Abstract
Objective
Tofacitinib is an oral JAK inhibitor that is used for the treatment of rheumatoid arthritis (RA). In previous clinical trials of tofacitinib, a Disease Activity Score in 28 joints (DAS28)–based analysis was used to assess outcomes. In this study, remission rates according to various remission criteria were evaluated across 5 phase III randomized controlled studies.
Methods
In all 5 studies, tofacitinib was administered at a dosage of 5 mg twice daily or 10 mg twice daily, either as monotherapy or with background methotrexate or other conventional synthetic disease‐modifying antirheumatic drugs. One of the studies included adalimumab 40 mg once every 2 weeks. In addition to the 4‐variable DAS28 using the erythrocyte sedimentation rate (DAS28‐4[ESR]), a primary efficacy variable used in the phase III studies, disease activity was assessed post hoc by the 4‐variable DAS28 using the C‐reactive protein level (DAS28‐4[CRP]), the Clinical Disease Activity Index (CDAI), the Simplified Disease Activity Index (SDAI), and Boolean‐based assessment.
Results
A total of 3,306 patients were analyzed (1,213 of these patients received tofacitinib 5 mg twice daily, 1,212 received tofacitinib 10 mg twice daily, 679 received placebo, and 202 received adalimumab 40 mg every 2 weeks). Remission rates varied according to the criteria used, with higher rates in the active‐treatment groups for the DAS28‐4(CRP) than for other scores. At month 3, remission rates with tofacitinib 5 mg twice daily were 18–22% using the DAS28‐4(CRP), 5–10% using the DAS28‐4(ESR), 4–7% using the SDAI, 5–6% using the CDAI, and 2–7% using the Boolean‐based method. In contrast, the remission rates with placebo varied from 0% to 7%, with small differences between the DAS28‐4(ESR) and the DAS28‐4(CRP).
Conclusion
Although tofacitinib at dosages of 5 mg twice daily and 10 mg twice daily was effective compared with placebo in achieving disease remission, regardless of the disease activity measure, remission rates were substantially higher when the DAS28‐4(CRP) was used. The presence or absence and type of acute‐phase reactants in remission criteria were significant contributors to remission rates across treatment groups. This finding has important consequences for trial design and clinical practice.
The aim of rheumatoid arthritis (RA) treatment is to decrease synovial inflammation, relieve symptoms, improve health‐related quality of life, and prevent joint damage. If remission (the absence of disease activity) is unattainable, particularly in patients with longstanding RA, a state of low disease activity is targeted 1, 2.
Several criteria for defining disease states are used in clinical practice, including the 28‐joint Disease Activity Score using the erythrocyte sedimentation rate (DAS28‐ESR), the DAS28 using the C‐reactive protein level (DAS28‐CRP) 3, 4, the Simplified Disease Activity Index (SDAI) 5, and the Clinical Disease Activity Index (CDAI) 4. In 2011, the American College of Rheumatology (ACR) and the European League Against Rheumatism (EULAR) devised new provisional definitions of remission: an index‐based approach using the SDAI definition or the CDAI definition of remission (≤3.3 and ≤2.8, respectively) and a Boolean‐based approach requiring scores of ≤1 for a number of individual measures of disease activity 6. Although traditional definitions of remission have included the DAS28‐ESR (<2.6) or the DAS28‐CRP (<2.6) 3, 4, these definitions have not been regarded as valid criteria by the ACR and EULAR 6. A state of low disease activity is typically defined by a higher cutoff point within a composite measure (e.g., SDAI low disease activity is defined as a score of ≤11.0, and DAS28 low disease activity is defined as a score of ≤3.2).
Tofacitinib is an oral JAK inhibitor used for the treatment of RA. In the phase III studies of tofacitinib, the 4‐variable DAS28‐ESR (DAS28‐4[ESR])–based analysis was consistently used to assess remission and low disease activity 7, 8, 9, 10, 11. Here, we investigated the rates of remission and low disease activity according to 5 different definitions in 5 phase III randomized controlled studies of tofacitinib and explored the consistency and reasons for possible inconsistencies in the reported rates.
