Abstract
Among the new psychoactive substances (NPS) that have recently emerged on the market, many of the new synthetic opioids have shown to be particularly harmful. A new synthetic analogue of fentanyl, N‐phenyl‐N‐[1‐(2‐phenethyl)piperidin‐4‐yl]prop‐2‐enamide (acrylfentanyl), was identified in powder from a seized capsule found at a forensic psychiatric ward in Denmark. Gas chromatography with mass spectrometry (GC‐MS) identified a precursor to synthetic fentanyls, N‐phenyl‐1‐(2‐phenylethyl)piperidin‐4‐amine; however, the precursor 1‐(2‐phenethyl)piperidin‐4‐one, was not detected. Analysis of the electron impact mass spectrum of the main, unknown chromatographic peak (GC) tentatively identified an acryloyl analogue of fentanyl. Further analyses by quadrupole time‐of‐flight high resolution mass spectrometry (QTOF‐MS), matrix‐assisted laser ionization Orbitrap mass spectrometry (MALDI‐Orbitrap‐MS), nuclear magnetic resonance spectroscopy (NMR), and infra‐red spectroscopy (IR) confirmed the presence of acrylfentanyl (also known as acryloylfentanyl). Quantitative analysis with liquid chromatography and triple quadrupole mass spectrometry (LC‐MS/MS) determined the content of acrylfentanyl in the powder, equal to 88.3 mass‐% acrylfentanyl hydrochloride. An impurity observed by NMR was identified as triethylamine hydrochloride. Acrylfentanyl is sold on the Internet as a ‘research chemical’. Like other synthetic fentanyls, such as acetylfentanyl, it poses a serious risk of fatal intoxication. Copyright © 2016 The Authors. Drug Testing and Analysis Published by John Wiley & Sons Ltd.
Keywords: new psychoactive substances, acryloylfentanyl, acrylfentanyl, fentanyl analogue, identification, seizure
Introduction
The rapid appearance of new psychoactive substances (NPS) that are not controlled under international and national drug laws present a serious problem for public health. This drug phenomenon is characterised by the high number of new substances emerging each year, which in Europe meant a continuous increase in the number of new substances recorded for the first time from 24 in 2009 to 98 in 2015.1, 2 Constantly changing, the transformation of the market is different from anything recorded historically,3 and has led to a global spread of new psychoactive substances.4
New synthetic opioids pose an especially serious concern for public health because of their high potency and because they are often sold under the guise of heroin to unsuspecting users.5, 6, 7 Of particular note is illicitly manufactured fentanyl and its derivatives that have been involved in hundreds of deaths worldwide. Since the first appearance on the market in the USA in the late 1970s, sold as ‘synthetic heroin’ and ‘China White’,8, 9, 10 fentanyls have been detected in Europe, Canada, Australia, Japan, and elsewhere, resulting in overdoses and outbreaks of deaths. Estonia faces a serious situation with hundreds of deaths involving use of illicitly produced fentanyls, accompanied by a growth in the number of seizures, overdoses, and treatment demand.11, 12 In 2015, 32 deaths reported in Germany, Poland, Sweden, and the UK related to acetylfentanyl.13 The proportion of illicit drug overdose deaths involving fentanyls has grown in Canada during the last few years to exceed 50% in some parts of the country.14, 15, 16 Since 2012, 12 deaths in Russia17 and more than 50 deaths in the USA have been associated with acetylfentanyl.18, 19, 20, 21, 22 Other fentanyl analogues, such as butyrfentanyl, 3‐methylfentanyl, and furanylfentanyl, have also been linked to serious adverse events.23, 24, 25 Additional risks arise from hazardous injecting behaviours, such as injecting fentanyls with used needles and syringes that can cause the spread of hepatitis C and HIV.12
Synthetic cannabinoids are currently the largest group of NPS monitored by the European Monitoring Centre for Drugs and Drug Addiction (EMCDDA), followed by synthetic cathinones.1 Since 2008, 160 synthetic cannabinoids have been detected in Europe; 103 synthetic cathinones have been recorded from 2004 onwards. The number of new opioids recorded since 2009 is much lower in comparison (less than 20)1 with several of these being highly potent fentanyls.1, 2 However, whilst these drugs appear to take up a small proportion of the market, they can be particularly harmful.
The use of fentanyls presents a complicated problem characterized by: (1) diversion from patients, tampering, and misuse of licensed medicines containing fentanyl (used as an analgesic drug); (2) availability of illicit fentanyl; (3) the emergence of a range of new fentanyl derivatives, sold illicitly as ‘research chemicals’, which may avoid detection by regulating authorities for long periods of time; (4) the availability of fentanyl and derivatives on the Internet and the dark net, which is one of the key drivers of the global spread of new psychoactive substances; (5) increasing use of fentanyls that are far more potent as an analgesic than morphine; (6) fentanyls sold in highly concentrated powder form where it is difficult to estimate strength and manage the dose, posing a serious risk of overdose; and (7) complex marketing, including fentanyls passed off as heroin6, 7, 26 and heroin as fentanyl,27 fentanyl mixed with cocaine, heroin, amphetamine, and other NPS such as U‐47700 (a synthetic opioid),28, 29 and fake medicines sold on the illicit market (e.g. Xanas and oxycodone) laced with fentanyl.5, 28 All of this relates to the supply and use of heroin and may contribute to the recent increase in overall estimates of opioid deaths in Europe.
In this dynamic market place, keeping up with manufactures and suppliers of NPS is essential and yet detecting new drugs on the market, including fentanyl analogues, is very challenging in both clinical settings and in post‐mortem forensic analysis. Fentanyl derivatives may escape detection because routine testing of these drugs is rarely performed. In this study we report the identification of the fentanyl analogue acrylfentanyl (also known as acryloylfentanyl) in a seized sample obtained from a psychiatric ward at a Danish hospital. To the best of our knowledge this is the first analytically confirmed non‐biological case published within Europe.
Materials
A capsule was seized in May 2016 during a smuggling attempt at the Forensic Psychiatric Department at Aalborg Psychiatric Hospital (Denmark). The sample consisted of a white to pale‐yellow powder inside a translucent capsule (Figure 1). Initially, a large part of the powder was used by the staff to prepare an ad hoc aqueous solution, to test with an immunoassay panel, ABC‐multi‐10 (Simoco Diagnostics, Hillerød, Denmark). This indicated a positive reaction for 3,4‐methylenedioxymethamphetamine (MDMA). Subsequently, the capsule and the remaining powder (less than 6 mg) was sent to the Department of Clinical Biochemistry, North Denmark Regional Hospital (Denmark), for further analysis.
Figure 1.

Seized capsule containing acrylfentanyl. The ruler shows cm.
