Summary
Background
The incidence of inflammatory bowel disease (IBD) is record high in the Faroe Islands, and many Faroese emigrate to Denmark, where the IBD incidence is considerably lower.
Aim
To study the IBD incidence in first‐, second‐ and third‐generation immigrants from the Faroe Islands to Denmark to assess the extent to which the immigrants adopt the lower IBD incidence of their new home country.
Methods
Data on Faroese‐born Danish residents and their children were retrieved from the Danish Central Population Register for 1980–2014. Incident IBD cases were identified from the Danish National Patient Register. Standardised Incidence Ratios (SIRs) were used to compare the IBD risk in immigrants with that of Danes. 95% confidence intervals (CI) were calculated using the square‐root transform.
Results
First‐generation Faroese immigrants had a higher IBD incidence than Danes, SIR 1.25 (95% CI, 0.97–1.59) for men and 1.28 (95% CI, 1.05–1.53) for women. This excess risk derived from ulcerative colitis (UC), SIR 1.44 (95% CI, 1.10–1.87) for men and 1.36 (95% CI, 1.09–1.68) for women. No excess risk was found for Crohn's disease (CD). The UC risk was nearly doubled during the immigrants’ first 10 years in Denmark; SIR 2.13 (95% CI, 1.52–2.92) for men and 1.63 (95% CI, 1.19–2.18) for women.
Conclusions
Although some impact of genetic dilution cannot be excluded, our findings indicate importance of gene‐environment interplay in UC, as the excess UC risk in Faroese immigrants to Denmark disappeared over time and over one generation in men and over two generations in women.
Introduction
Inflammatory bowel diseases (IBD) are generally hypothesised to be caused by a combination of genetic pre‐dispositions and environmental influences.1 Recent studies do, however, indicate that the genetic pre‐disposition may play a minor role than previously thought. In updated data for Swedish twins, Haldvarson2 found the genetic component in Crohn's disease (CD) to be weaker than reported before. Furthermore, comparison of CD patients, their first‐degree relatives, and healthy controls for known IBD genetic risk scores showed that the first‐degree relatives had an increased CD‐specific genetic risk, but their general IBD genetic risk was similar to that of the healthy controls.3
Large population‐based cohort studies from Canada4 and Sweden5 have shown that immigrants from low‐ and middle‐income countries have a lower incidence of IBD than that of long‐term inhabitants of these two high‐income countries. Children of immigrants to these countries had an incidence of CD that was closer to, but still significantly lower, than that of the long‐term inhabitants; while their incidence of ulcerative colitis (UC) tended to catch up with that of the long‐term inhabitants. As the low risks of immigrant groups approached the higher risk of the receiving country within two generations, the data indicated that the incidence of IBD, and in particular of UC, was strongly modifiable by the lifestyle and/or environment of the new country.
However, given that the introduction of an exposure is associated with an increased risk of a disease, a stronger support for the causation hypothesis may be revealed, if the risk of the disease also decreases once the exposure ceases.6 The IBD incidence has increased in the Faroe Islands during the past 50 years.7 In this respect, the development in the Faroe Islands resembles that of other countries undergoing Westernisation.8 At present, the Faroe Islands has the highest IBD incidence in Europe with 84 per 100 000,9, 10 a level clearly above that of other Nordic countries, for example, 24 per 100 000 in Denmark.9 At the same time, the Faroe Islands are part of the Danish realm, and many Faroese move to Denmark for education and employment. In 2015, a total of 48 600 persons lived in the Faroe Islands,11 while 11 500 persons of Faroese origin lived in Denmark.12
On this background, we hypothesised that persons of Faroese origin living in Denmark have a higher IBD incidence than presently found in Denmark.10 Furthermore, we hypothesised that children and grandchildren of immigrants have a lower incidence than their forefathers, thus we undertook a nationwide, population‐based cohort study of IBD incidence in first‐, second‐ and third‐generation of Faroese immigrants to Denmark.
Material and methods
Study population
In this nationwide, population‐based cohort study, data were retrieved from the Danish Central Population Register (CPR), which was founded in 1968 and includes data on all persons with a permanent address in Denmark at any point in time since 1968. The CPR includes information on country of birth, and for immigrants the country of emigration and immigration date, and a parent‐child link enabling the construction of families. We retrieved data on all Faroese‐born residents in Denmark, their children and their grandchildren, if living in Denmark. The CPR is daily updated on vital status and migration, and the unique 10‐digit Civil Personal Register (CPR) numbers enables linkage to other Danish registers.13
From the CPR, data were retrieved for Faroese immigrants defined as: The Faroe Islands being the country of birth or/and the Faroe Islands being the country of emigration (first‐generation immigrants); and for children of immigrants defined as: a person having at least one immigrated parent (second‐generation immigrants); and for grandchildren of immigrants defined as a person having at least one immigrated grandparent (third‐generation immigrants). For each person, information on gender, date of birth, dates of immigration(s) and emigration(s) and date of death was retrieved. Person years (pys) were accumulated from 1 January 1980 or from date of first immigration to Denmark whichever came last; until date of emigration, death, contraction of disease, or 31 December 2014 whichever came first. Re‐immigration was frequent and a given person could therefore accumulate pys in more than one risk period. Second‐ and third‐generation also started accumulating pys from date of birth. For the entire Danish population, the pys for each calendar year were calculated using the number of inhabitants in the middle of the calendar year. Population data for the Danish population was available from Statistics Denmark.
