been published. Therefore, a reassessment of the efficacy of this intervention in women with a twin gestation and a short cervix is justified.
The objective of this study was to update the previous IPD meta‐analysis on the efficacy of vaginal progesterone in asymptomatic women with a twin gestation and a sonographic CL ≤ 25 mm in the mid‐trimester for the prevention of preterm birth and neonatal morbidity and mortality.
METHODS
The study was conducted according to a prospectively prepared protocol and reported in accordance with the Preferred Reporting Items for Systematic reviews and Meta‐Analyses statement48. The review was registered with PROSPERO (number CRD42016039682).
Data sources and searches
We searched MEDLINE, EMBASE, POPLINE, CINAHL and LILACS (all from inception to 31 December 2016), the Cochrane Central Register of Controlled Trials and Research Registers of ongoing trials using a combination of keywords and text words related to ‘progesterone’, ‘preterm birth’, ‘randomized controlled trial’ and ‘twin gestation’. Google Scholar, proceedings of congresses on obstetrics, maternal‐fetal medicine and ultrasound in obstetrics, reference lists of identified studies, previously published systematic reviews and review articles were also searched. Experts in the field were contacted to identify further studies. No language restrictions were applied.
Study selection
RCTs in which asymptomatic women with a twin gestation and a sonographic short cervix (CL ≤ 25 mm) in the mid‐trimester were allocated randomly to receive vaginal progesterone or placebo/no treatment for the prevention of preterm birth and/or adverse perinatal outcomes were eligible for inclusion in the review. Trials were included if the primary aim of the study was to prevent preterm birth in women with a twin gestation and a short cervix, or to prevent preterm birth in women with an unselected twin gestation but for whom outcomes were available in those with a prerandomization CL ≤ 25 mm. We excluded quasirandomized trials, trials that evaluated vaginal progesterone in women with preterm labor, arrested preterm labor (as maintenance tocolysis), preterm rupture of membranes or second‐trimester bleeding, trials that assessed vaginal progesterone in the first trimester only to prevent miscarriage and studies that did not report clinical outcomes. Studies published only as abstracts were excluded if additional information on methodological issues and results could not be obtained.
All of the potentially relevant studies were retrieved and reviewed independently by two authors to determine inclusion. Disagreements were resolved by discussion amongst the reviewers.
Data collection
The corresponding author of each eligible trial was contacted and asked to provide anonymized data (without identifiers) about baseline characteristics and outcomes for every randomly assigned patient, as well as data on study characteristics and details of interventions and co‐interventions. All initial communications with authors were based on a template explaining the study and the data required. Data provided by the investigators were merged into a master database specifically constructed for the review. Data were checked for missing information, errors and inconsistencies by cross‐referencing with the publications of the original trials. Quality and integrity of the randomization processes were assessed by reviewing the chronological randomization sequence and pattern of assignment, as well as the balance of baseline characteristics across treatment groups. Inconsistencies or missing data were discussed with the authors and corrections were made when deemed necessary.
Informed consent was provided by the patients upon enrollment in each of the original trials. In the present study, the data were not used for any purposes other than those of the original trial and no new data were collected. Therefore, informed consent specifically for this project was not considered necessary. This study was exempted from review by the Human Investigation Committee Administration Office of Wayne State University.
Outcome measures
The primary outcome measure was preterm birth < 33 weeks' gestation. Secondary outcome measures included: preterm birth < 37, < 36, < 35, < 34, < 32, < 30 and < 28 weeks' gestation; spontaneous preterm birth < 33 and < 34 weeks' gestation; respiratory distress syndrome (RDS); necrotizing enterocolitis; intraventricular hemorrhage; proven neonatal sepsis; retinopathy of prematurity; fetal death; neonatal death; perinatal death; a composite outcome of neonatal morbidity and mortality (defined as the occurrence of any of the following events: RDS, intraventricular hemorrhage, necrotizing enterocolitis, proven neonatal sepsis or neonatal death); birth weight < 1500 g and < 2500 g; admission to the neonatal intensive care unit; use of mechanical ventilation; and long‐term neurodevelopmental outcomes (suspected or diagnosed developmental delay, cerebral palsy, intellectual disabilities, vision impairment, hearing loss, cognitive and behavioral impairments and motor, communication and learning disorders at any age in childhood).
Assessment of risk of bias
The risk of bias in each included trial was assessed independently by two authors using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions 49. This tool assesses seven domains related to risk of bias (random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and other bias) and categorizes studies by low, unclear or high risk of bias in each domain. Disagreements in risk of bias assessment were resolved through consensus.
Statistical analysis
We included all randomized women and their fetuses/infants and performed all analyses on an intention‐to‐treat basis. For outcomes dealing with gestational age at delivery, the unit of analysis was the pregnancy, whereas for perinatal outcomes, the unit of analysis was the fetus/neonate. IPD were combined in a two‐stage approach in which outcomes were analyzed in the original trial and then summary statistics were generated using standard summary data meta‐analysis techniques to give an overall measure of effect (pooled RR with 95% CI)50. Heterogeneity of the results among studies was tested51 with the quantity I 2. We pooled results from individual studies using a fixed‐effect model if substantial statistical heterogeneity was not present (< 50%). If I 2 values were ≥ 50%, a random‐effects model was used to pool data across studies. For adverse perinatal outcomes, we estimated pooled RRs using analytical methods that assumed independence between neonates. However, to avoid incorrect conclusions due to the non‐independence of newborns from twin gestations, we also used a generalized linear model with generalized estimating equations to estimate parameters while controlling for cluster correlations52, 53, 54. The number needed to treat for benefit or harm, with a 95% CI, was calculated for outcomes for which there was a statistically significant reduction or increase in risk difference based on control event rates in the trials55.
Subgroup analyses were performed to evaluate the effect of vaginal progesterone according to CL (<10, 10–20 and 21–25 mm), daily dose of vaginal progesterone (100, 200 and 400 mg) and obstetric history (no previous spontaneous preterm birth < 37 weeks' gestation and at least one previous spontaneous preterm birth < 37 weeks' gestation). A test for interaction between the treatment and subgroups was performed to examine whether treatment effects differed among subgroups56, 57, 58. An interaction P‐value ≥ 0.05 was considered to indicate that the effect of treatment did not differ significantly among subgroups. We planned to carry out sensitivity analyses to explore the effect of trial quality assessed by allocation concealment and random sequence generation (considering selection bias) and blinding (considering performance and detection biases), with studies rated as ‘high risk of bias’ or ‘unclear risk of bias’ for these domains being excluded from the analyses in order to assess whether this made any difference to the overall result. Subgroup and sensitivity analyses were only performed for the primary outcome of preterm birth < 33 weeks' gestation and for the secondary outcome of neonatal death. We also planned to explore potential sources of heterogeneity and to assess publication and related biases if at least 10 studies were included in a meta‐analysis, but these analyses were not undertaken due to the limited number of trials included in the review.
Quality of evidence
We used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach, as outlined in the GRADE Handbook 59, to assess the quality of evidence for primary and secondary outcome measures. We considered evidence from RCTs as high quality but downgraded the evidence by one level for serious (or two levels for very serious) limitations based upon the following: design (risk of bias), consistency across studies, directness of the evidence, precision of estimates and presence of publication bias. The GRADEpro Guideline Development Tool60 was used to import data from Review Manager in order to create a ‘Summary of findings’ table to report the quality of the evidence. The GRADE approach results in an assessment of the quality of a body of evidence in one of four grades: (i) high: we are very confident that the true effect lies close to that of the estimate of the effect; (ii) moderate: we are moderately confident in the effect estimate, the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different; (iii) low: our confidence in the effect estimate is limited, the true effect may be substantially different from the estimate of the effect; and (iv) very low: we have very little confidence in the effect estimate, the true effect is likely to be substantially different from the estimate of effect.
We performed all statistical analyses using Review Manager (RevMan, version 5.3.5; The Nordic Cochrane Centre, Copenhagen, Denmark) and SAS version 9.2 (SAS Institute, Cary, NC, USA) software.
