Abstract
Aim
To evaluate the effects of progressive muscle relaxation on the behavioral and psychological symptoms of dementia, activities of daily living, and immune function of elderly patients with dementia in group homes.
Methods
The participants were ranked by their group home unit. Odd ranks were assigned to the intervention group and even ranks to the control group. The intervention group participated in progressive muscle relaxation for 15 min each day for 90 days in the group environment; the control group members continued with their normal routine. All the participants’ secretory immunoglobulin A was measured and they were assessed with the Neuropsychiatric Inventory‐Nursing Home version, Nishimura Mental State Scale for the Elderly, and Nishimura Activities of Daily Living Scale.
Results
The intervention group comprised 18 participants from six units and the control group comprised 19 participants from five units. After the intervention, the Neuropsychiatric Inventory scores were significantly better in the intervention group, particularly for Agitation and Anxiety. The intervention group also showed significantly lower Apathy and Irritability scores and significant improvement in the Interest, Volition, and Social relationships scores on the Mental State Scale, with improvement in the activities of daily living total. However, there was no difference in the secretory immunoglobulin A level between the groups.
Conclusion
The results suggest that progressive muscle relaxation improves the behavioral and psychological symptoms of dementia and activities of daily living in group home residents with dementia, but does not affect their immune function.
Keywords: activities of daily living, behavioral and psychological symptoms of dementia, immune function, progressive muscle relaxation
INTRODUCTION
Japan is facing a rapid increase in the aging population. According to the Japanese Ministry of Health, Labour, and Welfare (2014), the average life expectancy in 2014 for Japanese men was 80.5 years and 86.8 years for women. By the year 2025, 30% of the total Japanese population will be aged ≥65 years (Hotta, 2007; Tsuji, 2007). About 15% of individuals aged >65 years have dementia and ∼4.39 million persons in Japan suffer from the disease (Ministry of Health, Labour, and Welfare, 2013).
Dementia is a progressive syndrome. There are several behavioral and psychological symptoms of dementia (BPSD), such as cognitive function decline, functional impairment, delusions, hallucinations, depression, anxiety, and aggression (Van Zadelhoff, Verbeed, Widdershoven, van Rossum, & Abma, 2011).
In Japan, the government introduced a long‐term care insurance (LTCI) program to the elderly care system in April 2000 (Traphagan & Nagasawa, 2008). A system was established in Sweden in 1986 (Hozumi, 2007), whereby elderly patients with dementia often live in group homes (Saiga & Saeki, 2012), which provide an environment for proactive and collaborative daily living with similar individuals (Yamada, 2010). In Japan, the number of group homes has increased following the LTCI program; in 2012, there were an extra 10,000 group homes and 160,000 elderly residents (Asakawa, 2012; Ministry of Health, Labour, and Welfare, 2012). Although the main focus of group homes is daily living, rehabilitation is also necessary for such residents (Funaki, Kaneko, & Okamura, 2005). In many group homes, elderly persons carry out daily recreational activities, including swallowing and gymnastic exercises, that maintain bodily functions.
Pharmacological therapy often shows incomplete efficacy for complex BPSD; therefore, non‐pharmacological options have been developed. These include validation treatment, reality orientation, reminiscence therapy, music therapy, cognitive stimulation therapy, and exercise. Of these, cognitive stimulation therapy has a reasonable evidence base (Japanese Society of Neurology, 2011), which suggests that other non‐pharmacological therapies for dementia might have beneficial effects on the cognitive symptoms and BPSD (Yamaguchi, Maki, & Yamaguchi, 2010). Improvements in BPSD can decrease the suffering and disease burden of patients and their family (Finkel, 2000) and improve individuals’ daily life (Kinoshita, 2008). For patients with mild‐to‐moderate dementia, non‐pharmacological therapies should be considered early in the disease course because the therapies could become more difficult to administer as the disease progresses (Japanese Society of Neurology). Some non‐pharmacological therapies have been used in clinical practice and research, such as aromatherapy, music therapy, touching, doll therapy, and progressive muscle relaxation (Fujii et al., 2008; Mariko, Matsuda, Takahashi, Fujii, & Sasaki, 2015; Mitchell & O'Donnell, 2013; Suhr, Anderson, & Tranel, 1999; Suzuki et al., 2010), in order to help conditions like BPSD and to improve activities of daily living (ADLs).
Progressive muscle relaxation is one such therapy. The method is used to control muscle tension and the physical and psychological symptoms of anxiety (Jacobson, 1974). Progressive muscle relaxation promotes the relaxation of the major muscle groups, such as the face, arms, legs, neck, and back, and is combined with deep breathing. Relaxation helps to reduce stress in the body and restores homeostasis (Gustainiene, Perminas, Peciuliene, & Jarasiunaite, 2015). Most individuals find progressive muscle relaxation easy to learn and can use it to attain a state of relaxation (Sheu, Irvin, Lin, & Mar, 2003; Suhr et al., 1999). Previous studies have found good evidence for the beneficial effects of relaxation. Arena, Hightower, and Chong (1988) found that an 8 week relaxation intervention improved headaches in 10 elderly persons. Renfroe (1988) used progressive relaxation to treat dyspnea and anxiety in 20 outpatients with chronic obstructive pulmonary disease. Rankin, Gilner, Gfeller, and Katz (1993) found that progressive muscle relaxation reduced the level of state anxiety in their elderly participants and Peck (1998) showed that progressive muscle relaxation had beneficial effects on pain, tension, mood, and satisfaction in elderly persons with arthritis. Moreover, progressive muscle relaxation is helpful in the management of anxiety and memory complaints in elderly persons (Brooks, Friedman, & Yesavage, 1993; Rickard, Scogin, & Keith, 1994; Scogin, Rickard, Keith, Wilson, & McElreath, 1992; Yesavage, Rose, & Spiegel, 1982). According to Suhr et al., patients with Alzheimer's disease showed a significant decrease in psychiatric and behavioral disturbances from baseline to the 2 month follow‐up testing. Progressive muscle relaxation is an effective technique for managing BPSD in Alzheimer's disease, especially for patients with mild‐to‐moderate dementia. However, most previous research has not included control groups and has not evaluated the long‐term effect of progressive muscle relaxation.
