Abstract
Residual cardiovascular risk persists despite statins, yet outcome studies of lipid‐targeted therapies beyond low‐density lipoprotein cholesterol (LDL‐C) have not demonstrated added benefit. Triglyceride elevation is an independent risk factor for cardiovascular events. High‐dose eicosapentaenoic acid (EPA) reduces triglyceride‐rich lipoproteins without raising LDL‐C. Omega‐3s have postulated pleiotropic cardioprotective benefits beyond triglyceride‐lowering. To date, no large, multinational, randomized clinical trial has proved that lowering triglycerides on top of statin therapy improves cardiovascular outcomes. The Reduction of Cardiovascular Events with Icosapent Ethyl–Intervention Trial (REDUCE‐IT; NCT01492361) is a phase 3b randomized, double‐blinded, placebo‐controlled trial of icosapent ethyl, a highly purified ethyl ester of EPA, vs placebo. The main objective is to evaluate whether treatment with icosapent ethyl reduces ischemic events in statin‐treated patients with high triglycerides at elevated cardiovascular risk. REDUCE‐IT enrolled men or women age ≥45 years with established cardiovascular disease or age ≥50 years with diabetes mellitus and 1 additional risk factor. Randomization required fasting triglycerides ≥150 mg/dL and <500 mg/dL and LDL‐C >40 mg/dL and ≤100 mg/dL with stable statin (± ezetimibe) ≥4 weeks prior to qualifying measurements. The primary endpoint is a composite of cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, coronary revascularization, or unstable angina. The key secondary endpoint is the composite of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke. Several secondary, tertiary, and exploratory endpoints will be assessed. Approximately 8000 patients have been randomized at approximately 470 centers worldwide. Follow‐up will continue in this event‐driven trial until approximately 1612 adjudicated primary‐efficacy endpoint events have occurred.
Keywords: Clinical trials, General clinical cardiology/adult, Lipidology
1. INTRODUCTION
Statin therapy has been well established as a cornerstone of cardiovascular prevention, and yet despite potent therapies for lowering low‐density lipoprotein cholesterol (LDL‐C), substantial residual risk remains.1, 2, 3 Clinical and epidemiological studies have demonstrated that triglyceride (TG) elevation is an independent risk factor for increased cardiovascular (CV) events, and therefore may represent one contributive factor of residual CV risk beyond statin therapy.1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11 More recently, elegant Mendelian randomization studies have supported a causal role for TG in the pathogenesis of cardiovascular disease (CVD), showing that elevated TG are not merely a risk marker, but rather a risk factor and thus potentially modifiable.3 Despite the available data, an important question that remains is whether treatment of modest degrees of TG elevation would decrease CV events, in particular in patients already receiving LDL‐C–lowering therapy with statins. Prior CV outcome studies that administered therapies with TG‐lowering effects (niacin or fenofibrate) on top of statin therapy did not reach their primary endpoints. Nonetheless, these studies also did not prospectively enroll patients with elevated TG levels despite statin therapy,6, 12, 13, 14, 15 and subgroup analyses suggested possible benefits to TG lowering in patients with dyslipidemia.5, 6
Outcome studies of relatively low doses of prescription omega‐3 therapies in Japan (the Japan EPA Lipid Intervention Study [JELIS])16 and Italy (Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico [GISSI])17, 18 have suggested that omega‐3 therapy may provide CV protection. However, these studies were performed in single countries prior to current treatment guidelines, and therefore provide supportive but not conclusive evidence of CV benefit. Other more recent omega‐3 therapy outcome studies conducted in the presence of statins have been less encouraging, but these studies were characterized by evaluating nonhypertriglyceridemic patient populations (eg, TG <200 mg/dL) and administering low doses of long‐chain omega‐3 fatty acids (eg, eicosapentaenoic acid [EPA] and/or docosoahexaenoic acid [DHA]).19, 20, 21, 22, 23, 24
Omega‐3 therapies, including EPA, have been postulated to have cardioprotective effects such as beneficial changes to TG and other lipid and lipoprotein parameters (eg, non–high‐density lipoprotein cholesterol [non‐HDL‐C], apolipoprotein CIII), as well as other potential benefits beyond plasma lipid modification.19, 25, 26, 27, 28, 29, 30 Icosapent ethyl (Vascepa; Amarin Pharma Inc., Bedminster, NJ) is a highly purified ethyl ester of EPA, which has been reported to improve atherogenic dyslipidemia characterized by reductions in TG, TG‐rich lipoproteins, and factors involved in their metabolism, without raising LDL‐C.25, 26, 27, 28, 29 Based on trials with TG lowering as the primary endpoint, this prescription therapy is currently approved for use in the United States by the US Food and Drug Administration (FDA) as an adjunct to diet to reduce TG levels in adult patients with severe hypertriglyceridemia (≥500 mg/dL).25, 26 In this range of very elevated TG levels, reduction is considered to be clinically necessary to decrease the risk of pancreatitis.
In addition to beneficial changes to TG‐rich lipoproteins and other plasma lipid markers, some clinical studies with higher‐dose EPA also suggest beneficial effects on markers of oxidation and inflammation, coronary plaque characteristics, and major CV events.16, 25, 26, 29, 31, 32, 33 For example, in contrast to the fenofibrate and niacin studies, JELIS found a 19% relative risk reduction in CV events in statin‐treated patients with relatively normal TG but a more pronounced 53% reduction in the subgroup with mixed dyslipidemia, specifically TG ≥150 mg/dL and HDL‐C <40 mg/dL.4, 16 Although confirmation of these results is needed in western populations, the reduction of CV events with EPA therapy in a patient population with relatively normal TG levels suggests that EPA may have pleiotropic effects beyond plasma‐lipid modification.
It is worth noting that the promising results from JELIS occurred with a high‐purity EPA preparation dosed at 1.8 g/d in a Japanese population, for whom the baseline EPA levels are higher than in western populations due to greater dietary intake of marine omega‐3 fatty acids. Icosapent ethyl 12‐week dosing at 4 g/d in a high‐risk population similar to that within the Reduction of Cardiovascular Events with Icosapent Ethyl–Intervention Trial (REDUCE‐IT) who had persistent elevations of TG after treatment with statins resulted in significant reductions in TG and atherogenic lipoproteins,26, 27, 28 as well as comparable plasma EPA levels as the 1.8 g/d dosing group in JELIS.34 Therefore, a dose of 4 g/d was selected as the dose for further study. In this context, REDUCE‐IT was designed to determine if treatment with icosapent ethyl 4 g/d vs placebo would reduce ischemic events in patients at increased CV risk already being treated with statins.