The standard of fentanyl used as reference was from Cerilliant (Round Rock, TX, USA). The standard of acrylfentanyl hydrochloride (CAS no. 79279‐03‐1) was from Cayman Chemical (Ann Arbor, MI, USA). The standard of N‐phenyl‐1‐(2‐phenylethyl)piperidin‐4‐amine was from Carbosynth (Compton, Berkshire, UK).
Methods
Gas chromatography‐mass spectrometry (GC‐MS)
Analysis was performed at the Department of Clinical Biochemistry, North Denmark Regional Hospital, on a 6890 gas chromatograph with a 5973 mass spectrometer (GC‐MS) from Agilent (Santa Clara, CA, USA) equipped with a Combi‐Pal auto sampler from CTC (Zwingen, Switzerland). Analysis was performed using a XTI‐5 capillary column from Restek (Bellefonte, PA, USA), 30 m, 0.25 mm i.d., film thickness 0.25 µm. The injection port temperature was 250 °C, the transfer line temperature was 280 °C and the MS source temperature was 230 °C. The initial column oven temperature was set to 50 °C and held constant for 1 min during injection. The oven temperature was ramped at 25 °C/min to 170 °C. Then the temperature was ramped at 15 °C/min to 300 °C, where it was held constant for 10 min. The carrier gas was helium at a constant flow rate of 1.3 mL/min. The sample powder was dissolved in methanol from Merck (Darmstadt, Germany). The injection volume was 1 μL in splitless mode (1 min).
The mass spectrometer was operated in positive full scan mode, with acquisition of electron impact (EI) mass spectra in the range m/z 20–550, and the threshold was 100. Data acquisition started at 4 min. Data were processed using MSD Chemstation (02.02) software. Mass spectral libraries searches were performed using: (1) NIST/EPA/NIH library 11 (2011); (2) Mass Spectral Library of Drugs, Poisons, pesticides, Pollutants and their Metabolites 2011 (MPW2011); and (3) SWGDRUG MS Library version 2.4 (2015).
Nuclear magnetic resonance (NMR) spectroscopy
Analysis by 1D and 2D NMR was performed at the Department of Drug Design and Pharmacology, University of Copenhagen. NMR spectra were recorded in CD3OD (CAS # 811‐98‐3), DMSO‐d6 (CAS # 2206‐27‐1) (VWR Chemicals, Leuven, Belgium) or CDCl3 (CAS # 865‐49‐6) (Cambridge Isotope Laboratories, Andover, MA, USA) on a 400 or 600 MHz Bruker instrument (Bremen, Germany). Triethylamine hydrochloride (CAS # 554‐68‐7) was from Fluka (Sigma‐Aldrich, Steinheim, Germany). The obtained FID‐files (Free induction decay) were processed with MestReNova 10 software using Whittaker smoother baseline correction and exponential apodization. Signals are reported in ppm (δ) using the solvent as reference. Coupling constants (J) are given in hertz (Hz). Coupling constants are rounded to the nearest 0.5 Hz. Multiplet patterns are designated the following abbreviations, or combinations thereof: m – multiplet, d – doublet, t – triplet, q – quartet. Signal assignments were made from unambiguous chemical shifts and COSY (Correlation spectroscopy), HSQC (Heteronuclear single quantum coherence), and DEPT (Distortionless enhancement by polarization transfer) spectroscopy experiments.
Infra‐red (IR) spectroscopy
Infra‐red (IR) spectroscopy was recorded on a Spectrum One IR spectrometer from Perkin‐Elmer (Waltham, MA, USA) using Spectrum One version 3.02 software. Samples were loaded as neat solids and signals (ν max) are reported in wavenumbers (cm‐1) in the range 3600‐600 cm‐1.
Orbitrap mass spectrometry
Accurate mass measurement was performed by matrix‐assisted laser ionization Orbitrap mass spectrometry (MALDI/Orbitrap MS) at the Department of Pharmacy, University of Copenhagen. Analysis was performed in positive ion mode with MALDI ionization on a Thermo QExactive Orbitrap mass spectrometer (Thermo Scientific, Bremen, Germany) equipped with an AP‐SMALDI 10 ion source (TransmitMIT, Giessen, Germany) and operated with mass resolving power 140 000 at m/z 200. 2,5‐Dihydroxybenzoic acid (CAS # 490‐79‐9) (Sigma‐Aldrich, Steinheim, Germany) was used as matrix and lock‐mass for internal mass calibration, providing a mass accuracy of 3 ppm or better. Samples were dissolved in a solution of 2,5‐dihydroxybenzoic acid in methanol (2 mg/mL) and 3 μL of the solution was loaded on a glass plate for analysis.
Quadrupole time‐of‐flight (QTOF)‐mass spectrometry
High resolution product ion spectra were acquired with quadrupole time‐of‐flight mass spectrometry (QTOF‐MS) at the Section for Forensic Chemistry, Aarhus University, using a maXis Impact QTOF from Bruker Daltonics (Bremen, Germany), equipped with an orthogonal electrospray ionization (ESI) source. The software used to acquire HR‐TOF‐MS data and instrument control was OTOFcontrol 3.2 (Bruker Daltonics, Bremen, Germany) and HyStar 3.2 (Bruker Daltonics, Bremen, Germany). Samples were introduced into the mass spectrometer using an ultra‐performance liquid chromatography (UPLC) method described by Telving et al.30 Mass spectrometry was performed in positive electrospray ionization mode using Broad Band Collision Induced Dissociation (bbCID). The mass range was m/z 50 to 1000. Nebulizer gas pressure was 4.0 bar, drying gas was set to 11 L/min at a temperature of 220 °C. Nitrogen was used for nebulizer, drying gas and collision gas. The mass spectrometer conditions were as follows: capillary voltage of the ion source, 4.0 kV; end plate offset, 500 V; Funnel 1 RF, 200 Vpp; Funnel 2 RF, 200 Vpp; isCID, 0.0 eV; Hexapole RF, 50 Vpp; Quadrupole Ion Energy, 4.0 eV; Quadrupole Low Mass, 50 m/z; Collision Energy, 4.0 eV; Pre Pulse Storage 6.0 µs. The spectra rate was set to 10 Hz. Stepping was enabled with the following settings: Mode, basic; Collision RF from 300 to 700 Vpp; Transfer Time from 30 to 70 µs. Analyte fragmentation was performed in bbCID mode with the settings described, except for the following MS/MS settings: Collision Energy MS, 4.0 eV and MS/MS 25 eV. The instrument was calibrated externally before each sequence with a 1 mM sodium formate/acetate solution. Thirty‐five clusters (Na(HCOONa)x, Na(CH3COONa)x and Na(COOHNa)x(COONa)x) were selected and used for the instrument calibration. Mass range of the chosen clusters was from 90.9766 to 948.8727 Da. For post‐run mass calibration and processing of the data the software DataAnalysis 4.1 and Target Analysis 1.3 (Bruker Daltonics, Bremen, Germany) were used.