Diagnoses of CD or UC are recorded in the Danish National Patient Register, which was founded in 1977 and includes information from hospitals on all in‐patients in somatic wards, and since 1995 also on all patients in psychiatric wards, and on all out‐ and emergency room patients. The International Classification of Diseases, version 8 (ICD‐8) was used until 1994, where it was replaced by the 10th version (ICD‐10).14 For CD the following codes were used 563.00–563.09 and K50–K50.9. The UC codes were; 563.19, 569.04 and K51–K51.9.15, 16 A person became a case on the first date within his/her risk period(s), he/she was registered with one of these diagnoses. To avoid inclusion of prevalent cases, we excluded cases in persons already known with an IBD diagnosis in the patient register prior to 1980.
Statistical analysis
Standardised Incidence Ratios (SIRs) were calculated as the observed numbers of IBD, CD and UC, respectively, in a given immigrant group divided by the expected numbers calculated from the accumulated pys and the incidence rates for IBD, CD and UC in the Danish population. Standard rates were calculated by gender, 5‐year age groups and 5‐year calendar periods. The 95% confidence intervals for SIRs were calculated using the square‐root transform.
In our study, a diagnosis of IBD at the age of 19 years or younger was defined as a paediatric/adolescent case, in correspondence with the limit set on almost half of the previous studies.17 SIRs were calculated from the age of 20 years and above. Furthermore, SIRs were calculated by time living in Denmark divided into <10 years and >10 years. It should be noted though that the CPR‐data were left censored to 1980. This means that there will be a certain degree of misclassification on this variable, because some persons can have immigrated to Denmark prior to 1980. It would not have helped to restrict the analysis to persons with a record of immigration after 1 January 1980, because these persons could easily have lived in Denmark also in one or more period(s) prior to 1980.
Ethics
This is a register‐based research project with no contact to patients, their relatives or treating physicians. The Danish Data Protection Agency approved the study, journal no: 2014‐41‐3538. Data from CPR and the Danish National Patient Register were obtainable via Statistics Denmark.
Results
The cohort included 57 373 persons of Faroese origin contributing 812 196 pys. First‐generation Faroese immigrants included 26 408 persons and 324 680 pys; second‐generation 19 987 persons and 330 109 pys; and third‐generation 10 978 persons and 157 381 pys (Table 1). Men and women in the first‐generation spent 86% and 88%, respectively, of their life in Denmark. In the second generation, both genders spent 91%, and in the third‐generation the percentage of time spent in Denmark was 98% for both genders (Table 1). In total, 180 incident IBD cases were observed in first‐generation immigrants, 122 in second generation and 42 in third‐generation (Tables 2 and 3).
Table 1.
First, second and third‐generation immigrants from the Faroe Islands to Denmark by gender and year of birth. Person years (pys) for the same groups by calendar period and age
| First‐generation | Second‐generation | Third‐generation | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Year of birth | Men (n) | Women (n) | Total (n) | Men (n) | Women (n) | Total (n) | Men (n) | Women (n) | Total (n) |
| ≤1949 | 2075 | 3291 | 5366 | 12 | 1 | 13 | 0 | 0 | 0 |
| 1950–59 | 1441 | 1660 | 3101 | 544 | 386 | 930 | 0 | 0 | 0 |
| 1960–69 | 2143 | 2328 | 4471 | 1320 | 1279 | 2599 | 4 | 2 | 6 |
| 1970–79 | 2228 | 2557 | 4785 | 1481 | 1390 | 2871 | 77 | 73 | 150 |
| 1980–89 | 2144 | 2450 | 4594 | 1661 | 1567 | 3228 | 850 | 790 | 1640 |
| ≥1990 | 1861 | 2230 | 4091 | 5226 | 5120 | 10346 | 4764 | 4418 | 9182 |