RESULTS
Selection, characteristics and risk of bias of studies
Figure 1 summarizes the process of identification and selection of studies. A total of 213 records were identified by the searches, of which nine were retrieved for full‐text review. Three studies, which evaluated vaginal progesterone in unselected twin gestations61, 62 or pregnancies conceived by in‐vitro fertilization or intracytoplasmic sperm injection63, were excluded because CL was not measured or collected before randomization or there were no data for women with a CL ≤ 25 mm at randomization. Six studies, including a total of 303 women (606 fetuses/infants) with a CL ≤ 25 mm, met the inclusion criteria64, 65, 66, 67, 68, 69; 159 women were assigned to vaginal progesterone and 144 to placebo/no treatment. Minimal differences were noted in baseline maternal characteristics between the vaginal progesterone and placebo/no treatment groups (Table 1).
Figure 1.
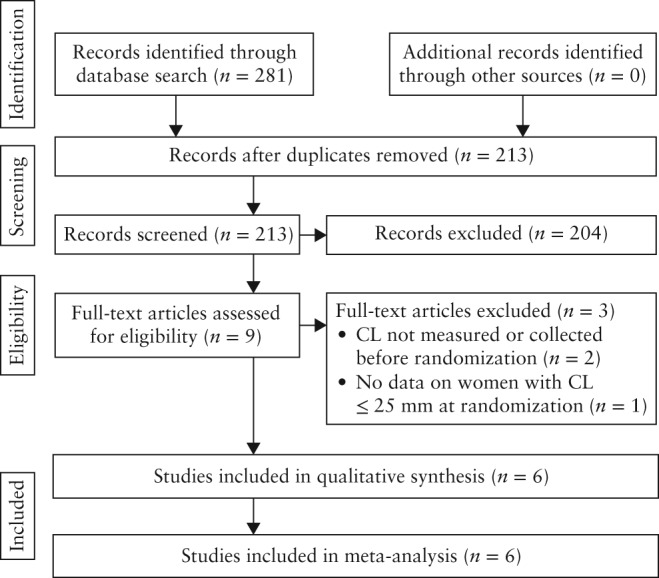
Study selection process. CL, cervical length.
Table 1.
Baseline characteristics of pooled women
| Characteristic | Vaginal progesterone (n = 159) | Placebo/no treatment (n = 144) |
|---|---|---|
| Maternal age (years) | 27 (25–30) | 28 (25–31) |
| Body mass index (kg/m2) | 22.4 (21.2–25.7)* | 22.9 (21.0–25.4)† |
| Smoker | 3 (1.9) | 3 (2.1) |
| Previous spontaneous PTB | 28 (17.6) | 28 (19.4) |
| Monochorionic pregnancy | 8 (5.0) | 6 (4.2) |
| GA at randomization (weeks) | 21.7 (20.6–23.1) | 22.1 (21.1–23.3) |
| CL at randomization (mm) | 22 (20–23) | 22 (20–23) |
| CL ≤ 20 mm at randomization | 49 (30.8) | 47 (32.6) |
Data are given as median (interquartile range) or n (%).
n = 41.
n = 36.
CL, cervical length; GA, gestational age; PTB, preterm birth.
The individual characteristics of the studies included in the meta‐analysis are shown in Table 2. Five studies were double‐blind, placebo‐controlled trials64, 65, 66, 67, 68. The remaining study compared vaginal progesterone with no treatment69. Three studies were performed in low/middle‐income countries65, 68, 69, two in high‐income countries66, 67 and one in both low/middle‐ and high‐income countries64. Two trials were specifically designed to evaluate the use of vaginal progesterone in women with a twin gestation and a sonographic short cervix (CL ≤ 15 mm64 and CL between 20 and 25 mm69). The remaining four studies tested the effect of vaginal progesterone in women with unselected twin gestations and their authors provided data relevant to women with a CL ≤ 25 mm before randomization65, 66, 67, 68. The trial that assessed vaginal progesterone in women with a CL between 20 and 25 mm69 provided data for 224 mothers and their 448 fetuses/infants. The other five studies provided data for 79 women and 158 fetuses/infants.
Table 2.
Characteristics of studies included in the systematic review
| Study | Country | Primary target population | Inclusion and exclusion criteria | Women with CL ≤ 25 mm (n)/fetuses or infants (n) | Intervention | Primary outcome measure |
|---|---|---|---|---|---|---|
| Fonseca (2007)64 | UK, Chile, Brazil, Greece | Women with short cervix |
Inclusion: women with singleton or twin gestation and transvaginal sonographic CL ≤ 15 mm Exclusion: major fetal abnormality, painful regular uterine contractions, history of ruptured membranes or cervical cerclage |
24/48 | Vaginal progesterone capsule (200 mg/day) or placebo from 24 to 33 + 6 weeks | Spontaneous PTB < 34 weeks |
| Cetingoz (2011)65 | Turkey | Women at high risk of PTB |
Inclusion: women with at least one previous spontaneous PTB, uterine malformation or twin gestation Exclusion: in‐place or planned cervical cerclage or serious fetal anomaly |
7/14 | Vaginal progesterone suppository (100 mg/day) or placebo from 24 to 34 weeks | PTB < 37 weeks |
| Rode (2011)66 | Denmark, Austria | Women with twin gestation |
Inclusion: women with a diamniotic twin gestation and chorionicity assessed by ultrasound before 16 weeks Exclusion: higher order multiple pregnancies, age < 18 years, known allergy to progesterone or peanuts as active treatment contained peanut oil, history of hormone‐associated thromboembolic disorders, rupture of membranes, pregnancies treated for or with signs of TTTS, intentional fetal reduction, known major structural or chromosomal fetal abnormality, known or suspected malignancy in genitals or breasts or known liver disease |
21/42 | Vaginal progesterone pessary (200 mg/day) or placebo from 20 to 23 + 6 up to 33 + 6 weeks | PTB < 34 weeks |
| Serra (2013)67 | Spain | Women with twin gestation |
Inclusion: women with dichorionic diamniotic twin gestation Exclusion: monochorionic twin gestation, triplet or higher order multiple gestation, elective cervical cerclage prior to 14 weeks, history of hepatic problem or gestational cholestasis, abnormal liver enzymes, abnormal kidney function, local allergy to micronized natural progesterone or peanuts, recurrent vaginal bleeding, recurrent vaginal infection, fetal anomaly, alcohol or illicit drug consumption or smoking ≥ 10 cigarettes/day |
6/12 | Vaginal progesterone pessary (200 or 400 mg/day) or placebo from 20 to 34 weeks | PTB < 37 weeks |
| Brizot (2015)68 | Brazil | Women with twin gestation |
Inclusion: women with naturally conceived diamniotic twin gestation, no previous PTB and gestational age between 18 + 0 and 21 + 6 weeks Exclusion: major fetal abnormality, allergy to progesterone or peanuts, hepatic dysfunction, porphyria, otosclerosis, malignant disease, severe depressive state, current or previous thromboembolic disease, uterine malformation, prophylactic cerclage or ovular infection |
21/42 | Vaginal progesterone ovule (200 mg/day) or placebo from 18 to 21 + 6 up to 34 + 6 weeks | Mean gestational age at delivery |
| El‐Refaie (2016)69 | Egypt | Women with twin gestation and short cervix |
Inclusion: women with dichorionic twin gestation, gestational age between 20 and 24 weeks, transvaginal sonographic CL between 20 and 25 mm, and without signs or symptoms of preterm labor Exclusion: known allergy or contraindication to progesterone therapy, monochorionic twin gestation, known major fetal structural or chromosomal abnormality, single fetal demise, fetal reduction in current pregnancy, cervical cerclage in current pregnancy, medical conditions that may lead to preterm labor, rupture of membranes or vaginal bleeding |
224/448 | Vaginal progesterone suppository (400 mg/day) from 20 to 24 up to 37 weeks or no treatment | PTB < 34 weeks |
Only the first author of each study is given.