PATIENTS AND METHODS
Study design and patients
The 5 phase III studies included in this analysis had a duration of 6–24 months, with tofacitinib (5 mg or 10 mg twice daily) administered as monotherapy (ORAL Solo, ClinicalTrials.gov identifier: NCT00814307 [7]); with background methotrexate (ORAL Scan, ClinicalTrials.gov identifier: NCT00847613 [8], ORAL Step, ClinicalTrials.gov identifier: NCT00960440 [9], and ORAL Standard, ClinicalTrials.gov identifier: NCT00853385 [10]); or with conventional synthetic disease‐modifying antirheumatic drugs (ORAL Sync, ClinicalTrials.gov identifier: NCT00856544 [11]). ORAL Standard 10 also included an active‐treatment control arm, with adalimumab 40 mg administered subcutaneously once every 2 weeks with background methotrexate. Patient inclusion and exclusion criteria were similar for all 5 studies and have been reported previously 7, 8, 9, 10, 11.
All studies were conducted in compliance with the Declaration of Helsinki and the International Conference on Harmonisation Good Clinical Practice Guidelines. All patients provided written informed consent. Final protocols, amendments, and consent documentation were reviewed and approved by the Institutional Review Board and/or Independent Ethics Committee of the study centers.
Remission and low disease activity assessments
Five disease activity measures were used for this analysis. The component variables included the following: 28‐joint tender joint count, 28‐joint swollen joint count, patient's global assessment determined using a visual analog scale (VAS [0–10 cm]), physician's global assessment determined using a VAS, the ESR, and the CRP level. The formulas for determining the DAS28, SDAI, and CDAI disease activity measures as well as cutoff scores for remission and low disease activity were previously developed 4, 6. Remission and low disease activity were defined as <2.6 and ≤3.2, respectively, for the 4‐variable DAS28 using the CRP level (DAS28‐4[CRP]), <2.6 and ≤3.2, respectively, for the DAS28‐4(ESR), ≤2.8 and ≤10.0, respectively, for the CDAI, and ≤3.3 and ≤11.0, respectively, for the SDAI. Achievement of a Boolean‐based definition of remission required that patients have a 28‐joint tender joint count of ≤1, a 28‐joint swollen joint count of ≤1, a CRP level of ≤1 mg/dl, and a patient's global assessment score of ≤1 cm (using a 0–10‐cm VAS).
Statistical analysis
The full analysis set included all randomized patients who received ≥1 dose of study drug and had ≥1 postbaseline assessment. Analyses were carried out using the data as observed, with last observation carried forward (LOCF) imputation for missing values; only postbaseline values were carried forward. The normal approximation for a difference in binomial proportions was used to test each tofacitinib dose against placebo. No preservation of Type I error was applied, and P values less than 0.05 were considered significant. Descriptive statistics for continuous variables were calculated using the observed data (for applicable studies, patients who advanced from placebo to tofacitinib had their month 6 values set to missing). For additional analyses of the CRP level and the ESR, values were pooled across the 5 studies; the actual change from baseline and the percent change from baseline were calculated from the pooled mean values, and changes are presented at the group level.
The primary analyses in the 5 studies presented here applied nonresponder imputation for missing values. Therefore, small differences in remission and low disease activity rates may be observed between this analysis and the primary analyses 7, 8, 9, 10, 11.
RESULTS
This analysis included 3,306 patients: 1,213 patients received tofacitinib 5 mg twice daily, 1,212 patients received tofacitinib 10 mg twice daily, 679 patients received placebo, and 202 patients received adalimumab 40 mg every 2 weeks. The baseline demographics and disease characteristics were generally consistent across the 5 studies 7, 8, 9, 10, 11.