| All persons | 11892 | 14516 | 26408 | 10244 | 9743 | 19987 | 5695 | 5283 | 10978 |
| Current period (pys) | |||||||||
| 1980–89 | 27047.7 | 41758.7 | 68806.4 | 30546.9 | 28036.1 | 58583.0 | 3779.1 | 3440.8 | 7219.9 |
| 1990–99 | 40322.7 | 56223.9 | 96546.6 | 45231.0 | 42641.4 | 87872.4 | 16838.8 | 15585.1 | 32423.9 |
| 2000–09 | 41926.6 | 60029.1 | 101956 | 57800.6 | 56355.8 | 114156 | 35757.5 | 33334.1 | 69091.6 |
| 2010–14 | 23940.4 | 33431.0 | 57371.4 | 34687.8 | 34809.6 | 69497.4 | 25220.0 | 23424.8 | 48644.7 |
| Current age (pys) | |||||||||
| 0–19 | 22291.2 | 24304.4 | 46595.5 | 83008.4 | 81377.1 | 164386 | 71507.2 | 66598.3 | 138106 |
| 20–29 | 30234.3 | 41132.0 | 71366.3 | 37147.3 | 37033.6 | 74179.9 | 8954.1 | 8116.6 | 17070.7 |
| 30–39 | 25359.5 | 33028.8 | 58388.2 | 27201.8 | 25503.4 | 52705.2 | 1088.1 | 1021.5 | 2109.6 |
| 40–49 | 19244.0 | 28825.2 | 48069.2 | 15637.0 | 14103.6 | 29740.6 | 42.2 | 47.8 | 90.0 |
| 50–59 | 15270.0 | 24133.0 | 39403.0 | 4990.4 | 3739.2 | 8729.7 | 3.8 | 0.6 | 4.3 |
| 60–69 | 11447.6 | 19450.4 | 30897.9 | 281.4 | 87.0 | 368.4 | 0 | 0 | 0 |
| 70–79 | 6630.4 | 13203.4 | 19833.7 | 0 | 0 | 0 | 0 | 0 | 0 |
| 80+ | 2760.5 | 7365.7 | 10126.2 | 0 | 0 | 0 | 0 | 0 | 0 |
| % of life in DK | Men | Women | Men | Women | Men | Women | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (n) | (%) | (n) | (%) | (n) | (%) | (n) | (%) | (n) | (%) | (n) | (%) | |
| 11892 | 86 | 14515* | 88 | 10237† | 91 | 9732‡ | 91 | 5688§ | 98 | 5275¶ | 98 | |
Individuals were excluded due to missing information on pys for *one woman, †seven men, ‡eleven women, §seven men, ¶eight women.
Table 2.
Standardised incidence ratio (SIR) of CD, UC and IBD for male first‐, second‐, and third‐generation immigrants from the Faroe Islands to Denmark
| Men | CD | UC | IBD | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Obs. | Exp. | SIR (95% CI) | Obs. | Exp. | SIR (95% CI) | Obs.a | Exp. | SIR (95% CI) | |
| First | 15 | 19 | 0.81 (0.45–1.34) | 56 | 39 | 1.44 (1.10–1.87) | 66 | 53 | 1.25 (0.97–1.59) |
| First: <10 years in DK | 7 | 10 | 0.72 (0.28–1.49) | 39 | 18 | 2.13 (1.52–2.92) | 45 | 26 | 1.74 (1.27–2.33) |
| >10 years in DK | 8 | 9 | 0.91 (0.39–1.80) | 17 | 21 | 0.82 (0.48–1.32) | 21 | 27 | 0.78 (0.48–1.19) |
| Second | 17 | 21 | 0.81 (0.47–1.30) | 37 | 36 | 1.01 (0.71–1.40) | 50 | 52 | 0.96 (0.71–1.26) |
| Second: one parent | 17 | 18 | 1.00 (0.60–1.53) | 32 | 31 | 1.03 (0.70–1.45) | 45 | 44 | 1.01 (0.74–1.35) |
| Second: two parents | 0 | 3 | NA | 5 | 5 | 0.94 (0.30–2.22) | 5 | 8 | 0.65 (0.21–1.54) |
| Third | 10 | 9 | 1.12 (0.53–2.10) | 9 | 10 | 0.87 (0.39–1.66) | 16 | 17 | 0.97 (0.55–1.57) |
| Age 0–19 | |||||||||
| First | 1 | 2 | 0.50 (0.000–2.84) | 1 | 2 | 0.43 (0.000–2.44) | 2 | 4 | 0.51 (0.05–1.86) |
| Second | 3 | 6 | 0.48 (0.09–1.41) | 8 | 7 | 1.14 (0.49–2.26) | 11 | 12 | 0.92 (0.45–1.64) |
| Second: one parent | 3 | 5 | 0.60 (0.11–1.70) | 7 | 6 | 1.18 (0.47–1.45) | 10 | 10 | 0.98 (0.47–1.81) |
| Second: two parents | 0 | 1 | NA | 1 | 1 | 0.93 (0.00–5.31) | 1 | 2 | 0.54 (0.00–3.10) |
| Third | 8 | 5 | 1.48 (0.63–2.92) | 5 | 6 | 0.86 (0.27–2.02) | 11 | 10 | 1.10 (0.54–1.97) |
| Age 20+ | |||||||||
| First | 14 | 17 | 0.85 (0.46–1.43) | 55 | 37 | 1.50 (1.13–1.95) | 64 | 49 | 1.31 (1.01–1.68) |
| Second | 14 | 15 | 0.95 (0.52–1.60) | 29 | 29 | 0.98 (0.66–1.41) | 39 | 40 | 0.97 (0.69–1.33) |
| Second: one parent | 14 | 13 | 1.11 (0.61–1.87) | 25 | 25 | 0.99 (0.64–1.46) | 35 | 34 | 1.02 (0.71–1.42) |
| Second: two parents | 0 | 2 | NA | 4 | 4 | 0.95 (0.25–2.45) | 4 | 6 | 0.69 (0.18–1.78) |
| Third | 2 | 3 | 0.73 (0.07–2.69) | 4 | 5 | 0.88 (0.23–2.29) | 5 | 7 | 0.77 (0.24–1.80) |
Cases of Crohn's disease and UC do not add to the number of IBD cases as some patients had both diagnoses. NA 95% CI cannot be calculated using the square‐root transform. DK, Denmark.