2. METHODS
REDUCE‐IT (NCT01492361) is a phase 3b, international, multicenter, prospective, randomized, double‐blinded, placebo‐controlled, parallel‐group trial of icosapent ethyl 4 g/d (2 g twice daily with food) vs placebo (Figure). The main objective is to evaluate whether treatment with icosapent ethyl reduces ischemic events in patients at elevated CV risk concurrently treated with statins. Inclusion and exclusion criteria are listed in Table 1 and Table 2, respectively. Men or women age ≥45 years with established CVD (CV risk stratum 1, Table 1) or age ≥50 years with diabetes mellitus in combination with 1 additional risk factor for CVD (CV risk stratum 2, Table 1) were eligible for inclusion. Fasting TG levels ≥150 mg/dL and <500 mg/dL were required. A study amendment was made during the early part of the trial, increasing the lower end of the fasting TG level from ≥150 mg/dL to ≥200 mg/dL, to increase enrollment of patients with more significant TG elevations. LDL‐C levels needed to be >40 mg/dL and ≤100 mg/dL, with patients on stable statin therapy (± ezetimibe) for ≥4 weeks prior to the LDL‐C and TG qualifying measurements for randomization.
Table 1.
Inclusion Criteria
| General inclusion criteria |
|---|
| 1. Men or women age ≥45 years with established CVD (CV Risk Stratum 1; see below) OR age ≥50 years with DM in combination with 1 additional risk factor for CVD (CV Risk Stratum 2; see below) |
| 2. Fasting TG levels ≥150 mg/dL and <500 mg/dL1 |
| 3. LDL‐C >40 mg/dL and ≤100 mg/dL and on stable statin therapy (± ezetimibe) for ≥4 weeks prior to the LDL‐C and TG qualifying measurements for randomization |
| 4. Women who are not pregnant, not breastfeeding, not planning on becoming pregnant, and using an acceptable form of birth control during the study (if of child‐bearing potential) |
| 5. Able to provide informed consent and adhere to study schedules |
| 6. Agree to follow and maintain a physician‐recommended diet during the study |
| CV Risk Stratum 1 inclusion criteria (men and women age ≥45 years with ≥1 of the following): |
| 1. Documented CAD (≥1 of the following primary criteria must be satisfied): |
| a. Documented multivessel CAD (≥50% stenosis in ≥2 major epicardial coronary arteries, with or without antecedent revascularization) |
| b. Documented prior MI |
| c. Hospitalization for high‐risk NSTE‐ACS (with objective evidence of ischemia: ST‐segment deviation or biomarker positivity) |
| 2. Documented cerebrovascular or carotid disease (1 of the following primary criteria must be satisfied): |
| a. Documented prior ischemic stroke |
| b. Symptomatic carotid artery disease with ≥50% carotid arterial stenosis |
| c. Asymptomatic carotid artery disease with ≥70% carotid arterial stenosis per angiography or duplex ultrasound |
| d. History of carotid revascularization (catheter‐based or surgical) |
| 3. Documented PAD (≥1 of the following primary criteria must be satisfied): |
| a. ABI <0.9 with symptoms of intermittent claudication |
| b. History of aortoiliac or peripheral arterial intervention (catheter‐based or surgical) |
| CV Risk Stratum 2 inclusion criteria (patients with the following): |
| 1. DM (type 1 or type 2) requiring treatment with medication AND |
| 2. Men and women age ≥50 years AND |
| 3. One of the following at Visit 1 (additional risk factor for CVD): |
| a. Men ≥55 years of age and women ≥65 years of age |
| b. Cigarette smoker or stopped smoking within 3 months before Visit 1 |
| c. HTN (BP ≥140 mm Hg systolic OR ≥90 mm Hg diastolic) or on antihypertensive medication |
| d. HDL‐C ≤40 mg/dL for men or ≤50 mg/dL for women |
| e. hsCRP >3.00 mg/L (0.3 mg/dL) |
| f. Renal dysfunction: CrCl >30 and <60 mL/min |
| g. Retinopathy, defined as any of the following: nonproliferative retinopathy, preproliferative retinopathy, proliferative retinopathy, maculopathy, advanced diabetic eye disease, or a history of photocoagulation |
| h. Micro‐ or macroalbuminuria. Microalbuminuria is defined as either a positive micral or other strip test (may be obtained from medical records), an albumin/Cr ratio ≥2.5 mg/mmol, or an albumin excretion rate on timed collection ≥20 mg/min all on ≥2 successive occasions. Macroalbuminuria is defined as Albustix or other dipstick evidence of gross proteinuria, an albumin/Cr ratio ≥25 mg/mmol, or an albumin excretion rate on timed collection ≥200 mg/min all on ≥2 successive occasions. |
| i. ABI <0.9 without symptoms of intermittent claudication (patients with ABI <0.9 with symptoms of intermittent claudication are counted under CV Risk Stratum 1) |
Abbreviations: ABI, ankle‐brachial index; BP, blood pressure; CAD, coronary artery disease; Cr, creatinine; CrCl, creatinine clearance; CV, cardiovascular; CVD, cardiovascular disease; DM, diabetes mellitus; HDL‐C, high‐density lipoprotein cholesterol; hsCRP, high‐sensitivity C‐reactive protein; HTN, hypertension; LDL‐C, low‐density lipoprotein cholesterol; MI, myocardial infarction; NSTE‐ACS, non–ST‐segment elevation acute coronary syndrome; PAD, peripheral arterial disease; TG, triglycerides.
Note: Patients with DM and CVD as defined above are eligible based on the CVD requirements and will be counted under CV Risk Stratum 1. Only patients with DM and no documented CVD as defined above need ≥1 additional risk factor as listed, and they will be counted under CV Risk Stratum 2.
A study amendment (May 2013) was made, increasing the lower end of the fasting TG level from ≥150 mg/dL to ≥200 mg/dL to increase enrollment of patients with TG ≥200 mg/dL; it is anticipated that mean and median qualifying TG levels will be >200 mg/dL.
Table 2.