CL, cervical length; PTB, preterm birth; TTTS, twin‐to‐twin transfusion syndrome.
Three studies used vaginal progesterone 200 mg/day (capsule64, pessary66 or ovule68), one used vaginal progesterone suppositories 100 mg/day65, one used vaginal progesterone suppositories 400 mg/day69 and the remaining study used vaginal progesterone suppositories 200 or 400 mg/day67. Treatment was started between 20 and 24 weeks' gestation in five trials64, 65, 66, 67, 69, and between 18 and 21 weeks' gestation in the remaining trial68. Five studies reported that participants received medication from the time of enrollment until ∼34 weeks' gestation64, 65, 66, 67, 68, and one study reported medication from enrollment until 37 weeks' gestation69. Two trials included only women with a dichorionic twin gestation67, 69. Major fetal abnormality, cervical cerclage in place or planned, allergy to progesterone and hepatic dysfunction were reported as exclusion criteria in most studies. The primary outcome measure was preterm birth < 34 weeks' gestation in two trials66, 69, preterm birth < 37 weeks' gestation in two trials65, 67, spontaneous preterm birth < 34 weeks' gestation in one trial64 and mean gestational age at delivery in the remaining study68. The study by El‐Refaie et al. 69 did not collect data for some neonatal morbidities, such as necrotizing enterocolitis, intraventricular hemorrhage, proven neonatal sepsis and retinopathy of prematurity.
The risk of bias in each included study is summarized in Figure 2. All studies had adequate generation of allocation sequence and concealment of allocation, and appeared to be free of selective outcome reporting and other sources of bias. Five studies were considered to be at low risk of selection, performance, detection, attrition and reporting biases64, 65, 66, 67, 68. The study by El‐Refaie et al. 69 had a high risk of performance and detection biases because patients, clinical staff and outcome assessors were not blinded to the allocated interventions. In addition, this trial was judged to be at unclear risk of attrition bias because the number of losses to follow‐up was not balanced across study groups (7.2% in the vaginal progesterone group and 13.6% in the no treatment group).
Figure 2.
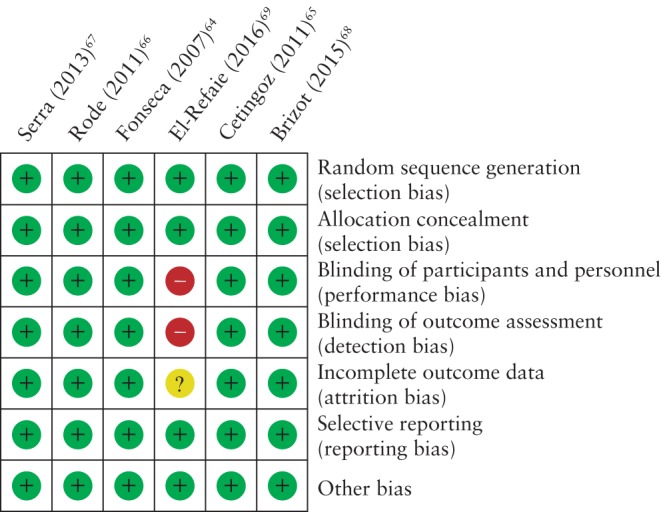
Risk of bias of studies included in the systematic review.  , low risk of bias;
, low risk of bias;  , high risk of bias;
, high risk of bias;  , unclear risk of bias.
, unclear risk of bias.
Effect of vaginal progesterone on preterm birth
Women allocated to receive vaginal progesterone had a significantly lower risk of preterm birth < 33 weeks' gestation (31.4% vs 43.1%; RR, 0.69 (95% CI, 0.51– 0.93); P = 0.01; I 2 = 0%; six studies, 303 women; moderate‐quality evidence) compared with those allocated to placebo/no treatment (Figure 3). In addition, vaginal progesterone was associated with a significant reduction in the risk of preterm birth < 35 weeks' gestation (RR, 0.83 (95% CI, 0.69–0.99); moderate‐quality evidence), < 34 weeks' gestation (RR, 0.71 (95% CI, 0.56–0.91); moderate‐quality evidence), < 32 weeks' gestation (RR, 0.51 (95% CI, 0.34–0.77); moderate‐quality evidence), < 30 weeks' gestation (RR, 0.47 (95% CI, 0.25–0.86); moderate‐quality evidence), and spontaneous preterm birth at < 33 weeks' gestation (RR, 0.67 (95% CI, 0.48–0.93); moderate‐quality evidence) and < 34 weeks' gestation (RR, 0.71 (95% CI, 0.54–0.93); moderate‐quality evidence) (Table 3). The number needed to treat to prevent one case of preterm birth occurring at < 30 to < 35 gestational weeks varied from 6 to 12. There were no significant differences between the study groups in the risk of preterm birth < 37 weeks' (moderate‐quality evidence), < 36 weeks' (moderate‐quality evidence) and < 28 weeks' (low‐quality evidence) gestation.
Figure 3.
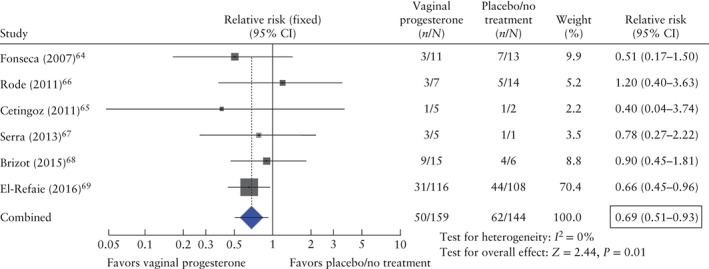
Forest plot of the effect of vaginal progesterone on the risk of preterm birth < 33 weeks' gestation. CI, confidence interval.
Table 3.
Effect of vaginal progesterone on the risk of preterm birth
| Events (n)/Total (N) | ||||||
|---|---|---|---|---|---|---|
| Outcome | Trials (n refs) | Vaginal progesterone | Placebo/no treatment | Pooled RR (95% CI) | I 2 (%) | NNT (95% CI) |
| Preterm birth < 37 weeks | 664, 65, 66, 67, 68, 69 | 137/159 | 131/144 | 0.94 (0.86–1.02) | 0 | — |
| Preterm birth < 36 weeks | 664, 65, 66, 67, 68, 69 | 112/159 | 110/144 | 0.92 (0.80–1.05) | 0 | — |
| Preterm birth < 35 weeks | 664, 65, 66, 67, 68, 69 | 90/159 | 98/144 | 0.83 (0.69–0.99) | 0 | 9 (5–147) |
| Preterm birth < 34 weeks | 664, 65, 66, 67, 68, 69 | 63/159 | 78/144 | 0.71 (0.56–0.91) | 0 | 6 (4–21) |
| Preterm birth < 32 weeks | 664, 65, 66, 67, 68, 69 | 29/159 | 46/144 | 0.51 (0.34–0.77) | 0 | 6 (5–14) |
| Preterm birth < 30 weeks | 664, 65, 66, 67, 68, 69 | 14/159 | 22/144 | 0.47 (0.25–0.86) | 0 | 12 (9–47) |
| Preterm birth < 28 weeks | 664, 65, 66, 67, 68, 69 | 9/159 | 12/144 | 0.51 (0.24–1.08) | 0 | — |
| Spontaneous preterm birth < 33 weeks | 664, 65, 66, 67, 68, 69 | 42/159 | 54/144 | 0.67 (0.48–0.93) | 0 | 8 (5–38) |
| Spontaneous preterm birth < 34 weeks | 664, 65, 66, 67, 68, 69 | 55/159 | 69/144 | 0.71 (0.54–0.93) | 0 | 7 (5–30) |
CI, confidence interval; NNT, number needed to treat; refs, reference numbers; RR, relative risk.