Table 3.
Standardised Incidence Ratio (SIR) of CD, UC and IBD for female first‐, second‐, and third‐generation immigrants from the Faroe Islands to Denmark
| Women | CD | UC | IBD | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Obs. | Exp. | SIR (95% CI) | Obs. | Exp. | SIR (95% CI) | Obs.a | Exp. | SIR (95% CI) | |
| First | 35 | 35 | 1.00 (0.70–1.40) | 86 | 63 | 1.36 (1.09–1.68) | 114 | 89 | 1.28 (1.05–1.53) |
| First: <10 year in DK | 20 | 18 | 1.12 (0.68–1.72) | 45 | 28 | 1.63 (1.19–2.18) | 63 | 42 | 1.51 (1.16–1.94) |
| >10 years in DK | 15 | 17 | 0.89 (0.50–1.47) | 41 | 36 | 1.15 (0.83–1.56) | 51 | 48 | 1.07 (0.80–1.41) |
| Second | 25 | 27 | 0.92 (0.60–1.36) | 53 | 41 | 1.29 (0.97–1.69) | 72 | 61 | 1.18 (0.92–1.48) |
| Second: one parent | 18 | 23 | 0.80 (0.47–1.26) | 42 | 35 | 1.21 (0.87–1.64) | 56 | 51 | 1.09 (0.82–1.41) |
| Second: two parents | 7 | 4 | 1.60 (0.63–3.32) | 11 | 6 | 1.71 (0.85–3.07) | 16 | 10 | 1.65 (0.94–2.69) |
| Third | 18 | 10 | 1.83 (1.08–2.90) | 13 | 11 | 1.18 (0.62–2.02) | 26 | 18 | 1.46 (0.95–2.14) |
| Age 0–19 | |||||||||
| First | 2 | 3 | 0.67 (0.06–2.46) | 5 | 3 | 1.49 (0.47–3.49) | 7 | 6 | 1.21 (0.48–2.51) |
| Second | 9 | 7 | 1.23 (0.56–2.34) | 9 | 8 | 1.09 (0.49–2.07) | 16 | 14 | 1.13 (0.64–1.84) |
| Second: one parent | 7 | 6 | 1.14 (0.45–2.37) | 5 | 7 | 0.73 (0.23–1.71) | 11 | 12 | 0.93 (0.46–1.67) |
| Second: two parents | 2 | 1 | 1.63 (0.15–5.98) | 4 | 1 | 2.86 (0.74–7.39) | 5 | 2 | 2.10 (0.66–4.94) |
| Third | 9 | 5 | 1.67 (0.76–3.18) | 8 | 6 | 1.27 (0.54–2.52) | 14 | 10 | 1.35 (0.73–2.26) |
| Age 20+ | |||||||||
| First | 33 | 32 | 1.04 (0.71–1.46) | 81 | 60 | 1.35 (1.08–1.68) | 107 | 84 | 1.28 (1.05–1.55) |
| Second | 16 | 20 | 0.81 (0.46–1.32) | 44 | 33 | 1.34 (0.98–1.80) | 56 | 47 | 1.19 (0.90–1.55) |
| Second: one parent | 11 | 16 | 0.66 (0.33–1.19) | 37 | 28 | 1.33 (0.94–1.84) | 45 | 40 | 1.13 (0.83–1.52) |
| Second: two parents | 5 | 4 | 1.38 (0.44–3.25) | 7 | 5 | 1.39 (0.55–2.89) | 11 | 7 | 1.50 (0.75–2.70) |
| Third | 7 | 4 | 1.98 (0.78–4.10) | 5 | 4 | 1.05 (0.33–2.46) | 12 | 7 | 1.61 (0.83–2.83) |
Cases of Crohn's disease and UC do not add to the number of IBD cases as some patients had both diagnoses. DK: Denmark.