Exclusion criteria
| General exclusion criteria |
|---|
| 1. Severe (NYHA class IV) HF |
| 2. Any life‐threatening disease expected to result in death within the next 2 years (other than CVD) |
| 3. Diagnosis or laboratory evidence of active severe liver disease |
| 4. HbA1c >10.0% at screening |
| 5. Poorly controlled HTN: SBP ≥200 mm Hg or DBP ≥100 mm Hg (despite antihypertensive therapy) |
| 6. Planned coronary intervention or any noncardiac major surgical procedure |
| 7. Known familial lipoprotein lipase deficiency (Fredrickson type I), apoCII deficiency, or familial dysbetalipoproteinemia (Fredrickson type III) |
| 8. Participation in another clinical trial involving an investigational agent within 90 days prior to screening |
| 9. Intolerance or hypersensitivity to statin therapy |
| 10. Known hypersensitivity to fish and/or shellfish, or ingredients of the study product or placebo |
| 11. History of acute or chronic pancreatitis |
| 12. Malabsorption syndrome and/or chronic diarrhea |
| 13. Use of non–study‐drug‐related, nonstatin lipid‐altering medications, dietary supplements, or foods during the screening period (after Visit 1) and/or plans for use during the treatment/follow‐up period, including: |
| a. Niacin (>200 mg/d) or fibrates (unless ≥28‐day washout) |
| b. Any OM‐3 fatty acid medications (unless ≥28‐day washout) |
| c. Dietary supplements containing OM‐3 fatty acids (eg, flaxseed, fish, krill, or algal oils; unless ≥28‐day washout) |
| d. Bile acid sequestrants (unless ≥7‐day washout) |
| e. PCSK9 inhibitors (unless ≥90‐day washout) |
| 14. Other medications (not indicated for lipid alteration): |
| a. Tamoxifen, estrogens, progestins, thyroid hormone therapy, systemic corticosteroids (local, topical, inhalation, or nasal corticosteroids are allowed), HIV‐protease inhibitors that have not been stable for ≥28 days prior to the qualifying lipid measurements (TG and LDL‐C) during screening |
| b. Cyclophosphamide or systemic retinoids during the screening period (unless ≥28‐day washout) and/or plans for use during the treatment/follow‐up period |
| 15. Known AIDS (HIV‐positive patients without AIDS are allowed) |
| 16. Requirement for peritoneal dialysis or hemodialysis for renal insufficiency or CrCl <30 mL/min |
| 17. Unexplained elevated CK concentration >5 × ULN or elevation due to known muscle disease |
| 18. Any condition or therapy which, in the opinion of the investigator, might pose a risk to the patient or make participation in the study not in the patient's best interest |
| 19. Drug or alcohol abuse within the past 6 months, and inability/unwillingness to abstain from drug abuse and excessive alcohol consumption during the study |
| 20. Mental/psychological impairment or any other reason to expect patient difficulty in complying with the requirements of the study or understanding the goal and potential risks of participating in the study |
Abbreviations: AIDS, acquired immunodeficiency syndrome; apoCII, apolipoprotein CII; CK, creatine kinase; CrCl, creatinine clearance; CVD, cardiovascular disease; DBP, diastolic blood pressure; HbA1c, glycated hemoglobin; HF, heart failure; HIV, human immunodeficiency virus; HTN, hypertension; LDL‐C, low‐density lipoprotein cholesterol; NYHA, New York Heart Association; OM‐3, omega‐3; PCSK9, proprotein convertase subtilisin/kexin type 9; SBP, systolic blood pressure; TG, triglyceride; ULN, upper limit of normal.
Figure 1.
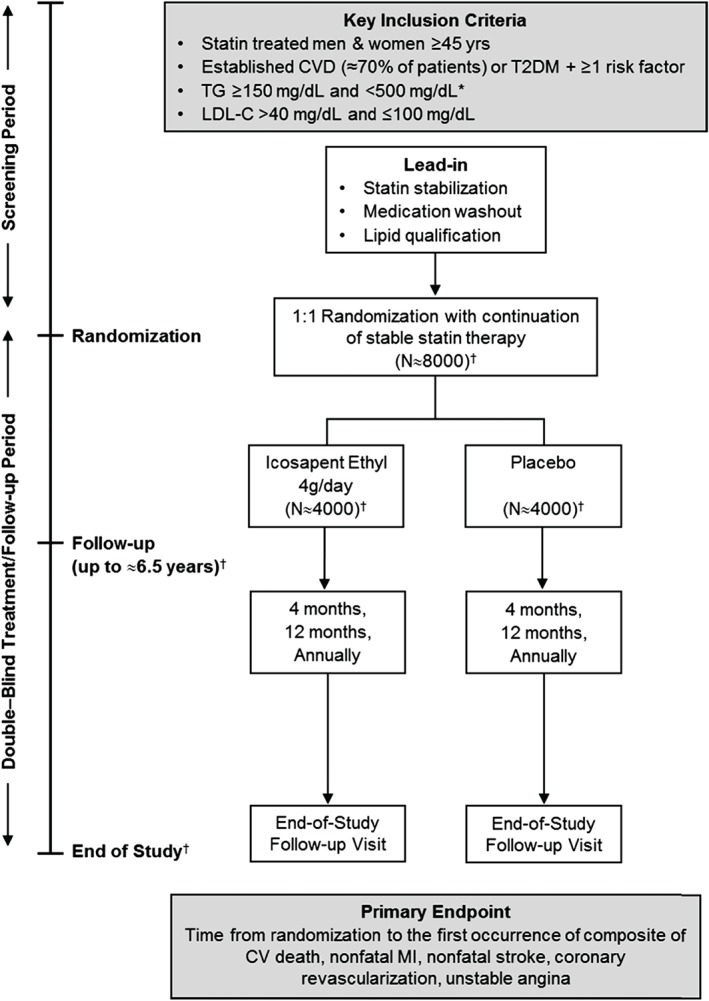
Study design for REDUCE‐IT. During the screening period, patients were evaluated for inclusion/exclusion criteria. If patients met the inclusion criteria at Visit 1, they were asked to return for the randomization visit (Visit 2) and entered the treatment/follow‐up period. Patients who were not eligible at Visit 1 but who became eligible in the next 28 days (such as patients whose statin dose changed at Visit 1 and/or needed to wash out prohibited medications) may have returned for an optional second screening visit (Visit 1.1). Such patients entered a statin stabilization/medication washout period of ≥28 days prior to rescreening. Patients who were eligible following screening/rescreening entered the treatment/follow‐up period, with follow‐up visits occurring at 4 months, 12 months, and annually thereafter. *A study amendment (May 2013) was made, increasing the lower end of the fasting TG level from ≥150 mg/dL to ≥200 mg/dL to increase enrollment of patients with TG ≥200 mg/dL; it is anticipated that mean and median qualifying TG levels will be >200 mg/dL. †Final values to be known at study unblinding. Event‐driven design: approximately 1612 primary efficacy events will be required during the study; study duration will vary accordingly. Abbreviations: CV, cardiovascular; CVD, cardiovascular disease; LDL‐C, low‐density lipoprotein cholesterol; MI, myocardial infarction; REDUCE‐IT, Reduction of Cardiovascular Events with Icosapent Ethyl–Intervention Trial; T2DM, type 2 diabetes mellitus; TG, triglycerides.
The primary endpoint is a composite of CV death, nonfatal myocardial infarction (MI), nonfatal stroke, coronary revascularization, or unstable angina. The key secondary endpoint is the composite of CV death, nonfatal MI, or nonfatal stroke. Several other secondary, tertiary, and exploratory endpoints are being assessed (Table 3), which were designed to provide additional insights into the potential effects of EPA therapy on various outcomes and in distinct high‐risk patient populations.
Table 3.