Comparison of remission rates across indices
Across all 5 studies, the DAS28‐4(CRP) criteria generated higher remission rates versus the Boolean‐based, the DAS28‐4(ESR), SDAI, and CDAI definitions (Figure 1). In all instances the DAS28‐4(CRP) remission rates during active therapy were at least 2‐fold higher, and up to 5‐fold higher, than the DAS28‐4(ESR) remission rates. This difference was also observed for the DAS28‐4(CRP) low disease activity rate versus the DAS28‐4(ESR) low disease activity rate (additional information is available upon request from the corresponding author). Low disease activity rates as defined by the SDAI, CDAI, and DAS28‐4(CRP) were similar and were higher than DAS28‐4(ESR) low disease activity rates.
Figure 1.
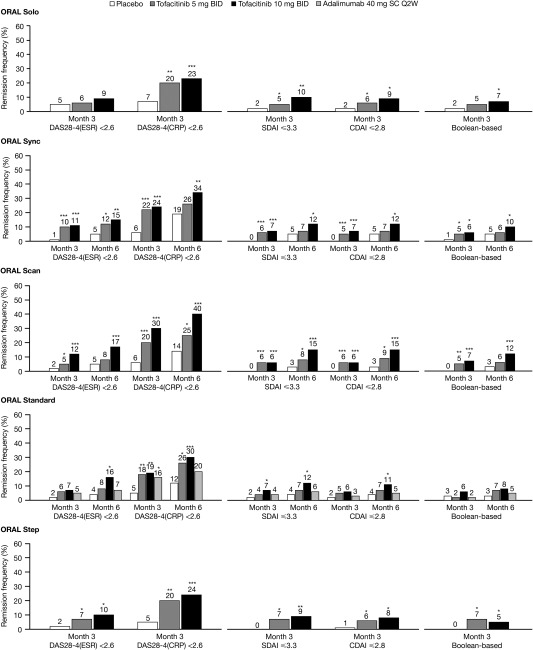
Frequency of remission across all 5 studies, defined as a 4‐variable Disease Activity Score in 28 joints using the erythrocyte sedimentation rate (DAS28‐4[ESR]) <2.6, a 4‐variable DAS28 using the C‐reactive protein level (DAS28‐4[CRP]) <2.6, a Simplified Disease Activity Index (SDAI) score ≤3.3, a Clinical Disease Activity Index (CDAI) score ≤2.8, and Boolean‐based remission, using the last observation carried forward method in the full analysis set. ∗ = P < 0.05; ∗∗ = P < 0.001; ∗∗∗ = P < 0.0001 versus placebo. BID = twice daily; Q2W = once every 2 weeks; SC = subcutaneous.
The cutoff points for defining remission and low disease activity were originally established using the DAS28‐4(ESR) and have not been sufficiently validated for the DAS28‐4(CRP). Alternative DAS28‐4(CRP) cutoff points have been estimated that correspond to the respective cutoff points for the DAS28‐4(ESR) and SDAI 12. As would be expected, lower remission response rates were observed when the alternative, lower cutoff point for remission was used (2.4 versus 2.6); however, the response rates were still substantially higher for the DAS28‐4(CRP) than for the other measures (additional information is available upon request from the corresponding author). A similar effect was observed for rates of low disease activity using the alternative cutoff point (2.9 versus 3.2).
To investigate whether the ESR or the CRP level was a primary component contributing to these differences, the mean changes from baseline in the ESR and CRP level at month 3 were compared (additional information is available upon request from the corresponding author). Clinically significant reductions in both the ESR and the CRP level were observed in the active‐treatment groups. However, the postbaseline values for acute‐phase reactants had differentially weighted effects on the DAS‐defined target thresholds. For example, after 3 months of treatment with tofacitinib 10 mg twice daily, the resulting mean CRP concentration of 5.4 mg/liter across the 5 studies contributed 0.67 points to the DAS28‐4(CRP) (or 1.63 points when accounting for “+0.96” in the formula), while the mean ESR value of 32.2 mm/hour contributed 2.43 points to the DAS28‐4(ESR).