Liquid chromatography and triple quadrupole mass spectrometry (LC‐MS/MS)
The amount of acrylfentanyl in the seized sample was quantified with high performance liquid chromatography and triple quadrupole mass spectrometry (LC‐MS/MS) at the Department of Clinical Biochemistry (North Denmark Regional Hospital). Parameters for dynamic multiple reaction monitoring (dMRM) were optimized by flow injection analysis using a standard of acrylfentanyl, and finally added to an existing routine LC‐MS/MS method for drugs‐of‐abuse (Table S1). As deuterated internal standard fentanyl‐d5 was used. The parameters for sample preparation, data acquisition and quantification are shown in the Supporting Information.
Results and discussion
GC‐MS analysis
Analysis by GC‐MS showed a chromatogram (Figure 2) with a major peak at retention time (RT) 17.949 min. The EI mass spectrum (Figure 3B) could not be identified by library searches. A peak at RT 15.476 min was identified as N‐phenyl‐1‐(2‐phenylethyl)piperidin‐4‐amine, also called 4‐anilino‐N‐phenethylpiperidine with abbreviation ANPP (A, Figure 4), by both the NIST11 and SWGDRUG MS library. ANPP is a precursor for illicit synthesis of fentanyl according to a method referred to as the ‘Siegfried method’ on the drug forum discussion forum Erowid.org31 and a controlled substance in the United States. However, the synthetic precursor to ANPP, 1‐(2‐phenethyl)piperidin‐4‐one (also called N‐phenethyl‐piperidone with abbreviation NPP), was not identified through library searches or from extracted ion chromatograms of the base peak m/z 112 after injection of a 2 mg/mL sample concentration. Minor peaks in the chromatogram (Figure 2) included impurities, which were also present in a blank solvent sample.
Figure 2.
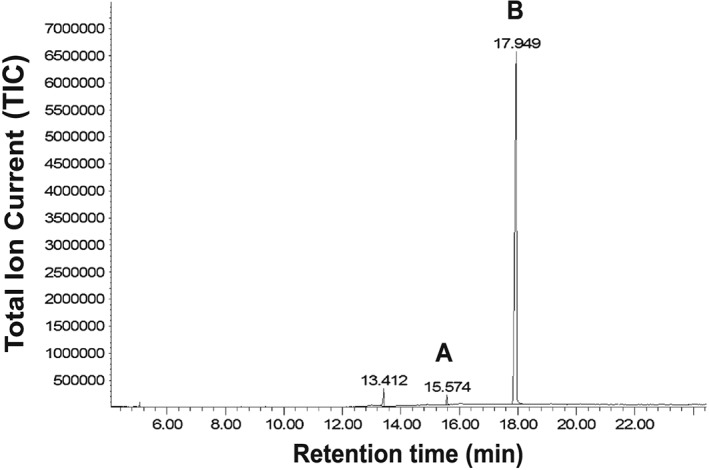
Total ion chromatogram from GC‐MS analysis of the seized sample powder, dissolved in methanol (0.2 mg/mL), showing (A) N‐phenyl‐1‐(2‐phenylethyl)piperidin‐4‐amine (precursor for synthesis of fentanyl analogues) at RT 15.576 min; and (B) the unknown compound at RT 17.949 min, identified as N‐phenyl‐N‐[1‐(2‐phenethyl)piperidin‐4‐yl]prop‐2‐enamide (synonym: acrylfentanyl). The peak at RT 13.412 min is also present in a blank solvent sample.
Figure 3.
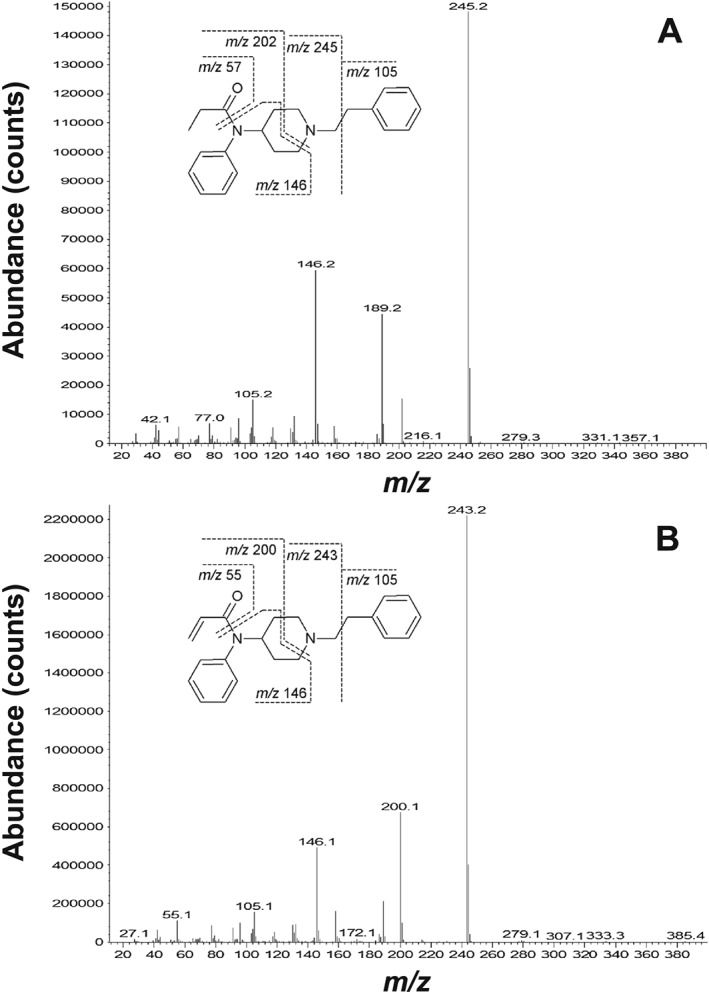
Electron impact (EI) mass spectra acquired during GC‐MS analysis of (A) a standard of N‐phenyl‐N‐[1‐(2‐phenethyl)piperidin‐4‐yl]propanamide (fentanyl) at RT 17.949 min; and (B) the unknown compound, identified as N‐phenyl‐N‐[1‐(2‐phenethyl)piperidin‐4‐yl]prop‐2‐enamide (acrylfentanyl) at RT 17.696 min. Background ions were subtracted for both spectra. Bond cleavage sites for key fragment ions are assigned according to Ohta et al.32
Figure 4.