There is a great need for research on ADLs in elderly persons with dementia (Cummings & Mega, 2003). In order to precisely evaluate the potential of non‐pharmacological therapy, its effects on BPSD and ADLs need to be measured separately (Takeda, 2013). The main purpose of this study was to clarify the effects of progressive muscle relaxation on BPSD, but its effects on ADLs and immune function also were measured because elderly persons are at increased risk of infections and ADL impairments (Okuno & Ohnishi, 2009). This study aimed to determine the effects of progressive muscle relaxation on BPSD, ADLs, and immune function.
METHODS
Study environment and participants
The research was conducted in group homes in Nagoya, Japan. Group home participation in the research was requested by sending a letter to group home associations between March and September 2012. The participants were required to meet the selection criteria and to provide informed consent. Exclusion criteria also were applied.
The selection criteria were: (i) aged ≥65 years; (ii) diagnosis of dementia; (iii) resident of a group home for ≥3 months; (iv) mild‐to‐moderate dementia with a Mini‐Mental State Examination (MMSE) total score of between ≥11 and ≤23 points; (v) the ability to participate in recreational activities; (vi) the ability to sit during vital sign measurements and progressive muscle relaxation; and (vii) the presence of BPSD, as defined by a total score of >1 on the Neuropsychiatric Inventory‐Nursing Home version (NPI‐NH). The pre‐intervention exclusion criteria were: (i) the presence of acute disease; and (ii) the presence of musculoskeletal diseases. In addition, the participants were excluded on observation of any of the following situations: (i) any initiation or change in the type or dose of oral antipsychotics, anti‐anxiety drugs, or antidepressants; (ii) any change from normal in their care, such as oral care or foot care; or (iii) the initiation of another type of recreation with the potential to induce a relaxation response, such as aromatherapy, massage, or music therapy.
Intervention
Those patients and their family who met the selection criteria and provided consent participated. They then were ranked according to their group home unit. Odd ranks were assigned to the intervention group and even ranks were assigned to the control group. The intervention group participated in progressive muscle relaxation for 15 min each day for 90 days in the group environment; the control group members continued with their normal routines. All the intervention group participants completed every session and a researcher and trained group home staff encouraged the participants to follow the instructions correctly. The following conditions had to be met for participation in progressive muscle relaxation: (i) a willingness to participate; (ii) stable vital signs; (iii) no viral symptom; and (iv) no acute pain.
A simple method of progressive muscle relaxation was adopted that divided the muscles into seven groups: forearm and upper arm; lower leg and front thigh; lower leg and rear thigh; chest; shoulder; forehead; and periorbital area and lower jaw. This simplified method has been found to have positive psychological and physiological effects in patients with cancer (Kondo, Koitabashi, Kaneko, & Kobayashi, 2011). Progressive muscle relaxation always was conducted by between three and five researchers and group home staff, comprising nurses, care managers, and care workers, with ~30 group home staff in total. The researcher, a nursewith a Master's qualification and skilled in the method, was always present. When directions were provided for tension and relaxation behaviors, clear verbal instructions and gestures were used that were easily mimicked. When the participants found the instruction difficult (e.g. because of hearing loss), either the group home staff or the researchers provided individual assistance. In order to aid facilitation, the group home staff members were taught progressive muscle relaxation by using digital media (i.e. a DVD) and a brochure. In addition, the technique that was used by the staff was checked and corrected when necessary, both before and during the intervention period.
Data collection
Data on the participants’ age, sex, level of care required (scored between 1 and 5 based on an assessment of care requirements), and MMSE scores were collected from interviews with the group home staff and participants in the pre‐intervention stage in order to obtain the baseline characteristics of all the participants.
Scores for the NPI‐NH, the Nishimura Mental State Scale for the Elderly (NM scale), and the Nishimura Activities of Daily Living Scale (N‐ADL) were obtained through interviews with the group home staff who were involved in the day‐to‐day care of the participants. The scores were recorded at pre‐intervention, 30 days after the intervention's initiation, and 90 days after the intervention's initiation. As a physiological measurement, the participants’ secretory immunoglobulin A (S‐IgA) was measured pre‐intervention and 90 days after the intervention's initiation.
Measures
Neuropsychiatric Inventory‐Nursing Home version
The BPSD was evaluated by using the internationally validated NPI‐NH that was developed by Cummings et al. (1994). This measure includes 10 questions that are related to mental symptoms that are found often in patients with dementia, such as delusions, hallucinations, agitation, depression, anxiety, euphoria, apathy, disinhibition, irritability, and aberrant motor behavior (Shigenobu, Tabuse, Hirono, & Ikeda, 2008). The respondents, group home staff, and those who were connected with the participants’ daily well‐being rated the frequency of the symptoms from 1 to 4 as follows: 1 = “sometimes” (less than once per week) to 4 = “very often” (several times per day or always). The level of symptom severity was graded from 1 to 3 (1 = “mild” to 3 = “severe”). The sum of the frequency and severity scores could range between 0 and 120 points.