Effect of vaginal progesterone on adverse perinatal outcomes
Infants whose mothers received vaginal progesterone had a significantly lower risk of neonatal death (RR, 0.53 (95% CI, 0.35–0.81); moderate‐quality evidence), perinatal death (RR, 0.58 (95% CI, 0.39–0.84); moderate‐quality evidence), RDS (RR, 0.70 (95% CI, 0.56–0.89); moderate‐quality evidence), composite neonatal morbidity and mortality (RR, 0.61 (95% CI, 0.34–0.98); moderate‐quality evidence), birth weight < 1500 g (RR, 0.53 (95% CI, 0.35–0.80); moderate‐quality evidence) and use of mechanical ventilation (RR, 0.54 (95% CI, 0.36–0.81); moderate‐quality evidence) (Table 4). The number needed to treat to prevent one case of these adverse perinatal outcomes varied from 6 to 8. There was no evidence of an effect of vaginal progesterone on necrotizing enterocolitis (low‐quality evidence), intraventricular hemorrhage (low‐quality evidence), proven neonatal sepsis (low‐quality evidence), retinopathy of prematurity (low‐quality evidence), fetal death (very low‐quality evidence), birth weight < 2500 g (moderate‐quality evidence) and admission to the neonatal intensive care unit (moderate‐quality evidence).
Table 4.
Effect of vaginal progesterone on the risk of adverse perinatal outcomes
| Pooled RR (95% CI) | |||||||
|---|---|---|---|---|---|---|---|
| Events (n)/Total (N) | |||||||
| Outcome | Trials (n refs) | Vaginal progesterone | Placebo/no treatment | Assuming independence between twins | Adjustment for non‐independence between twins | I 2 (%) | NNT (95% CI) |
| Respiratory distress syndrome | 664, 65, 66, 67, 68, 69 | 102/311 | 131/280 | 0.67 (0.55–0.82) | 0.70 (0.56–0.89) | 0 | 6 (4–16) |
| Necrotizing enterocolitis | 564, 65, 66, 67, 68 | 1/82 | 0/68 | 1.00 (0.04–22.43) | 1.07 (0.05–22.25) | NA | — |
| Intraventricular hemorrhage | 564, 65, 66, 67, 68 | 2/80 | 2/68 | 0.93 (0.15–5.75) | 1.47 (0.22–9.63) | 0 | — |
| Proven neonatal sepsis | 564, 65, 66, 67, 68 | 4/80 | 7/68 | 0.44 (0.13–1.46) | 0.59 (0.18–1.93) | 0 | — |
| Retinopathy of prematurity | 564, 65, 66, 67, 68 | 1/80 | 1/68 | 0.42 (0.07–2.56) | 0.45 (0.08–2.59) | 17 | — |
| Fetal death | 664, 65, 66, 67, 68, 69 | 9/318 | 9/288 | 0.57 (0.23–1.42) | 0.68 (0.26–1.84) | 0 | — |
| Neonatal death | 664, 65, 66, 67, 68, 69 | 34/318 | 63/288 | 0.50 (0.34–0.71) | 0.53 (0.35–0.81) | 25 | 8 (5–19) |
| Perinatal death | 664, 65, 66, 67, 68, 69 | 43/318 | 72/288 | 0.51 (0.36–0.70) | 0.58 (0.39–0.84) | 24 | 7 (5–20) |
| Composite neonatal morbidity/mortality* | 564, 65, 66, 67, 68 | 23/84 | 28/70 | 0.57 (0.36–0.93) | 0.61 (0.34–0.98) | 0 | 6 (3–109) |
| Birth weight < 1500 g | 664, 65, 66, 67, 68, 69 | 48/315 | 73/280 | 0.52 (0.38–0.72) | 0.53 (0.35–0.80) | 17 | 7 (5–17) |
| Birth weight < 2500 g | 664, 65, 66, 67, 68, 69 | 244/315 | 223/280 | 0.97 (0.89–1.06) | 0.99 (0.89–1.10) | 0 | — |
| Admission to the NICU | 664, 65, 66, 67, 68, 69 | 211/315 | 209/282 | 0.92 (0.83–1.02) | 0.95 (0.84–1.08) | 0 | — |
| Mechanical ventilation | 664, 65, 66, 67, 68, 69 | 49/311 | 76/280 | 0.52 (0.37–0.71) | 0.54 (0.36–0.81) | 0 | 7 (5–17) |
Occurrence of any of the following events: respiratory distress syndrome, intraventricular hemorrhage, necrotizing enterocolitis, proven neonatal sepsis or neonatal death.
CI, confidence interval; NA, not applicable; NICU, neonatal intensive care unit; NNT, number needed to treat; refs, reference numbers; RR, relative risk.
Subgroup and sensitivity analyses
Subgroup analyses of the effect of vaginal progesterone on preterm birth < 33 weeks' gestation and neonatal death, according to CL, daily dose of vaginal progesterone and obstetric history, are shown in Table 5. There was no evidence that women in any one of the prespecified subgroups benefited more or less from the use of vaginal progesterone than those in any other subgroup (all, interaction P‐value ≥ 0.40). Nonetheless, vaginal progesterone was associated with a statistically significant reduction in the risk of preterm birth < 33 weeks' gestation and neonatal death in women with a CL between 10 and 20 mm (RR, 0.44 (95% CI, 0.22–0.87) and 0.20 (95% CI, 0.05–0.86), respectively) and women who were administered 400 mg of daily vaginal progesterone (RR, 0.66 (95% CI, 0.46–0.95) and 0.42 (95% CI, 0.23–0.76), respectively). Moreover, vaginal progesterone significantly decreased the risk of neonatal death in women with a CL between 21 and 25 mm (RR, 0.57 (95% CI, 0.36–0.90)) and women with no previous spontaneous preterm birth (RR, 0.58 (95% CI, 0.36–0.93)).
Table 5.
Subgroup analyses of the effect of vaginal progesterone on preterm birth < 33 weeks' gestation and neonatal death
| Preterm birth < 33 weeks' gestation | Neonatal death | |||||
|---|---|---|---|---|---|---|
| Subgroup | n | Pooled RR (95% CI) | Interaction P‐value | n | Pooled RR (95% CI)* | Interaction P‐value |
| Cervical length | 0.40 | 0.40 | ||||
| < 10 mm | 14 | 0.74 (0.37–1.49) | 28 | 0.67 (0.12–3.70) | ||
| 10–20 mm | 82 | 0.44 (0.22–0.87) | 164 | 0.20 (0.05–0.86) | ||
| 21–25 mm | 207 | 0.74 (0.51–1.06) | 414 | 0.57 (0.36–0.90) | ||
| Daily dose of vaginal progesterone | 0.77 | 0.60 | ||||
| 100 mg | 7 | 0.40 (0.04–3.74) | 14 | 0.09 (0.00–3.59) | ||
| 200 mg | 69 | 0.79 (0.48–1.30) | 138 | 0.66 (0.15–2.86) | ||
| 400 mg | 227 | 0.66 (0.46–0.95) | 454 | 0.42 (0.23–0.76) | ||
| Obstetric history | 0.40 | 0.62 | ||||
| No previous preterm birth | 247 | 0.72 (0.52–1.01) | 494 | 0.58 (0.36–0.93) | ||
| ≥ 1 previous preterm birth | 56 | 0.50 (0.22–1.11) | 112 | 0.45 (0.18–1.10) | ||
Adjusted for non‐independence between twins.
CI, confidence interval; RR, relative risk.
When the sensitivity analysis was restricted to the five trials with adequate blinding of patients, clinical staff and outcome assessors64, 65, 66, 67, 68, the effect of vaginal progesterone on the reduction in the risk of preterm birth < 33 weeks' gestation and neonatal death was non‐significant (RR, 0.77 (95% CI, 0.48–1.24) and 0.56 (95% CI, 0.21–1.48), respectively). However, it should be noted that the sensitivity analyses did not substantially change the magnitude and direction of effect sizes obtained in the overall analyses. Sensitivity analyses based on allocation concealment and random sequence generation were not performed because there were no trials at unclear or high risk of bias for these domains.