The relative change from baseline in the CRP levels, based on pooled mean values, was stable in the placebo group (−1.3%) and ranged from −69.1% to −54.0% in the active‐treatment groups. Similar trends were observed for change from baseline in the ESR, which was comparably stable in the placebo group (−9.1%) but ranged from only −37.8% to −31.9% for the active‐treatment groups. Therefore, given this different weighting of the acute‐phase reactants and the same cutoff point for remission (<2.6), a much smaller proportion of patients will achieve DAS28‐4(ESR) remission versus DAS28‐4(CRP) remission, if the other score components (joint counts and patient's global assessments) are equal, as is the case in the individual trials 13, 14.
Frequencies of remission
Remission and low disease activity rates are described at the time point at which the primary efficacy end point was assessed for each study: month 3 for ORAL Step and ORAL Solo, and month 6 for ORAL Scan, ORAL Standard, and ORAL Sync. The remission rates based on the DAS28, CDAI, and SDAI using LOCF were generally statistically significantly greater for both tofacitinib doses versus placebo and were generally numerically higher for tofacitinib 10 mg twice daily than for 5 mg twice daily (Figure 1). Remission rates were lower for the DAS28‐4(ESR) versus the DAS28‐4(CRP), while CDAI‐ and SDAI‐defined rates of remission and low disease activity were similar, despite the use of the CRP level in the SDAI formula. Mean values for the DAS‐based measures, the CDAI, and the SDAI across the 5 studies are available upon request from the corresponding author.
Of note, a co‐primary end point in ORAL Solo was DAS28‐4(ESR) remission at 3 months, which was not significantly different between the placebo and active‐treatment groups (Figure 1). In contrast, the rate of DAS28‐4(CRP) remission was significantly higher in patients treated with tofacitinib compared with those treated with placebo. Importantly, although the SDAI and CDAI remission rates were much lower than the DAS28‐4(CRP) remission rates, the SDAI and CDAI remission rates in the active‐treatment groups were significantly different from the rates in the placebo group (Figure 1). Consistent with this, similar results were observed when low disease activity was used as an end point (additional information is available upon request from the corresponding author).
Boolean‐based remission rates were statistically significantly greater for tofacitinib 10 mg twice daily than for placebo in all studies except ORAL Standard, and for tofacitinib 5 mg twice daily versus placebo for ORAL Step only (Figure 1). Remission rates were generally numerically greater for tofacitinib 10 mg twice daily than for 5 mg twice daily, with the exception of the ORAL Step study.
DISCUSSION
In this analysis, we used data from 5 phase III clinical studies of tofacitinib in RA to compare remission rates using 5 disease status criteria. Across the 5 studies, DAS28‐4(CRP) criteria generated 2‐fold to 5‐fold higher remission rates compared with the DAS28‐4(ESR). At month 3, remission rates with tofacitinib 5 mg twice daily were 18–22% using the DAS28‐4(CRP) and 5–10% using the DAS28‐4(ESR) and with tofacitinib 10 mg twice daily were 19–30% and 7–12%, respectively. Remission rates determined using the SDAI and the CDAI were consistently similar to each other. At month 3, remission rates with tofacitinib 5 mg twice daily were 4–7% using the SDAI and 5–6% using the CDAI and with tofacitinib 10 mg twice daily were 6–10% and 6–9%, respectively. As expected, these rates were also similar to rates using the Boolean‐based method. At month 3, remission rates with tofacitinib 5 mg twice daily were 2–7% and with tofacitinib 10 mg twice daily were 5–7%.
It has been noted that the DAS28‐4(CRP) conveys lower scores than the DAS28‐4(ESR) at identical joint counts and global assessments 12, and with a remission cutoff of <2.6, the DAS28‐4(CRP) may underestimate disease activity more than the DAS28‐4(ESR). However, the extent of the discrepancy between these 2 variants of the same type of score is surprising. In addition, previous studies have shown similar low disease activity response rates when using these DAS28 variants 12. In the current analysis, however, low disease activity response rates were ∼2‐fold higher for the DAS28‐4(CRP) than for the DAS28‐4(ESR). Given that the tender joint count, the swollen joint count, and the global assessment scores were identical using the DAS28‐4(CRP) and the DAS28‐4(ESR) and the ESR and CRP level are transformed in a similar manner in the 2 formulas, the likely explanations for these differences may be the differential effects of drugs with distinct mechanisms of action on the ESR and CRP level. Alternatively, the term “0.36*ln(CRP+1) + 0.96” of the DAS28‐4(CRP) may not adequately replace the term “0.70*ln(ESR)” of the DAS28‐ESR in patients with lower levels of disease activity when agents affecting the acute‐phase response are used. As a consequence, a greater reduction in the ESR term than in the CRP term is needed to achieve the same score for the 2 DAS28 instruments.