Nishimura Mental State Scale for the Elderly and Nishimura Activities of Daily Living Scale
The NM scale is a behavior rating scale that is used to evaluate mental status in daily life. Its items are rated on a 0–10 point scale and include Housework and the Arrangement of personal belongings, Interest, Volition, and Social relationships, Conversation, Memory, and Orientation (Fukunaga, Ukai, Kobayashi, & Nishimura, 2006). The N‐ADL is a behavioral assessment scale that is used in conjunction with the NM scale and helps to capture the overall practical capabilities of elderly persons in daily life (Kobayashi, Hariguchi, Nishimura, Takeda, & Hukunaga, 1988). This scale assesses functional status with five items that measure walking, living area, bathing, putting on and taking off clothes, feeding, and excretion. The possible scores are between 0 and 10 points, according to the severity level.
Secretory immunoglobulin A
The S‐IgA has an important role in host defense (Lamm, 1998) and it can indicate prolonged stress and immune function (Izawa et al., 2007). The S‐IgA was used in this study as a long‐term indicator of stress. The sample collection is simple and uninvasive, as only a small volume of saliva is required for testing. Hence, this method is regarded as the gold standard of stress measurement in elderly persons (Sugiyama, 2011). The S‐IgA is central to oral cavity immune function and prevents viral and bacterial infection of the upper respiratory tract (Kawai, 1973). In order to control for the effects of dietary intake and diurnal variation, the saliva was collected between 10.00 hours and 11.00 hours and the participants were asked to rinse their mouth ~30 min prior to its collection. With the patient in a sitting position and at rest, the sample was obtained over 1 min by using an oral cotton saliva collection device (Salivette; Sarstedt, Nümbrecht, Germany) and it was stored immediately in a cooling box. Later, the samples were separated from the sterile cotton in a centrifuge (5 min at 3500G) and freeze‐stored at −30°C. All the samples were analyzed by Corporation SRL, Tokyo, Japan.
Statistical analyses
After testing for normal distributions, the unpaired t‐test and the χ2‐test were used to compare the baseline differences between the intervention group and the control group. In addition, the NPI‐NH, NM scale, and N‐ADL scores, as well as the S‐IgA levels, were analyzed by using a one‐way ANOVA with repeated measures, followed by a Bonferroni post‐hoc test, at the three time points of pre‐intervention, 30 days after the intervention's initiation, and 90 days after the intervention's initiation. Tests of normal distribution and equal variance were carried out in order to confirm the use of parametric analyses. All the P‐values that were <0.05 were considered to be statistically significant.
Ethical considerations
The purpose of this research was explained both in writing and verbally. Consent then was obtained from the participants, family members, and group home staff. The autonomy of the patients was respected as to whether to choose to participate or not in the study and their personal information was protected. This study was approved by the ethical review boards at the authors’ institutions.
RESULTS
Sample characteristics
In total, patients from seven group homes with 11 units were assigned randomly to the intervention group (n = 21) or the control group (n = 23). However, three participants in the intervention group and four in the control group were excluded because of changes in oral medications or after fractures following falls. Therefore, the final analysis included 18 participants in the intervention group and 19 in the control group (Fig. 1). There was no significant difference between the two groups in age, sex, and level of care required, the NPI‐NH, NM scale, and N‐ADL scores, or in the S‐IgA levels at baseline (Table 1).
Figure 1.
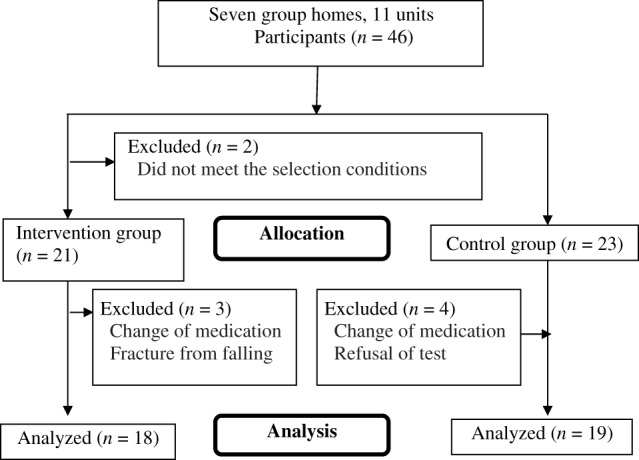
Flow chart showing the participants, group allocation, and analysis.
Table 1.
Participants’ characteristics
| Item | Intervention group (n = 18) Mean ± SD or N (%) |
Control group (n = 19) | t‐Value | χ2‐Value | P‐value |
|---|---|---|---|---|---|
| Age | 86.89 ± 4.19 | 86.74 ± 6.68 | 0.082 | – | 0.935 |
| Sex | |||||
| Male | 4 (22) | 3 (16) | – | 0.249 | 0.618 |
| Female | 14 (78) | 16 (84) | – | ||
| Level of care required | |||||
| 1 | 7 (39) | 5 (26) | – | 1.485 | 0.686 |
| 2 | 7 (39) | 8 (42) | – | ||
| 3 | 4 (22) | 5 (26) | – | ||
| 4 | 0 (0) | 1 (5) | – | ||
| Type of dementia | |||||
| Alzheimer's | 5 (28) | 3 (16) | – | – | – |
| Cerebrovascular | 0 (0) | 1 (5) | – | – | – |
| Mixed type | 0 (0) | 1 (5) | – | – | – |
| No definitive diagnosis | 13 (72) | 14 (74) | – | – | – |
| Measure | |||||
| NPI‐NH | 8.94 ± 6.74 | 7.63 ± 6.22 | 0.616 | – | 0.542 |
| NM scale | 31.67 ± 8.24 | 31.11 ± 9.77 | 0.188 | – | 0.852 |
| N‐ADL | 37.94 ± 7.37 | 37.68 ± 10.12 | 0.089 | – | 0.930 |
| S‐IgA | 178.20 ± 129.72 | 123.47 ± 49.18 | 1.714 | – | 0.095 |
| MMSE | 16.61 ± 3.47 | 16.84 ± 3.20 | −0.211 | – | 0.834 |
MMSE, Mini‐Mental State Examination; N‐ADL, Nishimura Activities of Daily Living Scale; NM, Nishimura Mental State Scale for the Elderly; NPI‐NH, Neuropsychiatric Inventory‐Nursing Home version; SD, standard deviation; S‐IgA, secretory immunoglobulin A.