Efficacy endpoints
| Primary Efficacy Endpoint1 | Secondary Efficacy Endpoints2 | Tertiary/Exploratory Efficacy Endpoints2 |
|---|---|---|
| Time from randomization to the first occurrence of the following: | ||
| Composite of the following clinical events: | Key secondary endpoint: | Total CV events3 |
| CV death | Composite of CV death, nonfatal MI,4 or nonfatal stroke | Primary endpoint in patient subsets: DM, metabolic syndrome, impaired glucose metabolism at baseline |
| Nonfatal MI4 | Key secondary composite endpoint in patients with impaired glucose metabolism at baseline | |
| Nonfatal stroke | Additional individual or composite endpoints (tested in order listed): | Additional composite endpoints5 |
| Coronary revascularization | Composite of CV death or nonfatal MI4 | New CHF, new CHF as the primary cause of hospitalization, TIA, amputation for PVD, and carotid revascularization |
| UA determined to be caused by myocardial ischemia by invasive/noninvasive testing and requiring emergent hospitalization | Fatal or nonfatal MI4 | All coronary revascularizations (defined as the composite of emergent, urgent, elective, or salvage) and each subtype of coronary revascularization (emergent, urgent, elective, and salvage) |
| Nonelective coronary revascularization (defined as emergent or urgent) | Cardiac arrhythmias requiring hospitalization ≥24 h | |
| CV death | Cardiac arrest | |
| UA determined to be caused by myocardial ischemia by invasive/noninvasive testing and requiring emergent hospitalization | Ischemic stroke, hemorrhagic stroke, and fatal or nonfatal stroke (with prior history of stroke) | |
| Fatal or nonfatal stroke | New‐onset type 2 DM or HTN | |
| Composite of total mortality, nonfatal MI4, or nonfatal stroke | Fasting TG, TC, LDL‐C, HDL‐C, non–HDL‐C, VLDL‐C, apoB, hsCRP, hsTnT, and RLP‐C6 | |
| Total mortality | Change in body weight and waist circumference |
Abbreviations: apoB, apolipoprotein B; CHF, coronary heart failure; CV, cardiovascular; DM, diabetes mellitus; ECG, electrocardiography; HDL‐C, high‐density lipoprotein cholesterol; hsCRP, high‐sensitivity C‐reactive protein; hsTnT, high‐sensitivity troponin T; HTN, hypertension; LDL‐C, low‐density lipoprotein cholesterol; MI, myocardial infarction; non–HDL‐C, non–high‐density lipoprotein cholesterol; PVD, peripheral vascular disease; RLP‐C, remnant lipoprotein cholesterol; TC, total cholesterol; TG, triglycerides; TIA, transient ischemic attack; UA, unstable angina; VLDL‐C, very low‐density lipoprotein cholesterol.
The first occurrence of any of these major adverse vascular events during the follow‐up period of the study will be included in the incidence.
For the secondary and tertiary endpoints that count a single event, the time from randomization to the first occurrence of this type of event will be counted for each patient. For secondary and tertiary endpoints that are composites of ≥2 types of events, the time from randomization to the first occurrence of any of the event types included in the composite will be counted for each patient.
The time from randomization to occurrence of the first and all recurrent major CV events defined as CV death, nonfatal MI (including silent MI), nonfatal stroke, coronary revascularization, or UA determined to be caused by myocardial ischemia by invasive/noninvasive testing and requiring emergent hospitalization.
Including silent MI; ECG will be performed annually for the detection of silent MI.
Composite endpoints include: composite of CV death, nonfatal MI (including silent MI), nonfatal stroke, cardiac arrhythmia requiring hospitalization of ≥24 hours, or cardiac arrest; composite of CV death, nonfatal MI (including silent MI), nonelective coronary revascularizations (defined as emergent or urgent classifications), or UA determined to be caused by myocardial ischemia by invasive/noninvasive testing and requiring emergent hospitalization; composite of CV death, nonfatal MI (including silent MI), nonelective coronary revascularizations (defined as emergent or urgent classifications), UA determined to be caused by myocardial ischemia by invasive/noninvasive testing and requiring emergent hospitalization, nonfatal stroke, or PVD requiring intervention such as angioplasty, bypass surgery, or aneurysm repair; and composite of CV death, nonfatal MI (including silent MI), nonelective coronary revascularizations (defined as emergent or urgent classifications), UA determined to be caused by myocardial ischemia by invasive/noninvasive testing and requiring emergent hospitalization, PVD requiring intervention, or cardiac arrhythmia requiring hospitalization of ≥24 hours.
Assessment of the relationship between baseline biomarker values and treatment effects within the primary and key secondary composite endpoints; assessment of the effect of study drug on each marker; and assessment of the relationship between post‐baseline biomarker values and treatment effects within the primary and key secondary composite endpoints by including post‐baseline biomarker values (for example, at 4 months, or at 1 year) as a covariate.
The sample‐size calculation was based on a hazard ratio assumption of 0.85. Therefore, 1612 events would be required to have approximately 90% power with a 1‐sided α‐level of 2.5% and with 2 interim analyses. This results in a total target sample size of 7990 patients. Approximately 70% of randomized patients were to be in CV risk stratum 1 (established CVD) and approximately 30% of randomized patients were to be in CV risk stratum 2 (high‐risk primary prevention defined by diabetes mellitus and other risk factors). Randomization was stratified by CV risk strata, ezetimibe use, and by geographical region.
The first patient was randomized on November 28, 2011. Protocol amendment 1 (May 2013) changed the lower limit of TG levels for entry into the trial from 150 mg/dL to 200 mg/dL, as a majority of the steering committee members felt that those were the patients most likely to benefit from TG lowering. Protocol amendment 2 (July 2016) designated the composite of hard major adverse cardiovascular events (CV death, nonfatal MI, nonfatal stroke) as the “key secondary endpoint” per suggestions from the FDA with steering committee concordance. The last patient was randomized on August 4, 2016. Approximately 8000 patients have been randomized at approximately 470 centers worldwide (see Supporting Information, Appendix, in the online version of this article). Follow up will continue in this event‐driven trial until approximately 1612 adjudicated primary efficacy endpoint events have occurred. This study is being conducted in accordance with a special protocol assessment agreement with the FDA.
3. DISCUSSION
Despite CV risk reduction through potent LDL‐C–lowering therapies such as statins, substantial residual CV risk remains. Epidemiological, biological, and genetic studies have provided robust evidence of a strong association between elevated TG levels and higher rates of CV events.1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11 Furthermore, TG reduction lowers several inflammatory markers associated with CV risk, and subgroup and post hoc analyses of outcome studies suggest possible reductions in major CV events with TG‐lowering therapy.3, 4, 5, 6, 7, 8, 9, 10, 11 Finally, studies administering higher‐dose EPA suggest additional beneficial effects beyond lipid‐lowering that may be unique to EPA relative to other TG‐lowering therapies, such as beneficial changes in coronary plaque characteristics, which may lead to reductions in major CV events.4, 16, 25, 26, 27, 28, 29, 30, 31, 32, 33
However, randomized data from large outcome studies across broad populations regarding pharmacological TG lowering and effect on CV outcomes have been mixed (Table 4).1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24 Part of the reason may involve differences between the classes of drugs studied, such as fibrates, niacin, and omega‐3 fatty acids. Even among omega‐3 fatty acid studies, there are marked differences with respect to the relatively low doses of omega‐3 administered and the ratio of EPA to DHA.16, 17, 18, 19, 20, 21, 22, 23, 24 In addition, different TG‐lowering therapies may exert differential effects across lipid profiles. For example, fibrates and DHA‐containing omega‐3 fatty acid mixtures have been shown to increase LDL‐C, which in turn might adversely influence trial results. Among outcome studies administering TG‐lowering agents beyond statin therapy, only the JELIS trial using pure EPA demonstrated a significant reduction in CV events in patients with relatively normal TG levels.16 The subgroup data (Table 5) from the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Lipid,6 Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides and Impact on Global Health Outcomes (AIM‐HIGH),5 and JELIS4 trials further support a prospective study of EPA in a broader patient population with hypertriglyceridemia, as exemplified in REDUCE‐IT, as a potential add‐on to statin therapy to reduce residual CV risk. That the lipid changes in JELIS were relatively modest (ie, approximately a 5% TG reduction) raises the possibility that other pleiotropic effects beyond lipid lowering may have also contributed to the reduction in CV risk.4, 16 Finally, any benefits to TG‐lowering therapies may be most pronounced among statin‐treated patients in the higher range of TG elevation (ie, ≥200 mg/dL),4, 5, 6 for whom randomized prospectively designed outcome studies have not been previously conducted prior to the REDUCE‐IT study.