While tofacitinib, as a JAK inhibitor, interferes with interleukin‐6 (IL‐6) signaling, and IL‐6 is the major activator of acute‐phase reactants 15, it appears to have different effects on the CRP level and the ESR. Tofacitinib reduces CRP concentrations to a level that, when entered into the DAS28‐4(CRP) formula, may have resulted in values below the remission threshold, despite residual joint counts and high patient's global assessment, while the ESR was not affected to a similar extent. Therefore, it is likely that the differing extents of changes in acute‐phase reactant levels, regardless of treatment, contribute significantly to the disparate rates of remission and low disease activity achieved using the DAS28‐4(ESR) and the DAS28‐4(CRP), especially when compared with the other criteria; previous studies that have assessed remission rates using both the DAS28 and the index‐based ACR/EULAR criteria demonstrated SDAI and CDAI remission rates that were consistently lower than both DAS28‐based definitions 16. Here, DAS28‐4(CRP) clearly overestimated remission rates.
The interaction between treatment effects on acute‐phase reactants and the criteria used to assess rates of remission and low disease activity have been described previously in the context of IL‐6 inhibition 15. Indices in which acute‐phase reactants have higher weight may lead to discordances in remission rates when compared with other outcomes across treatments. This study also reveals the limitations of using DAS28‐defined remission as an end point, consistent with the conclusions of the ACR/EULAR provisional definition of remission 6 and expands this information to use of the DAS28 to determine a state of low disease activity.
The differential effect on the ESR and CRP level deserves further investigation, bearing in mind that the CRP level constitutes a measure of a single protein, while the ESR is a complex reaction of various acute‐phase reactants and presumably other factors 17. Indeed, in ORAL Solo, DAS28‐4(ESR) remission rates (the primary end point) were not significantly different with tofacitinib than with placebo, while DAS28‐4(CRP) remission and, of special note, CDAI and SDAI remission rates (i.e., index‐based ACR/EULAR criteria) were significantly different with tofacitinib than with placebo. These and other previous findings reveal the importance of selecting appropriate measures of disease activity and efficacy end points in clinical trials. Results that are more consistent are obtained when indices are used in which clinical components are more highly weighted than acute‐phase reactants 15.
Although it is clearly necessary to select appropriate instruments in clinical trial design, our study also has important implications for clinical practice, because the DAS28 is widely used in daily practice during care of RA patients. Rheumatologists may make little distinction between the DAS28‐ESR and the DAS28‐CRP, given the availability of the respective calculators and programs; however, today, when both the ACR and EULAR strongly recommend use of a treat‐to‐target approach in the management of RA to achieve remission or low disease activity 1, 2, use of an appropriate instrument is crucial. If a score such as the DAS28‐4(CRP) conveys high rates of remission and low disease activity when a particular therapy is used, rheumatologists may be inclined to continue the respective treatment despite persistently active disease. On the other hand, if a score such as the DAS28‐4(ESR), in the case of tofacitinib use, reveals an erroneously low frequency of remission, effective therapy may be regarded as insufficient and may be stopped despite a state of inactive disease. Because of the huge number of differences in outcomes, it is alarming when these scores are used interchangeably, as the term “DAS28‐4” would suggest. The importance of our findings in terms of clinical practice must be considered in the context of increasing use of tofacitinib and the advent of additional JAK inhibitors.
This analysis confirmed that treatment with tofacitinib in patients with moderate to severe RA resulted in greater remission and low disease activity rates versus placebo when a variety of disease activity indices were used. Irrespective of the instrument used, the rates of remission and low disease activity continued to increase to month 6 in the respective studies (ORAL Sync, ORAL Scan, and ORAL Standard), and rates were generally numerically higher for tofacitinib 10 mg twice daily versus 5 mg twice daily.