Changes in the measures
Neuropsychiatric Inventory‐Nursing Home version scores
There was a difference between the intervention group and the control group in the total NPI‐NH score. With regard to the NPI‐NH subscores, there was a significant difference between the intervention and the control groups in the Apathy and Irritability scores. For the intervention group, the scores 30 days after the initiation of the intervention and 90 days after the initiation of the intervention were significantly better than at pre‐intervention. A significant improvement in scores also was observed between 30 days after the initiation of the intervention and 90 days after the initiation. However, the control group showed no significant difference in scores between the two periods. For the NPI‐NH subscores, the intervention group's Agitation scores were significantly better 90 days after the intervention's initiation than 30 days after initiation of the intervention. Similarly, there was a significant improvement in their Anxiety scores between pre‐intervention and 90 days after the intervention's initiation and between 30 days after the intervention's initiation and 90 days after the initiation of the intervention (Tables 2, 3). There was a significant improvement in NPI‐NH scores in the intervention group, particularly for Agitation, Anxiety, Apathy, and Irritability.
Table 2.
Changes in the scale scores in the intervention group
| Subscale | Intervention group (n = 18) | ||
|---|---|---|---|
| Pre‐intervention | 30 days after intervention initiation | 90 days after intervention initiation | |
| NPI‐NH total score | 8.94 ± 6.74 | 7.28 ± 6.50 | 4.78 ± 5.07 |
|
|||
| Delusions | 0.83 ± 1.58 | 0.78 ± 1.52 | 1.00 ± 2.28 |
| Hallucinations | 0.17 ± 0.71 | 0.22 ± 0.73 | 0.39 ± 0.98 |
| Agitation | 1.94 ± 2.41 | 1.89 ± 2.17 | 1.00 ± 1.28 |

|
|||
| Depression/dysphoria | 0.94 ± 1.21 | 0.39 ± 0.61 | 0.33 ± 0.69 |
| Anxiety | 1.22 ± 1.70 | 0.83 ± 1.25 | 0.28 ± 0.67 |

|
|||
| Euphoria/elation | 0.56 ± 1.65 | 0.22 ± 0.73 | 0.22 ± 0.94 |
| Apathy/indifference | 1.17 ± 3.00 | 0.94 ± 2.92 | 0.44 ± 1.42 |
| Disinhibition | 0.61 ± 1.42 | 0.83 ± 1.47 | 0.67 ± 1.03 |
| Irritability/lability | 1.22 ± 2.02 | 0.89 ± 1.75 | 0.28 ± 0.57 |
| Aberrant motor behavior | 0.28 ± 0.96 | 0.28 ± 0.96 | 0.17 ± 0.51 |
| NM scale total score | 31.67 ± 8.24 | 32.06 ± 8.29 | 33.56 ± 7.91 |
| Housework and arrangement of personal belongings | 4.78 ± 2.26 | 4.94 ± 2.13 | 5.11 ± 2.00 |
| Interest, volition, and social relationships | 6.06 ± 2.29 | 6.22 ± 2.37 | 7.22 ± 1.90 |

|
|||
| Conversation | 8.17 ± 2.46 | 8.17 ± 2.46 | 8.22 ± 2.32 |
| Memory | 5.67 ± 2.06 | 5.67 ± 2.06 | 6.33 ± 1.94 |
| Orientation | 7.00 ± 2.03 | 7.00 ± 2.03 | 6.67 ± 2.17 |
| N‐ADL total score | 37.94 ± 7.37 | 38.00 ± 7.86 | 39.00 ± 7.22 |

|
|||
Values are the mean ± standard deviation. N‐ADL, Nishimura Activities of Daily Living Scale; NM, Nishimura Mental State Scale for the Elderly; NPI‐NH, Neuropsychiatric Inventory‐Nursing Home version.
Table 3.
Changes in the scale scores in the control group
| Subscale | Control group (n = 19) | ||
|---|---|---|---|
| Pre‐intervention | 30 days after intervention initiation | 90 days after intervention initiation | |
| NPI‐NH total score | 7.63 ± 6.22 | 8.37 ± 7.27 | 8.58 ± 7.37 |
| Delusions | 0.63 ± 1.50 | 0.63 ± 1.54 | 0.68 ± 1.53 |
| Hallucinations | 0.16 ± 0.37 | 0.26 ± 0.93 | 0.11 ± 0.32 |
| Agitation | 1.58 ± 2.19 | 1.37 ± 1.89 | 1.37 ± 1.89 |
| Depression/dysphoria | 1.26 ± 1.63 | 1.32 ± 2.06 | 0.95 ± 1.65 |
| Anxiety | 1.32 ± 1.49 | 1.21 ± 2.04 | 1.00 ± 1.45 |
| Euphoria/elation | 0.00 | 0.11 ± 0.46 | 0.05 ± 0.23 |
| Apathy/indifference | 1.11 ± 2.13 | 1.21 ± 2.27 | 1.63 ± 2.67 |
| Disinhibition | 0.16 ± 0.50 | 0.26 ± 0.81 | 0.42 ± 1.12 |
| Irritability/lability | 0.53 ± 0.84 | 0.74 ± 1.24 | 1.16 ± 1.38 |
| Aberrant motor behavior | 0.89 ± 1.76 | 1.26 ± 2.62 | 1.21 ± 2.18 |
| NM scale total score | 31.11 ± 9.77 | 30.95 ± 9.56 | 30.00 ± 10.88 |
| Housework and arrangement of personal belongings | 4.68 ± 1.92 | 4.68 ± 1.92 | 4.68 ± 1.80 |
| Interest, volition, and social relationships | 6.05 ± 2.25 | 6.16 ± 2.24 | 5.53 ± 3.04 |
| Conversation | 7.42 ± 2.52 | 7.37 ± 2.48 | 7.26 ± 2.70 |
| Memory | 6.16 ± 2.14 | 6.05 ± 2.04 | 5.84 ± 2.61 |
| Orientation | 6.79 ± 2.30 | 6.68 ± 2.24 | 6.68 ± 2.24 |
| N‐ADL total score | 37.68 ± 10.12 | 37.84 ± 9.87 | 37.21 ± 10.55 |
Values are the mean ± standard deviation. N‐ADL, Nishimura Activities of Daily Living Scale; NM, Nishimura Mental State Scale for the Elderly; NPI‐NH, Neuropsychiatric Inventory‐Nursing Home version.