For first‐generation male immigrants from the Faroe Islands to Denmark, the SIR for CD was 0.81 (95% CI, 0.45–1.34), for UC, it was 1.44 (95% CI, 1.10–1.87), and for IBD 1.25 (95% CI, 0.97–1.59) (Table 2). The SIRs were 1.74 (95% CI, 1.27–2.33); 0.72 (95% CI, 0.28–1.49); and 2.13 (95% CI, 1.52–2.92), respectively, for the immigrants during their first 10 years in Denmark, and 0.78 (95% CI, 0.48–1.19); 0.91 (95% CI, 0.39–1.80); and 0.82 (95% CI, 0.48–1.32); respectively, for stays beyond 10 years (Figure 1). In second‐generation male immigrants, the SIRs were 0.81 (95% CI, 0.47–1.30), 1.01 (95% CI, 0.71–1.40), and 0.96 (95% CI, 0.71–1.26) respectively. The majority of second‐generation immigrants had one Faroese parent only, but those with two Faroese parents also had lower point estimates than first‐generation immigrants. The third‐generation male immigrants had SIRs of 1.12 (95% CI, 0.53–2.10), 0.87 (95% CI, 0.39–1.66), and 0.97 (95% CI, 0.55–1.57) respectively. The excess risks of UC and the borderline excess risk of IBD in first‐generation immigrants were found only in men 20 years or above.
Figure 1.
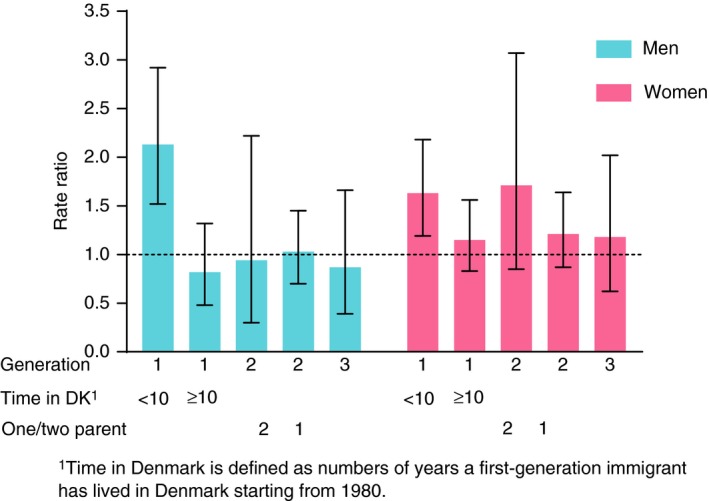
Standardised incidence ratio (SIR) of ulcerative colitis in Faroese immigrants to Denmark. Notes: DK, Denmark; One/two parent, one or two parent(s) of Faroese origin.
For first‐generation female immigrants, the SIR for CD was 1.00 (95% CI, 0.70–1.40); for UC, it was 1.36 (95% CI, 1.09–1.68) and for IBD, it was 1.28 (95% CI, 1.05–1.53) (Table 3).
The SIRs were 1.51 (95% CI, 1.16–1.94); 1.12 (95% CI, 0.68–1.72); and 1.63 (95% CI, 1.19–2.18), respectively, for the immigrants during their first 10 years in Denmark, and 1.07 (95% CI, 0.80–1.41); 0.89 (95% CI, 0.50–1.47); and 1.15 (95% CI, 0.83–1.56); respectively, for stays beyond 10 years. In the second generation, the SIRs were 0.92 (95% CI, 0.60–1.36), 1.29 (95% CI, 0.97–1.69), and 1.18 (95% CI, 0.92–1.48) respectively. In contrast to the pattern in men, second‐generation immigrants with two Faroese parents had higher point estimates than those with one Faroese parent only. In the third‐generation female immigrants, the SIRs were 1.83 (95% CI, 1.08–2.90), 1.18 (95% CI, 0.62–2.02), and 1.46 (95% CI, 0.95–2.14) respectively. The excess risks of UC and IBD in first‐generation immigrants were found in women both below and above 20 years of age. The borderline significant excess risk of UC in second‐generation immigrants was found only in women 20 years and above. The excess risk of CD in third‐generation immigrants was found in women both below and above the age of 20 years, but the numbers did not reach statistical significance when divided by age.
Discussion
Our study based on nationwide data demonstrated that first‐generation Faroese immigrants to Denmark had an about 25% higher incidence of IBD than the Danish population. This excess risk derived exclusively from UC, where the Faroese immigrants had a 44% higher incidence than Danes, while there was no difference between the two populations in the incidence of CD. The excess risk of UC was nearly doubled during the immigrants’ first 10 years in Denmark. After 10 years in Denmark the immigrants’ UC risk was similar to that of Danes. For women, but not for men, the excess risk of UC prevailed among second‐generation immigrants in adult age, especially among those with two Faroese parents. All third‐generation immigrants had the same risk of UC as the Danish population. The immigrants of Faroese origin over three generations thus adapted the lower UC risk of their new home country. Surprisingly, third‐generation female, but not male, immigrants had an excess risk of CD as compared to Danes.