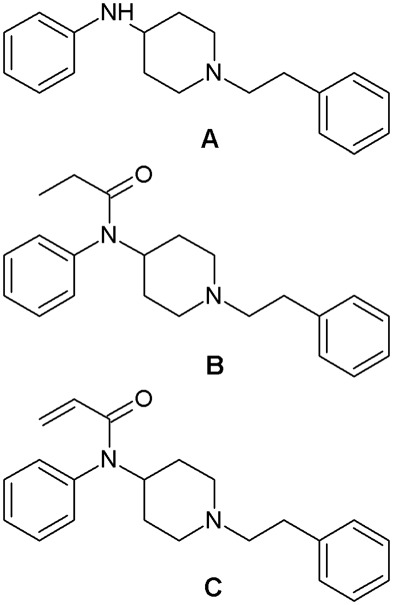
Chemical structures. A) N‐phenyl‐1‐(2‐phenylethyl)piperidin‐4‐amine (synthetic precursor); B) N‐phenyl‐N‐[1‐(2‐phenethyl)piperidin‐4‐yl]propanamide (Fentanyl); C) N‐phenyl‐N‐[1‐(2‐ phenethyl)piperidin‐4‐yl]prop‐2‐enamide (synonym: acrylfentanyl).
The presence of ANPP suggested that the unknown compound at RT 17.949 min could be a fentanyl analogue with a modification on the exocyclic amine (substitution of the propanoyl group in fentanyl with another moiety). A study by Ohta et al. on GC‐MS analysis of fentanyl and its analogues found two important mass spectrometric characteristics for EI spectra: (1) an absence of molecular ions for most fentanyl analogues; and (2) diagnostic ions formed by loss of a tropylium ion (M‐91), which in most cases formed the base peaks of the mass spectra.32
The base peak in the EI mass spectrum of RT 17.949 min was m/z 243 (Figure 3). This theoretically corresponds to a molecular ion of m/z 334 (243 + 91). Accordingly, the difference in molecular mass between fentanyl and the unknown compound was determined to 2 Da. This could be explained by introduction of a double bond in fentanyl (B, Figure 4) to give an α,β‐unsaturated fentanyl analogue (C, Figure 4). The mass spectrum of the unknown compound is remarkably similar to the mass spectrum of fentanyl (Figure 3A), acquired on the same apparatus. Based on the GC‐MS data alone, we arrived at the hypothesis, that the unknown compound was the acryloyl derivative of fentanyl (acrylfentanyl). Following the EI fragmentation patterns for fentanyl and related compounds published by Ohta et al.,32 the bond cleavage points can be assigned (Figure 3). Three fragment ions for acrylfentanyl (Figure 3B) differ with 2 Da from those of fentanyl (Figure 3A): m/z 243 (base peak), m/z 200 (from the piperidine ring), and m/z 55 (from the acryloyl group).
A new standard, the hydrochloride salt of acrylfentanyl with CAS # 79279‐03‐1, was acquired from Cayman Chemical (Ann Arbor, MI, USA). GC‐MS analysis confirmed the RT and EI mass spectrum of acrylfentanyl in the seized powder. The IUPAC name of acrylfentanyl (as free base) is N‐phenyl‐N‐[1‐(2‐phenethyl)piperidin‐4‐yl]prop‐2‐enamide with CAS # 82003‐75‐6.
A standard of N‐phenyl‐1‐(2‐phenylethyl)piperidin‐4‐amine from Carbosynth (Compton, Berkshire, UK) was used to confirm RT and EI mass spectrum of the detected ANPP in the sample. Acrylfentanyl can be synthesized by a reaction of acryloyl chloride (2‐propenoyl chloride) with ANPP. The acylation of ANPP to synthesise fentanyl and its analogues is well described and results in high yields (typically >94%).33
Pyrolytic degradation of fentanyl to ANPP during GC analysis is possible at high injection port temperatures. In a study of pyrolytic products from fentanyl, using a probe temperature of 750 °C under anaerobic conditions, minor signals from ANPP could be detected (1.9 area% of largest chromatographic peak).34 To test for pyrolytic degradation with the GC‐MS methods applied in the present study (injection port temperature 250 °C), standards of both fentanyl and acrylfentanyl (100 µg/mL) were injected. However, no ANPP could be detected. It should be noted that one of the major human metabolites of fentanyl is ANPP, in this context known as despropionylfentanyl, hence detection of this compound in biological matrices does not indicate a specific synthetic pathway.
Fentanyl and acrylfentanyl can be fully baseline separated by GC analysis using the methods described above with RT 17.653 min for fentanyl and RT 17.839 min for acrylfentanyl.
The chemical structures and IUPAC names of fentanyl, acrylfentanyl and ANPP are depicted in Figure 4.
Analysis by NMR and IR spectroscopy
The seized sample of acrylfentanyl was analysed by 1H‐ and 13C‐NMR and signals were assigned based on COSY, HSQC and DEPT spectroscopy experiments. In addition, standards of fentanyl and acrylfentanyl hydrochloride were analysed by 1H‐NMR and compared to that of the seized sample (Supporting Information). Based on the recorded NMR data, the seized compound was unambiguously identified as acrylfentanyl. Peaks arising from the terminal alkene are clearly observed by 1H NMR (Figure 5). The 1H and 13C NMR spectra are identical to that of the standard (Figures S6–S9) with the exception of an impurity in the seized sample that was identified as triethylamine hydrochloride.
Figure 5.
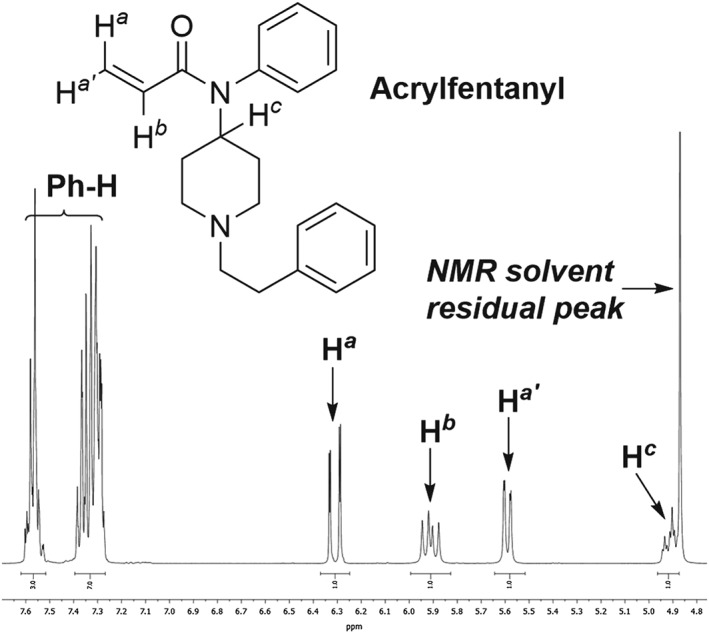
1H‐NMR spectrum of acrylfentanyl (CD3OD, 400 MHz). Enlargement of the region where signals arising from the terminal alkene are observed. See supporting information for the complete 1H‐ and 13C‐NMR spectra for acrylfentanyl and 1H‐NMR data for fentanyl.