Nishimura Mental State Scale for the Elderly and Nishimura Activities of Daily Living Scale scores
There was a significant difference between the intervention group and the control group in the total NM scale scores and a significant interaction between the two groups on the NM scale's Interest subscores. For the intervention group, the NM scale's Interest, Volition, and Social relationships subscores improved significantly from pre‐intervention to 90 days after the intervention's initiation and from 30 days after the intervention's initiation to 90 days after the initiation of the intervention. There was a significant difference in the N‐ADL total scores between the intervention and the control groups. In the intervention group, the N‐ADL total score significantly improved between 30 days after the initiation of the intervention and 90 days after the initiation of the intervention (Tables 2, 3).
For the intervention group, there was a significant improvement in the NM scale scores and in the NM scale subscores for Interest, Volition, and Social relationships. There was a significant difference in the N‐ADL scores between the two groups.
Secretory immunoglobulin A
For both the intervention group and the control group, the S‐IgA levels decreased from pre‐intervention to 30 days after the initiation of the intervention, but then increased at 90 days after initiation (Table 4). There was no significant change in the S‐IgA levels between the two groups.
Table 4.
Changes in the secretory immunoglobulin A (S‐IgA) levels of the participants
| Subscale | Intervention group (n = 18) | Control group (n = 19) | ||||
|---|---|---|---|---|---|---|
| Pre‐intervention | 30 days after intervention initiation | 90 days after intervention initiation | Pre‐intervention | 30 days after intervention initiation | 90 days after intervention initiation | |
| S‐IgA | 178.20 ± 129.72 | 122.16 ± 40.28 | 190.49 ± 127.42 | 123.47 ± 49.18 | 111.29 ± 56.11 | 138.79 ± 69.09 |
Values are the mean ± standard deviation.
DISCUSSION
The present study evaluated the effects of progressive muscle relaxation in elderly persons with mild‐to‐moderate dementia in group home environments. Continuing the practice of progressive muscle relaxation tended to improve the NPI‐NH scores, particularly for Anxiety and Agitation. In addition, there was a significant difference in the scores on Apathy and Irritability between the intervention group and the control group. The NM scale scores and N‐ADL scale scores tended to be better in the intervention group; the NM scale's Interest, Volition, and Social relationships subscores improved in the intervention group, compared with the control group.
Characteristics of the participants
According to the Japanese Dementia Group Home Association (2010), the average age of residents in 2010 was 84.9 years; 81.8% of these were women. In this study, the participants were 2 years older on average than the national trend, but overall, the group was considered to be average. The Japanese Dementia Group Home Association reported that level 3 long‐term care was most prevalent in this group, with 29.4% of the group home residents in Japan receiving such care. In this study, there were many residents receiving long‐term care at level 1 and level 2 in both the intervention and the control group. As the participants had to be able to participate recreationally unaided to take part in the study, it is likely that there was a selection bias toward patients with low‐care needs.
Changes in the measures
Neuropsychiatric Inventory‐Nursing Home version scores
The total NPI‐NH score significantly improved in the intervention group following progressive muscle relaxation, which appeared to suppress BPSD. Progressive muscle relaxation aims to recreate a relaxed state and the perception of comfort, attenuation of habitual strain reactions, and to strengthen the relaxation response. Significantly better scores were observed on Agitation and Anxiety at both 30 and 90 days after the initiation of the intervention, which is consistent with research on progressive muscle relaxation for mental anxiety and physical tension (Koitabashi, 2001). In this study, a downward trend was observed for both the Agitation and Anxiety levels, as expected with this technique. Progressive muscle relaxation produces gradual relaxation and relief from stress and enhances coping ability in stressful situations (Molassiotis, 2000). The Progressively Lowered Stress Threshold model has been used for planning individualized care for elderly persons with dementia (Hall & Buckwalter, 1987) and this model can help to manage BPSD, control the factors related to stress, minimize catastrophic reactions, and improve the daily functions of patients with dementia (Suhr et al., 1999). The present findings suggest that progressive muscle relaxation improved BPSD. This suggests that relaxation in elderly persons with dementia can change stress thresholds, relieve stress progressively, and reduce BPSD.
Nishimura Mental State Scale for the Elderly and Nishimura Activities of Daily Living Scale scores
There was a difference between the intervention group and the control group in the NM scale's total scores and a tendency for the scores in the intervention group to improve from pre‐intervention to 30 and 90 days after the initiation of the intervention. For the NM subscales, there were differences between the intervention and the control groups on the Interest, Volition, and Social relationships scores and between the three time points (for the intervention group) following progressive muscle relaxation, suggesting different reactions in each group.