A limitation of this analysis was that all patients had a uniform baseline disease status of moderate to severe RA; ∼90% of these patients met the criteria for high disease activity (baseline DAS28‐4[ESR] >5.1); patients with less‐active RA or early RA may be expected to achieve higher remission rates. This should be taken into account when applying these data to clinical practice.
In summary, the rates of remission and low disease activity observed using SDAI and CDAI criteria were consistent across studies, while, in line with previous observations, DAS28‐based criteria did not convey consistent results. In the current study, this was attributable to differential contributions of acute‐phase reactant measures to the observed remission and low disease activity rates. These data reveal the limitations of the DAS28 as a definition of remission due to its heavy dependence on changes in acute‐phase reactants. This is of particular relevance when agents that interfere with the acute‐phase response are used, such as those that lead to cytokine blockade or JAK inhibition; this should be considered during the design of clinical trials and taken into account in clinical practice. The data also show that the DAS28‐4(ESR) and DAS28‐4(CRP) are not interchangeable. Consequently, this study confirms the usefulness of the ACR/EULAR provisional definition of remission, both Boolean‐based and index‐based.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Smolen had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Smolen, Aletaha, Gruben, Zwillich, Krishnaswami, Mebus.
Analysis and interpretation of data
Smolen, Aletaha, Gruben, Zwillich, Krishnaswami, Mebus.
ROLE OF THE STUDY SPONSOR
This study was sponsored by Pfizer, Inc. Editorial support was provided by Complete Medical Communications and funded by Pfizer, Inc. Publication of this article was not contingent upon approval by Pfizer, Inc.
Supporting information
Supporting Information Figure 1
Supporting Information Figure 2
Supporting Information Table 1
Supporting Information Table 2
Supporting Information Table 3
Supporting Information Table 4
ACKNOWLEDGMENTS
The authors would like to thank the patients, the investigators, and the study teams that were involved in the ORAL Step, ORAL Scan, ORAL Solo, ORAL Sync, and ORAL Standard studies. We thank Martin Goulding and Alice Palmer of Complete Medical Communications, who provided editorial support (under the direction of the authors).
Supported by Pfizer, Inc.
Dr. Smolen has received consulting fees, speaking fees, and/or honoraria from AbbVie, Amgen, AstraZeneca, Astro, Celgene, Celtrion, Glaxo, ILTOO, Janssen, Lilly, MedImmune, MSD, Novartis‐Sandoz, Pfizer, Roche, Samsung, Sanofi, and UCB (less than $10,000 each) and has received institutional grants from AbbVie, Janssen, Lilly, MSD, Pfizer, and Roche. Dr. Aletaha has received consulting fees, speaking fees, and/or honoraria from AbbVie, BMS, Eli Lilly, Janssen, MSD, Pfizer, Roche, and UCB (less than $10,000 each). Drs. Gruben, Zwillich, Krishnaswami, and Mebus own stock or stock options in Pfizer, Inc.