In addition to acrylfentanyl (major component) the seized sample contained an aliphatic impurity that could not be identified when recorded in CD3OD (400 MHz) due to overlapping 1H‐NMR signals with those of acrylfentanyl. At 400 MHz in CD3OD a triplet could be observed at 1.37 ppm, which couples to an overlapping signal (COSY) at ~3.26 ppm (Figure S2). Most likely the triplet represents a CH3‐group coupling to a CH2‐group connected to an electronegative group. To identify the unknown entity a series of NMR experiments were performed in DMSO‐d6 and CDCl3 (600 MHz instrument) in the hope that signal separation of acrylfentanyl and the unknown compound might be achieved. When the spectrum was recorded in CDCl3 there was no useful separation of the signals, however a broad singlet integrating for one proton at 12.75 ppm (Figure S6) indicated that the compound was an ammonium ion, most probable the hydrochloride salt as described in the Siegfried method. Of note is that another smaller peak from an ammonium ion was observed at 12.87 ppm suggesting that the unknown compound might also be a hydrochloride salt. When the spectrum was recorded in DMSO‐d6 separation of the overlapping peaks was achieved (Figure S10). With full separation of the peaks we determined that the impurity was triethylamine hydrochloride (TEA × HCl). This was confirmed by recording the 13C‐NMR spectrum of a standard of TEA × HCl in DMSO‐d6 and comparing the spectrum to that of the seized sample (Figure S11). Furthermore, the 1H‐NMR sample of the seized powder of acrylfentanyl in DMSO‐d6 was spiked with a standard of TEA × HCl to unambiguously establish the identity of the impurity (Figure S12). Based on integration of the triplet arising from TEA × HCl at 1.19 ppm and the peak at 1.65 ppm arising from acrylfentanyl (Figure S12A), an acrylfentanyl to TEA × HCl ratio of 1:0.37 was determined. Provided that there are no significant impurities that cannot be detected by 1H‐NMR this translates to approximately 14 mass% of TEA × HCl in the seized sample.
The IR spectra of the seized and commercially acquired samples were recorded on the neat solids. Both samples clearly show the presence of an ammonium ion at ~2400‐2600 cm‐1 (Figures S13 and S14).
The presence of TEA × HCl in the seized sample most likely arises from a modification of the Siegfried method by utilising triethylamine as the base for the acetylation reaction instead of pyridine. In the last step of the Siegfried method the hydrochloride salt of fentanyl is generated. During the equivalent step in the synthesis of acrylfentanyl we hypothesise that the undesired TEA × HCl residue was generated in the final product. According to the Siegfried synthesis lactose is recommended as the dilution agent but no lactose or other dilution agents were observed by NMR or IR.
MALDI/Orbitrap MS
Accurate mass measurement by MALDI/Orbitrap MS found m/z 335.2114 for the protonated ion of the unknown compound [M + H]+ (Figure S3). This matches with the theoretical value for acrylfentanyl (m/z 335.2118 for C22H27N2O+) within 1.1 ppm.
Product ion spectra (QTOF‐MS)
High resolution precursor and product ion spectrum of fentanyl (C22H28N2O), acquired with quadrupole time‐of‐flight mass (QTOF), are shown in Figure 6. The major fragment was m/z 188.1448. Figure 7 shows the precursor and product ion spectrum for acrylfentanyl (C22H26N2O). A major fragment with m/z 188.1437 was found. In silico fragmentation of acrylfentanyl using the software ACD/MS Fragmenter (Advanced Chemistry Development, Toronto, Canada) shows a fragment with m/z 188.1434 with the proposed formula C13H18N. According to a study by Thevis et al. on analysis of fentanyl this fragmentation is a charge‐driven elimination of N‐phenyl‐propionimidic acid (M‐149).35
Figure 6.
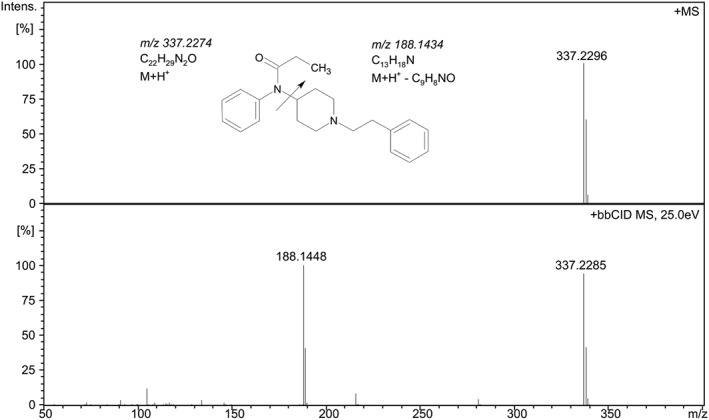
Mass spectrum for precursor (+MS) (top) and product ions (+bbCID) (bottom) for fentanyl, (C22H29N2O). The product ion at m/z 188.1448 corresponds to C13H18N, which is a well‐known fragmentation pathway for fentanyl (cf.35).
Figure 7.
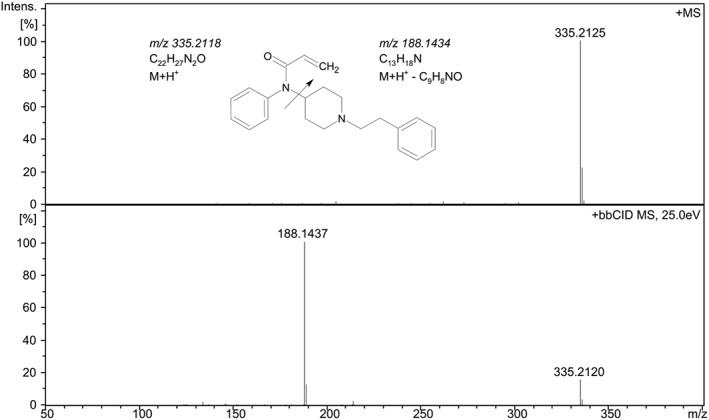
Mass spectrum for precursor (+MS) (top) and product ions (+bbCID) (bottom) for acrylfentanyl, (C22H27N2O). The product ion at m/z 188.1437 corresponds to C13H18N, which is a predicted fragmentation pathway for acrylfentanyl (cf.35).