Takemura (2007) found that color therapy that was used for 60–90 min twice per month for nine group home residents did not cause postintervention improvement; however, it improved their housework, interest, conversation, and memory domains. The color therapy probably encouraged interaction (smiles, mutual recognition, and connection with others), resulting in a greater sense of security and confidence. It is believed that the Interest, Volition, and Social relationships scores improved because progressive muscle relaxation was carried out in a group setting within each unit. The participants had the opportunity to relax their muscles and decrease tension (Sun, Kang, Wang, & Zeng, 2013).
There was a significant improvement in the N‐ADL scores between 30 days and 90 days after the initiation of the intervention. It is possible that the stress response decreased through the incorporation of progressive muscle relaxation into daily life, which improved the participants’ mental health, relaxed their body, and helped in maintaining and improving ADLs. According to Norton, Malloy, and Salloway (2001), BPSD is associated with poorer ADL levels. Hence, the improvement in BPSD resulting from progressive muscle relaxation probably improved the ADL levels in the group home residents with dementia.
Secretory immunoglobulin A levels
No significant change was observed in the S‐IgA levels between the two groups. According to Valdimarsdottir and Stone (1997), chronic stress can reduce the S‐IgA level and progressive muscle relaxation is an effective way to increase it (Lowe, Bland, Greenman, Kirkpatrick, & Lowe, 2001). However, the participants’ levels decreased 30 days after the initiation of the intervention in both the intervention and the control groups, before recovering to just above baseline at 90 days after the intervention's initiation. At pre‐intervention, the levels for the intervention group were higher than those for the control group and therefore a greater decrease was observed in the former group. However, the S‐IgA levels only decreased after progressive muscle relaxation in nine participants of the intervention group, making it difficult to determine causality. The results also were limited by the fact that the individual variation was large and there was a change in the individual comparison values between the groups. The S‐IgA is a simple biochemical index of the stress response in elderly patients with dementia, which requires only a small amount of saliva (Sugiyama, 2011), thus decreasing the burden on participants. However, when used as an indicator, the saliva test should reflect both interindividual and intra‐individual variabilities (Sawada, Inamizu, Taitou, Sekikawa, & Kawaguchi, 2008). The present findings did not provide clear evidence of increases in S‐IgA levels and immune system effects following progressive muscle relaxation in the elderly persons with dementia.
Implications for the care of patients with dementia in group homes
Incorporating progressive muscle relaxation into the daily recreational activities of patients with dementia in group home environments is useful and easily achieved. It was found that a daily routine of progressive muscle relaxation suppressed the appearance of BPSD and reduced anxiety and agitation.
In order to promote the continuation of progressive muscle relaxation and to motivate the participants, care workers need to develop methods of persuasion and promote the recreational and enjoyable aspects of the exercises. Sometimes, individual attention was given to a participant in order to concentrate their mind on the procedure. It was important to encourage the participation and cooperation of the group home staff members who were involved with the participants on a daily basis.
Limitations of the study
As a result of the group home staff, acting as evaluators, and the researchers who assigned the participants into the respective intervention and control groups, maintaining a double‐blind study design was difficult. This could have affected the changes in the NPI‐NH, NM scale, and N‐ADL scores. As the intervention group members received an additional recreational activity, progressive muscle relaxation, they had a greater opportunity to communicate with the researchers and group home staff. The relationships between the participants and the group home staff and/or the researchers in this study might have affected the results. However, it is difficult to control the amount of communication in this type of intervention study.
CONFLICTS OF INTEREST
No potential conflict of interest was disclosed.
AUTHOR CONTRIBUTIONS
S. I. conducted the whole study; Y. M. supervised the study process.
ACKNOWLEDGMENTS
The authors wish to thank all the residents, families, and group home staff members for participating in this study. The study was supported by Japan Society for the Promotion of Science, Tokyo, Japan (KAKENHI Grant No. 24792598).
References
- Arena, J. G. , Hightower, N. E. & Chong, G. C. (1988). Relaxation therapy for tension headache in the elderly: A prospective study. Psychology and Aging, 3, 96–98. [DOI] [PubMed] [Google Scholar]
- Asakawa, S. (2012). Recommendations for care site. Good Morning, 21, 28–29 (in Japanese). [Google Scholar]
- Brooks, J. D. , Friedman, L. & Yesavage, J. A. (1993). A study of the problems older adults encounter when using a mnemonic technique. International Psychogeriatrics, 5, 57–65. [DOI] [PubMed] [Google Scholar]