Effect of vaginal progesterone on long‐term neurodevelopmental outcomes
No study has reported the effects of vaginal progesterone on long‐term neurodevelopmental outcomes in twin gestations with a short cervix. Thus far, two trials have reported the effects of prenatal exposure to vaginal progesterone on long‐term neurodevelopmental outcomes in unselected twin gestations70, 71. In 2015, a follow‐up study of one of the excluded trials61 reported that there was no significant difference in developmental delay (assessed using the Child Development Inventory tool) between twins exposed to either vaginal progesterone (42/140) or placebo (65/184) at a mean age of 55.5 months (odds ratio (OR), 0.87 (95% CI, 0.46–1.63))70. Recently, one of the studies included in the review66 reported on the developmental performance of children exposed prenatally to vaginal progesterone (n = 225) or placebo (n = 212), at a mean age of 57 months71. The developmental performance was evaluated by the parent‐completed Ages and Stages Questionnaire (ASQ) screening tool. Overall, mean ASQ total scores were significantly higher in the vaginal progesterone‐exposed group (269 ± 28) than in the placebo‐exposed group (262 ± 31) (P = 0.03), although there was no statistically significant difference in the risk of low ASQ score (< 10th percentile) between the study groups (OR, 0.47 (95% CI, 0.21–1.06)). A subgroup analysis showed that dichorionic twins who were exposed prenatally to vaginal progesterone had a significantly lower risk of having a low total ASQ score than those who were exposed to placebo (OR, 0.34 (95% CI, 0.14–0.86)).
DISCUSSION
Principal findings
The main finding in this updated IPD meta‐analysis is that the administration of vaginal progesterone to asymptomatic women with a twin gestation and a mid‐trimester sonographic short cervix significantly reduces the risk of preterm birth < 33 weeks' gestation (primary outcome) by 31% and neonatal death by 47%. In addition, patients who received vaginal progesterone had a significantly decreased risk of preterm birth < 35, < 34, < 32 and < 30 weeks, spontaneous preterm birth < 33 and < 34 weeks, perinatal death, composite neonatal morbidity and mortality, RDS, birth weight < 1500 g and use of mechanical ventilation. Moreover, evidence from two trials that assessed vaginal progesterone in unselected twin gestations showed that there were no significant differences in the risk of neurodevelopmental disability at 4–5 years of age between children exposed prenatally to vaginal progesterone and those exposed to placebo.
Quality of the evidence
Evidence for most critical outcomes assessed with GRADE methodology was considered to be of moderate quality (Table S1). We downgraded the evidence from high quality to moderate quality because most of the pooled effect was provided by one study with moderate risk of bias. A judgment of moderate quality means that we have some confidence that our results approach the true impact of vaginal progesterone on preterm birth and adverse neonatal outcomes in twin gestations with a short cervix; at the same time, we acknowledge that future trials may change these results.
Subgroup analyses
We evaluated several clinically important subgroups based on CL, daily dose of vaginal progesterone and history of spontaneous preterm birth. Overall, subgroup analyses indicated that the beneficial effects of vaginal progesterone did not differ significantly across patient groups, as the interaction tests for subgroup differences were non‐significant. Patients with a CL between 10 and 20 mm or those who received vaginal progesterone 400 mg/day seemed to have a greater‐than‐average reduction in the risk of preterm birth < 33 weeks' gestation and neonatal death. However, analyses of categories such as CL < 10 mm, daily dose of vaginal progesterone of 100 or 200 mg and history of spontaneous preterm birth were based on small numbers of women, reflecting the pattern of recruitment to the original trials, in which most women had a CL between 10 and 25 mm, used vaginal progesterone 400 mg/day and did not have a history of spontaneous preterm birth. As a result, our analysis was limited in its power to estimate effects within those groups of patients. Thus, although prespecified and clinically interesting, these subgroup analyses should be interpreted with caution.
Lack of long‐term adverse neurodevelopmental outcomes in twins exposed to vaginal progesterone during pregnancy
Current evidence suggests that in‐utero exposure to vaginal progesterone, administered in twin gestations for the prevention of preterm birth, has no detrimental effects on long‐term neurodevelopmental outcomes. A total of 761 surviving children who participated in two placebo‐controlled trials of vaginal progesterone to prevent preterm birth in unselected twin gestations61, 66 were evaluated at a mean age of ∼56 months for neurodevelopmental outcomes70, 71. Both studies reported no significant differences in the risk of developmental delay70 or suspected developmental delay71 between children whose mothers received vaginal progesterone and those whose mothers received placebo. It should be noted that vaginal progesterone had no effect on gestational age at delivery in both trials, which allowed the assessment of the direct effect of vaginal progesterone on childhood neurodevelopmental outcomes independent of any effect of vaginal progesterone on preterm birth. Interestingly, a subgroup analysis of one of these studies70 found that dichorionic twins who were exposed prenatally to progesterone had a significantly reduced risk of a low total ASQ score, a higher total mean ASQ score and higher mean ASQ scores in communication, gross motor skills and personal/social skills in comparison with dichorionic twins who were exposed to placebo. These findings suggest a potential long‐term benefit related to prenatal exposure to vaginal progesterone, which would not be surprising because there is some evidence indicating that progesterone could act as a neuroprotectant for brain disorders, mainly traumatic brain injury72. Thereby, a direct beneficial effect of vaginal progesterone on childhood neurodevelopment would be plausible. This issue deserves further investigation.
Lack of long‐term adverse health outcomes in twins exposed to vaginal progesterone during pregnancy
With regard to the effects of the prenatal exposure to vaginal progesterone on childhood health outcomes in twins, the follow‐up study by McNamara et al.70 reported that there were no significant differences between vaginal progesterone‐exposed and placebo‐exposed twins with respect to death, congenital malformations, growth, health service utilization and global health status at 3–6 years of age. The follow‐up study by Vedel et al.71 reported that the rates of diagnoses related to 10 organ systems, the median number of hospital admissions and the median length of hospital stay did not differ significantly between the vaginal progesterone‐ and placebo‐exposed twins up to 8 years of age. Notwithstanding, in subgroup analyses restricted to dichorionic twins and diagnoses made solely during hospital admission, the investigators found that diagnoses related to structural and functional abnormalities of the heart were significantly more frequent among children who were exposed prenatally to vaginal progesterone. However, these differences became non‐significant after Bonferroni adjustment for multiple comparisons. In conclusion, second‐ and third‐trimester exposure to vaginal progesterone does not seem to have harmful effects on the childhood health of twins.
Lack of adverse maternal events
In our previous IPD meta‐analysis47, in which all included studies used vaginal progesterone 90–200 mg/day, the rates of maternal adverse effects, such as vaginal discharge, vaginal pruritus and discontinuation of treatment because of adverse effects, were similar between the vaginal progesterone and placebo groups. In 2013, the three‐armed trial by Serra et al.67 comparing placebo with two different daily doses of vaginal progesterone (200 and 400 mg) reported a dose‐dependent, non‐significant trend towards a higher rate of intrahepatic cholestasis of pregnancy (0% in the placebo group, 1% in the group receiving 200 mg and 5% in the group receiving 400 mg). Nonetheless, the larger study by El‐Refaie et al.69 reported that there was no significant difference in the rate of intrahepatic cholestasis of pregnancy between the group using 400 mg of daily vaginal progesterone (1%) and the no treatment group (0%). Moreover, this study found that the rates of vaginal pruritus, vaginal discharge, headache, skin rash and gastrointestinal symptoms did not differ significantly between the study groups. Thus, it appears that a 400‐mg daily dose of vaginal progesterone is not associated with an increased risk of adverse maternal effects as compared with a 200‐mg daily dose of vaginal progesterone or placebo/no treatment.