To our knowledge, this is the first nationwide study of IBD incidence among immigrants from a high‐risk country to a country with a lower risk, although in an international perspective Denmark is also a high‐risk country.10 Studies of IBD in migrants date back to the famous study by Probert et al., from 1992 on IBD in migrants of South Asian descent into the city of Leicester in the United Kingdom.18 Recently, two nationwide studies have assessed the IBD incidence in immigrants from low‐risk to high‐risk countries. A Canadian study showed that the incidence of UC among immigrants was 0.42 (95% CI, 0.33–0.53) as compared to that of the Canadian population, but this relative incidence increased to 0.78 (95% CI, 0.60–1.01) in their children. The same trend was observed for CD, where immigrants had a relative incidence of 0.25 (95% CI, 0.19–0.32), which increased to 0.49 (95% CI, 0.38–0.63) in their children.4 A study from Sweden found similar results, as the incidence of UC in immigrants was 0.81 (95% CI, 0.77–0.85) as compared to that of the Swedish population, but 0.95 (95% CI, 0.89–1.02) in their children. For CD, the relative incidence was 0.75 (95% CI, 0.71–0.80), and for their children, it was 0.91 (95% CI, 0.85–0.98).5
The Canadian and Swedish studies covered immigrants from low‐risk to high‐risk countries. In these studies, first‐generation immigrants had a deficit risk both for CD and UC, and these deficit risks diminished among their children. The introduction of an exposure thus manifested itself over one generation. In our study, first‐generation immigrants had an excess risk of UC during their first 10 years in Denmark, and this excess risk disappeared over time and over one generation in men and over two generations in women. The removal of an exposure thus manifested itself over one‐to‐two generations. Together these studies form a strong evidence for the importance of – so far unidentified – environmental exposures in the aetiology of UC. In the analysis, we controlled for sex, age, and calendar period, but we were not able to control for other risk factors as these data are not available in Danish registers. It should also be noted that the decreased risk from first to second‐generation immigrants could in part be due to genetic dilution as the majority of second‐generation immigrants had one parent only of Faroese origin. In second‐generation immigrants with two parents of Faroese origin, the men had no excess IBD risk as compared with Danes, but there was an indication of an excess risk in women. A longer follow‐up of the cohort will show whether this reflects true differences or a random variation due to small numbers.
It is noteworthy that in our previous study of time trends in IBD incidence in the Faroe Islands, we found a rapid increase over time in the UC incidence, while the trend for CD incidence showed a more modest increase. Furthermore, for CD patients’ disease was primarily located in the colon (L2)7 and the average age at onset is higher in the Faroe Islands than in the rest of Europe.10 IBD is believed to occur in genetically pre‐disposed individuals.19 The genetic background for the high incidence in the Faroe Islands is still unknown, but the innate risk of developing IBD is likely to be higher compared to other populations. The Faroe Islands is an autonomous country within the Kingdom of Denmark with Danish rule dating back to the mid 1500s, and Danish trade monopoly until the mid 1800s. The Faroese population originates in part from Scandinavia, and – in particular for the female part – from the British Isles.20 Nevertheless, the Faroese population has been isolated and is genetically relatively homogeneous, with some rare disease syndromes attributable to founder mutations, as for instance carnitine transporter deficiencies,21 glycogen storage disease type IIIA,22 and cystic fibrosis.23
However, genetics alone cannot explain the recent steep increase in IBD incidence in the Faroe Islands.7 Differences in diet are considered to be the most topical and likely factors in explaining the geographical variation and rising incidence of IBD.24 The intake of fish in adults is higher in the Faroe Islands25 than in Denmark.26 Therefore, despite the difference in latitude between the Faroe Islands (62° 00′ N) and Denmark (55–57° 00′ N) and the consequential difference in solar exposure, vitamin D deficiency (= <50 nmol/L) in elderly people has been found to be at the same level in the two countries; 54% in the Faroe Islands,25 and 55% in Denmark.27 While IBD patients have been found to have high prevalence of vitamin D deficiency,28 this factor is unlikely to explain the higher IBD incidence in the Faroe Islands than in Denmark.