Table 4.
CV outcome trials administering therapies that can be used for TG lowering
| Study | Publication Date | Patient Population | Statin Use | Baseline LDL‐C, mg/dL1 | Baseline TG, mg/dL1 | Interventions | Duration, y | Primary Endpoint | Outcomes, HR or OR (95% CI) or RRR (P Value)2 |
|---|---|---|---|---|---|---|---|---|---|
| Omega‐acid mixture studies | |||||||||
| GISSI‐P17 | 1999 | N = 11 324, recent MI (≤3 mo) | Cholesterol‐lowering: BL = 5%, EOS = 46% | 137 | 162 | 850 mg EPA + DHA vs Vit E vs n‐3 + Vit E vs PBO | 3.5 | Death or nonfatal MI or nonfatal stroke | HR = 0.85 (0.74‐0.98), 4‐way analysis |
| GISSI‐HF18 | 2008 | N = 6975, chronic HF (NYHA class II–IV) | 22.3%–23.0% | Not provided; TC = 188 | 126 | 850 mg EPA + DHA vs PBO | 3.9 | Co‐primary of death, and death or CV hospitalization | Death: HR: 0.91 (0.833‐0.998); death or CV hospitalization: HR: 0.92 (0.849‐0.999) |
| OMEGA22 | 2010 | N = 3851, recent MI (≤2 wk) | 94%–95% | Not provided; EOS = 95 | Not provided; EOS = 121 vs 127 | 840 mg EPA + DHA vs PBO | 1 | SCD | OR: 0.95 (0.56‐1.60) |
| Alpha‐Omega20 | 2010 | N = 4837, prior MI (median, 3.7 y) | BL lipid‐lowering = 85%–87% | 99–102 | 144–150 | 400 mg EPA + DHA vs PBO and ALA (2 g) combined | 3.3 | Expanded MACE | HR: 1.01 (0.87‐1.17) |
| SU.FOL.OM324 | 2010 | N = 2501, recent CVD event (median, 101 d) | BL lipid‐lowering = 83%–87% | 101–104 | 97–115 | 600 mg EPA + DHA vs PBO and B vitamin | 4.7 | MACE | HR: 1.08 (0.79‐1.47) |
| ORIGIN21 | 2012 | N = 12 536, dysglycemia + prior or high‐risk CVD | 53%–54% | 112 | 140–142 | 840 mg EPA + DHA vs PBO | 6.2 | CV death | HR: 0.98 (0.87‐1.10) |
| Risk & Prevention23 | 2013 | N = 12 513, high‐risk CVD | 41% | 132 | 150 | 850 mg EPA + DHA vs PBO | 5 | CV death or CV hospitalization | HR: 0.97 (0.88‐1.08) |
| Pure EPA study | |||||||||
| JELIS16 | 2007 | N = 18 645, hypercholesterolemic | 100% | 182 | 151 | 1800 mg EPA + statin vs statin | 4.6 | Expanded MACE | HR: 0.81 (0.69‐0.95) |
| Fibrate studies | |||||||||
| HHS10, 11 | 1987 | N = 4081, dyslipidemia + primary prevention | 0% | 188–189 | 175–177 | 1200 mg gemfibrozil vs PBO | 5 | Cardiac death, or fatal or nonfatal MI | RRR: −34% (P < 0.02) |
| VA‐HIT7 | 1999 | N = 2531, prior CHD + HDL‐C ≤40 mg/dL | 0% | 111 | 160 | 1200 mg gemfibrozil vs PBO | 5.1 | CHD death or nonfatal MI | RRR: −22% (P = 0.006) |
| BIP9 | 2000 | N = 3090, prior MI or stable angina | 0% | 148–149 | 145 | 400 mg bezafibrate vs PBO | 6.2 | Sudden death or fatal or nonfatal MI | RRR: −9.4% (P = 0.26) |
| FIELD8 | 2005 | N = 9795, T2DM | BL = 0%; EOS: PBO = 16%, feno = 8% | 119 | 153–154 | 200 mg fenofibrate vs PBO | 5 | CHD death or nonfatal MI | HR: 0.89 (0.75‐1.05) |
| ACCORD Lipid6 | 2010 | N = 5518, T2DM + high CV risk | 100% | 101 | 162 | 160 mg fenofibrate vs PBO | 4.7 | MACE | HR: 0.92 (0.79‐1.08) |
| Niacin studies | |||||||||
| AIM‐HIGH15 | 2011 | N = 3414, prior CVD | 100% | 72–73 | 163–168 | 1500–2000 mg ER niacin vs PBO | 3 | Expanded MACE | HR: 1.02 (0.87‐1.21) |
| HPS2‐THRIVE12, 14 | 2014 | N = 25 673, prior vascular disease | 100% | 63 | 108 | 2000 mg ER niacin + laropiprant vs PBO | 3.9 | Expanded MACE | HR: 0.96 (0.90‐1.03) |
Abbreviations: ACCORD, Action to Control Cardiovascular Risk in Diabetes; AIM‐HIGH, Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides: Impact on Global Health Outcomes; ALA, alpha‐linolenic acid; Alpha‐Omega, Study of Omega‐3 Fatty Acids and Coronary Mortality; BIP, Bezafibrate Infarction Prevention; BL, baseline; CHD, coronary heart disease; CI, confidence interval; CV, cardiovascular; CVD, cardiovascular disease; DHA, docosahexaenoic acid; EOS, end of study; EPA, eicosapentaenoic acid; ER, extended release; feno, fenofibrate; FIELD, Fenofibrate Intervention and Event Lowering in Diabetes; GISSI‐P, Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico‐Prevenzione; GISSI‐HF, Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico‐Heart Failure; HDL‐C, high‐density lipoprotein cholesterol; HF, heart failure; HPS2‐THRIVE, Heart Protection Study 2–Treatment of HDL to Reduce the Incidence of Vascular Events; HR, hazard ratio; JELIS, Japan EPA Lipid Intervention Study; MACE, major adverse cardiac events; MI, myocardial infarction; NYHA, New York Heart Association; OAE, omega acid esters; OMEGA, Effect of Omega 3‐Fatty Acids on the Reduction of Sudden Cardiac Death After Myocardial Infarction; OR, odds ratio; ORIGIN, Outcome Reduction with an Initial Glargine Intervention; PBO, placebo; RRR, relative risk reduction; SCD, sudden cardiac death; SU.FOL.OM3, Supplémentation en Folates et Omega‐3; T2DM, type 2 diabetes mellitus; TC, Total cholesterol; TG, triglycerides; VA‐HIT, Veterans Affairs Cooperative Studies Program High‐Density Lipoprotein Cholesterol Intervention Trial; Vit E, vitamin E.