Strengths and limitations
The main strengths of our meta‐analysis include: (i) the use of patient‐level data, which offer several advantages over study‐level analysis, including the ability to use more appropriate statistical methods not always feasible using study‐level analysis, define outcome measures consistently across studies, investigate subgroups in which treatment may be either more or less effective, address questions that have not been satisfactorily resolved by individual trials, minimize publication and reporting biases and adjust for prognostic variables that may have confounded the original treatment comparisons; (ii) the baseline balance in prognostic factors between the two study groups, which reduces the possibility of causing bias in the intervention effect estimates; (iii) the absence of substantial heterogeneity in most of the meta‐analyses performed; indeed, all meta‐analyses on the effect of vaginal progesterone on preterm birth had no observed heterogeneity (I 2 = 0%), whereas the majority of meta‐analyses regarding adverse perinatal outcomes had low heterogeneity or no heterogeneity; and (iv) the sensitivity analyses restricted to trials at low risk of bias that were consistent with (and thus supportive of) the overall findings.
Some potential limitations must also be considered. First, only two trials were specifically designed to assess the efficacy of vaginal progesterone in women with a twin gestation and a sonographic short cervix. Second, 74% of the total sample size of the IPD meta‐analysis was provided by one study69, which included women with a CL between 20 and 25 mm and was not placebo‐controlled. However, it should be highlighted that assessment and measurement of most outcomes included in our review are considered objective in nature, and therefore not likely to be influenced by lack of blinding49. It is noteworthy that estimates of pooled RRs obtained after excluding this study were not significantly different from those obtained in the overall analyses. Moreover, the significant 39% reduction in the risk of composite neonatal morbidity and mortality associated with vaginal progesterone administration was obtained without including data from the study by El‐Refaie et al.69 in the meta‐analysis. Third, the larger study69 did not collect information about several neonatal morbidities, such as necrotizing enterocolitis, intraventricular hemorrhage, proven neonatal sepsis and retinopathy of prematurity. Finally, some subgroup analyses included a small number of patients, which limits the statistical power to estimate the effects within these subgroups.
Implications for practice and research
This updated IPD meta‐analysis indicates that vaginal progesterone reduces the risk of preterm birth and neonatal morbidity and mortality in patients with a twin gestation and a sonographic short cervix, without any deleterious effects on childhood neurodevelopment. Although the results of our meta‐analysis appear promising, further research is required before conclusive advice can be provided with regard to the benefits of using vaginal progesterone in women with a twin gestation and a short cervix. Evidence from this updated IPD meta‐analysis and three ongoing RCTs comparing vaginal progesterone with placebo (NCT02697331 and NCT02518594) or no treatment (NCT02329535) in ∼750 women with a twin gestation and a sonographic short cervix will help to determine whether vaginal progesterone can be recommended to these patients with the aim of preventing preterm birth and improving perinatal outcomes.
Supporting information
Table S1 Summary of findings of the quality of evidence for each outcome measure
ACKNOWLEDGMENT
This research was supported, in part, by the Perinatology Research Branch, Program for Perinatal Research and Obstetrics, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services (NICHD/NIH/DHHS); and, in part, with Federal funds from NICHD/NIH/DHHS under Contract No. HHSN275201300006C.
The copyright line for this article was changed on 21 March 2017 after original online publication.
REFERENCES
- 1. Ananth CV, Chauhan SP. Epidemiology of twinning in developed countries. Semin Perinatol 2012; 36: 156–161. [DOI] [PubMed] [Google Scholar]
- 2. Martin JA, Hamilton BE, Osterman MJ. Three decades of twin births in the United States, 1980–2009. NCHS Data Brief 2012; 80: 1–8. [PubMed] [Google Scholar]
- 3. Chauhan SP, Scardo JA, Hayes E, Abuhamad AZ, Berghella V. Twins: prevalence, problems, and preterm births. Am J Obstet Gynecol 2010; 203: 305–315. [DOI] [PubMed] [Google Scholar]
- 4. Hamilton BE, Martin JA, Osterman MJ, Curtin SC, Matthews TJ. Births: Final Data for 2014. Natl Vital Stat Rep 2015; 64: 1–64. [PubMed] [Google Scholar]
- 5. Conde‐Agudelo A, Belizán JM, Lindmark G. Maternal morbidity and mortality associated with multiple gestations. Obstet Gynecol 2000; 95: 899–904. [PubMed] [Google Scholar]
- 6. Blickstein I. Maternal mortality in twin gestations. J Reprod Med 1997; 42: 680–684. [PubMed] [Google Scholar]
- 7. Kinzler WL, Ananth CV, Vintzileos AM. Medical and economic effects of twin gestations. J Soc Gynecol Investig 2000; 7: 321–327. [PubMed] [Google Scholar]
- 8. Walker MC, Murphy KE, Pan S, Yang Q, Wen SW. Adverse maternal outcomes in multifetal pregnancies. BJOG 2004; 111: 1294–1296. [DOI] [PubMed] [Google Scholar]
- 9. Vogel JP, Torloni MR, Seuc A, Betrán AP, Widmer M, Souza JP, Merialdi M. Maternal and perinatal outcomes of twin pregnancy in 23 low‐ and middle‐income countries. PLoS One 2013; 8: e70549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Burgess JL, Unal ER, Nietert PJ, Newman RB. Risk of late‐preterm stillbirth and neonatal morbidity for monochorionic and dichorionic twins. Am J Obstet Gynecol 2014; 210: 578.e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Blickstein I. Do multiple gestations raise the risk of cerebral palsy? Clin Perinatol 2004; 31: 395–408. [DOI] [PubMed] [Google Scholar]
- 12. Pharoah PO. Risk of cerebral palsy in multiple pregnancies. Clin Perinatol 2006; 33: 301–313. [DOI] [PubMed] [Google Scholar]
- 13. Lorenz JM. Neurodevelopmental outcomes of twins. Semin Perinatol 2012; 36: 201–212. [DOI] [PubMed] [Google Scholar]
- 14. Luke B, Brown MB, Alexandre PK, Kinoshi T, O'Sullivan MJ, Martin D, Misiunas RB, Nugent C, van de Ven C, Newman RB, Mauldin JG, Witter FR. The cost of twin pregnancy: maternal and neonatal factors. Am J Obstet Gynecol 2005; 192: 909–915. [DOI] [PubMed] [Google Scholar]
- 15. Henderson J, Hockley C, Petrou S, Goldacre M, Davidson L. Economic implications of multiple births: inpatient hospital costs in the first 5 years of life. Arch Dis Child Fetal Neonatal Ed 2004; 89: F542–F545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Crowther CA, Han S. Hospitalisation and bed rest for multiple pregnancy. Cochrane Database Syst Rev 2010; 7: CD000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yamasmit W, Chaithongwongwatthana S, Tolosa JE, Limpongsanurak S, Pereira L, Lumbiganon P. Prophylactic oral betamimetics for reducing preterm birth in women with a twin pregnancy. Cochrane Database Syst Rev 2015; 12: CD004733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Goodnight W, Newman R; Society of Maternal‐Fetal Medicine . Optimal nutrition for improved twin pregnancy outcome. Obstet Gynecol 2009; 114: 1121–1134. [DOI] [PubMed] [Google Scholar]