Nevertheless, westernisation has changed the living conditions in the Faroe Islands during the past 50 years. The traditional diet of fish, lamb and pilot whale meat has changed to include a broader variety of imported foods. This means, however, that the present dietary pattern in the Faroe Islands is fairly similar to the present dietary pattern in Denmark. It is therefore unlikely that the westernisation of the dietary pattern in the Faroe Islands can explain both the increasing IBD incidence in the Faroe Islands, and the decreasing IBD incidence in Faroese immigrants to Denmark. At present, we have no suggestion for the possible aetiological agent(s) behind the high IBD risk in the Faroese population. We are currently investigating the IBD risk in the Faroese mother‐child cohorts,29 and we hope that this further characterisation of high‐risk subgroups may provide clues to this puzzle. It should also be taken into account that risk factors may affect native and migrant populations differently, as seen for instance in the different risk profiles of IBD patients in Australia for migrants of middle east origin and for natives of caucasian origin.30
It is a strength of the study that all risk periods could be measured accurately due to the availability of complete im‐ and emigration histories in the Danish population register. The use of Danish National Patient Register data for identification of incident IBD cases both among the immigrants and among the Danes ensured that the same case definitions were used for the numerator and for the denominator. Our study had, however, also some limitations. We defined an incident IBD case as the first time a person had either an in‐ or an out‐patient contact with the relevant diagnosis in the Danish National Patient Register. This definition does, however, lead to a higher IBD incidence than in the ECCO‐EpiCom study,9 where patients had to meet the Copenhagen Diagnostic Criteria based on clinical symptoms, endoscopic or radiological evidence or mucosal biopsies. The ECCO‐EpiCom age‐standardised rate (European Standard Population) was 24 per 100 000 for Denmark, while this rate was 45 per 100 000 in our data. For this reason, we were not able to compare our IBD incidence data for the immigrants with the available rates from the Faroese population, as these rates were generated based on the Copenhagen Diagnostic Criteria.7 This reflects the inaccuracy of using the routine hospital discharge diagnosis. But diagnoses according to the Copenhagen Criteria were not available in the register data. Furthermore, the purpose of the present study was to compare the IBD risk of Faroese immigrants with that of Danes in general. Using the register data only, we ensured that potential diagnostic misclassifications affected the numerator (observed cases in Faroese immigrants) and the denominator (expected cases in Faroese immigrants) to the same extent.
Although we included all Faroese immigrants to Denmark, some numbers were small, which could result in random findings. It will in particular be important to follow‐up on the observed difference between second‐generation men and women with two parents of Faroese origin, and on the excess risk of CD in third‐generation female immigrants.
In conclusion, this population‐based study showed an excess risk of UC incidence in Faroese first‐generation immigrants to Denmark. This excess risk diminished over time and over generations, faster for men than for women, and the UC incidence of third‐generation immigrants was in line with that of the Danish population. Although the fact that the IBD risk was still high in female second‐generation immigrants with two Faroese parents could point to some genetic dilution, the general pattern supports the causation hypothesis of environmental risk factors in IBD and stresses that the gene‐environment interaction in the Faroese population is of importance. Future research should focus on identification of these risk factors.
Authorship
Guarantor of the article: Elsebeth Lynge.
Author contributions: All authors contributed significantly to the study. JB and KRN suggested the study. TH and EL designed the study, interpreted the data, obtained funding and drafted the manuscript. MvEC defined register data for the study. SNL performed the statistical analysis and contributed to the interpretation. JB, PM, PW and KRN contributed to the interpretation and writing of the manuscript. All authors critically revised and approved the final manuscript.
Acknowledgements
Declaration of personal interests: JB has served as a speaker for AbbVie, Takeda and MSD, as well as an advisory board member for AbbVie, Celgene, and Janssen.
Declaration of funding interests: This work was supported by the Faroese Research Council; European Crohn's and Colitis Organisation (ECCO); Beckett Foundation; The Danish Colitis‐Crohn Patients Organisation (CCF); and Aage and Johanne Louis‐Hansen Foundation.
The Handling Editor for this article was Professor Peter Gibson, and it was accepted for publication after full peer‐review.
References
- 1. Loftus EV. Clinical epidemiology of inflammatory bowel disease: incidence, prevalence, and environmental influences. Gastroenterology 2004; 126: 1504–17. [DOI] [PubMed] [Google Scholar]
- 2. Halfvarson J. Genetics in twins with Crohn's disease: less pronounced than previously believed? Inflamm Bowel Dis 2011; 17: 6–12. [DOI] [PubMed] [Google Scholar]
- 3. Kevans D, Silverberg MS, Borowski K, et al IBD genetic risk profile in healthy first‐degree relatives of Crohn's disease patients. J Crohn's Colitis 2016; 10: 209–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Benchimol EI, Mack DR, Guttmann A, et al Inflammatory bowel disease in immigrants to Canada and their children: a population‐based cohort study. Am J Gastroenterol 2015; 110: 553–63. [DOI] [PubMed] [Google Scholar]
- 5. Li X, Sundquist J, Hemminki K, et al Risk of inflammatory bowel disease in first‐ and second‐generation immigrants in Sweden: a nationwide follow‐up study. Inflamm Bowel Dis 2011; 17: 1784–91. [DOI] [PubMed] [Google Scholar]
- 6. Hill AB. The environment and disease: association or causation? J R Soc Med 2015; 108: 32–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hammer T, Nielsen KR, Munkholm P, et al The Faroese IBD Study: incidence of inflammatory bowel disease across 54 years of population‐based data. J Cronhs Colitis 2016; 10: 934–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kaplan GG, Ng SC. Understanding and preventing the global increase in inflammatory bowel disease. Gastroenterology 2017; 152: 313–21. [DOI] [PubMed] [Google Scholar]
- 9. Vegh Z, Burisch J, Pedersen N, et al Incidence and initial disease course of inflammatory bowel diseases in 2011 in Europe and Australia: results of the 2011 ECCO‐EpiCom inception cohort. J Crohns Colitis 2014; 8: 1506–15. [DOI] [PubMed] [Google Scholar]
- 10. Burisch J, Pedersen N, Čuković‐Čavka S, et al East‐West gradient in the incidence of inflammatory bowel disease in Europe: the ECCO‐EpiCom inception cohort. Gut 2014; 63: 588–97. [DOI] [PubMed] [Google Scholar]
- 11. Statistics Faroe Islands . Population by sex, age and village/city, 1th January (1985‐2016). Available at: https://statbank.hagstova.fo/pxweb/en/H2/H2__IB__IB01/fo_aldbygd.px/table/tableViewLayout1/?rxid=533fdf8a-8327-4e98-bfee-2dc2753e5448. (accessed 31 May 2016).