Mean or median values are presented. Where available, medians are preferentially presented.
Bolded values approached/achieved statistical significance.
Table 5.
Subgroup analyses of patients with dyslipidemia from CV outcome trials administering TG‐lowering therapies added to statin therapy vs statin monotherapy
| Study | Publication Date | Intervention | Statin Use in Study (at Baseline) | Subgroup Criterion | Subgroup Primary Endpoint | Subgroup Outcome (P Value)1 |
|---|---|---|---|---|---|---|
| JELIS4 | 2008 | EPA | 100% (100% initiated at BL) | TG ≥150 mg/dL; HDL‐C <40 mg/dL | Expanded MACE | −53% (0.043) |
| ACCORD Lipid6 | 2010 | Fenofibrate | 100% (40% initiated at BL) | TG ≥204 mg/dL; HDL‐C ≤34 mg/dL | MACE | −31% (0.0567) |
| AIM‐HIGH5 | 2013 | Niacin ER | 100% (statin‐stabilized at BL) | TG ≥200 mg/dL; HDL‐C <32 mg/dL | Expanded MACE | −36% (0.032) |
| HPS2‐THRIVE13 | 2013 | Niacin ER + laropiprant | 100% (statin‐stabilized at BL) | TG ≥151 mg/dL; HDL‐C <35 mg/dL | Expanded MACE | 0% (0.95) |
Abbreviations: ACCORD, Action to Control Cardiovascular Risk in Diabetes; AIM‐HIGH, Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides: Impact on Global Health Outcomes; BL, baseline; CV, cardiovascular; EPA, eicosapentaenoic acid; ER, extended release; HDL‐C, high‐density lipoprotein cholesterol; HPS2‐THRIVE, Heart Protection Study 2–Treatment of HDL to Reduce the Incidence of Vascular Events; JELIS, Japan EPA Lipid Intervention Study; MACE, major adverse cardiac events; TG, triglycerides.
Bolded values approached/achieved statistical significance.
REDUCE‐IT is designed to evaluate whether treating at‐risk patients with high‐dose EPA will lower the rates of important ischemic events beyond statin therapy. However, this trial alone will not validate whether lowering TG specifically in patients with elevated TG levels will result in lower rates of important ischemic events, because the effects of EPA may be broader than TG reduction alone. Several trials, including REDUCE‐IT, are ongoing or planned to determine if different TG‐lowering therapies in patients with elevated TG levels lower the rate of important ischemic events.35, 36, 37, 38 The use of different therapeutic agents across these trials may in aggregate help us better understand the relative importance of TG lowering alone and may also help define which potential effects observed in REDUCE‐IT might be uniquely attributable to EPA therapy. Several lines of data, including comparison of the JELIS study results to those of fibrate and niacin outcome studies, suggest that EPA may be differentiated from other TG‐lowering agents as statin add‐on therapy, by potentially providing unique pleiotropic cardioprotective benefits in addition to TG lowering.
Other changes in the lipid‐lowering field may also affect the interpretation of the ongoing REDUCE‐IT trial. For example, the proprotein convertase subtilisin/kexin type 9 inhibitors are being tested in large CV outcome trials of patients for whom LDL‐C control from statin (± ezetimibe) therapy may be insufficient or poorly tolerated. If these LDL‐C–lowering agents are found to be beneficial, EPA therapy could potentially serve as a complementary approach to reduce residual CV risk even further, though this specific combination would not have been studied well in terms of incremental effects on CV events. Importantly, residual CV risk remains high in patients with LDL‐C well controlled by statins, and many of these patients will likely need to be treated from multiple angles. The growing body of TG‐related evidence suggests that TG‐rich lipoproteins may be a causal factor in such residual risk. Consequently, TG lowering represents a target of great interest to optimize further CV risk reduction beyond the LDL‐C–lowering benefits attained with statin use. EPA‐specific studies suggest that EPA may provide unique CV benefits through favorable effects on plasma lipid parameters, as well as on other pleiotropic pathways.
4. CONCLUSION
A major remaining question is how to achieve CV risk reduction beyond the benefits realized from effective management of LDL‐C. For patients with persistently high TG levels despite statin therapy, an agent that improves atherogenic dyslipidemia without raising LDL‐C and provides other potentially pleiotropic benefits may improve CV outcomes. The addition of EPA to statin therapy may thus provide additional CV benefit. The REDUCE‐IT trial with high‐dose EPA is designed to address this long‐standing scientific gap and to provide physicians with this much‐needed information to guide clinical care of patients at high CV risk.
Supporting information
SUPPORTING APPENDIX
ACKNOWLEDGMENTS
The authors thank members of the Amarin team, including: Lisa Jiao, PhD, for statistical support; Katelyn Diffin, MBA, for operational support; Peggy Berry, BA, for regulatory support; and Craig Granowitz, MD, PhD, Joy Bronson, MA, CMPP, and Sephy Philip, RPh, PharmD, for editorial support.