- 19. Schuit E, Stock S, Rode L, Rouse DJ, Lim AC, Norman JE, Nassar AH, Serra V, Combs CA, Vayssiere C, Aboulghar MM, Wood S, Çetingöz E, Briery CM, Fonseca EB, Worda K, Tabor A, Thom EA, Caritis SN, Awwad J, Usta IM, Perales A, Meseguer J, Maurel K, Garite T, Aboulghar MA, Amin YM, Ross S, Cam C, Karateke A, Morrison JC, Magann EF, Nicolaides KH, Zuithoff NP, Groenwold RH, Moons KG, Kwee A, Mol BW; Global Obstetrics Network (GONet) collaboration . Effectiveness of progestogens to improve perinatal outcome in twin pregnancies: an individual participant data meta‐analysis. BJOG 2015; 122: 27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rafael TJ, Berghella V, Alfirevic Z. Cervical stitch (cerclage) for preventing preterm birth in multiple pregnancy. Cochrane Database Syst Rev 2014; 9: CD009166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liem S, Schuit E, Hegeman M, Bais J, de Boer K, Bloemenkamp K, Brons J, Duvekot H, Bijvank BN, Franssen M, Gaugler I, de Graaf I, Oudijk M, Papatsonis D, Pernet P, Porath M, Scheepers L, Sikkema M, Sporken J, Visser H, van Wijngaarden W, Woiski M, van Pampus M, Mol BW, Bekedam D. Cervical pessaries for prevention of preterm birth in women with a multiple pregnancy (ProTWIN): a multicentre, open‐label randomised controlled trial. Lancet 2013; 382: 1341–1349. [DOI] [PubMed] [Google Scholar]
- 22. Nicolaides KH, Syngelaki A, Poon LC, de Paco Matallana C, Plasencia W, Molina FS, Picciarelli G, Tul N, Celik E, Lau TK, Conturso R. Cervical pessary placement for prevention of preterm birth in unselected twin pregnancies: a randomized controlled trial. Am J Obstet Gynecol 2016; 214: 3.e1–9. [DOI] [PubMed] [Google Scholar]
- 23. Iams JD, Goldenberg RL, Meis PJ, Mercer BM, Moawad A, Das A, Thom E, McNellis D, Copper RL, Johnson F, Roberts JM. The length of the cervix and the risk of spontaneous premature delivery. N Engl J Med 1996; 334: 567–572. [DOI] [PubMed] [Google Scholar]
- 24. Hassan SS, Romero R, Berry SM, Dang K, Blackwell SC, Treadwell MC, Wolfe HM. Patients with an ultrasonographic cervical length < or =15 mm have nearly a 50% risk of early spontaneous preterm delivery. Am J Obstet Gynecol 2000; 182: 1458–1467. [DOI] [PubMed] [Google Scholar]
- 25. Honest H, Bachmann LM, Coomarasamy A, Gupta JK, Kleijnen J, Khan KS. Accuracy of cervical transvaginal sonography in predicting preterm birth: a systematic review. Ultrasound Obstet Gynecol 2003; 22: 305–322. [DOI] [PubMed] [Google Scholar]
- 26. Crane JM, Hutchens D. Transvaginal sonographic measurement of cervical length to predict preterm birth in asymptomatic women at increased risk: a systematic review. Ultrasound Obstet Gynecol 2008; 31: 579–587. [DOI] [PubMed] [Google Scholar]
- 27. Honest H, Forbes CA, Durée KH, Norman G, Duffy SB, Tsourapas A, Roberts TE, Barton PM, Jowett SM, Hyde CJ, Khan KS. Screening to prevent spontaneous preterm birth: systematic reviews of accuracy and effectiveness literature with economic modelling. Health Technol Assess 2009; 13: 1–627. [DOI] [PubMed] [Google Scholar]
- 28. Domin CM, Smith EJ, Terplan M. Transvaginal ultrasonographic measurement of cervical length as a predictor of preterm birth: a systematic review with meta‐analysis. Ultrasound Q 2010; 26: 241–248. [DOI] [PubMed] [Google Scholar]
- 29. Barros‐Silva J, Pedrosa AC, Matias A. Sonographic measurement of cervical length as a predictor of preterm delivery: a systematic review. J Perinat Med 2014; 42: 281–293. [DOI] [PubMed] [Google Scholar]
- 30. Goldenberg RL, Iams JD, Miodovnik M, Van Dorsten JP, Thurnau G, Bottoms S, Mercer BM, Meis PJ, Moawad AH, Das A, Caritis SN, McNellis D. The preterm prediction study: risk factors in twin gestations. National Institute of Child Health and Human Development Maternal‐Fetal Medicine Units Network. Am J Obstet Gynecol 1996; 175: 1047–1053. [DOI] [PubMed] [Google Scholar]
- 31. Gibson JL, Macara LM, Owen P, Young D, Macauley J, Mackenzie F. Prediction of preterm delivery in twin pregnancy: a prospective, observational study of cervical length and fetal fibronectin testing. Ultrasound Obstet Gynecol 2004; 23: 561–566. [DOI] [PubMed] [Google Scholar]
- 32. Sperling L, Kiil C, Larsen LU, Qvist I, Bach D, Wøjdemann K, Bladh A, Nikkilä A, Jørgensen C, Skajaa K, Bang J, Tabor A. How to identify twins at low risk of spontaneous preterm delivery. Ultrasound Obstet Gynecol 2005; 26: 138–144. [DOI] [PubMed] [Google Scholar]
- 33. Arabin B, Roos C, Kollen B, van Eyck J. Comparison of transvaginal sonography in recumbent and standing maternal positions to predict spontaneous preterm birth in singleton and twin pregnancies. Ultrasound Obstet Gynecol 2006; 27: 377–386. [DOI] [PubMed] [Google Scholar]
- 34. To MS, Fonseca EB, Molina FS, Cacho AM, Nicolaides KH. Maternal characteristics and cervical length in the prediction of spontaneous early preterm delivery in twins. Am J Obstet Gynecol 2006; 194: 1360–1365. [DOI] [PubMed] [Google Scholar]
- 35. Conde‐Agudelo A, Romero R, Hassan SS, Yeo L. Transvaginal sonographic cervical length for the prediction of spontaneous preterm birth in twin pregnancies: a systematic review and metaanalysis. Am J Obstet Gynecol 2010; 203: 128.e1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lim AC, Hegeman MA, Huis In ‘T Veld MA, Opmeer BC, Bruinse HW, Mol BW. Cervical length measurement for the prediction of preterm birth in multiple pregnancies: a systematic review and bivariate meta‐analysis. Ultrasound Obstet Gynecol 2011; 38: 10–17. [DOI] [PubMed] [Google Scholar]
- 37. Kindinger L, Ashrafian H, Poon L, Fox NS, Nicolaides K, Darzi K, Teoh TG, Bennett P. Prediction of preterm delivery with cervical length in twin pregnancy: A meta‐analysis and systematic review. Reprod Sci 2014; 21: 256A. [Google Scholar]
- 38. Conde‐Agudelo A, Romero R. Prediction of preterm birth in twin gestations using biophysical and biochemical tests. Am J Obstet Gynecol 2014; 211: 583–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Conde‐Agudelo A, Romero R. Predictive accuracy of changes in transvaginal sonographic cervical length over time for preterm birth: a systematic review and metaanalysis. Am J Obstet Gynecol 2015; 213: 789–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pagani G, Stagnati V, Fichera A, Prefumo F. Cervical length at mid‐gestation in screening for preterm birth in twin pregnancy. Ultrasound Obstet Gynecol 2016; 48: 56–60. [DOI] [PubMed] [Google Scholar]
- 41. Kindinger LM, Poon LC, Cacciatore S, MacIntyre DA, Fox NS, Schuit E, Mol BW, Liem S, Lim AC, Serra V, Perales A, Hermans F, Darzi A, Bennett P, Nicolaides KH, Teoh TG. The effect of gestational age and cervical length measurements in the prediction of spontaneous preterm birth in twin pregnancies: an individual patient level meta‐analysis. BJOG 2016; 123: 877–884. [DOI] [PubMed] [Google Scholar]