- Cummings, J. L. & Mega, M. (2003). Neuropsychiatry and behavioral neuroscience (1st edn). Oxford: Oxford University Press. [Google Scholar]
- Cummings, J. L. , Mega, M. , Gray, K. , Rosenberg, T. S. , Carusi, D. A. & Gornbein, J. (1994). The Neuropsychiatric Inventory: Comprehensive assessment of psychopathology in dementia. Neurology, 44, 2308–2314. [DOI] [PubMed] [Google Scholar]
- Finkel, S. (2000). Introduction to behavioral and psychological symptoms of dementia (BPSD). International Journal of Geriatric Psychiatry, 15, S2–S4. [DOI] [PubMed] [Google Scholar]
- Fujii, M. , Hatakeyama, R. , Fukuoka, Y. , Yamamoto, T. , Sasaki, R. , Moriya, M. et al. (2008). Lavender aroma therapy for behavioral and psychological symptoms in dementia patients. Geriatrics Gerontology International, 8, 136–138. [DOI] [PubMed] [Google Scholar]
- Fukunaga, T. , Ukai, S. , Kobayashi, T. & Nishimura, T. (2006). Neuropsychological test for the detection of dementia in elderly individuals: The Nishimura Dementia Test. Psychogeriatrics, 6, 159–167. [Google Scholar]
- Funaki, Y. , Kaneko, F. & Okamura, H. (2005). Study on factors associated with changes in quality of life of demented elderly persons in group homes. Scandinavian Journal of Occupational Therapy, 12, 4–9. [DOI] [PubMed] [Google Scholar]
- Gustainiene, L. , Perminas, A. , Peciuliene, L. & Jarasiunaite, G. (2015). Effectiveness of progressive muscle relaxation and biofeedback relaxation in lowering physiological arousal among students with regard to personality features. International Journal of Psychology: A Biopsychosocial Approach, 16, 67–91. [Google Scholar]
- Hall, G. R. & Buckwalter, K. C. (1987). Progressively lowered stress threshold: A conceptual model for care of adults with Alzheimer's disease. Archives of Psychiatric Nursing, 1, 399–406. [PubMed] [Google Scholar]
- Hotta, S. (2007). Toward maintaining and improving the quality of long‐term care: The current state and issues regarding home helpers in Japan under the Long‐Term Care Insurance System. Social Science Japan Journal, 10, 265–279. [Google Scholar]
- Hozumi, I. (2007). Care and care‐education system for elderly people with dementia in Japan and Sweden. Geriatrics & Gerontology International, 7, 89–90. [Google Scholar]
- Izawa, S. , Shirotsuki, K. , Sugaya, N. , Ogawa, N. , Suzuki, K. & Nomura, S. (2007). The application of saliva to an assessment of stress: Procedures for collecting and analyzing saliva and characteristics of salivary substances. Japanese Journal of Complementary and Alternative Medicine, 4, 91–101 (in Japanese). [Google Scholar]
- Jacobson, E. (1974). Progressive relaxation (3rd edn). Chicago, IL: University of Chicago Press. [Google Scholar]
- Japanese Dementia Group Home Association . (2010). Research report by Heisei 21 year health promotion. The Japan Dementia Group Home Cooperation Meeting, 81, 127–134 (in Japanese). [Google Scholar]
- Dementia diseases treatment guidelines created joint committee (Ed.) . (2011). Choice principles and correspondence to treatment of dementia In: Dementia diseases treatment guidelines 2010 (1st edn, pp. 74–77). Tokyo: Igakusyoin; (in Japanese). [Google Scholar]
- Kawai, T. (1973). Immunoserological test (1st edn). Tokyo: Igakusyoin; (in Japanese). [Google Scholar]
- Kinoshita, T. (2008). Role of the home visit medical service for patients with behavioral and psychological symptoms of dementia (BPSD) living in the community. Psychogeriatrics, 8, 142–147. [Google Scholar]
- Kobayashi, T. , Hariguchi, S. , Nishimura, K. , Takeda, M. & Hukunaga, T. (1988). A new clinical scale for rating of mental states and activities of daily living of the elderly (NM scale and N‐ADL). Japanese Journal of Clinical Psychiatry, 17, 1653–1668. [Google Scholar]
- Koitabashi, K. (2001). Progressive muscle relaxation In: Arakawa S. (Ed.), Make use of relaxation techniques in nursing (1st edn, pp. 30–52). Tokyo: Igakusyoin; (in Japanese). [Google Scholar]
- Kondo, Y. , Koitabashi, K. , Kaneko, Y. & Kobayashi, S. (2011). Development of simplified progressive muscle relaxation method and efficacy of its application to cancer patients. Japan Society of Nursing Research, 34, 87–93 (in Japanese). [Google Scholar]
- Lamm, M. E. (1998). Current concepts in mucosal immunity IV. How epithelial transport of IgA antibodies relates to host defense. The American Journal of Physiology, 274, G614–G617. [DOI] [PubMed] [Google Scholar]
- Lowe, G. , Bland, R. , Greenman, J. , Kirkpatrick, N. & Lowe, G. (2001). Progressive muscle relaxation and secretory immunoglobulin A. Psychological Reports, 88, 912–914. [DOI] [PubMed] [Google Scholar]
- Mariko, A. , Matsuda, H. , Takahashi, M. , Fujii, M. & Sasaki, H. (2015). Touch on the acupoint of Shinchuu of Alzheimer's disease patient. Geriatrics Gerontology International, 15, 385–386. [DOI] [PubMed] [Google Scholar]
- Ministry of Health, Labour, and Welfare . (2012). Nursing service facilities and offices Survey in Heisei 23 [Cited 23 December 2012.] Available from URL: http://www.mhlw.go.jp/toukei/saikin/hw/kaigo/service11/index.html (in Japanese).
- Ministry of Health, Labour, and Welfare . (2013). Dementia prevalence investigation [Cited 28 March 2016.] Available from URL: http://www.mhlw.go.jp/file.jsp?id=146270&name=2r98520000033t9m_1.pdf (in Japanese).
- Ministry of Health, Labour, and Welfare . (2014). Annual changes in the main countries of the average life expectancy [Cited 28 March 2016.] Available from URL: http://www.mhlw.go.jp/toukei/saikin/hw/life/life14/dl/life14‐04.pdf (in Japanese).