Conflicts of Interest
Dr. Bhatt has served on advisory boards for Cardax, Elsevier Practice Update Cardiology, Medscape Cardiology, and Regado Biosciences; has served on the board of directors for Boston VA Research Institute and Society of Cardiovascular Patient Care; has been chair of the American Heart Association Quality Oversight Committee; has served on data monitoring committees for Duke Clinical Research Institute, Harvard Clinical Research Institute, Mayo Clinic, and the Population Health Research Institute; has received honoraria from the American College of Cardiology (senior associate editor, clinical trials and news, for acc.org), Belvoir Publications (editor in chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees), Harvard Clinical Research Institute (clinical trial steering committee), HMP Communications (editor in chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (guest editor, associate editor), Population Health Research Institute (clinical trial steering committee), Slack Publications (chief medical editor, Cardiology Today's Intervention), Society of Cardiovascular Patient Care (secretary/treasurer), and WebMD (CME steering committees); has served as deputy editor of Clinical Cardiology, as chair of the NCDR‐ACTION Registry Steering Committee, and as chair of the VA CART Research and Publications Committee; has received research funding from Amarin (for his role as chair of the steering committee and principal investigator of REDUCE‐IT), Amgen, AstraZeneca, Bristol‐Myers Squibb, Eisai, Ethicon, Forest Laboratories, Ischemix, Lilly, Medtronic, Pfizer, Roche, Sanofi‐Aventis, and The Medicines Company; has received royalties from Elsevier (editor, Cardiovascular Intervention: A Companion to Braunwald's Heart Disease); has served as site co‐investigator for Biotronik, Boston Scientific, and St. Jude Medical; has served as a trustee of the American College of Cardiology; and reports unfunded research with FlowCo, PLx Pharma, and Takeda. Dr. Steg has received research grants from Merck, Sanofi, and Servier and has received speaking or consulting fees from Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol‐Myers Squibb, CSL‐Behring, Daiichi‐Sankyo, GlaxoSmithKline, Janssen, Lilly, Merck, Novartis, Pfizer, Regeneron, Sanofi, Servier, and The Medicines Company. Dr. Brinton has received speaking and/or consulting honoraria from Alexion, Amarin, Amgen, Aralez, Arisaph, AstraZeneca, Janssen, Kastle, Kowa, Merck, PTS Diagnostics, Regeneron, and Sanofi‐Aventis and has received research funding from Amarin (for his role as steering committee member of REDUCE‐IT) and Kowa (for his role as steering committee member of PROMINENT). Dr. Jacobson has served as a consultant for Amarin, Amgen, AstraZeneca, Merck, Regeneron, and Sanofi and has done research for Amgen and Regeneron/Sanofi. Dr. Miller has served as a consultant for Amarin, Akcea, Gemphire, and Pfizer. Dr. Tardif has received research grants from Amarin, AstraZeneca, DalCor, Eli Lilly, Esperion, Merck, Pfizer, Sanofi, and Servier; has received honoraria from Amarin, AstraZeneca, DalCor, Sanofi, and Servier; and holds equity (modest position) in DalCor. Drs. Ketchum, Soni, Braeckman, and Juliano, and Mr. Doyle, are current or former Amarin employees and shareholders. Ms. Murphy has served as a consultant for Amarin and received honoraria from Merck. Dr. Ballantyne discloses grant/research support (all paid to the institution, not individual) from Amarin, Amgen, Eli Lilly, Esperion, Ionis, Novartis, Pfizer, Regeneron, Sanofi‐Synthelabo, the National Institutes of Health, the American Heart Association, and the American Diabetes Association, and has served as a consultant for Amarin, Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Esperion, Ionis, Matinas BioPharma Inc, Merck, Novartis, Pfizer, Regeneron, and Sanofi‐Synthelabo.
Bhatt DL, Steg PG, Brinton EA, Jacobson TA, Miller M, Tardif J‐C, Ketchum SB, Doyle RT Jr, Murphy SA, Soni PN, Braeckman RA, Juliano RA, Ballantyne CM and on behalf of the REDUCE‐IT Investigators . Rationale and design of REDUCE‐IT: Reduction of Cardiovascular Events with Icosapent Ethyl–Intervention Trial. Clin Cardiol. 2017;40:138–148. 10.1002/clc.22692
Funding information The trial is sponsored by Amarin Pharma Inc.
REFERENCES
- 1. Libby P. Triglycerides on the rise: should we swap seats on the seesaw? Eur Heart J. 2015;36:774–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Klempfner R, Erez A, Sagit BZ, et al. Elevated triglyceride level is independently associated with increased all‐cause mortality in patients with established coronary heart disease: twenty‐two‐year follow‐up of the Bezafibrate Infarction Prevention Study and Registry [published correction appears in Circ Cardiovasc Qual Outcomes. 2016;9:613]. Circ Cardiovasc Qual Outcomes . 2016;9:100–108. [DOI] [PubMed] [Google Scholar]
- 3. Nordestgaard BG. Triglyceride‐rich lipoproteins and atherosclerotic cardiovascular disease: new insights from epidemiology, genetics, and biology. Circ Res. 2016;118:547–563. [DOI] [PubMed] [Google Scholar]
- 4. Saito Y, Yokoyama M, Origasa H, et al; JELIS Investigators . Effects of EPA on coronary artery disease in hypercholesterolemic patients with multiple risk factors: sub‐analysis of primary prevention cases from the Japan EPA Lipid Intervention Study (JELIS) [published correction appears in Atherosclerosis. 2009;204:233]. Atherosclerosis . 2008;200:135–140. [DOI] [PubMed] [Google Scholar]
- 5. Guyton JR, Slee AE, Anderson T, et al. Relationship of lipoproteins to cardiovascular events: the AIM‐HIGH Trial (Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides and Impact on Global Health Outcomes). J Am Coll Cardiol. 2013;62:1580–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ginsberg HN, Elam MB, Lovato LC, et al; ACCORD Study Group . Effects of combination lipid therapy in type 2 diabetes mellitus [published correction appears in N Engl J Med. 2010;362:1748]. N Engl J Med . 2010;362:1563–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rubins HB, Robins SJ, Collins D, et al. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high‐density lipoprotein cholesterol. Veterans Affairs High‐Density Lipoprotein Cholesterol Intervention Trial Study Group. N Engl J Med . 1999;341:410–418. [DOI] [PubMed] [Google Scholar]
- 8. Keech A, Simes RJ, Barter P, et al; FIELD Study Investigators . Effects of long‐term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial [published correction appears in Lancet. 2006;368:1420]. Lancet . 2005;366:1849–1861. [DOI] [PubMed] [Google Scholar]
- 9. BIP Study Group . Secondary prevention by raising HDL cholesterol and reducing triglycerides in patients with coronary artery disease. Bezafibrate Infarction Prevention (BIP) study. Circulation. 2000;102:21–27. [DOI] [PubMed] [Google Scholar]
- 10. Manninen V, Tenkanen L, Koskinen P, et al. Joint effects of serum triglyceride and LDL cholesterol and HDL cholesterol concentrations on coronary heart disease risk in the Helsinki Heart Study: implications for treatment. Circulation. 1992;85:37–45. [DOI] [PubMed] [Google Scholar]
- 11. Frick MH, Elo O, Haapa K, et al. Helsinki Heart Study: primary‐prevention trial with gemfibrozil in middle‐aged men with dyslipidemia. Safety of treatment, changes in risk factors, and incidence of coronary heart disease. N Engl J Med . 1987;317:1237–1245. [DOI] [PubMed] [Google Scholar]
- 12. HPS2‐THRIVE Collaborative Group . HPS2‐THRIVE randomized placebo‐controlled trial in 25 673 high‐risk patients of ER niacin/laropiprant: trial design, pre‐specified muscle and liver outcomes, and reasons for stopping study treatment. Eur Heart J . 2013;34:1279–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Armitage J; on behalf of the HPS2‐THRIVE Collaborative Group . HPS2‐THRIVE: randomized placebo‐controlled trial of ER niacin and laropiprant in 25 673 patients with pre‐existing cardiovascular disease. http://www.hps2‐thrive.org/hps2‐thrive_ACC_slides.ppt. Slides presented at: American College of Cardiology Annual Scientific Sessions; March 9–11, 2013; San Francisco, CA. Accessed January 23, 2017.