- 12. Statistics Denmark. People born in the Faroe Islands and living in Denmark 1. January by sex, age and parents place of birth. Available at: http://www.statistikbanken.dk/BEF5F. (accessed 31 May 2016).
- 13. Schmidt M, Pedersen L, Sørensen HT. The Danish Civil Registration System as a tool in epidemiology. Eur J Epidemiol 2014; 29: 541–9. [DOI] [PubMed] [Google Scholar]
- 14. Lynge E, Sandegaard JL, Rebolj M. The Danish National Patient Register. Scand J Public Health 2011; 39: 30–3. [DOI] [PubMed] [Google Scholar]
- 15. International Classification of Diseases, Revision 8 (1965). Available at: http://www.wolfbane.com/icd/icd8h.htm. (accessed 29 August 2016).
- 16. International Statistical Classification of Diseases and Related Health Problems 10th Revision. Chapter XI Diseases of the digestive system. Available at: http://apps.who.int/classifications/icd10/browse/2016/en#/K50. (accessed 29 August 2016).
- 17. Benchimol EI, Fortinsky KJ, Gozdyra P, et al Epidemiology of pediatric inflammatory bowel disease: a systematic review of international trends. Inflamm Bowel Dis 2011; 17: 423–39. [DOI] [PubMed] [Google Scholar]
- 18. Probert CS, Jayanthi V, Pinder D, et al Epidemiological study of ulcerative protocolitis in Indian migrants and the indigenous population of Leicestershire. Gut 1992; 33: 687–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Podolsky DK. Inflammatory bowel disease. N Engl J Med 2002; 347: 417–29. [DOI] [PubMed] [Google Scholar]
- 20. Als TD, Jorgensen TH, Borglum AD, et al Highly discrepant proportions of female and male Scandinavian and British Isles ancestry within the isolated population of the Faroe Islands. Eur J Hum Genet 2006; 14: 497–504. [DOI] [PubMed] [Google Scholar]
- 21. Poulsen SD, Lund AM, Christensen E, et al Carnitine transporter deficiency is a hereditary disease with a high incidence in the Faroe Islands. Danish Med J 2012; 174: 1217–9 [In Danish]. [PubMed] [Google Scholar]
- 22. Santer R, Kinner M, Steuerwald U, et al Molecular genetic basis and prevalence of glycogen storage disease type IIIA in the Faroe Islands. Eur J Hum Genet 2001; 9: 388–91. [DOI] [PubMed] [Google Scholar]
- 23. Schwartz M, Sorensen N, Brandt NJ, et al High incidence of cystic fibrosis on the Faroe Islands: a molecular and genealogical study. Hum Genet 1995; 95: 703–6. [DOI] [PubMed] [Google Scholar]
- 24. Ng SC, Bernstein CN, Vatn MH, et al Geographical variability and environmental risk factors in inflammatory bowel disease. Gut 2013; 62: 630–49. [DOI] [PubMed] [Google Scholar]
- 25. Dalgård C, Petersen MS, Schmedes AV, et al High latitude and marine diet: vitamin D status in elderly Faroese. Br J Nutr 2010; 104: 914–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Roswall N, Olsen A, Boll K, et al Consumption of predefined “Nordic” dietary items in ten European countries – an investigation in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort. Public Health Nutr 2014; 17: 2650–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Andersen R, Mølgaard C, Skovgaard LT, et al Teenage girls and elderly women living in northern Europe have low winter vitamin D status. Eur J Clin Nutr 2005; 59: 533–41. [DOI] [PubMed] [Google Scholar]
- 28. Kabbani TA, Koutroubakis IE, Schoen RE, et al Association of vitamin D level with clinical status in inflammatory bowel disease: a 5‐year longitudinal Study. Am J Gastroenterol 2016; 111: 712–9. [DOI] [PubMed] [Google Scholar]
- 29. Childern's health and the environment of the Faroes. Available at: http://www.chef-project.dk. (accessed 29 November 2016).
- 30. Ko Y, Kariyawasam V, Karnib M, et al Inflammatory bowel disease environmental risk factors: a population‐based case‐control study in Middle Eastern migration to Australia. Clin Gastroenterol Hepatol 2015; 13: 1453–63. [DOI] [PubMed] [Google Scholar]