Quantitative analysis (LC‐MS/MS)
Analysis by LC‐MS/MS determined the amount of acrylfentanyl (free base) in the seized powder, equal to 88.3 mass% of acrylfentanyl hydrochloride. This is in acceptable agreement with the impurity estimate from NMR spectroscopy (14 mass%).
The use of LC‐MS/MS with multiple reaction monitoring (MRM) is complicated due to the co‐elution of fentanyl (RT 5.102 min) and acrylfentanyl (RT 5.103 min). Within the resolution limits of a triple quadrupole mass spectrometer acrylfentanyl may cause interference with the detection of fentanyl due to the [M + H]+ + 2 isotope signals. The metabolites expected after human metabolism, norfentanyl and noracrylfentanyl, can also be difficult to separate by liquid chromatography, and hence necessitate the use of high‐resolution mass spectrometry if both compounds are present in the sample.
Previous studies
Compounds equal in molecular structure to acrylfentanyl have previously been reported in the literature for opiate receptor affinity after synthesis,36 as a theoretical derivative in mathematic modelling for drug design,37 and for in vivo activity in mice after custom synthesis.38 In the latter study, with the use of mouse hot plate tests, it was shown that acrylfentanyl was more potent than fentanyl and had a longer duration of action. A study by Zhu et al. on the synthesis and analgesic activity of 22 derivatives of fentanyl, including acrylfentanyl, is only available in Chinese.39 No clinical study data are available for the pharmacokinetic and pharmacodynamic properties of acrylfentanyl in humans.
Immunoassay screening
An aqueous solution of the seized powder (20 000 ng/mL) was tested with the immunoassay drug test described herein. All test results were negative. When the powder was tested on‐site after the seizure, and a positive result for MDMA was displayed, the aqueous solution may have been saturated, not free from particles or otherwise incompatible with requirements of the urine testing device. Although some immunoassays for fentanyl may cross‐react with acrylfentanyl (and prove useful), only a limited number of fentanyl assays have been critically validated for clinical use,40 hence screening with LC‐MS/MS or equivalent techniques are recommendable.
Conclusion
The new synthetic analogue (acrylfentanyl) of the potent opioid fentanyl was detected in a seized sample (powder) in Denmark. New synthetic opioids present a serious problem for public health due to their potency and risk of a fatal intoxication. Users of new synthetic opioids introduced to the illicit market have no certain knowledge about the presence of contaminants or cutting agents, which poses an additional risk to the opioid effect of the drug. Fentanyls can be absorbed through the skin and inhaled, introducing additional risks to individuals who come in contact with these drugs, such as package handlers and couriers who encounter fentanyls ordered online, family and friends of users, where these drugs are stored in peoples’ homes, law enforcement personnel and healthcare professionals in hospitals and drug treatment services. Our findings suggest that testing should be carried out for acrylfentanyl to monitor its emergence in samples presented by individual users and in post‐mortem forensic analysis under circumstances suggesting intoxication by an opioid. This could possibly prevent a spike in deaths as previously seen with acetylfentanyl.
Supporting information
Figure S1: Acrylfentanyl (seized sample), 1H NMR (400 MHz) in CD3OD
Figure S2: Acrylfentanyl (seized sample), COSY (400 MHz) in CD3OD
Figure S3: Acrylfentanyl (seized sample), MALDI‐TOF HRMS spectrum
Figure S4: Fentanyl (standard), 1H NMR (400 MHz) in CD3OD
Figure S5: Fentanyl (standard), MALDI‐TOF HRMS spectrum
Figure S6: 1H NMR (600 MHz) in CDCl3
Figure S7: 1H NMR (600 MHz) in CDCl3 : Enlargement (4.7–7.7 ppm)
Figure S8: 1H NMR (600 MHz) in CDCl3 : Enlargement (1.1–3.8 ppm)
Figure S9: DEPT (600 MHz) in CDCl3
Figure S10: Identification of the impurity as triethylamine hydrochloride by 1H NMR (600 MHz) in DMSO‐d6. Acrylfentanyl (seized sample),
Figure S11: Identification of the triethylamine hydrochloride impurity by 13C NMR (600 MHz) in DMSO‐d6
Figure S12: Identification of the impurity as triethylamine hydrochloride. Enlargement of aliphatic region for A) Acrylfentanyl (seized sample); and B) Acrylfentanyl (seized sample) spiked with triethylamine hydrochloride, 1H NMR (600 MHz) in DMSO‐d6
Figure S13: IR spectrum of acrylfentanyl (seized sample)
Figure S14: IR spectrum of acrylfentanyl (standard)
Figure S15: LC‐MS/MS calibration curve
Figure S16: MRM signal for m/z 335 → m/z 188 (acrylfentanyl in seized sample)
Figure S17: Qualifying ion transitions (overlaid) for acrylfentanyl in seized sample
Figure S18: MRM signal for m/z 343 → m/z 105 (internal standard, fentanyl‐d5) spiked to seized sample during sample preparation
Figure S19: MRM signal for m/z 335 → m/z 188 Acrylfentanyl calibrator, 156.25 ng/mL (free base)
Figure S20: Qualifying ion transitions (overlaid) Acrylfentanyl calibrator, 156.25 ng/mL (free base)
Table S1: Multiple Reaction Monitoring (MRM) parameters
Supporting info item
Acknowledgements
DSP is grateful to the Carlsberg and Lundbeck Foundations for financial support. TB and AK are part of the project CASSANDRA (Computer Assisted Solutions for Studying the Availability aNd DistRibution of novel psychoActive substances), which has received funding from the European Union under the ISEC programme ‐ Prevention of and fight against crime [JUST2013/ISEC/DRUGS/AG/6414]. Dr Helle Gravesen, Head of Forensic Psychiatric department (Aalborg Psychiatric Hospital, Denmark) is thanked for her outstanding collaboration in our common effort to identify new psychoactive substances. Laboratory technician Kirsten Andreasen at the Department of Clinical Biochemistry (North Denmark Regional Hospital, Denmark) is thanked for her skilful assistance during analysis.
Breindahl, T. , Kimergård, A. , Andreasen, M. F. , and Pedersen, D. S. (2017) Identification of a new psychoactive substance in seized material: the synthetic opioid N‐phenyl‐N‐[1‐(2‐phenethyl)piperidin‐4‐yl]prop‐2‐enamide (Acrylfentanyl). Drug Test. Analysis, 9: 415–422. doi: 10.1002/dta.2046.