REFERENCES
- 1. Smolen JS, Landewé R, J Bijlsma, G Burmester, K Chatzidionysiou, M Dougados, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease‐modifying antirheumatic drugs: 2016 update. Ann Rheum Dis 2017. In press. [DOI] [PubMed] [Google Scholar]
- 2. Singh JA, KG Saag, SL Bridges Jr, EA Akl, RR Bannuru, MC Sullivan, et al. 2015 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Care Res (Hoboken) 2016;68:1–25. [DOI] [PubMed] [Google Scholar]
- 3. Prevoo ML, van't Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty‐eight–joint counts: development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 1995;38:44–8. [DOI] [PubMed] [Google Scholar]
- 4. Aletaha D, Smolen JS. The definition and measurement of disease modification in inflammatory rheumatic diseases. Rheum Dis Clin North Am 2006;32:9–44, vii. [DOI] [PubMed] [Google Scholar]
- 5. Smolen JS, Breedveld FC, Schiff MH, Kalden JR, Emery P, Eberl G, et al. A simplified disease activity index for rheumatoid arthritis for use in clinical practice. Rheumatology (Oxford) 2003;42:244–57. [DOI] [PubMed] [Google Scholar]
- 6. Felson DT, Smolen JS, Wells G, Zhang B, van Tuyl LH, Funovits J, et al. American College of Rheumatology/European League Against Rheumatism provisional definition of remission in rheumatoid arthritis for clinical trials. Arthritis Rheum 2011;63:573–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fleischmann R, Kremer J, Cush J, Schulze‐Koops H, Connell CA, Bradley JD, et al. Placebo‐controlled trial of tofacitinib monotherapy in rheumatoid arthritis. N Engl J Med 2012;367:495–507. [DOI] [PubMed] [Google Scholar]
- 8. Van der Heijde D, Tanaka Y, Fleischmann R, Keystone E, Kremer J, Zerbini C, et al. Tofacitinib (CP‐690,550) in patients with rheumatoid arthritis receiving methotrexate: twelve‐month data from a twenty‐four–month phase III randomized radiographic study. Arthritis Rheum 2013;65:559–70. [DOI] [PubMed] [Google Scholar]
- 9. Burmester GR, Blanco R, Charles‐Schoeman C, Wollenhaupt J, Zerbini C, Benda B, et al. Tofacitinib (CP‐690,550) in combination with methotrexate in patients with active rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitors: a randomised phase 3 trial. Lancet 2013;381:451–60. [DOI] [PubMed] [Google Scholar]
- 10. Van Vollenhoven RF, Fleischmann R, Cohen S, Lee EB, García Meijide JA, Wagner S, et al. Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. N Engl J Med 2012;367:508–19. [DOI] [PubMed] [Google Scholar]
- 11. Kremer J, Li ZG, Hall S, Fleischmann R, Genovese M, Martin‐Mola E, et al. Tofacitinib in combination with nonbiologic disease‐modifying antirheumatic drugs in patients with active rheumatoid arthritis: a randomized trial. Ann Intern Med 2013;159:253–61. [DOI] [PubMed] [Google Scholar]
- 12. Fleischmann R, van der Heijde D, Koenig AS, Pedersen R, Szumski A, Marshall L, et al. How much does Disease Activity Score in 28 joints ESR and CRP calculations underestimate disease activity compared with the Simplified Disease Activity Index? Ann Rheum Dis 2015;74:1132–7. [DOI] [PubMed] [Google Scholar]
- 13. Aletaha D, Smolen JS. Joint damage in rheumatoid arthritis progresses in remission according to the Disease Activity Score in 28 joints and is driven by residual swollen joints. Arthritis Rheum 2011;63:3702–11. [DOI] [PubMed] [Google Scholar]
- 14. Makinen H, Kautiainen H, Hannonen P, Sokka T. Is DAS28 an appropriate tool to assess remission in rheumatoid arthritis? Ann Rheum Dis 2005;64:1410–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Smolen JS, Aletaha D. Interleukin‐6 receptor inhibition with tocilizumab and attainment of disease remission in rheumatoid arthritis: the role of acute‐phase reactants. Arthritis Rheum 2011;63:43–52. [DOI] [PubMed] [Google Scholar]
- 16. Gaujoux‐Viala C, Mouterde G, Baillet A, Claudepierre P, Fautrel B, Le Loet X, et al. Evaluating disease activity in rheumatoid arthritis: which composite index is best? A systematic literature analysis of studies comparing the psychometric properties of the DAS, DAS28, SDAI and CDAI. Joint Bone Spine 2012;79:149–55. [DOI] [PubMed] [Google Scholar]
- 17. Westergren A. Diagnostic tests: the erythrocyte sedimentation rate range and limitations of the technique. Triangle 1957;3:20–5. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Figure 1
Supporting Information Figure 2
Supporting Information Table 1
Supporting Information Table 2
Supporting Information Table 3
Supporting Information Table 4