- 14. Landray MJ, Haynes R, Hopewell JC, et al; HPS2‐THRIVE Collaborative Group . Effects of extended‐release niacin with laropiprant in high‐risk patients. N Engl J Med . 2014;371:203–212. [DOI] [PubMed] [Google Scholar]
- 15. Boden WE, Probstfield JL, Anderson T, et al; AIM‐HIGH Investigators . Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy [published correction appears in N Engl J Med. 2012;367:189]. N Engl J Med . 2011;365:2255–2267. [DOI] [PubMed] [Google Scholar]
- 16. Yokoyama M, Origasa H, Matsuzaki M, et al; JELIS Investigators . Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open‐label, blinded endpoint analysis [published correction appears in Lancet. 2007;370:220]. Lancet . 2007;369:1090–1098. [DOI] [PubMed] [Google Scholar]
- 17. GISSI‐Prevenzione Investigators . Dietary supplementation with n‐3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI‐Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico [published corrections appear in Lancet. 2001;357:642 and 2007;369:106]. Lancet . 1999;354:447–455. [PubMed] [Google Scholar]
- 18. Tavazzi L, Maggioni AP, Marchioli R, et al; GISSI‐HF Investigators . Effect of n‐3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI‐HF trial): a randomised, double‐blind, placebo‐controlled trial. Lancet . 2008;372:1223–1230. [DOI] [PubMed] [Google Scholar]
- 19. Wu JH, Mozaffarian D. Omega‐3 fatty acids, atherosclerosis progression and cardiovascular outcomes in recent trials: new pieces in a complex puzzle. Heart. 2014;100:530–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kromhout D, Giltay EJ, Geleijnse JM; Alpha Omega Trial Group . n‐3 fatty acids and cardiovascular events after myocardial infarction. N Engl J Med . 2010;363:2015–2026. [DOI] [PubMed] [Google Scholar]
- 21. Bosch J, Gerstein HC, Dagenais GR, et al; ORIGIN Trial Investigators . n‐3 fatty acids and cardiovascular outcomes in patients with dysglycemia. N Engl J Med . 2012;367:309–318. [DOI] [PubMed] [Google Scholar]
- 22. Rauch B, Schiele R, Schneider S, et al; OMEGA Study Group . OMEGA, a randomized, placebo‐controlled trial to test the effect of highly purified omega‐3 fatty acids on top of modern guideline‐adjusted therapy after myocardial infarction. Circulation. 2010;122:2152–2159. [DOI] [PubMed] [Google Scholar]
- 23. Risk and Prevention Study Collaborative Group . n‐3 fatty acids in patients with multiple cardiovascular risk factors [published correction appears in N Engl J Med. 2013;368:2146]. N Engl J Med . 2013;368:1800–1808. [DOI] [PubMed] [Google Scholar]
- 24. Galan P, Kesse‐Guyot E, Czernichow S, et al. Effects of B vitamins and omega 3 fatty acids on cardiovascular diseases: a randomised placebo controlled trial. BMJ. 2010;341:c6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bays HE, Ballantyne CM, Kastelein JJ, et al. Eicosapentaenoic acid ethyl ester (AMR101) therapy in patients with very high triglyceride levels (from the Multi‐center, Placebo‐controlled, Randomized, Double‐blind, 12‐week study with an open‐label Extension [MARINE] trial). Am J Cardiol. 2011;108:682–690. [DOI] [PubMed] [Google Scholar]
- 26. Ballantyne CM, Bays HE, Kastelein JJ, et al. Efficacy and safety of eicosapentaenoic acid ethyl ester (AMR101) therapy in statin‐treated patients with persistent high triglycerides (from the ANCHOR study). Am J Cardiol. 2012;110:984–992. [DOI] [PubMed] [Google Scholar]
- 27. Ballantyne CM, Bays HE, Philip S, et al. Icosapent ethyl (eicosapentaenoic acid ethyl ester): effects on remnant‐like particle cholesterol from the MARINE and ANCHOR studies. Atherosclerosis. 2016;253:81–87. [DOI] [PubMed] [Google Scholar]
- 28. Ballantyne CM, Bays HE, Braeckman RA, et al. Icosapent ethyl (eicosapentaenoic acid ethyl ester): effects on plasma apolipoprotein C‐III levels in patients from the MARINE and ANCHOR studies. J Clin Lipidol . 2016;10:635.e1–645.e1. [DOI] [PubMed] [Google Scholar]
- 29. Bays HE, Ballantyne CM, Braeckman RA, et al. Icosapent ethyl, a pure ethyl ester of eicosapentaenoic acid: effects on circulating markers of inflammation from the MARINE and ANCHOR studies. Am J Cardiovasc Drugs. 2013;13:37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Muhammad KI, Morledge T, Sachar R, et al. Treatment with ω‐3 fatty acids reduces serum C‐reactive protein concentration. Clin Lipidol. 2011;6:723–729. [Google Scholar]
- 31. Niki T, Wakatsuki T, Yamaguchi K, et al. Effects of the addition of eicosapentaenoic acid to strong statin therapy on inflammatory cytokines and coronary plaque components assessed by integrated backscatter intravascular ultrasound. Circ J. 2016;80:450–460. [DOI] [PubMed] [Google Scholar]
- 32. Nishio R, Shinke T, Otake H, et al. Stabilizing effect of combined eicosapentaenoic acid and statin therapy on coronary thin‐cap fibroatheroma. Atherosclerosis. 2014;234:114–119. [DOI] [PubMed] [Google Scholar]
- 33. Nosaka K, Miyoshi T, Iwamoto M, et al. Early initiation of eicosapentaenoic acid and statin treatment is associated with better clinical outcomes than statin alone in patients with acute coronary syndromes: 1‐year outcomes of a randomized controlled study. Int J Cardiol. 2017;228:173–179. [DOI] [PubMed] [Google Scholar]
- 34. Bays HE, Ballantyne CM, Doyle RT Jr, et al. Icosapent ethyl: eicosapentaenoic acid concentration and triglyceride‐lowering effects across clinical studies. Prostag Oth Lipid M. 2016;125:57–64. [DOI] [PubMed] [Google Scholar]
- 35. Reduction of Cardiovascular Events With EPA–Intervention Trial (REDUCE‐IT) . http://www.clinicaltrials.gov NCT01492361.
- 36.Outcomes Study to Assess Statin Residual Risk Reduction With EpaNova in High CV Risk Patients With Hypertriglyceridemia (STRENGTH). http://www.clinicaltrials.gov NCT02104817.
- 37.Pemafibrate to Reduce Cardiovascular Outcomes by Reducing Triglycerides In Diabetic Patients (PROMINENT). http://www.prnewswire.com/news‐releases/landmark‐trial‐entitled‐prominent‐to‐explore‐the‐prevention‐of‐heart‐disease‐in‐diabetic‐patients‐with‐high‐triglycerides‐and‐low‐hdl‐c‐300201581.html. Accessed January 21, 2017.
- 38.Vitamin D and Omega‐3 Trial (VITAL). http://www.clinicaltrials.gov NCT01169259.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPORTING APPENDIX