References
- 1. EMCDDA (European Monitoring Centre for Drugs and Drug Addiction). European Drug Report 2016 Trends and Developments Available at: http://www.emcdda.europa.eu/edr2016 [4 July 2016].
- 2. EMCDDA (European Monitoring Centre for Drugs and Drug Addiction). New psychoactive substances in Europe: An update from the EU Early Warning System Available at: http://www.emcdda.europa.eu/publications/2015/new‐psychoactive‐substances [4 July 2016].
- 3. Brandt S. D., King L. A., Evans‐Brown M.. The new drug phenomenon. Drug Test. Anal. 2014, 6, 587. [DOI] [PubMed] [Google Scholar]
- 4. UNODC (United Nations Office on Drug and Crime). The challenge of new psychoactive substances Available at: https://www.unodc.org/documents/scientific/NPS_2013_SMART.pdf [4 July 2016].
- 5. Carroll County Health Department. Alert: Counterfeit Street Pills and fentanyl‐Related Overdoses in Carroll County Available at: http://cchd.maryland.gov/alert‐counterfeit‐street‐pills‐and‐fentanyl‐related‐overdoses‐in‐carroll‐county/ [4 July 2016].
- 6. WEDINOS (Welsh Emerging Drugs and Identification of Novel Substances Project). Sample W005255 Available at: http://wedinos.org/db/samples/ [4 July 2016].
- 7. Quintana P., Ventura M.. Ocfentanil: a novel fentanyl derivative detected as an adulterant of heroin. 2016. Poster presented at IV International Conference on Novel Psychoactive Substances. Budapest, 30–31 May, 2016.
- 8. Kram T. C., Cooper D. A., Allen A. C.. Behind the identification of China White. Anal. Chem. 1981, 53, 1379A. [DOI] [PubMed] [Google Scholar]
- 9. Henderson G. L.. Designer drugs: past history and future prospects. J. Forensic Sci. 1988, 33, 569. [PubMed] [Google Scholar]
- 10. Henderson G. L.. Fentanyl‐related deaths: demographics, circumstances, and toxicology of 112 cases. J. Forensic Sci. 1991, 36, 422. [PubMed] [Google Scholar]
- 11. Tuusov J., Vals K., Tõnisson M., Riikoja A., Denissov G., Väli M.. Fatal poisoning in Estonia 2000–2009. Trends in illegal drug‐related deaths. J. Forensic Leg. Med. 2012, 20, 51. [DOI] [PubMed] [Google Scholar]
- 12. Talu A., Rajaleid K., Abel‐Ollo K., Rüütel K., Rahu M., Rhodes T., Platt L., Bobrova N., Uusküla A.. HIV infection and risk behaviour of primary fentanyl and amphetamine injectors in Tallinn, Estonia: implications for intervention. Int. J. Drug Pol. 2009, 21, 56. [DOI] [PubMed] [Google Scholar]
- 13. EMCDDA (European Monitoring Centre for Drugs and Drug Addiction) – Europol). Acetylfentanyl. EMCDDA–Europol Joint Report on a new psychoactive substance: N‐phenyl‐N‐[1‐(2‐phenylethyl)piperidin‐4‐yl] acetamide (acetylfentanyl). Available at: http://www.emcdda.europa.eu/publications/joint‐reports/acetylfentanyl [4 July 2016].
- 14. CCENDU (Canadian Centre on Substance Misuse). CCENDU Bulletin. Novel synthetic opioids in Counterfeit Pharmaceuticals and other illicit street drugs Available at: http://www.ccsa.ca/Resource%20Library/CCSA‐CCENDU‐Novel‐Synthetic‐Opioids‐Bulletin‐2016‐en.pdf [4 July 2016].
- 15. Office of the Chief Coroner. Illicit Drug Overdose Deaths in BC January 1, 2007 – May 31, 2016. Available at: http://www2.gov.bc.ca/assets/gov/public‐safety‐and‐ emergency‐services/death‐investigation/statistical/illicit‐drug.pdf.[4 July 2016]
- 16. Amlani A., McKee G., Khamis N., Raghukumar G., Tsang E., Buxton J. A.. Why the FUSS (Fentanyl Urine Screen Study)? A cross‐sectional survey to characterize an emerging threat to people who use drugs in British Columbia, Canada. Harm Reduct.J. 2015, 12, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Melent'ev A. B., Kataev S. S., Dvorskaya O. N.. Identification and analytical properties of acetyl fentanyl metabolites. J. Analyt. Chem. 2015, 70, 240. [Google Scholar]
- 18. WHO (World Health Organization). Acetylfentanyl. Critical Review Report. Agenda item 5.2. Expert Committee on Drug Dependence. Thirty‐seventh Meeting, Geneva, 2015. Available at: http://www.who.int/medicines/access/controlled‐substances/5.2_Acetylfentanyl_CRev.pdf [4 July 2016].
- 19. Lozier M. J., Boyd M., Stanley C., Ogilvie L., King E., Martin C., Lewis L.. Acetyl fentanyl, a novel fentanyl analog, causes 14 overdose deaths in Rhode Island, March–May 2013. J. Med. Toxicol. 2015, 11, 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McIntyre I. M., Trochta A., Gary R. D., Malamatos M., Lucas J. R.. An acute acetyl fentanyl fatality: a case report with postmortem concentrations. J. Analyt. Toxicol. 2015, 39, 490 Erratum in: J Anal Toxicol 2016, 88. [DOI] [PubMed] [Google Scholar]
- 21. DEA (Drug Enforcement Administration). Office of Diversion Control. Drug & Chemical Evaluation Section. Acetyl fentanyl (N‐(1‐phenethylpiperidin‐4‐yl)‐N‐phenylacetamide) (DEA/OD/ODE, July 2015). Available at: http://www.deadiversion.usdoj.gov/drug_chem_info/acetylfentanyl.pdf [4 July 2016].
- 22. Cunningham S. M., Haikal N. A., Kraner J. C.. Fatal intoxication with acetyl fentanyl. J. Forensic Sci. 2015, 61, S276. [DOI] [PubMed] [Google Scholar]
- 23. Helander A., Bäckberg M., Beck O.. Intoxications involving the fentanyl analogs acetylfentanyl, 4‐methoxybutyrfentanyl and furanylfentanyl: results from the Swedish STRIDA project. Clin. Toxicol. 2016, 54, 324. [DOI] [PubMed] [Google Scholar]
- 24. Cole J. B., Dunbar J. F., McIntire S. A., Regelmann W. E., Slusher T. M.. Butyrfentanyl overdose resulting in diffuse alveolar hemorrhage. Pediatrics. 2015, 135, e740. [DOI] [PubMed] [Google Scholar]
- 25. Bäckberg M., Beck O., Jönsson K.‐H., Helander A.. Opioid intoxications involving butyrfentanyl, 4‐fluorobutyrfentanyl, and fentanyl from the Swedish STRIDA project. Clin. Toxicol. 2015, 53, 609. [DOI] [PubMed] [Google Scholar]
- 26. Mounteney J., Giraudon I., Denissov G., Griffiths P.. Fentanyls: Are we missing the signs? Highly potent and on the rise in Europe. Int. J. Drug Pol. 2015, 26, 626. [DOI] [PubMed] [Google Scholar]
- 27. WEDINOS (Welsh Emerging Drugs and Identification of Novel Substances Project). Sample W004251. Available at: http://wedinos.org/db/samples/ [4 July 2016].