- 42. Melamed N, Pittini A, Hiersch L, Yogev Y, Korzeniewski SS, Romero R, Barrett J. Serial cervical length determination in twin pregnancies reveals 4 distinct patterns with prognostic significance for preterm birth. Am J Obstet Gynecol 2016; 215: 476.e1–476.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Melamed N, Pittini A, Hiersch L, Yogev Y, Korzeniewski SJ, Romero R, Barrett J. Do serial measurements of cervical length improve the prediction of preterm birth in asymptomatic women with twin gestations? Am J Obstet Gynecol 2016; 215: 616.e1–616.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Romero R, Nicolaides KH, Conde‐Agudelo A, O'Brien JM, Cetingoz E, Da Fonseca E, Creasy GW, Hassan SS. Vaginal progesterone decreases preterm birth ≤ 34 weeks of gestation in women with a singleton pregnancy and a short cervix: an updated meta‐analysis including data from the OPPTIMUM study. Ultrasound Obstet Gynecol 2016: 48: 308–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Conde‐Agudelo A, Romero R. Vaginal progesterone to prevent preterm birth in pregnant women with a sonographic short cervix: clinical and public health implications. Am J Obstet Gynecol 2016; 214: 235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Romero R, Yeo L, Miranda J, Hassan SS, Conde‐Agudelo A, Chaiworapongsa T. A blueprint for the prevention of preterm birth: vaginal progesterone in women with a short cervix. J Perinat Med 2013; 41: 27–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Romero R, Nicolaides K, Conde‐Agudelo A, Tabor A, O'Brien JM, Cetingoz E, Da Fonseca E, Creasy GW, Klein K, Rode L, Soma‐Pillay P, Fusey S, Cam C, Alfirevic Z, Hassan SS. Vaginal progesterone in women with an asymptomatic sonographic short cervix in the midtrimester decreases preterm delivery and neonatal morbidity: a systematic review and metaanalysis of individual patient data. Am J Obstet Gynecol 2012; 206: 124.e1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 2009; 62: e1–34. [DOI] [PubMed] [Google Scholar]
- 49. Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies In Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0 (updated March 2011), Higgins JPT, Green S. (eds). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org [Google Scholar]
- 50. Simmonds MC, Higgins JP, Stewart LA, Tierney JF, Clarke MJ, Thompson SG. Meta‐analysis of individual patient data from randomized trials: a review of methods used in practice. Clin Trials 2005; 2: 209–217. [DOI] [PubMed] [Google Scholar]
- 51. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003; 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gates S, Brocklehurst P. How should randomised trials including multiple pregnancies be analysed? BJOG 2004; 111: 213–219. [DOI] [PubMed] [Google Scholar]
- 53. Yelland LN, Salter AB, Ryan P, Makrides M. Analysis of binary outcomes from randomised trials including multiple births: when should clustering be taken into account? Paediatr Perinat Epidemiol 2011; 25: 283–297. [DOI] [PubMed] [Google Scholar]
- 54. Higgins JPT, Deeks JJ, Altman DG. Chapter 16: Special topics in statistics In Cochrane Handbook for Systematic Reviews of Interventions, version 5.1.0 (updated March 2011), Higgins JPT, Green S. (eds). The Cochrane Collaboration, 2011. Available at http://handbook.cochrane.org. [Google Scholar]
- 55. Altman DG. Confidence intervals for the number needed to treat. BMJ 1998; 317: 1309–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Rothwell PM. Treating individuals 2. Subgroup analysis in randomized controlled trials: importance, indications, and interpretation. Lancet 2005; 365: 176–186. [DOI] [PubMed] [Google Scholar]
- 57. Klebanoff MA. Subgroup analysis in obstetrics clinical trials. Am J Obstet Gynecol 2007; 197: 119–122. [DOI] [PubMed] [Google Scholar]
- 58. Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analyzing data and undertaking meta‐analyses In Cochrane Handbook for Systematic Reviews of Interventions, version 5.1.0 (updated March 2011), Higgins JPT, Green S. (eds). The Cochrane Collaboration, 2011. Available at http://handbook.cochrane.org. [Google Scholar]
- 59. Schünemann H, Brożek J, Guyatt G, Oxman A (eds). GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations. Updated October 2013. The GRADE Working Group, 2013. Available at https://gdt.gradepro.org/app/handbook/handbook.html. [Google Scholar]
- 60. GRADEpro GDT: GRADEpro Guideline Development Tool [Software] . McMaster University, 2015. (developed by Evidence Prime, Inc.). Available from gradepro.org
- 61. Norman JE, Mackenzie F, Owen P, Mactier H, Hanretty K, Cooper S, Calder A, Mires G, Danielian P, Sturgiss S, MacLennan G, Tydeman G, Thornton S, Martin B, Thornton JG, Neilson JP, Norrie J. Progesterone for the prevention of preterm birth in twin pregnancy (STOPPIT): a randomised, double‐blind, placebo‐controlled study and meta‐analysis. Lancet 2009; 373: 2034–2040. [DOI] [PubMed] [Google Scholar]
- 62. Wood S, Ross S, Tang S, Miller L, Sauve R, Brant R. Vaginal progesterone to prevent preterm birth in multiple pregnancy: a randomized controlled trial. J Perinat Med 2012; 40: 593–599. [DOI] [PubMed] [Google Scholar]
- 63. Aboulghar MM, Aboulghar MA, Amin YM, Al‐Inany HG, Mansour RT, Serour GI. The use of vaginal natural progesterone for prevention of preterm birth in IVF/ICSI pregnancies. Reprod Biomed Online 2012; 25: 133–138. [DOI] [PubMed] [Google Scholar]
- 64. Fonseca EB, Celik E, Parra M, Singh M, Nicolaides KH; Group Fetal Medicine Foundation Second Trimester Screening. Progesterone and the risk of preterm birth among women with a short cervix. N Engl J Med 2007; 357: 462–469. [DOI] [PubMed] [Google Scholar]
- 65. Cetingoz E, Cam C, Sakalli M, Karateke A, Celik C, Sancak A. Progesterone effects on preterm birth in high‐risk pregnancies: a randomized placebo‐controlled trial. Arch Gynecol Obstet 2011; 283: 423–429. [DOI] [PubMed] [Google Scholar]
- 66. Rode L, Klein K, Nicolaides KH, Krampl‐Bettelheim E, Tabor A; PREDICT Group . Prevention of preterm delivery in twin gestations (PREDICT): a multicenter, randomized, placebo‐controlled trial on the effect of vaginal micronized progesterone. Ultrasound Obstet Gynecol 2011; 38: 272–280. [DOI] [PubMed] [Google Scholar]
- 67. Serra V, Perales A, Meseguer J, Parrilla JJ, Lara C, Bellver J, Grifol R, Alcover I, Sala M, Martínez‐Escoriza JC, Pellicer A. Increased doses of vaginal progesterone for the prevention of preterm birth in twin pregnancies: a randomised controlled double‐blind multicentre trial. BJOG 2013; 120: 50–57. [DOI] [PubMed] [Google Scholar]
- 68. Brizot ML, Hernandez W, Liao AW, Bittar RE, Francisco RP, Krebs VL, Zugaib M. Vaginal progesterone for the prevention of preterm birth in twin gestations: a randomized placebo‐controlled double‐blind study. Am J Obstet Gynecol 2015; 213: 82.e1–9. [DOI] [PubMed] [Google Scholar]
- 69. El‐Refaie W, Abdelhafez MS, Badawy A. Vaginal progesterone for prevention of preterm labor in asymptomatic twin pregnancies with sonographic short cervix: a randomized clinical trial of efficacy and safety. Arch Gynecol Obstet 2016; 293: 61–67. [DOI] [PubMed] [Google Scholar]
- 70. McNamara HC, Wood R, Chalmers J, Marlow N, Norrie J, MacLennan G, McPherson G, Boachie C, Norman JE. STOPPIT Baby Follow‐up Study: the effect of prophylactic progesterone in twin pregnancy on childhood outcome. PLoS One 2015; 10: e0122341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Vedel C, Larsen H, Holmskov A, Andreasen KR, Uldbjerg N, Ramb J, Bødker B, Skibsted L, Sperling L, Krebs L, Zingenberg H, Laursen L, Christensen JT, Tabor A, Rode L. Long‐term effects of prenatal progesterone exposure: neurophysiological development and hospital admissions in twins up to 8 years of age. Ultrasound Obstet Gynecol 2016; 48: 382–389. [DOI] [PubMed] [Google Scholar]
- 72. Schumacher M, Denier C, Oudinet JP, Adams D, Guennoun R. Progesterone neuroprotection: The background of clinical trial failure. J Steroid Biochem Mol Biol 2016; 160: 53–66. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Summary of findings of the quality of evidence for each outcome measure