- Mitchell, G. & O'Donnell, H. (2013). The therapeutic use of doll therapy in dementia. British Journal of Nursing, 22, 329–334. [DOI] [PubMed] [Google Scholar]
- Molassiotis, A. (2000). A pilot study of the use of progressive muscle relaxation training in the management of post‐chemotherapy nausea and vomiting. European Journal of Cancer Care, 9, 230–234. [DOI] [PubMed] [Google Scholar]
- Norton, L. E. , Malloy, P. F. & Salloway, S. (2001). The impact of behavioral symptoms on activities of daily living in patients with dementia. The American Journal of Geriatric Psychiatry, 9, 41–48. [PubMed] [Google Scholar]
- Okuno, S. & Ohnishi, K. (2009). Geriatric nursing. Introduction to the practice of nursing (4th edn). Tokyo: Nouvel Hirokawa; (in Japanese). [Google Scholar]
- Peck, S. D. (1998). The efficacy of therapeutic touch for improving functional ability in elders with degenerative arthritis. Nursing Science Quarterly, 11, 123–132. [DOI] [PubMed] [Google Scholar]
- Rankin, E. J. , Gilner, F. H. , Gfeller, J. D. & Katz, B. M. (1993). Efficacy of progressive muscle relaxation for reducing state anxiety among elderly adults on memory tasks. Perceptual and Motor Skills, 77, 1395–1402. [DOI] [PubMed] [Google Scholar]
- Renfroe, K. (1988). Effect of progressive relaxation on dyspnea and state anxiety in patients with chronic obstructive pulmonary disease. Heart Lung, 17, 408–413. [PubMed] [Google Scholar]
- Rickard, H. C. , Scogin, F. & Keith, S. (1994). A one‐year follow‐up of relaxation training for elders with subjective anxiety. Gerontologist, 34, 121–122. [DOI] [PubMed] [Google Scholar]
- Saiga, M. & Saeki, T. (2012). Actual situation of the dementia patients and evaluation of donepezil hydrochloride (Aricept) at the dementia nursing home. Clinical Pharmacology and Therapy, 22, 21–24. [Google Scholar]
- Sawada, M. , Inamizu, T. , Taitou, S. , Sekikawa, K. & Kawaguchi, K. (2008). Variabilities of salivary secretory immunoglobulin A among elderly in long‐term care facilities. Japanese Journal of Physical Fitness and Sports Medicine, 57, 241–248. [Google Scholar]
- Scogin, F. , Rickard, H. C. , Keith, S. , Wilson, J. & McElreath, L. (1992). Progressive and imaginal relaxation for elders with subjective anxiety. Psychology and Aging, 7, 419–424. [DOI] [PubMed] [Google Scholar]
- Sheu, S. , Irvin, B. L. , Lin, H. S. & Mar, C. L. (2003). Effects of progressive muscle relaxation on blood pressure and psychosocial status for clients with essential hypertension in Taiwan. Holistic Nursing Practice, 17(1), 41–47. [DOI] [PubMed] [Google Scholar]
- Shigenobu, K. , Tabuse, K. , Hirono, S. & Ikeda, M. (2008). Validity and reliability of the Japanese version of the Neuropsychiatric Inventory‐Nursing Home version (NPI‐NH). Brain Nerve, 60, 1463–1469 (in Japanese). [PubMed] [Google Scholar]
- Sugiyama, T. (2011). The stress measurement method of elderly with dementia. Stress Science Research, 26, 26–32 (in Japanese). [Google Scholar]
- Suhr, J. , Anderson, S. & Tranel, D. (1999). Progressive muscle relaxation in the management of behaviour disturbance in Alzheimer's disease. Neuropsychological Rehabilitation, 9, 31–44. [Google Scholar]
- Sun, J. , Kang, J. , Wang, P. & Zeng, H. (2013). Self‐relaxation training can improve sleep quality and cognitive functions in the older: A one‐year randomised controlled trial. Journal of Clinical Nursing, 22, 1270–1280. [DOI] [PubMed] [Google Scholar]
- Suzuki, M. , Tatsumi, A. , Otsuka, T. , Kikuchi, K. , Mizuta, A. , Makino, K. et al. (2010). Physical and psychological effects of 6‐week tactile massage on elderly patients with severe dementia. American Journal of Alzheimer's Disease and Other Dementias, 25, 680–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda, M. (2013). For nonpharmacological therapy for dementia. Spring Mind, 12, 2–5 (in Japanese). [Google Scholar]
- Takemura, M. (2007). A study of the elderly: Reactions and effects to color therapy. Bulletin of Aichi Kiwami College of Nursing, 3, 143–150 (in Japanese). [Google Scholar]
- Traphagan, J. W. & Nagasawa, T. (2008). Group homes for elders with dementia in Japan. Care Management Journals, 9, 89–96. [DOI] [PubMed] [Google Scholar]
- Tsuji, T. (2007). Health‐care issues: Japan's aging society and appropriate countermeasures. Japan Journal of Nursing Science, 4, 71–73. [Google Scholar]
- Valdimarsdottir, H. B. & Stone, A. A. (1997). Psychosocial factors and secretory immunoglobulin A. Critical Reviews in Oral Biology and Medicine, 8, 461–474. [DOI] [PubMed] [Google Scholar]
- Van Zadelhoff, E. , Verbeed, H. , Widdershoven, G. , van Rossum, E. & Abma, T. (2011). Good care in group home living for people with dementia. Experiences of residents, family and nursing staff. Journal of Clinical Nursing, 20, 2490–2500. [DOI] [PubMed] [Google Scholar]
- Yamada, R. (2010). Social security and aging society In: Kitagawa K. (Ed.), System nursing geriatric nursing course (7th edn, pp. 32–42). Tokyo: Igakusyoin; (in Japanese). [Google Scholar]
- Yamaguchi, H. , Maki, Y. & Yamaguchi, T. (2010). Overview of non‐pharmacological intervention for dementia and principles of brain‐activating rehabilitation. Psychogeriatrics, 10, 206–213. [DOI] [PubMed] [Google Scholar]
- Yesavage, J. A. , Rose, T. H. & Spiegel, D. (1982). Relaxation training and memory improvement in elderly normal. Correlation of anxiety rating and recall improvement. Experimental Aging Research, 8, 195–198. [DOI] [PubMed] [Google Scholar]