- 28. Marinetti L. J., Ehlers B. J.. A series of forensic toxicology and drug seizure cases involving illicit fentanyl alone and in combination with heroin, cocaine or heroin and cocaine. J. Anal. Toxicol. 2014, 38, 592. [DOI] [PubMed] [Google Scholar]
- 29. Coopman V., Blanckaert P., Van Parys G., Van Calenbergh S., Cordonnier J.. A case of acute intoxication due to combined use of fentanyl and 3,4‐dichloro‐N‐[2‐(dimethylamino)cyclohexyl]‐N‐methylbenzamide (U‐47700). Forensic Sci Int. 2016, 266, 68. [DOI] [PubMed] [Google Scholar]
- 30. Telving R., Hasselstrøm J. B., Andreasen M. F.. Targeted toxicological screening for acidic, neutral and basic substances in postmortem and antemortem whole blood using simple protein precipitation and UPLC‐HR‐TOF‐MS. J. Forensic Sci. 2016, 266, 453. [DOI] [PubMed] [Google Scholar]
- 31. Anonymous author . A description of the synthesis of fentanyl. Available at: https://www.erowid.org/archive/rhodium/chemistry/fentanyl.html [24 June 2016].
- 32. Ohta H., Suzuki S., Ogasawara K.. Studies on fentanyl and related compounds IV. Chromatographic and spectrometric discrimination of fentanyl and its derivatives. J. Anal. Toxicol. 1999, 23, 280. [DOI] [PubMed] [Google Scholar]
- 33. Valdez C. A., Leif R. N., Mayer B. P.. An efficient, optimized synthesis of fentanyl and related analogs. PLoS One. 2014, 9, e108250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nishikawa R. K., Bell S. C., Kraner J. C., Callery P. S.. Potential biomarkers of smoked fentanyl utililizing pyrolysis gas chromatography‐mass spectrometry. J. Anal. Toxicol. 2009, 33, 418. [DOI] [PubMed] [Google Scholar]
- 35. Thevis M., Geyer H., Bahr D., Schänzer W.. Identification of fentanyl, alfentanil, sufentanil, remifentanil and their major metabolites in human urine by liquid chromatography/tandem mass spectrometry for doping control purposes. Eur. J. Mass Spectrom. 2005, 11, 419. [DOI] [PubMed] [Google Scholar]
- 36. Maryanoff B. E., Simon E. J., Gioannini T., Gorissen H.. Potential affinity labels for the opiate receptor based on fentanyl and related compounds. J. Med. Chem. 1982, 25, 913. [DOI] [PubMed] [Google Scholar]
- 37. Dong N., Lu W. C., Chen N. Y., Zhu Y. C., Chen K. X.. Using support vector classification for SAR of fentanyl derivatives. Acta Pharmacol. Sin. 2005, 26, 107. [DOI] [PubMed] [Google Scholar]
- 38. Essawi M. Y. H.. Fentanyl analogues with a modified propanamido group as potential affinity labels: synthesis and in vivo activity. Pharmazie. 1999, 54, 307. [PubMed] [Google Scholar]
- 39. Zhu Y.‐Q., Ge B.‐L., Fang S.‐N., Zhu Y.‐C., Dai Q.‐Y., Tan Q.‐Y., Huang Z.‐M., Chen X.‐J.. Studies on potent analgesics. I. Synthesis and analgesic activity of fentanyl derivatives. Yaoxue Xuebao. 1981, 16, 199. [PubMed] [Google Scholar]
- 40. Wang B.‐T., Colby J. M., Wu A. H. B., Lynch K. L.. Cross‐reactivity of acetylfentanyl and risperidone with a fentanyl immunoassay. J. Analytic. Toxicol. 2014, 38, 672. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Acrylfentanyl (seized sample), 1H NMR (400 MHz) in CD3OD
Figure S2: Acrylfentanyl (seized sample), COSY (400 MHz) in CD3OD
Figure S3: Acrylfentanyl (seized sample), MALDI‐TOF HRMS spectrum
Figure S4: Fentanyl (standard), 1H NMR (400 MHz) in CD3OD
Figure S5: Fentanyl (standard), MALDI‐TOF HRMS spectrum
Figure S6: 1H NMR (600 MHz) in CDCl3
Figure S7: 1H NMR (600 MHz) in CDCl3 : Enlargement (4.7–7.7 ppm)
Figure S8: 1H NMR (600 MHz) in CDCl3 : Enlargement (1.1–3.8 ppm)
Figure S9: DEPT (600 MHz) in CDCl3
Figure S10: Identification of the impurity as triethylamine hydrochloride by 1H NMR (600 MHz) in DMSO‐d6. Acrylfentanyl (seized sample),
Figure S11: Identification of the triethylamine hydrochloride impurity by 13C NMR (600 MHz) in DMSO‐d6
Figure S12: Identification of the impurity as triethylamine hydrochloride. Enlargement of aliphatic region for A) Acrylfentanyl (seized sample); and B) Acrylfentanyl (seized sample) spiked with triethylamine hydrochloride, 1H NMR (600 MHz) in DMSO‐d6
Figure S13: IR spectrum of acrylfentanyl (seized sample)
Figure S14: IR spectrum of acrylfentanyl (standard)
Figure S15: LC‐MS/MS calibration curve
Figure S16: MRM signal for m/z 335 → m/z 188 (acrylfentanyl in seized sample)
Figure S17: Qualifying ion transitions (overlaid) for acrylfentanyl in seized sample
Figure S18: MRM signal for m/z 343 → m/z 105 (internal standard, fentanyl‐d5) spiked to seized sample during sample preparation
Figure S19: MRM signal for m/z 335 → m/z 188 Acrylfentanyl calibrator, 156.25 ng/mL (free base)
Figure S20: Qualifying ion transitions (overlaid) Acrylfentanyl calibrator, 156.25 ng/mL (free base)
Table S1: Multiple Reaction Monitoring (MRM) parameters
Supporting info item


